Significance
Social cognition critically impacts the development, progression, and treatment of psychiatric disorders. However, social cognition skills are insufficiently targeted by current treatment approaches. By applying a multimodal brain imaging strategy, the present study demonstrated the importance of the serotonin 2A/1A receptor system in the modulation of social exclusion processing. Understanding the biochemical underpinnings of the social rejection experience is important for increasing our knowledge about social/emotional processing and the related neural responses. The identification of relevant neural responses is in turn crucial for the efficacious management of disorders influenced by social factors. Our findings may help to diminish a knowledge gap that currently restrains the development of pharmacotherapies for sociocognitive deficits in psychiatric disorders.
Keywords: social cognition, serotonin, psilocybin, functional magnetic resonance imaging, magnetic resonance spectroscopy
Abstract
Social ties are crucial for physical and mental health. However, psychiatric patients frequently encounter social rejection. Moreover, an increased reactivity to social exclusion influences the development, progression, and treatment of various psychiatric disorders. Nevertheless, the neuromodulatory substrates of rejection experiences are largely unknown. The preferential serotonin (5-HT) 2A/1A receptor agonist, psilocybin (Psi), reduces the processing of negative stimuli, but whether 5-HT2A/1A receptor stimulation modulates the processing of negative social interactions remains unclear. Therefore, this double-blind, randomized, counterbalanced, cross-over study assessed the neural response to social exclusion after the acute administration of Psi (0.215 mg/kg) or placebo (Pla) in 21 healthy volunteers by using functional magnetic resonance imaging (fMRI) and resting-state magnetic resonance spectroscopy (MRS). Participants reported a reduced feeling of social exclusion after Psi vs. Pla administration, and the neural response to social exclusion was decreased in the dorsal anterior cingulate cortex (dACC) and the middle frontal gyrus, key regions for social pain processing. The reduced neural response in the dACC was significantly correlated with Psi-induced changes in self-processing and decreased aspartate (Asp) content. In conclusion, 5-HT2A/1A receptor stimulation with psilocybin seems to reduce social pain processing in association with changes in self-experience. These findings may be relevant to the normalization of negative social interaction processing in psychiatric disorders characterized by increased rejection sensitivity. The current results also emphasize the importance of 5-HT2A/1A receptor subtypes and the Asp system in the control of social functioning, and as prospective targets in the treatment of sociocognitive impairments in psychiatric illnesses.
Dysfunctional social cognition represents a central characteristic of various psychiatric disorders and critically impacts the development, progression, and treatment of psychiatric illnesses (1–3). Impairments in social cognition are leading causes of disability and compromise real-world functioning, including independent living and productivity at work (2, 4, 5). However, the neuronal and pharmacological bases of both normal and dysfunctional social cognition lack sufficient investigation, severely limiting current treatment approaches (1, 2). Given the broad clinical relevance of dysfunctional social cognition in diverse psychiatric disorders, a better understanding of the neurobiological foundations of social cognition is urgently required for the development of novel and targeted therapies (6).
Pharmacological neuroimaging offers the opportunity to investigate the roles of specific neurotransmitter and receptor systems in a constrained hypothesis-driven manner (7, 8). Recent evidence suggests that the serotonin [5-hydroxytryptamine (5-HT)] system encompassing 14 subtypes of 5-HT receptors not only plays a key role in the regulation of mood, affect, learning, and memory (2, 9, 10), but is also implicated in social cognition (11, 12). Psilocybin [4-phosphoryloxy-N,N-dimethyltryptamine (Psi)] is a serotonergic hallucinogen that induces an altered state of consciousness characterized by changes in sensory perception, emotion, thought, and the sense of self in a dose-dependent manner (13). Psi binds with high affinity to 5-HT1A, 5-HT2A/C, 5-HT6, and 5-HT7 receptors (Psychoactive Drug Screening Program database at kidbdev.med.unc.edu/databases/kidb.php). In humans, Psi is rapidly dephosphorylated to psilocin (4-N,N-dimethyltryptamine), which acts as a partial agonist at 5-HT2A and 5-HT1A receptors (14, 15). Therefore, the use of Psi provides a distinctive opportunity to explore the relative contribution of 5-HT receptors to social cognition.
Notably, Psi modulates neural activity in prefrontal brain areas involved in social cognition (16–18). In addition, recent evidence suggests that Psi at moderate doses can enhance mood and attenuate the processing of negative emotional stimuli (e.g., negative facial expressions or threat-related scenes) (10, 19–21) via 5-HT2A receptor activation (20). Thus, Psi may have antidepressant properties (10, 19–22). Nevertheless, it is unclear whether this shift in emotional processing translates into the social domain, particularly regarding negative social interaction processing, and is therefore of relevance to real-life functioning in patients suffering from psychiatric disorders. To date, research efforts into the effects of Psi on emotional processing and cognition have solely focused on the individual’s response to external stimulus manipulations (10, 20), as opposed to truly interactive, real-time social encounters. Indeed, studies investigating the neuropharmacological and neurochemical substrates of social interaction processing are rare.
The present study set out to assess the effect of 5-HT2A/1A receptor stimulation by Psi (0.215 mg/kg orally) vs. placebo (Pla) on social interaction processing via a multimodal brain imaging approach. Specifically, we used functional magnetic resonance imaging (fMRI) and proton magnetic resonance spectroscopy (1H-MRS) to investigate the processing of ostracism generated by an interactive paradigm termed “Cyberball” (23). Notably, an increased reactivity to social exclusion is clinically relevant in depression, borderline personality disorder, social anxiety disorder, and other psychiatric disorders (24–26). Given previous reports of the capacity of Psi to attenuate negative stimulus processing (10, 20), we predicted a reduced response to social exclusion after Psi administration.
“Social pain,” the painful feelings resulting from social exclusion, rejection, or loss (27), is consistently associated with increased brain activity. The increased activity is primarily observed in the anterior cingulate cortex (ACC) (17), but is also seen in the insula, the inferior orbitofrontal cortex (OFC), and the middle frontal gyrus (MFG) (28, 29). Therefore, we hypothesized that these social pain-related brain regions would show less pronounced activation after Psi treatment. Furthermore, given that 5-HT1A receptor stimulation has been associated with decreased, and 5-HT2A receptor stimulation has been associated with both decreased and increased, neuronal excitation of medial prefrontal neurons at rest (30–32), we also used 1H-MRS to determine whether Psi modulates the concentration of excitatory neurotransmitters and/or neurometabolic markers in the ACC. Our findings, presented below, assist in understanding the neurobiology of social processes relevant to the psychopathology of psychiatric disorders and contribute to a better mechanistic view of social cognition.
Results
Subjective Effects and Physical Effects.
An ANOVA (treatment × scale) was conducted for the Altered States of Consciousness (5D-ASC) questionnaire, and revealed significant main effects for treatment [F(1, 20) = 93.54, P < 0.001] and scale [F(10, 200) = 18.98, P < 0.001] and a significant interaction of treatment × scale [F(10, 200) = 19.67, P < 0.001]. Simple main effect analyses showed increased ratings on all 5D-ASC scales after Psi vs. Pla treatment (all P < 0.05; Fig. S1). No order effects with regard to the sequence of substance administration were observed (SI Results). For Positive and Negative Affect Schedule (PANAS) ratings, see SI Results and Fig. S2. Systolic and diastolic blood pressure as well as pulse were slightly but significantly increased after Psi administration compared with Pla (all P < 0.05; Table S1).
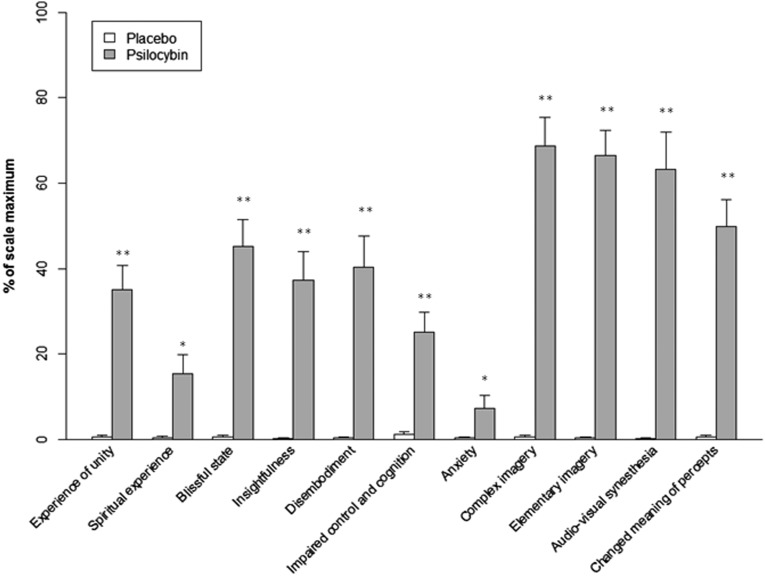
Fig. S1.
Effect of Psi and Pla on 5D-ASC scores. Scores are expressed as a percent of the scale maximum. Ratings on all scales were significantly increased after Psi compared with Pla treatment. Data are expressed as means + the SEM (n = 21 subjects). Asterisks indicate significant differences between Psi and Pla conditions (**P < 0.001; *P < 0.05).
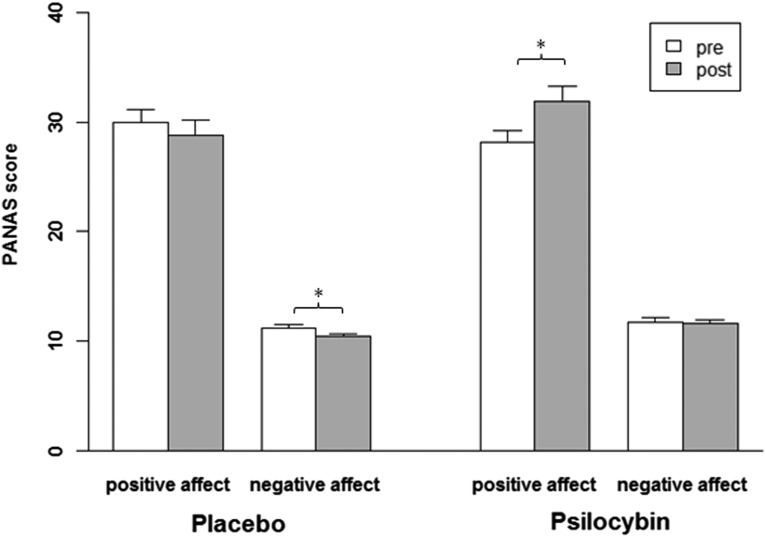
Fig. S2.
Effect of Psi and Pla on mood state as assessed by PANAS. Mood state was assessed 75 min before (pre) and 360 min after (post) each drug treatment. Negative mood affects were significantly reduced after Pla treatment, whereas positive mood affects were increased after Psi treatment. Data are expressed as means + the SEM. Asterisks indicate significant differences between pre- and posttreatment conditions (*P < 0.05, n = 20).
Table S1.
Physical effects
| Physical effects | Pla | Psi | t value | df | P value |
| Systolic blood pressure (mm Hg) | 114.67 (14.40) | 131.76 (13.45) | −7.46 | 20 | <0.001 |
| Diastolic blood pressure (mm Hg) | 75.57 (12.48) | 83.14 (11.90) | −2.69 | 20 | 0.01 |
| Pulse | 65.38 (12.50) | 70.62 (12.53) | −2.15 | 20 | 0.04 |
Significant P values are shown in boldface type. Means and SDs are given in parentheses. Pla, placebo; Psi, psilocybin.
Cyberball Task.
Posttask questionnaire.
Participants reported a reduced feeling of exclusion after Psi vs. Pla treatment [t(20) = 2.71, P < 0.01; Fig. 1A]. Additional posttask questionnaire (PTQ) items revealed no significant differences between Psi and Pla conditions (all P > 0.1). In particular, participants accurately gauged the number of throws received in each run, indicating equal awareness of exclusion under both treatment conditions (Table S2).
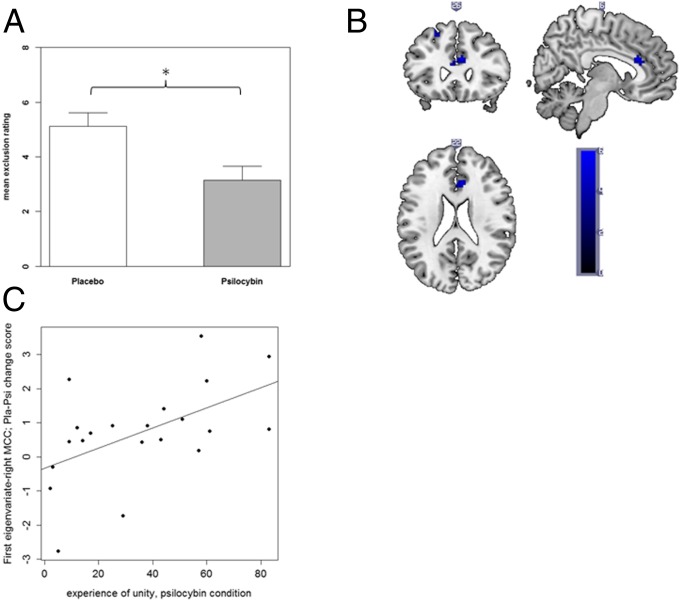
Fig. 1.
(A) Social exclusion feelings were significantly reduced after Psi vs. Pla administration. Data are expressed as means + the SEM (n = 21 subjects). Asterisks indicate significant differences between Psi and Pla conditions (*P < 0.05). (B) fMRI activation data for Pla > Psi in the “not receiving ESE > receiving INCL” contrast. Blue shades represent significantly reduced activation in the Psi condition displayed at uncorrected P < 0.001 at peak aMCC voxel (x = 6, y = 26, z = 22). (C) Positive association between the experience of unity assessed by the 5D-ASC questionnaire and right MCC activation in the “not receiving ESE > receiving INCL” contrast (r = 0.53, P < 0.02, n = 21; first eigenvariate; Pla–Psi change score).
Table S2.
PTQ results
| Item | Pla | Psi | t value | df | P value |
| Self-esteem (1–9) | 6.14 (1.39) | 6.62 (2.13) | −0.90 | 20 | 0.38 |
| Meaningful existence (1–9) | 1.76 (1.34) | 1.81 (1.86) | −0.15 | 20 | 0.88 |
| Control (1–9) | 7.38 (1.24) | 7.71 (1.10) | −1.05 | 20 | 0.31 |
| Belongingness (1–9) | 5.00 (2.00) | 5.71 (2.53) | −1.09 | 20 | 0.29 |
| Feeling of exclusion (1–9) | 5.12 (2.27) | 3.14 (2.39) | 2.71 | 20 | 0.01 |
| Feeling of inclusion (1–9) | 5.40 (1.71) | 5.83 (2.09) | −0.77 | 20 | 0.45 |
| Received throws (ISE) | 0.00 (0.00) | 1.67 (4.83) | −1.58 | 20 | 0.13 |
| Received throws (INCL) | 22.71 (9.59) | 26.00 (21.53) | −0.90 | 20 | 0.38 |
| Received throws (ESE) | 4.53 (2.69) | 8.14 (11.01) | −1.72 | 20 | 0.10 |
| Liking of left player (1–5) | 3.90 (0.83) | 4.14 (0.85) | −1.42 | 20 | 0.17 |
| Liking of right player (1–5) | 3.62 (0.67) | 3.71 (1.06) | −0.35 | 20 | 0.73 |
| Liked by left player (1–5) | 3.57 (0.68) | 3.76 (0.70) | −1.07 | 20 | 0.30 |
| Liked by right player (1–5) | 3.38 (0.67) | 3.52 (0.87) | −0.50 | 20 | 0.62 |
Significant P values are shown in boldface type. Means and SDs are given in parentheses. ESE, explicit social exclusion; INCL, inclusion; ISE, implicit social exclusion; Pla, placebo; Psi, psilocybin; PTQ, posttask questionnaire.
fMRI data.
The “not receiving explicit social exclusion (ESE) > receiving inclusion (INCL)” contrast in the Pla condition revealed significant activation in brain regions of interest (ROIs) commonly associated with social exclusion processing: the bilateral anterior midcingulate cortex (aMCC), bilateral posterior MCC (pMCC), left inferior OFC, and bilateral MFG (see Table S3 for whole-brain results). To assess whether social exclusion was processed differently after Psi vs. Pla administration, we compared the “not receiving ESE > receiving INCL” contrast between Pla and Psi conditions. Brain activation was significantly less pronounced in the right aMCC and left MFG for Psi vs. Pla (Fig. 1B; see Table S4 for whole-brain results). Because these areas have been shown to represent key regions for social exclusion processing, these results suggest that Psi administration reduced the processing of social pain. No significant differences in activation were found for the inverse comparison (Psi > Pla) for the “not receiving ESE > receiving INCL” contrast.
Table S3.
Whole-brain analysis under Pla condition for the contrasts not receiving ESE > receiving INCL, not receiving ISE > receiving INCL, and not receiving INCL > receiving INCL
| Brain region | Hemisphere | x | y | z | k | t |
| Not receiving ESE > receiving INCL | ||||||
| Anterior midcingulate cortex | R | 6 | 23 | 19 | 13 | 4.66* |
| Anterior midcingulate cortex | L | 0 | 26 | 19 | 10 | 4.52* |
| Posterior midcingulate cortex | L | 0 | −37 | 43 | 11 | 4.49* |
| Posterior midcingulate cortex | R | 3 | −40 | 43 | 7 | 4.45* |
| Inferior orbitofrontal cortex | L | −54 | 35 | −5 | 20 | 4.91* |
| Middle frontal gyrus | R | 48 | 44 | 7 | 57 | 5.17* |
| Middle frontal gyrus | R | 33 | 23 | 49 | 5 | 4.03 |
| Middle frontal gyrus | L | −39 | 44 | 1 | 13 | 4.97* |
| Middle frontal gyrus | L | −24 | 23 | 52 | 94 | 6.38* |
| Caudate nucleus | R | 15 | 2 | 19 | 6 | 4.35 |
| Temporal pole | R | 27 | 5 | −20 | 8 | 4.46 |
| Fusiform gyrus | L | −39 | −67 | −20 | 5 | 3.95 |
| Inferior occipital gyrus | R | 45 | −79 | −8 | 46 | 4.80 |
| Inferior frontal gyrus | L | −48 | 47 | −2 | 117 | 5.61 |
| Putamen | R | 27 | −16 | 4 | 64 | 5.55 |
| Putamen | L | −27 | −19 | 4 | 6 | 4.68 |
| Superior occipital gyrus | L | −15 | −91 | 4 | 82 | 5.55 |
| Middle temporal gyrus | L | −57 | −64 | −2 | 4 | 3.70 |
| Middle occipital gyrus | L | −48 | −76 | 4 | 17 | 4.38 |
| Cuneus | R | 18 | −85 | 7 | 16 | 4.61 |
| Precuneus | L | −6 | −52 | 13 | 7 | 4.48 |
| Superior frontal gyrus | L | −6 | 65 | 19 | 19 | 4.38 |
| Supramarginal gyrus | R | 63 | −46 | 31 | 40 | 4.99 |
| Inferior parietal lobule | L | −51 | −46 | 37 | 43 | 4.55 |
| Angular gyrus | L | −42 | −67 | 37 | 5 | 3.72 |
| Paracentral lobule | L | −15 | −31 | 64 | 29 | 4.97 |
| Postcentral gyrus | R | 15 | −34 | 67 | 11 | 4.73 |
| Not receiving ISE > receiving INCL | ||||||
| Anterior midcingulate cortex | L | −3 | 20 | 22 | 93 | 5.17* |
| Pregenual anterior cingulate cortex | R | 3 | 47 | 19 | 82 | 5.54* |
| Posterior midcingulate cortex | L | 0 | −40 | 40 | 19 | 4.39* |
| Posterior midcingulate cortex | R | 3 | −43 | 37 | 9 | 4.12 |
| Middle frontal gyrus | L | −18 | 23 | 49 | 54 | 3.94 |
| Middle frontal gyrus | R | 48 | 44 | 10 | 4 | 3.96 |
| Superior frontal gyrus | L | −21 | 50 | 25 | 7 | 4.37 |
| Inferior frontal gyrus | L | −48 | 38 | 4 | 36 | 5.42 |
| Inferior frontal gyrus | R | 48 | 35 | 13 | 21 | 4.46 |
| Temporal pole | R | 33 | 17 | −26 | 6 | 4.78 |
| Middle temporal gyrus | L | −57 | −46 | −8 | 56 | 5.22 |
| Cuneus | L | −15 | −91 | 4 | 19 | 4.53 |
| Precuneus | L | −6 | −58 | 22 | 85 | 4.50 |
| Postcentral gyrus | L | −48 | −10 | 19 | 7 | 3.92 |
| Postcentral gyrus | R | 30 | −28 | 64 | 7 | 4.22 |
| Precentral gyrus | R | 18 | −31 | 67 | 43 | 4.29 |
| Angular gyrus | L | −45 | −70 | 34 | 109 | 5.29 |
| Not receiving INCL > receiving INCL | ||||||
| Subgenual anterior cingulate cortex | L | 0 | 20 | −8 | 8 | 5.28* |
| Pregenual anterior cingulate cortex | L | −3 | 44 | 13 | 7 | 4.16 |
| Posterior midcingulate cortex | L | 0 | −40 | 43 | 8 | 4.19 |
| Middle frontal gyrus | R | 48 | 47 | 7 | 8 | 4.76 |
| Middle frontal gyrus | L | −21 | 29 | 43 | 11 | 3.92 |
| Inferior orbitofrontal cortex | L | −51 | 29 | −5 | 8 | 4.00 |
| Medial orbitofrontal cortex | R | 6 | 50 | −5 | 5 | 3.97 |
| Superior frontal gyrus | L | −15 | 38 | 49 | 102 | 5.08 |
| Superior frontal gyrus | R | 27 | 23 | 55 | 25 | 4.41 |
| Temporal pole | L | −39 | 14 | −26 | 17 | 4.02 |
| Superior temporal gyrus | L | −45 | −1 | −11 | 12 | 4.68 |
| Inferior temporal gyrus | R | 51 | −70 | −5 | 21 | 4.47 |
| Middle temporal gyrus | L | −60 | −46 | −8 | 24 | 4.84 |
| Middle temporal gyrus | L | −57 | −61 | −2 | 26 | 4.41 |
| Middle temporal gyrus | R | 54 | −58 | 16 | 26 | 4.56 |
| Calcarine gyrus | R | 18 | −88 | 4 | 81 | 5.06 |
| Superior occipital gyrus | L | −12 | −91 | 4 | 86 | 6.22 |
| Superior medial gyrus | R | 6 | 56 | 10 | 80 | 4.84 |
| Precuneus | L | −6 | −58 | 16 | 5 | 3.84 |
| Angular gyrus | L | −42 | −67 | 31 | 42 | 4.54 |
| Angular gyrus | L | −39 | −73 | 40 | 5 | 4.52 |
| Superior parietal lobule | R | 30 | −67 | 55 | 13 | 4.93 |
| Paracentral lobule | L | −9 | −31 | 76 | 20 | 5.28 |
Statistical threshold: P < 0.001 (uncorrected), k > 3; *P < 0.05, FWE after SVC. MNI coordinates of peak voxels for each cluster are given. ESE, explicit social exclusion; FWE, family-wise error; INCL, inclusion; ISE, implicit social exclusion; MNI, Montreal Neurological Institute; Pla, placebo; SVC, small-volume correction.
Table S4.
Whole-brain analyses for the Pla > Psi comparison for the contrasts not receiving ESE > receiving INCL, not receiving ISE > receiving INCL, and not receiving INCL > receiving INCL
| Brain region | Hemisphere | x | y | z | k | t |
| Not receiving ESE > receiving INCL | ||||||
| Anterior midcingulate cortex | R | 6 | 26 | 22 | 34 | 5.79* |
| Anterior midcingulate cortex | L | 0 | 23 | 22 | 13 | 3.91 |
| Middle frontal gyrus | L | −27 | 44 | 7 | 27 | 4.98* |
| Middle frontal gyrus | L | −24 | 29 | 49 | 33 | 4.71 |
| Middle frontal gyrus | L | −33 | 59 | 7 | 8 | 3.83 |
| Middle frontal gyrus | L | −27 | −1 | 67 | 52 | 4.68 |
| Middle frontal gyrus | R | 30 | −4 | 64 | 18 | 4.22 |
| Caudate nucleus | L | −9 | 20 | −8 | 6 | 3.93 |
| Middle temporal gyrus | R | 54 | −61 | 13 | 26 | 4.01 |
| Middle temporal gyrus | R | 33 | −58 | 13 | 20 | 5.03 |
| Middle temporal gyrus | L | −57 | −64 | 1 | 37 | 4.15 |
| Thalamus | R | 21 | −28 | 1 | 21 | 5.34 |
| Putamen | R | 30 | −16 | 10 | 11 | 4.00 |
| Supramarginal gyrus | R | 57 | −43 | 34 | 22 | 5.23 |
| Inferior parietal lobule | L | −54 | −43 | 46 | 18 | 4.09 |
| Inferior parietal lobule | R | 45 | −37 | 46 | 15 | 4.45 |
| Postcentral gyrus | L | −48 | −31 | 52 | 15 | 4.07 |
| Not receiving ISE > receiving INCL | ||||||
| Pregenual anterior cingulate cortex | L | 0 | 47 | 16 | 8 | 4.33* |
| Anterior midcingulate cortex | R | 6 | 29 | 22 | 35 | 5.69* |
| Anterior midcingulate cortex | L | 0 | 23 | 25 | 12 | 4.15 |
| Posterior midcingulate cortex | L | 0 | −40 | 43 | 26 | 5.19* |
| Posterior midcingulate cortex | R | 3 | −40 | 43 | 7 | 4.42* |
| Middle frontal gyrus | L | −33 | 44 | 25 | 5 | 4.08 |
| Middle frontal gyrus | R | 30 | 17 | 55 | 6 | 4.24 |
| Precuneus | R | 24 | −55 | 43 | 5 | 3.99 |
| Precuneus | R | 33 | −70 | 40 | 6 | 3.98 |
| Fusiform gyrus | L | −45 | −61 | −17 | 4 | 3.90 |
| Middle temporal gyrus | R | 57 | −64 | −2 | 149 | 5.76 |
| Middle occipital gyrus | L | −48 | −73 | 1 | 96 | 5.79 |
| Thalamus | R | 21 | −31 | 7 | 10 | 4.07 |
| Calcarine gyrus | L | 0 | −94 | 13 | 10 | 5.46 |
| Calcarine gyrus | L | −12 | −52 | 10 | 39 | 5.10 |
| Superior temporal gyrus | R | 36 | −52 | 13 | 49 | 4.79 |
| Superior temporal gyrus | R | 66 | −31 | 19 | 11 | 5.59 |
| Inferior parietal lobule | L | −51 | −22 | 28 | 194 | 5.57 |
| Superior parietal lobule | L | −30 | −64 | 49 | 13 | 4.30 |
| Not receiving INCL > receiving INCL | ||||||
| Middle frontal gyrus | L | −30 | 14 | 58 | 6 | 4.45 |
| Inferior occipital gyrus | R | 39 | −85 | −8 | 17 | 4.71 |
| Middle temporal gyrus | L | −48 | −70 | 10 | 29 | 4.36 |
| Superior frontal gyrus | L | −21 | 5 | 70 | 4 | 3.92 |
Statistical threshold: P < 0.001 (uncorrected), k > 3; *P < 0.05, FWE after SVC. MNI coordinates of peak voxels for each cluster are given. ESE, explicit social exclusion; FWE, family-wise error; INCL, inclusion; ISE, implicit social exclusion; MNI, Montreal Neurological Institute; Pla, placebo; Psi, psilocybin; SVC, small-volume correction.
The “not receiving implicit social exclusion (ISE) > receiving INCL” contrast in the Pla condition revealed significant activation in the left anterior and posterior MCC and the right pregenual ACC (Table S3). Comparison of the “not receiving ISE > receiving INCL” contrast revealed significantly reduced activation in the bilateral anterior and posterior MCC and the left pregenual ACC in the Psi vs. Pla condition (Table S4). No significant differences in activation were found for the Psi > Pla comparison for the “not receiving ISE > receiving INCL” contrast.
Context-specific effects (33) were investigated by computing the “not receiving INCL > receiving INCL” contrast. This contrast revealed significant activation in the left subgenual ACC for Pla (Table S3). The same contrast showed no significant differences in activation for Psi vs. Pla administration (Table S4), indicating that Psi did not significantly modulate the processing of “not receiving the ball” without an exclusion context. Again, no significant differences in activation were found for the Psi > Pla comparison for the “not receiving INCL > receiving INCL” contrast.
Relationship Between Social Exclusion Processing and Subjective Effects.
A correlation analysis was conducted to evaluate the association between the difference between Pla > Psi conditions in (i) BOLD responses to the “not receiving ESE > receiving INCL” contrast in the right MCC and left MFG, and (ii) subjective drug effects (5D-ASC, PANAS) and PTQ items. A significant positive correlation was found between the difference in activation in the right MCC and the 5D-ASC scale “experience of unity“ in the Psi condition (r = 0.53, P < 0.02; Fig. 1C). No other significant correlations between BOLD responses and subjective drug effects were observed (all P > 0.08), and no significant correlations were found between differences in BOLD responses and differences in PANAS scores or PTQ items (all P > 0.1).
Relationship Between Social Exclusion Processing and Metabolite Concentrations.
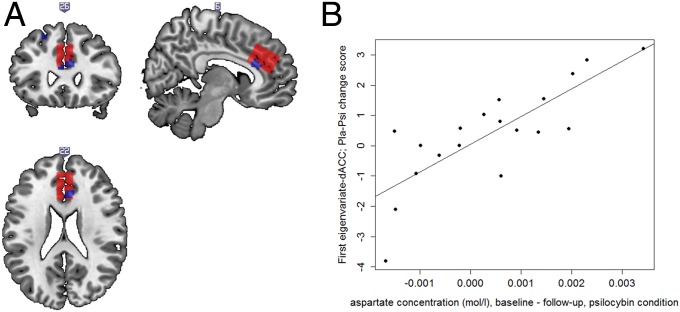
Brain region activation after Psi treatment was significantly reduced in response to the “not receiving ESE > receiving INCL” contrast in the aMCC, and overlapped with the dorsal ACC (dACC) voxel established for MRS acquisition (Fig. 2A). To explore the relationship between Psi-induced differences in social exclusion processing and metabolite concentrations, correlational analyses were computed for the difference in activity between the Pla and Psi conditions in the dACC voxel used for MRS measurement and the baseline-corrected metabolite concentration in the Psi condition as well as the metabolite concentrations in the Psi follow-up measurement. A significant correlation was obtained between activity in the dACC voxel computed for the “not receiving ESE > receiving INCL” contrast and baseline-corrected aspartate (Asp) concentration (r = 0.80, P < 0.001, n = 19; Fig. 2B). Congruently, a significant correlation was observed between Asp concentration in the Psi follow-up measurement and activity in the dACC voxel (r = −0.56, P < 0.02, n = 19). No significant correlations were found for other baseline-corrected or follow-up metabolite concentrations (all P > 0.17). For data and fit quality measures, see SI Results, MRS Data Quality and Fig. S3.
Fig. 2.
(A) Overlap between activation for Pla > Psi in the “not receiving ESE > receiving INCL” contrast and the voxel established for MRS acquisition (red box). Blue shades represent significantly reduced activation in the Psi condition displayed at uncorrected P < 0.001. (B) Positive association between BOLD responses in the “not receiving ESE > receiving INCL” contrast (first eigenvariate extracted from the dACC ROI established for MRS acquisition; Pla–Psi change score) and changes in Asp concentration (baseline to follow-up) in the Psi condition (r = 0.80, P < 0.001, n = 19).
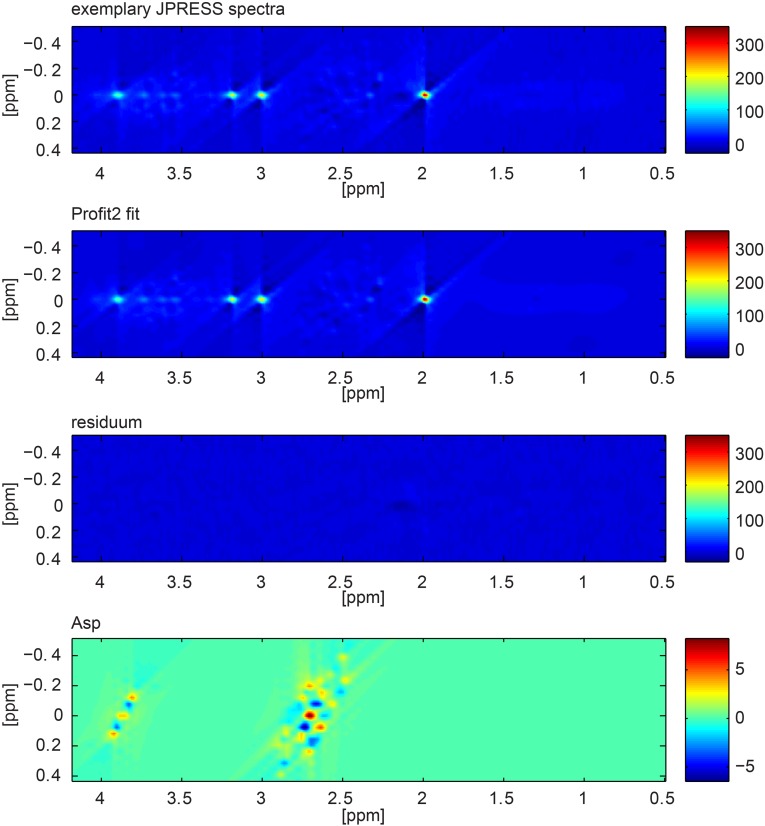
Fig. S3.
Two-dimensional JPRESS data from one exemplary participant, the Profit2 fit, the residuum of the fit, and the Asp component.
SI Results
5D-ASC.
To test for potential order effects with regard to the sequence of substance administration, MANOVAs were performed for the ratings on the 11 scales of the 5D-ASC in the Psi and the Pla condition. The analyses did not reveal significant differences in the experience of drug effects between participants who received Psi in the first session and those who received Psi in the second session [Psi condition ratings: F(11, 9) = 1.18, P > 0.40; Pla condition ratings: F(7, 13) = 0.76, P > 0.62].
PANAS.
A repeated-measures ANOVA (time × treatment × scale) for the PANAS revealed a significant main effect for scale [F(1, 19) = 307.83, P < 0.001], indicative of higher ratings on the positive affect scale than on the negative affect scale. Furthermore, significant interactions were obtained for time × treatment [F(1, 19) = 13.85, P < 0.01] and time × treatment × scale [F(1, 19) = 8.67, P < 0.01]. Simple main effect analyses showed that negative affect was significantly reduced after Pla treatment (P < 0.01), whereas positive affect was increased after Psi treatment (P < 0.05; Fig. S2). No other main effects or interactions reached significance (all P > 0.05). PANAS scores of one subject could not be analyzed due to missing data.
MRS Data Quality.
No artifacts were identified in the analyzed 2D plots and in the projections of the JPRESS spectra by the visual artifact inspection. N-acetylaspartate, creatine, choline, glutathione, Asp, Glu, glutamine, glycine, myo-inositol, scyllo-inositol, taurine, and ascorbate passed the stringent Cramer Rao lower bounds (CRLBs) quality criteria (all CRLBs <20% and mean CRLBs <15%). Mean CRLBs of glucose, γ-aminobutyric acid, lactate, N-acetylaspartylglutamate, and phosphoethanolamine were also below 15%, but some outliers exceeded 20%. Mean and SD of CRLBs of Asp of all measurements were 6.10 ± 0.87% (Psi) and 5.99 ± 0.79% (Pla). Fig. S3 shows the 2D JPRESS data from one exemplary participant, the Profit2 fit, the residuum of the fit and the Asp component.
Discussion
The present study used a multimodal brain imaging approach to show that the processing of social pain is reduced after 5-HT2A/1A receptor stimulation by Psi; furthermore, this reduced response to social exclusion involved changes in cingulate Asp concentrations and changes in the experience of self. In agreement with previous studies of social exclusion and rejection (28, 29, 33–35; for reviews see refs. 27 and 36), we demonstrated that experiences of social exclusion vs. inclusion in the Pla condition involved the aMCC, pMCC, MFG, and inferior OFC. These brain areas are commonly associated with feelings of exclusion. Importantly, 5-HT2A/1A receptor stimulation by Psi significantly reduced activation of the aMCC and MFG in response to social exclusion. The aMCC, also termed the dACC (17, 37), represents a key region in social exclusion processing (17, 36). In particular, dACC activation is related to experiences of fear and anxiety, emotional distress, and social pain (17, 29, 37). Consistent with these observations, the current findings strongly suggest that Psi decreases the experience of social pain. Moreover, the frontal cortex also plays a regulatory role in social exclusion processing (29, 38). More specifically, activation in the MFG can lead to inhibition of affective distress and social pain (29, 39). Because Psi seems to reduce affective distress following social rejection, the decreased BOLD signal in the MFG observed herein after Psi administration possibly stemmed from a diminished need to block stressful experiences.
Here, implicit social exclusion relative to inclusion significantly recruited the aMCC, pMCC, and ventral ACC in the Pla condition. Activation in the MFG was not significant for ISE, contrary to ESE. This result supports previous findings showing increased ACC activity, but not self-regulatory responses, in frontal areas for ISE (29). Psi treatment reduced the response to ISE in the aMCC, pMCC, and ventral ACC, suggesting that Psi diminishes reactions to social exclusion even in an implicit, and therefore possibly more subtle, context. To investigate whether Psi-induced alterations in neural responses to the event of “not receiving the ball” are attributable to this specific event or are instead sensitive to the game context, we also analyzed the “not receiving > receiving the ball” contrast while participants were included in the game (33). In line with a previous study applying an event-related analysis to the Cyberball paradigm (33), this contrast recruited the subgenual ACC in the Pla condition. However, no significant differences were found between Pla and Psi for this contrast, indicating that Psi modulates the response to “not receiving the ball” only in conditions where the participant is actually excluded from the game, corroborating the interpretation that 5-HT2A/1A receptor stimulation by Psi apparently weakens the distressing affective response to social rejection.
In agreement with a decreased neural response to social exclusion, participants reported that they felt less excluded in the Psi vs. Pla condition on the PTQ. However, other ratings (e.g., self-esteem, meaningful existence, control, inclusion, belongingness, and liking) were not significantly altered by Psi; this suggests that Psi-mediated stimulation of the 5-HT2A/1A receptor may specifically reduce the feeling of being excluded, which is apparently not influenced by other social parameters. Additionally, no significant differences were found between Pla and Psi conditions for estimates of received throws, indicating that participants were equally aware of exclusion in both treatment conditions. Therefore, the reduced response to social exclusion is unlikely due to decreased attentiveness to the exclusion.
In the present study, the Psi-induced alterations on the 5D-ASC and increased scores for basic positive mood were generally similar to those observed in previous studies using comparable drug doses (10, 22). The low ratings on the 5D-ASC scale “anxiety” (mean score: 7.5%, maximum score: 46%) are in accordance with previous studies suggesting that even during peak effects Psi is well tolerated and rarely produces profound or psychotic anxiety in a controlled clinical setting in healthy human subjects (13). Moreover, the Psi-induced reduction in dACC activity in response to social exclusion showed significant correlations with scores for the feeling of unity. The 5D-ASC feeling of unity scale incorporates items assessing alterations in the sense of self/self-boundaries, such as feelings of being connected and/or one with the environment (40). The correlation between experience of unity and social pain processing may indicate that alterations in the sense of self after Psi administration are important for changes in social interaction processing, adding empirical evidence to the notion that concepts of self and others are closely intertwined (41), which suggests that the sense of self can profoundly impact the experience of interpersonal relationships.
The Psi-induced feeling of being connected with and embedded in the environment may also lead to an intensified connection between oneself and other human beings, as well as stronger identification and empathic encounters with others. This feeling may assist in reducing egocentric bias and consequently render negative experiences more bearable. This interpretation is also supported by previous studies reporting that the ACC is particularly involved in self/other representations and theory of mind (42). Moreover, the Psi-mediated feeling of being embedded in the environment may even be beneficial in therapeutic settings, supporting the relationship between patient and therapist, and facilitating the discussion of painful experiences in a protective and secure environment. In contrast to earlier work investigating the effect of Psi on nonsocial negative stimuli (10), the current data did not reveal any significant associations between BOLD responses and social exclusion or mood. Therefore, modulations of social interaction processing may be mostly independent of Psi-induced increases in positive mood.
To further examine the neurochemical substrates of altered social exclusion processing, changes in metabolite concentrations obtained by 1H-MRS measurements were correlated with changes in the BOLD signal responses to social exclusion. These analyses showed that a reduction in Asp content between baseline (before drug administration) and follow-up (Psi condition, measured after subjects had completed the Cyberball task) was significantly correlated with a reduced BOLD signal in the dACC in response to social exclusion (Psi vs. Pla condition). Even though the complex resonance pattern of Asp showing overlaps with various other metabolites is difficult to quantify with in vivo MRS, the used JPRESS acquisition together with Profit2 fitting allowed an Asp quantification with CRLBs around 6% and low SD, indicating good and homogeneous data quality. Together, these findings may indicate that changes in Asp levels are related to social pain processing.
Glutamate (Glu) and Asp are present in high concentrations in the central nervous system; both have excitatory effects on neurons, with Asp preferentially activating NMDA receptors (43). Moreover, functionally or pharmacologically induced changes in neurotransmission and brain energy metabolism underlying the origin of the BOLD signal are linked to changes in Glu and Asp concentrations as a consequence of an increased rate of the malate–aspartate shuttle, which is associated with the increased flux into the tricarboxylic acid cycle (44, 45). Interestingly, the structurally related psychotropic 5-HT2A/1A agonist lysergic acid diethylamide increases extracellular medial prefrontal Glu release and prefrontal pyramidal cell activity in rodents (46, 47). However, in our study, BOLD reductions to social exclusion in the dACC were related to changes in Asp content exclusively, and no correlations with changes in Glu concentrations were found. Hence, the interpretation of our findings remains somewhat speculative because the effects of Psi on Asp release are currently novel and have not yet been reported in animal and human studies and therefore require further investigation. Because extrasynaptic and intrasynaptic pools of Asp and Glu cannot be distinguished by spectroscopic imaging, no general conclusions can be drawn from metabolite concentrations to the process of neurotransmission. However, recent studies have consistently reported small concentration changes in lactate, Glu, Asp, and glucose in the human cortex during prolonged stimulation (48, 49). Positive linear relationships between metabolic and BOLD responses in the presence of excitatory sensory inputs could be found for Glu and lactate concentrations (48). In contrast, inverse correlations between BOLD responses and MRS measures were found for GABA concentrations (48, 50, 51). In line with these findings, we argue in favor of an emerging conceptual framework to interpret multimodal imaging findings including changes in metabolite concentrations and BOLD signals in terms of brain energetics and neurotransmission. In addition, a recent study proposed the existence of a neuron-to-astrocyte Asp transcellular pathway required for astrocyte Glu synthesis and subsequent glutamine formation (52). Accordingly, the relationship among decreased Asp levels, reduced BOLD responses, and social pain might indicate a possible role of glial Glu metabolism in the processing of social pain signals in the dACC. In conclusion, further studies would be needed to corroborate the hypothetical functional links between Asp levels and BOLD responses that we observed in our study.
To our knowledge, the current study represents the first multimodal brain imaging approach to investigate the neuropharmacological grounds of social exclusion processing, and particularly the influence of 5-HT receptor stimulation on negative social interactions. Evidence from animal studies suggests that the opioid system may similarly participate in the experience of pain and distress in response to maternal–infant separation (53). Furthermore, another study showed that chronic administration of the analgesic acetaminophen reduced neural responses to social rejection in the dACC and anterior insula, but not self-reported social distress after exclusion (54). However, the exact action mechanism of acetaminophen is still unclear. Additional behavioral analyses suggested that oxytocin does not buffer against the experience of social pain after social rejection (55). Moreover, recent work (56) found that administration of 3,4-methylenedioxy-methamphetamine (MDMA), a 5-HT, dopamine, and norepinephrine releasing agent/reuptake inhibitor (57), decreased the effect of social exclusion on mood and self-esteem, but not on physiological measures. The authors proposed that MDMA selectively decreased the perceived intensity of rejection, as indicated by increased estimates of received throws in the exclusion condition. Therefore, whereas MDMA seems to affect the perception of social exclusion, Psi may actually reduce the experience of social pain itself without significantly influencing the awareness of being excluded. The current study therefore represents to our knowledge the first indication that stimulation of the 5-HT2A/1A receptor system can regulate social pain processing and experience. The results are consistent with previous reports suggesting that the 5-HT system plays a role in emotional regulation (10, 20, 21), and may also be relevant for social cognition and behavior (11, 12); they likewise support earlier studies showing that 5-HT2A receptors are highly expressed in prefrontal brain areas and emphasize the importance of these receptors for prefrontal brain function (58). Considering that most previous studies investigating the role of 5-HT in social processes were conducted by using selective serotonin reuptake inhibitors to increase 5-HT levels, the current study extends these findings by showing that direct receptor simulation contributes to modulations of social cognition. Furthermore, our work highlights the importance of the 5-HT2A/1A receptor system not only in perception but also in the actual experience of social encounters, as indicated by the interactive nature of the Cyberball paradigm (27).
The multimodal brain imaging approach applied in this study offers the possibility of exploring neurobiological processes underlying the experience of social pain. Our findings indicate that Psi acts on brain areas associated with the experience of social pain via alterations in Glu/Asp metabolism. The current results must be interpreted with the following limitation in mind—namely, that the results of pharmacological MRI studies may be susceptible to drug effects on cerebral vasoactivity (8). However, two previous fMRI investigations of Psi actions found no effect of nonneural physiological changes or Psi itself on the vascular system of the brain (10, 16). Furthermore, psilocybin has been shown to reduce the BOLD signal in the dACC during resting state (16). It is therefore possible that the reduction of BOLD signal in the dACC reported in the current study might relate to a general reduction of neuronal activity within this area; however, this is unlikely considering that no decrease in BOLD signal was found for the “not receiving INCL > receiving INCL” contrast after Psi administration compared with Pla, indicating that the decreased BOLD response is related to the exclusion context. Furthermore, conflicting results have been obtained between PET and fMRI regarding resting state activity in the ACC after Psi administration, with a previous study showing increases in glucose metabolism in the dACC PET (18). However, the current study reports alterations in regional brain activity that are task specific and rely on contrasts between two conditions of this task. Therefore, we cannot make any conclusions about a general increase or decrease in neuronal activity in the ACC after Psi administration.
In summary, the present results suggest that 5-HT2A/1A receptor stimulation by Psi attenuates social pain processing, possibly in parallel with alterations in Glu/Asp metabolism and release. Furthermore, receptor stimulation is also associated with changes in self-processing, particularly the feeling of being connected with the environment. Social ties have repeatedly been shown to be crucial for physical and mental health (59, 60), and psychiatric patients often encounter social rejection (61). Additionally, perceived social isolation has a negative impact on social and general cognition, often leading to negative social interactions and a vicious cycle of social rejection (62). Patients suffering from major depressive disorder exhibit information acquisition and processing that is biased toward negative stimuli, which features predominantly in the development and maintenance of depression (63). This negative emotional bias is related inter alia to functional alterations in the ACC and frontal brain areas (63). Therefore, the current study validates reports suggesting that Psi may have antidepressant characteristics (10, 16), possibly by mitigating negative emotional bias (10, 20, 21). Last, the present results show that Psi may normalize the processing of negative social interaction in disorders characterized by increased rejection sensitivity through the adjustment of dACC and frontal brain activity, and by changes in self-processing. However, because this study was conducted in healthy volunteers, it has to be interpreted with the limitation in mind that the results may not directly translate to psychiatric patients with severe social exclusion experiences, such as schizophrenia patients (3).
Understanding the neural and biochemical foundations of rejection experiences is important for increasing our knowledge about social and emotional processes, and is crucial for the treatment of conditions influenced by social factors (3, 35, 54). Therefore, our findings may assist in diminishing a knowledge gap that restrains the development of pharmacotherapies for sociocognitive deficits in psychiatric disorders. The current results also emphasize the contribution of 5-HT2A/1A receptor subtypes and the Glu/Asp system in the regulation of social functioning, and their utility as prospective targets in the management of sociocognitive impairments.
Methods
Study Participants.
The data of 21 healthy participants were included in the statistical analyses (n = 12 males and 9 females; mean age = 26.48 y; SD = 4.76 y; range = 20–37 y). For further details, see SI Methods. The MRS data of two subjects were excluded in the corresponding analyses due to premature termination of MRS acquisition. Subjects received written and oral descriptions of the study procedures, as well as details regarding the effects and possible risks of Psi treatment. All participants provided written informed consent statements in accordance with the declaration of Helsinki before participation in the study. The Swiss Federal Office of Public Health, Bern, Switzerland, authorized the use of Psi in humans, and the study was approved by the Cantonal Ethics Committee of Zurich.
Study Design.
This study was designed as a randomized, double-blind, placebo-controlled, within-subject investigation. Subjects received either Pla (maltose) or oral Psi (0.215 mg/kg) in two separate sessions spaced at least 10 d apart. The dose of 0.215 mg/kg was chosen based on previous studies showing that it is well tolerated in healthy subjects (13) and attenuates the processing of negative emotional stimuli (20). For details, see SI Methods.
Cyberball Task.
While undergoing fMRI, participants completed an interactive virtual ball-tossing game called Cyberball that simulates a real-life interactive experience of social exclusion (23). For details, see SI Methods and Fig. S4. Briefly, participants played three rounds of Cyberball during separate fMRI scans, each representing one of the following conditions: (i) ISE, where participants were informed that due to technical difficulties the internet connection could not be established, but that they could watch the other participants playing; (ii) INCL, where participants were told that they were connected and could join in with the other players; and (iii) ESE, where participants received five throws and were then excluded from the game (i.e., the other players stopped throwing the ball to the participant for the remainder of the scan). Immediately after the scanning session, participants completed the PTQ. For details, see SI Methods.
Fig. S4.
Cyberball task display (inclusion and explicit social exclusion run). Participants were informed about the connection status on the top of the screen, which displayed “You are NOT connected” during the implicit social exclusion run; they also observed photographs and names of the other players, as well as their own name and photo at the bottom of the screen.
MRI Data Acquisition and Preprocessing.
MRI data were acquired by using a Philips Achieva 3.0T whole-body scanner (Philips Healthcare). Care was taken to ensure the comfort of the study participants on the scanner bed. To this end, the study protocol used inflatable Multipad pillows (Pearltec). A 32-channel receive-only phased-array head coil and MultiTransmit parallel radio frequency transmission technology were used to collect the fMRI data. For further details, see SI Methods. A birdcage transmit–receive passed-array head coil with a maximum B1 = 20 µT was used for collection of MRS data. Therefore, subjects were repositioned in different coils between MRS and fMRI scans to gain optimal data quality for the different modalities. During the 1H-MRS measurements, participants were not engaged in a task, but were instead instructed to lie as still as possible in the scanner. A 1-mm3 isotropic high-resolution T1-weighted image was used for voxel planning. A maximum echo J-resolved 1H-MRS protocol (64) was established to acquire spectra from an anatomical ROI of 32 (AP) × 21 (RL) × 24 (FH) mm3 in the dACC. The ROI was selected based on previous work highlighting the role of the ACC in social cognition (17), as well as changes in brain perfusion and glucose metabolism after Psi administration detected by PET (18). Measured data were quantified by using Profit2 software (65) to enable the detection of up to 18 different metabolites (N-acetylaspartate, creatine, choline, myo-inositol, Glu, glutamine, N-acetylaspartylglutamate, gamma-aminobutyric acid, glucose, lactate, scyllo-inositol, taurine, glycine, glutathione, phosphoethanolamine, Asp, ascorbate, and acetate). For details, see SI Methods.
Statistical Analysis.
Questionnaires and physical effects.
Repeated-measures ANOVAs with treatment (Pla vs. Psi) and scale as within-subject factors were conducted to analyze 5D-ASC ratings. For analysis of PANAS ratings, time (pre-drug administration vs. post-drug administration) was introduced as an additional within-subjects factor. Paired t tests were applied to analyze PTQ items as well as pulse and systolic/diastolic blood pressure. Significant main effects or interactions were followed by Bonferroni-corrected pairwise comparisons and simple main effects analyses, respectively. The confirmatory statistical comparisons of all data were carried out with a significance level of P < 0.05 (two-tailed test). Analyses were conducted by using IBM SPSS Statistics 21 software (IBM). For details, see SI Methods.
fMRI data.
The fMRI images were analyzed by using a general linear model implemented in the SPM8 software package and an event-related design (33). For details, see SI Methods.
Correlation analyses.
Correlation analyses were conducted to further investigate the relationship between Psi-induced differences in social exclusion processing and subjective effects. Furthermore, the relationship between Pla–Psi differences in social exclusion processing and changes in metabolite concentrations was explored. For details, see SI Methods.
SI Methods
Participants.
Participants were recruited through advertisements in local universities. All subjects were healthy according to medical history, physical examination, blood analysis, and electrocardiography, and showed normal or corrected-to-normal vision. The Mini-International Neuropsychiatric Interview (MINI-SCID) (66), the DSM-IV self-rating questionnaire for axis II personality disorders (SCID-II) (67), and the Hopkins Symptom Checklist (SCL-90-R) (68) were used to exclude subjects with present or previous psychiatric disorders or a history of major psychiatric disorders in first-degree relatives. Participants were asked to abstain from the use of any prescription or illicit drug for a minimum of 2 wk before the first test day and for the duration of the entire study, and to abstain from drinking alcohol for at least 24 h before test days. Urine tests and self-report questionnaires were used to verify the absence of drug and alcohol use. Urine tests were also used to exclude pregnancy. Further exclusion criteria included left-handedness, poor knowledge of the German language, cardiovascular disease, history of head injury or neurological disorder, history of alcohol or illicit drug dependence, MRI exclusion criteria including claustrophobia, and previous significant adverse reactions to a hallucinogenic drug.
The initial study population consisted of 29 participants. Completion of the task was not possible for one participant because of technical problems. Two participants were excluded because they declared familiarity with the task and therefore did not complete the task appropriately. Five additional participants were excluded due to excessive head movement during scanning (>3 mm), either at the first or second assessment. Finally, 21 participants were included in the study.
Study Design.
The Cyberball paradigm was conducted 75 min after Psi/Pla administration, during the plateau of peak subjective Psi effects (40). Two MRS scans were assessed per subject and per MRI session. A 40-min baseline MRS measurement [including survey scan, high-resolution T1, preparation scans (noise determination, resonance frequency determination, power optimization, higher order shimming) and JPRESS MRS] was conducted starting 50 min before substance administration. An additional (follow-up) MRS was assessed after completion of the Cyberball paradigm (i.e., 100 min after Psi/Pla administration). Pulse and blood pressure were measured after scanning (i.e., 150 min after Psi/Pla administration). Mood state was assessed by using the PANAS (69) 75 min before and 360 min after each drug treatment. The 5D-ASC (a retrospective self-report questionnaire) (40) was administered to participants 360 min after drug treatment to assess subjective experience after drug intake. Participants were required to abstain from smoking for at least 60 min before MRI assessments and from drinking caffeine during the test day.
Cyberball Task.
Before administration of Psi or Pla, participants were told that they would be connected over the Internet with two other players (one male and one female). The participants were then shortly introduced to the other players. Two different players were introduced at the second assessment. The actions of the players were controlled by the computer. To ensure that the participants believed that the other players were real, they were told that the task was used to investigate mental visualization skills, and that they should try to imagine the game as vividly as possible (23, 29).
The Cyberball paradigm was presented in the scanner with MRI-compatible video goggles (Resonance Technology). Responses (from the right hand) were collected by using a two-button response box. Participants were represented as animated cartoon images at the bottom of the screen. The two other players were also shown as animated cartoon images on the left and right side of the screen. The female coplayer was always shown on the right side, whereas the male coplayer was always shown on the left side. Black-and-white photographs of all three players and the players’ names were depicted beside each player’s icon. The connection status (“You are NOT connected” for the ISE condition and “You are connected” for the INCL and ESE conditions) was displayed at the top of the screen (Fig. S4).
To prevent expectation effects, the order of the conditions was varied. At the first assessment, the participants completed the scans in the following order: (i) ISE, (ii) INCL, and (iii) ESE. At the second assessment, the order was (i) INCL, (ii) ESE, and (iii) ISE. Participants were only informed about changes in connection status, i.e., between ISE and INCL at assessment 1 and ESE and ISE at assessment 2, but not about changes in condition.
Each run was initiated with a loading screen informing the participants that the computer was trying to connect to the other players. The duration of each ball toss was fixed at 1.4 s. The reaction time of the computerized players varied randomly between 500 and 3,000 ms. The ISE condition consisted of 60 ball tosses between the computerized players, and lasted for 200 s. During the INCL condition, 90 ball tosses in total were administered, with participants receiving 33% of the throws. After receiving the ball, participants could throw the ball to one of the other players by pressing the left or right button on the response box with the right index finger. The INCL run lasted for 262.5 s. The ESE condition consisted of a total of 60 ball tosses and lasted for 200 s.
Posttask Questionnaire.
The PTQ contained four questions of the Need Threat Scale (69) assessing participants’ socially orientated self-esteem (“To what extent do you think the other participants value you as a person?”); meaningful existence (“How true is the statement: ‘Life is meaningless’?”); control (“How true is the statement: I am in control of my life?”); and belongingness (“How much do you feel that you belonged to the group?”). Furthermore, the intensity of ostracism was measured by two questions (“To what extent did you feel that you were ignored or excluded by the other participants?” and “To what extent did you feel that you were noticed or included by the other participants?”). These questions were answered on a nine-point scale, ranging from 1 (not at all) to 9 (very much).
To assess the participants’ awareness of social exclusion, they were asked to gauge how many throws they received during each of the runs. Participants were also asked to answer the questions “How much did you like the other player?” and “How much do you think the other player liked you?” for each of the other players on a five-point scale (1 = not at all; 5 = very much). Last, participants were permitted to submit any thoughts regarding the game on blank lines of the PTQ.
MRI Data Acquisition and Preprocessing.
fMRI were acquired by using a whole-brain, gradient-echo planar imaging (EPI) sequence (repetition time = 2,500 ms; echo time = 30 ms; slice thickness = 2.75 mm; 40 axial slices; no slice gap; field of view = 220 × 220 mm2; in-plane resolution = 2.75 × 2.75 mm2; sensitivity-encoding reduction factor = 2.0). Additionally, high-resolution anatomical images (voxel size = 1 × 1 × 1 mm3) were acquired by using a standard T1-weighted 3D MPRAGE sequence. Images were analyzed with SPM8 software (www.fil.ion.ucl.ac.uk). Preprocessing consisted of slice-time correction, realignment, spatial normalization to the standard EPI template of the Montreal Neurological Institute (MNI), and spatial smoothing with a Gaussian kernel of 8 mm full width at half maximum to meet the statistical requirements of the general linear model.
For MRS data, a minimum echo time of TE = 28 ms and a repetition time of TR = 1,600 ms was used. The echo increment to encode the indirect dimension was set to 2 ms. One hundred steps were acquired by obtaining eight average measurements plus one additional interleaved acquired non–water-suppressed spectrum with identical TE. The system’s built in projection-based automatic second-order B0 shimming routine (described in detail in ref. 70) was used without electrocardiogram triggering. Water suppression was achieved by the variable pulse power and optimized relaxation delay, or VAPOR, method (71), and polynomial phase saturation pulses (72) were applied with interleaved inner-volume saturation (IVS). Due to IVS, the resulting voxel size was 29 (AP) × 19.8 (RL) × 21.8 (FH) mm3. Based on high-resolution 3D T1 images, cerebral spinal fluid, gray matter, and white matter fractions were calculated by using SPM8 software, and the metabolite concentrations were corrected*. Visual assessment of the line shape of the unsuppressed water peak of the MRS preparation scan was used to guarantee good and consistent shim quality. In addition, a visual artifact inspection of 2D plots and deduced projections (65) was used for spectral quality control. CRLBs were used as quality criteria, but no exclusion of concentration values based on CRLBs was made to avoid bias toward higher concentrations (73). Instead, a metabolite was marked as “reliably detected” if all CRLB values of all JPRESS measurements were below 20% and the mean concentration was below 15%.
Statistical Analysis of Questionnaires.
The 5D-ASC comprises 94 items to be answered on visual analog scales. Scores were calculated for 11 recently validated scales (40): experience of unity, spiritual experience, blissful state, insightfulness, disembodiment, impaired control and cognition, anxiety, complex imagery, elementary imagery, audiovisual synesthesia, and changed meaning of percepts. For PANAS ratings, scores were calculated for the positive and negative affect scales.
Statistical Analysis of fMRI Data.
The onsets of ball movement were modeled as stick functions and convolved with a canonical hemodynamic response function in the first-level analysis for each subject. Low-frequency signal drifts were filtered with a 128-s high-pass filter. Events were divided into the following conditions. INCL and ESE: receiving, not receiving, or playing the ball; and ISE: not receiving the ball. The following contrasts were computed for each participant: (i) not receiving ESE > receiving INCL, (ii) not receiving ISE > receiving INCL, and (iii) not receiving INCL > receiving INCL. The individual contrasts were then entered into a second-level group analysis by using a paired t test for the comparison between Psi and Pla conditions, and one-sample t tests for the within-Pla analysis with a threshold of uncorrected P < 0.001 and k > 3. Due to our a priori interest in social exclusion sensitive-areas, bilateral ROIs (ACC, MCC, insula, inferior OFC, and MFG) were defined that were previously identified to be involved in social rejection processing by meta-analysis (28). Condition effects were analyzed by using small-volume correction (SVC) based on these anatomical ROIs (74). Family-wise error (FWE) corrections were used in all SVC ROI analyses at a peak level-corrected threshold of P < 0.05. Uncorrected whole-brain results (P < 0.001) are shown in Table S3 (Pla) and Table S4 (Pla vs. Psi). All brain coordinates are reported in the MNI atlas space.
Correlational Analyses.
Correlation analyses were conducted to further investigate the relationship between Psi-induced differences in social exclusion processing and subjective effects. BOLD signal responses (first eigenvariate) were extracted from the right dACC and left MFG anatomical ROIs for the “not receiving ESE > receiving INCL” contrast for Psi and Pla by using the same anatomical masks as described above. Pearson’s product–moment correlations were conducted between the extracted BOLD responses in these ROIs (Pla–Psi change score) and the 5D-ASC scale scores (Psi condition, because 5D-ASC scores represent percent deviations from the normal waking state), PANAS ratings (Pla–Psi change score), and PTQ items (Pla–Psi change score). Finally, the relationship between Pla–Psi differences in social exclusion processing and changes in metabolite concentrations was explored. The BOLD response (first eigenvariate) was extracted from the dACC ROI established for MRS acquisition (Fig. 2A) for the “not receiving ESE > receiving INCL” contrast for Psi and Pla. Pearson’s product–moment correlation analyses were computed between the extracted BOLD responses (Pla–Psi change score) and the baseline-corrected metabolite concentrations in the Psi condition (baseline−follow-up) as well as metabolite concentrations in the Psi follow-up measurement.
Acknowledgments
The study was supported by grants from the Heffter Research Institute and the Swiss Neuromatrix Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.M.-L. is a guest editor invited by the Editorial Board.
*Zoelch N, et al. (2015) Necessity of Tissue Volume Composition Correction for Internal Referencing (Proc Intl Soc Mag Reson Med, Toronto).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524187113/-/DCSupplemental.
References
- 1.Patin A, Hurlemann R. Social cognition. Handbook Exp Pharmacol. 2015;228:271–303. doi: 10.1007/978-3-319-16522-6_10. [DOI] [PubMed] [Google Scholar]
- 2.Millan MJ, et al. Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 3.Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16(10):620–631. doi: 10.1038/nrn4005. [DOI] [PubMed] [Google Scholar]
- 4.Fett AK, et al. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Cusi AM, Macqueen GM, Spreng RN, McKinnon MC. Altered empathic responding in major depressive disorder: Relation to symptom severity, illness burden, and psychosocial outcome. Psychiatry Res. 2011;188(2):231–236. doi: 10.1016/j.psychres.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Crockett MJ, Fehr E. Social brains on drugs: Tools for neuromodulation in social neuroscience. Soc Cogn Affect Neurosci. 2014;9(2):250–254. doi: 10.1093/scan/nst113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anticevic A, et al. Connectivity, pharmacology, and computation: Toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. 2013;4:169. doi: 10.3389/fpsyt.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honey G, Bullmore E. Human pharmacological MRI. Trends Pharmacol Sci. 2004;25(7):366–374. doi: 10.1016/j.tips.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Meneses A. Serotonin, neural markers, and memory. Front Pharmacol. 2015;6:143. doi: 10.3389/fphar.2015.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraehenmann R, et al. Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol Psychiatry. 2015;78(8):572–581. doi: 10.1016/j.biopsych.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc Natl Acad Sci USA. 2010;107(40):17433–17438. doi: 10.1073/pnas.1009396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canli T, Lesch KP. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10(9):1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- 13.Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J Psychopharmacol. 2011;25(11):1434–1452. doi: 10.1177/0269881110382466. [DOI] [PubMed] [Google Scholar]
- 14.Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61(3):364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carhart-Harris RL, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA. 2012;109(6):2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rotge JY, et al. A meta-analysis of the anterior cingulate contribution to social pain. Soc Cogn Affect Neurosci. 2015;10(1):19–27. doi: 10.1093/scan/nsu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vollenweider FX, et al. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16(5):357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 19.Bernasconi F, et al. Spatiotemporal brain dynamics of emotional face processing modulations induced by the serotonin 1A/2A receptor agonist psilocybin. Cereb Cortex. 2014;24(12):3221–3231. doi: 10.1093/cercor/bht178. [DOI] [PubMed] [Google Scholar]
- 20.Kometer M, et al. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012;72(11):898–906. doi: 10.1016/j.biopsych.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt A, Kometer M, Bachmann R, Seifritz E, Vollenweider F. The NMDA antagonist ketamine and the 5-HT agonist psilocybin produce dissociable effects on structural encoding of emotional face expressions. Psychopharmacology (Berl) 2013;225(1):227–239. doi: 10.1007/s00213-012-2811-0. [DOI] [PubMed] [Google Scholar]
- 22.Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11(9):642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- 23.Williams KD, Jarvis B. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behav Res Methods. 2006;38(1):174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- 24.Platt B, Cohen Kadosh K, Lau JY. The role of peer rejection in adolescent depression. Depress Anxiety. 2013;30(9):809–821. doi: 10.1002/da.22120. [DOI] [PubMed] [Google Scholar]
- 25.Domsalla M, et al. Cerebral processing of social rejection in patients with borderline personality disorder. Soc Cogn Affect Neurosci. 2014;9(11):1789–1797. doi: 10.1093/scan/nst176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levinson CA, Langer JK, Rodebaugh TL. Reactivity to exclusion prospectively predicts social anxiety symptoms in young adults. Behav Ther. 2013;44(3):470–478. doi: 10.1016/j.beth.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenberger NI. Social pain and the brain: Controversies, questions, and where to go from here. Annu Rev Psychol. 2015;66:601–629. doi: 10.1146/annurev-psych-010213-115146. [DOI] [PubMed] [Google Scholar]
- 28.Cacioppo S, et al. A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep. 2013;3:2027. doi: 10.1038/srep02027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 30.Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Front Integr Nuerosci. 2013;7:25. doi: 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30(6):2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riga MS, Soria G, Tudela R, Artigas F, Celada P. The natural hallucinogen 5-MeO-DMT, component of Ayahuasca, disrupts cortical function in rats: Reversal by antipsychotic drugs. Int J Neuropsychopharmacol. 2014;17(8):1269–1282. doi: 10.1017/S1461145714000261. [DOI] [PubMed] [Google Scholar]
- 33.Moor BG, et al. Social exclusion and punishment of excluders: Neural correlates and developmental trajectories. Neuroimage. 2012;59(1):708–717. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci USA. 2011;108(15):6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo CW, et al. Separate neural representations for physical pain and social rejection. Nat Commun. 2014;5:5380. doi: 10.1038/ncomms6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenberger NI. The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13(6):421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- 37.Shackman AJ, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurage P, et al. Disrupted regulation of social exclusion in alcohol-dependence: An fMRI study. Neuropsychopharmacology. 2012;37(9):2067–2075. doi: 10.1038/npp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. J Cogn Neurosci. 2007;19(6):945–956. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- 40.Studerus E, Gamma A, Vollenweider FX. Psychometric evaluation of the altered states of consciousness rating scale (OAV) PLoS One. 2010;5(8):e12412. doi: 10.1371/journal.pone.0012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decety J, Sommerville JA. Shared representations between self and other: A social cognitive neuroscience view. Trends Cogn Sci. 2003;7(12):527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Vogeley K, et al. Mind reading: Neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14(1 Pt 1):170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- 43.Cavallero A, Marte A, Fedele E. L-aspartate as an amino acid neurotransmitter: Mechanisms of the depolarization-induced release from cerebrocortical synaptosomes. J Neurochem. 2009;110(3):924–934. doi: 10.1111/j.1471-4159.2009.06187.x. [DOI] [PubMed] [Google Scholar]
- 44.Mangia S, et al. Metabolic and hemodynamic events after changes in neuronal activity: Current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cereb Blood Flow Metab. 2009;29(3):441–463. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: Current evidence and pharmacological tools. Biochem Pharmacol. 2006;71(4):399–407. doi: 10.1016/j.bcp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Lambe EK, Aghajanian GK. Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: Role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology. 2006;31(8):1682–1689. doi: 10.1038/sj.npp.1300944. [DOI] [PubMed] [Google Scholar]
- 47.Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA. Lysergic acid diethylamide and [-]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 2004;1023(1):134–140. doi: 10.1016/j.brainres.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 48.Bednařík P, et al. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J Cereb Blood Flow Metab. 2015;35(4):601–610. doi: 10.1038/jcbfm.2014.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangia S, et al. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: Evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27(5):1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- 50.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA. 2009;106(20):8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Northoff G, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10(12):1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 52.Pardo B, et al. Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J Cereb Blood Flow Metab. 2011;31(1):90–101. doi: 10.1038/jcbfm.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barr CS, et al. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci USA. 2008;105(13):5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dewall CN, et al. Acetaminophen reduces social pain: Behavioral and neural evidence. Psychol Sci. 2010;21(7):931–937. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- 55.Alvares GA, Hickie IB, Guastella AJ. Acute effects of intranasal oxytocin on subjective and behavioral responses to social rejection. Exp Clin Psychopharmacol. 2010;18(4):316–321. doi: 10.1037/a0019719. [DOI] [PubMed] [Google Scholar]
- 56.Frye CG, Wardle MC, Norman GJ, de Wit H. MDMA decreases the effects of simulated social rejection. Pharmacol Biochem Behav. 2014;117:1–6. doi: 10.1016/j.pbb.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hysek CM, et al. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS One. 2012;7(5):e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amargós-Bosch M, et al. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14(3):281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- 59.Eisenberger NI, Cole SW. Social neuroscience and health: Neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15(5):669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- 60.Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164(10):1521–1529. doi: 10.1176/appi.ajp.2007.06091564. [DOI] [PubMed] [Google Scholar]
- 61.Feldman DB, Crandall CS. Dimensions of mental illness stigma: What about mental illness causes social rejection? J Soc Clin Psychol. 2007;26(2):137–154. [Google Scholar]
- 62.Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn Sci. 2009;13(10):447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 64.Schulte RF, Lange T, Beck J, Meier D, Boesiger P. Improved two-dimensional J-resolved spectroscopy. NMR Biomed. 2006;19(2):264–270. doi: 10.1002/nbm.1027. [DOI] [PubMed] [Google Scholar]
- 65.Fuchs A, Boesiger P, Schulte RF, Henning A. ProFit revisited. Magn Reson Med. 2014;71(2):458–468. doi: 10.1002/mrm.24703. [DOI] [PubMed] [Google Scholar]
- 66.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33, quiz 34–57. [PubMed] [Google Scholar]
- 67. Fydrich T, Renneberg B, Schmitz B, Wittchen H-U (1997) SKID-II Strukturiertes Klinisches Interview für DSM-IV, Achse II: Persönlichkeitsstörungen (Hogrefe, Göttingen, Germany) [SCID-II Structured Cinical Interview for DSM-IV, Axis II: Personality Disorders.]
- 68.Franke G. Die Symptom-Check-Liste von Derogatis: Deutsche Version. Beltz Test Gesellschaft; Göttingen, Germany: 1995. [Google Scholar]
- 69.Williams KD, Cheung CK, Choi W. Cyberostracism: Effects of being ignored over the Internet. J Pers Soc Psychol. 2000;79(5):748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- 70.Hock A, Fuchs A, Boesiger P, Kollias SS, Henning A. Electrocardiogram-triggered, higher order, projection-based B₀ shimming allows for fast and reproducible shim convergence in spinal cord ¹H MRS. NMR Biomed. 2013;26(3):329–335. doi: 10.1002/nbm.2852. [DOI] [PubMed] [Google Scholar]
- 71.Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 72.Schulte RF, Henning A, Tsao J, Boesiger P, Pruessmann KP. Design of broadband RF pulses with polynomial-phase response. J Magn Reson. 2007;186(2):167–175. doi: 10.1016/j.jmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Kreis R. The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magn Reson Med. 2016;75(1):15–18. doi: 10.1002/mrm.25568. [DOI] [PubMed] [Google Scholar]
- 74.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]