Significance
Although long postulated as a behavioral therapy for drug addiction, environmental enrichment (EE) applied alone achieves only marginal and transient anti-addiction effects. Here, we report that, after cocaine withdrawal, EE alone did not affect the amygdala-to-nucleus accumbens projection, which has been critically implicated in cocaine craving and seeking. However, in vivo optogenetic long-term depression (LTD) at this projection primed a portion of affected synapses to remodel upon EE, leading to long-lasting anti-relapse effects. Thus, projection-specific LTD unmasks the anti-relapse power of EE, indicating the synergy of combinatory approaches for treating addiction.
Keywords: environmental enrichment, cocaine, incubation, silent synapse, accumbens
Abstract
Environmental enrichment (EE) has long been postulated as a behavioral treatment for drug addiction based on its preventive effects in animal models: rodents experiencing prior EE exhibit increased resistance to establishing drug taking and seeking. However, the therapeutic effects of EE, namely, the effects of EE when applied after drug exposure, are often marginal and transient. Using incubation of cue-induced cocaine craving, a rat relapse model depicting progressive intensification of cocaine seeking after withdrawal from cocaine self-administration, our present study reveals that after cocaine withdrawal, in vivo circuit-specific long-term depression (LTD) unmasks the therapeutic power of EE to achieve long-lasting anti-relapse effects. Specifically, our previous results show that cocaine self-administration generates AMPA receptor (AMPAR)-silent excitatory synapses within the basolateral amygdala (BLA) to nucleus accumbens (NAc) projection, and maturation of these silent synapses via recruiting calcium-permeable (CP) AMPARs contributes to incubation of cocaine craving. Here, we show that after cocaine withdrawal and maturation of silent synapses, the BLA-to-NAc projection became highly resistant to EE. However, optogenetic LTD applied to this projection in vivo transiently re-silenced these silent synapses by removing CP-AMPARs. During this transient window, application of EE resulted in the insertion of nonCP-AMPARs, thereby remodeling the “incubated” BLA-to-NAc projection. Consequently, incubation of cocaine craving was decreased persistently. These results reveal a mechanistic basis through which the persistent anti-relapse effects of EE can be unleashed after drug withdrawal.
Environmental enrichment (EE) promotes neurocircuit development and improves brain recovery from disease conditions and has long been hypothesized as a behavioral therapy for drug addiction (1–4). Whereas animal studies demonstrate clear preventive effects of EE [i.e., animals experiencing prior EE exhibit increased resistance to establishing drug taking and seeking (5–7)], the therapeutic effects of EE, namely, the effects of EE when applied after drug exposure, are less consistent. For example, in the incubation of cue-induced cocaine-craving model in rats, where cocaine seeking progressively intensifies during prolonged withdrawal from cocaine self-administration (8, 9), EE applied after cocaine self-administration only marginally reduces incubation of cocaine craving, and any anti-incubation effect observed disappears upon termination of EE treatment (10–13). These results raise both opportunities and challenges for EE-based treatment of drug addiction.
Incubation of cocaine craving is partially mediated by synaptic accumulation of calcium permeable (CP) AMPA receptors (AMPARs) in the nucleus accumbens (NAc) (14, 15). A portion of these CP-AMPARs move into cocaine-generated silent excitatory synapses within the projection from the basolateral amygdala (BLA) pyramidal neurons to NAc medium spiny neurons (MSNs) (16), a critical projection in cue-induced drug seeking (17, 18). Abundant in the developing brain, silent synapses express stable NMDA receptors (NMDARs), with AMPARs that are either absent or highly labile. Silent synapses are potentially immature nascent synapses that can mature by recruiting AMPARs to strengthen a particular circuit (19–23). In the adult brain, exposure to cocaine generates silent synapses (24, 25), a process that may involve reopening of developmental mechanisms (26). Cocaine-induced generation and subsequent maturation of silent synapses profoundly remodel excitatory projections to NAc from BLA and likely other brain regions, contributing to incubated cocaine seeking (16, 27).
A clear link between EE and AMPAR trafficking has long been established in hippocampal and cortical regions, in which EE promotes synaptic insertion of nonCP-AMPARs, increases the size of dendritic spines, and facilitates long-term potentiation (LTP) (4, 28–31). In the NAc, EE induces several proteomic alterations that promote strengthening and maturation of excitatory synapses (32). However, the synaptic-strengthening effects tend to be robust only in developing or recently damaged brains, in which relatively weak and immature synapses are abundant (29, 31, 33, 34). After cocaine withdrawal, excitatory synapses are substantially strengthened in the NAc, partially through the insertion of CP-AMPARs to cocaine-generated silent synapses (14, 16, 27, 35). This withdrawal-associated synaptic strengthening may compromise insertion of additional nonCP-AMPARs by subsequent EE, thus undermining EE’s anti-relapse effects.
In an attempt to reveal potential anti-relapse effects of EE, we combined a 7-d EE treatment with an optogenetic protocol to induce long-term depression (LTD) in the BLA-to-NAc projection after cocaine withdrawal. Our results suggest that, after LTD-induced internalization of CP-AMPARs from matured silent synapses, and re-silencing of cocaine-generated silent synapses during cocaine withdrawal, subsequent EE promotes the insertion of nonCP-AMPARs to those LTD–re-silenced synapses in the BLA-to-NAc projection. These combined approaches achieve a prolonged anti-incubation effect. We propose that re-silencing of silent synapses unleashes the anti-relapse effect of EE, resulting in reduced cocaine craving after withdrawal.
Results
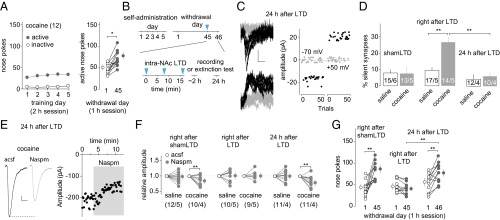
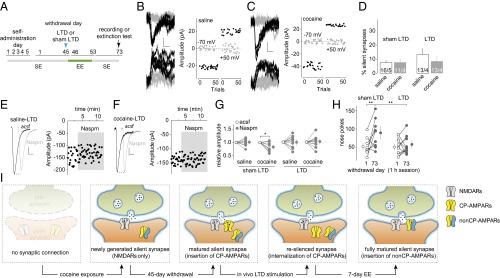
After 6 d of cocaine self-administration, rats developed incubation of cue-induced cocaine craving (16), which was evident in a 1-h extinction test where rats exhibited significantly higher levels of cocaine seeking on withdrawal day 45 than withdrawal day 1 (measured by nose pokes for cocaine; Fig. 1A).
Fig. 1.
Transient anti-incubation effects of BLA-to-NAc LTD. (A) Summarized results showing that, after 5 d of cocaine self-administration (2 h/d at 0.75 mg/kg) following an overnight training, cue-induced cocaine seeking (measured by nose pokes in a 1-h extinction) was increased 45 d after withdrawal from cocaine compared with withdrawal day 1 [t(11) = 4.87; P < 0.01, paired t test; the same rats tested on withdrawal days 1 and 45]. (B) Time line of behavioral procedure showing that in vivo LTD induction (blue arrows, 5 Hz × 3 min × 3 times with 5-min intervals) was applied to BLA-to-NAc synapses 45 d after saline or cocaine self-administration. Electrophysiology or extinction tests were performed either right after or 24 h after BLA-to-NAc LTD. (C) Example EPSCs evoked at −70 or +50 mV from BLA-to-NAc synapses by optogenetic minimal stimulations (Left) over 100 trials (Right) 24 h after in vivo LTD in rats after 45 d of cocaine withdrawal. (D) Summarized results showing that, similar to previous results (16), on withdrawal day 45, whereas the percentage of silent synapses in BLA-to-NAc projection in cocaine-exposed rats returned to basal (saline) levels, this percentage was significantly increased upon LTD induction (LTD: saline, 9.5 ± 1.8; cocaine, 26.3 ± 2.9; shamLTD: saline, 8.1 ± 2.0; cocaine, 8.1 ± 5.1, measured right after LTD/shamLTD), and this LTD effect on the percentage of silent synapses diminished after 24 h [saline, 4.3 ± 3.1; cocaine, 6.2 ± 1.9; F(2,23) = 4.7, P < 0.05, two-way ANOVA; P < 0.01, right after LTD vs. 24 h after LTD in cocaine groups; P < 0.01, saline right after LTD vs. cocaine right after LTD; Bonferroni posttest]. n/m, n cells from m rats. (E) Example EPSCs (Left) and the time course (Right) of BLA-to-NAc synapses before and during perfusion of Naspm in a cocaine-exposed rat 24 h after the withdrawal day 45 LTD. (F) Summarized results showing that on withdrawal day 45, in vivo LTD, but not shamLTD, abolished the cocaine withdrawal-induced increase in the sensitivity of EPSCs at BLA-to-NAc synapses to Naspm when tested right after LTD induction. This LTD effect diminished after 24 h. Evoked EPSCs at BLA-to-NAc synapses in saline-exposed rats were insensitive to Naspm right after shamLTD, right after LTD, or 24 h after LTD [F(5,21) = 4.2, P < 0.01, two-way ANOVA with repeated measures; P < 0.01, before vs. during Naspm in cocaine rats right after shamLTD; P < 0.01, before vs. during Naspm in cocaine rats 24 h after LTD]. (G) Summarized results showing that incubation of cocaine craving (increased number of nose pokes for cocaine on withdrawal day 45 or 46 compared with withdrawal day 1) was not affected right after shamLTD to BLA-to-NAc synapses and was abolished right after LTD, but this LTD effect diminished after 24 h [F(2,30) = 10.9, P < 0.01, two-way ANOVA with repeated measures; P < 0.01, withdrawal day 1 vs. day 45 in rats right after shamLTD or 24 h after LTD, Bonferroni posttest]. *P < 0.05; **P < 0.01.
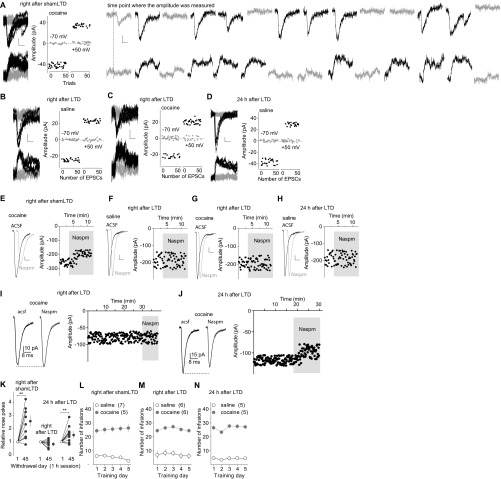
Our previous results suggest that incubation of cocaine craving is initiated in part by cocaine-induced generation of silent synapses in the BLA-to-NAc projection, followed by maturation of a subset of these cocaine-generated silent synapses, whereas in vivo application of an optogenetic LTD protocol targeting the BLA-to-NAc projection reverses maturation of silent synapses and reverses the development of cocaine incubation (16, 26). These results reveal a therapeutic potential of projection-specific in vivo LTD; however, our present results show that such anti-relapse effects were relatively transient. After 45-d withdrawal, the percentage of silent synapses among BLA-to-NAc synapses returned to basal levels (saline-exposed rats) in cocaine-exposed rats without LTD (sham LTD), accompanied by increased sensitivity of BLA-to-NAc synapses to the CP-AMPAR–selective antagonist 1-naplhthylacetyl spermine trihydrochloride (Naspm) (Fig. 1 C–F and Fig. S1 A–J and L–N). These results are consistent with previous findings suggesting that cocaine-generated silent synapses within the BLA-to-NAc projection are “unsilenced” through a maturation process involving the recruitment of CP-AMPARs (16). The percentage of silent synapses at this cocaine withdrawal time point was increased immediately (1–6 h) after the BLA-to-NAc–specific LTD in vivo, which was accompanied by a loss of sensitivity of BLA-to-NAc synapses to Naspm (Fig. 1 C–F and Fig. S1 A–J and L–N), suggesting that this LTD protocol induces internalization of CP-AMPARs and re-silences matured silent synapses (16). The correlations between disappearance/reappearance of silent synapses and appearance/disappearance of Naspm sensitivity suggest that internalization and reinsertion of CP-AMPARs mediate the re-silencing and rematuration of silent synapses, respectively. However, the re-silenced synapses within the BLA-to-NAc projection disappeared 24 h after LTD, accompanied by recovered sensitivity of BLA-to-NAc synapses to the CP-AMPAR–selective antagonist Naspm (Fig. 1 C–F and Fig. S1 A–J and L–N), indicating that the cellular effects of in vivo LTD are rather transient (lasting <24 h). The re-silenced synapses may quickly remature by reinsertion of CP-AMPARs shortly after in vivo LTD.
Fig. S1.
Additional results associated with Fig. 1. (A–D) EPSCs evoked at −70 or +50 mV by optogenetic minimal stimulations (Left) over 100 trials (Right) in example recordings from rats right after in vivo shamLTD at BLA-to-NAc synapses on withdrawal day 45 after cocaine self-administration (A), right after in vivo LTD at BLA-to-NAc synapses on day 45 after saline (B) or cocaine self-administration (C), and 24 h after in vivo LTD at BLA-to-NAc synapses on withdrawal day 45 after saline self-administration (D). (A, Right) Individual traces from A show clear separation of successes vs. failures. Successful responses exhibited similar peak amplitudes. (E–H) Example EPSCs (Left) and the time course (Right) before and during perfusion of Naspm right after in vivo shamLTD at BLA-to-NAc synapses on withdrawal day 45 after cocaine self-administration (E), right after in vivo LTD at BLA-to-NAc synapses on withdrawal day 45 after self-administration of saline (F) or cocaine (G), and 24 h after in vivo LTD at BLA-to-NAc synapses on withdrawal day 45 after saline self-administration (H). (I and J) Examples showing the typical recording time course for baseline and local perfusion of Naspm. The baselines in other related figures were truncated for clarity. (K) Normalized results showing that after 45 d of cocaine withdrawal, incubation of cocaine craving was intact in rats tested right after in vivo shamLTD on withdrawal day 45, was abolished right after in vivo LTD on withdrawal day 24, and was recovered 24 h after in vivo LTD on withdrawal day 45. Three independent groups of rats were used for these three experiments. In each experiment, the same group of rats was tested on withdrawal days 1 and 45. In each rat, the number of nose pokes on withdrawal day 45 was normalized to the number of nose pokes on withdrawal day 1 [right after shamLTD: n = 12, t(11) = 3.68, P < 0.01; right after LTD: n = 10, t(9) = 1.38, P = 0.20; 24 h after LTD: n = 11, t(10) = 3.47, P < 0.01; paired t test]. (L–N) Summarized results showing saline or cocaine self-administration training of rats used for A and E and Fig. 1 D and F (L); B, C, F, and G and Fig. 1 D and F (M); and D and H and Fig. 1 C–F (N).
Consistent with these cellular changes, the anti-incubation effects of in vivo LTD were also transient; 45 d after cocaine self-administration, sham control rats (without delivery of LTD) exhibited a significantly higher level of cue-induced cocaine seeking compared with withdrawal day 1, indicating incubation (Fig. 1G and Fig. S1K). As previously demonstrated (16), rats right after (∼1 min) in vivo LTD induction within the BLA-to-NAc projection on withdrawal day 45 exhibited reduced cocaine seeking, suggesting reversal of incubation (Fig. 1G and Fig. S1K). In contrast, a separate group of cocaine-exposed rats receiving the same in vivo LTD but tested 24 h later exhibited, once again, incubated cocaine seeking (Fig. 1G and Fig. S1K). These cellular and behavioral results taken together suggest that the re-silenced synapses in the BLA-to-NAc projection after in vivo LTD quickly rematured by rerecruiting CP-AMPARs, leading to restoration of incubated cocaine craving.
In an attempt to obtain sustained anti-relapse effects, we incorporated an EE treatment right after in vivo LTD. One mechanism by which EE regulates excitatory synapses is to promote synaptic insertion of nonCP-AMPARs: those that contain the GluA2 subunit (31). We therefore hypothesized that, after cocaine withdrawal, if EE is applied immediately after LTD-induced internalization of CP-AMPARs and re-silencing of silent synapses, EE may promote insertion of nonCP-AMPARs to silent synapses, compromising subsequent reinsertion of CP-AMPARs.
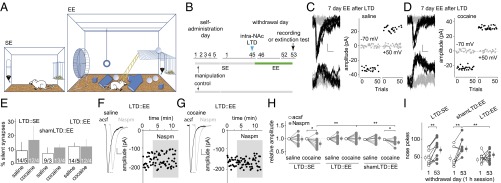
To test this hypothesis, 45 d after cocaine self-administration, we induced LTD at BLA-to-NAc synapses and then placed the rats into EE cages for 7 d (Fig. 2 A and B). In all manipulation groups, silent synapses in the BLA-to-NAc projection were at basal (saline control) levels after 7-d EE or standard environment (SE) housing (Fig. 2 C–E and Fig. S2 A–D and J–L), but the Naspm sensitivity of excitatory postsynaptic currents (EPSCs) at BLA-to-NAc synapses differed (Fig. 2 F–H and Fig. S2 E–H and J–L), revealing that SE vs. EE promoted synaptic insertion of different types of AMPARs to LTD-regenerated silent synapses after cocaine withdrawal. First, EE did not affect the percentage of silent synapses or Naspm sensitivity of BLA-to-NAc synapses in saline-exposed rats (controls) either with LTD or sham protocols, suggesting that EE alone did not affect the basal synaptic state in this projection. Second, in vivo LTD partially restored cocaine-generated silent synapses in the BLA-to-NAc projection on withdrawal day 45 (16) (Fig. 1D). After LTD and subsequent 7-d SE, silent synapses returned to basal levels, accompanied by restored Naspm sensitivity of BLA-to-NAc synapses in cocaine-exposed rats, suggesting that LTD-regenerated silent synapses rematured into CP-AMPAR–containing synapses in SE rats. Third, in cocaine-exposed rats with EE but without LTD, silent synapses remained at low levels, and the Naspm sensitivity of BLA-to-NAc synapses remained high, suggesting that EE alone (without LTD) minimally affected the “incubated” synapses. Finally, after in vivo LTD and subsequent 7-d EE, silent synapses returned to low levels, accompanied by a low Naspm sensitivity of BLA-to-NAc synapses in cocaine-exposed rats. Two possible mechanisms may underlie this combined effect of LTD and EE: LTD-regenerated silent synapses in these rats were either eliminated or rematured by recruiting nonCP-AMPARs. Although both possibilities are conceivable, the previously demonstrated effects of EE on promoting synaptic strengthening and AMPAR insertion support the latter. Collectively, these results suggest that 7-d EE treatment compromised the “default” rematuration process of LTD-regenerated silent synapses in the BLA-to-NAc projection after cocaine withdrawal, likely by inserting nonCP-AMPARs and preventing reinsertion of CP-AMPARs.
Fig. 2.
EE after BLA-to-NAc LTD decreases incubation of cocaine craving. (A) Diagrams showing housing conditions with SE or EE. (B) Time line showing the time points when manipulations were made. (C and D) Example EPSCs evoked at −70 or +50 mV by optogenetic minimal stimulations (Left) over 100 trials (Right) from a saline-exposed (C) or cocaine-exposed (D) rat with 7-d EE after BLA-to-NAc LTD on withdrawal day 45. (E) Summarized results showing that the percentage of silent synapses in BLA-to-NAc projection was at basal levels in all manipulation groups [saline-LTD-SE, 9.2 ± 4.9; cocaine-LTD-SE, 17.0 ± 9.8; saline-shamLTD-EE, 7.4 ± 4.1; cocaine-shamLTD-EE, 11.1 ± 1.7; saline-LTD-EE, 12.0 ± 2.2; cocaine-LTD-EE, 12.1 ± 3.9; F(2,19) = 0.31, P = 0.74, two-way ANOVA]. (F and G) Example EPSCs from BLA-to-NAc synapses (Left) and the time course (Right) before and during perfusion of Naspm in a saline-exposed (F) or a cocaine-exposed (G) rat with 7-d EE after in vivo LTD on withdrawal day 45. (H) Summarized results showing that the cocaine withdrawal-induced increase in Naspm sensitivity of BLA-to-NAc synapses, which was abolished by in vivo LTD on withdrawal day 45 (Fig. 1F), recovered in rats with 7-d SE after LTD and in rats with 7-d EE after shamLTD but not in rats with 7-d EE after LTD [F(5,20) = 2.9, P < 0.05, two-way ANOVA with repeated measures; P < 0.05, before vs. during Naspm in cocaine-exposed rats with either shamLTD-EE or LTD-SE; P < 0.01, LTD-SE vs. LTD-EE and shamLTD-EE vs. LTD-EE during Naspm in cocaine-exposed rats]. (I) Summarized results showing incubation of cocaine craving, tested in the same groups of rats on withdrawal day 53, was affected neither by in vivo LTD on withdrawal day 45 followed by 7-d SE nor by shamLTD on withdrawal day 45 followed by 7-d EE but was decreased by LTD on withdrawal day 45 followed by 7-d EE [F(2,28) = 3.5, P < 0.05, two-way ANOVA with repeated measures; P < 0.01, withdrawal day 1 vs. day 53 in LTD-SE or shamLTD-EE rats, P = 0.97, withdrawal day 1 vs. day 53 in LTD-EE rats, Bonferroni posttest]. *P < 0.05; **P < 0.01.
Fig. S2.
Additional results associated with Fig. 2. (A–D) EPSCs evoked at −70 or +50 mV by optogenetic minimal stimulations (Left) over 100 trials (Right) in example recordings from rats with 7-d SE after in vivo LTD at BLA-to-NAc synapses on day 45 after saline (A) or cocaine self-administration (B) and rats with 7-d EE after shamLTD on day 45 after saline (C) or cocaine self-administration (D). (E–H) Example EPSCs (Left) and the time course (Right) before and during perfusion of Naspm in rats with 7-d SE after in vivo LTD at BLA-to-NAc synapses on day 45 after saline (E) or cocaine self-administration (F) and rats with 7-d EE after shamLTD on day 45 after saline (G) or cocaine self-administration (H). (I) Normalized results showing that after 45 d of cocaine withdrawal, incubation of cocaine craving was significant in rats with in vivo LTD and subsequent 7-d SE and in rats with shamLTD and subsequent 7-d EE but was abolished in rats with LTD and subsequent 7-d EE. [LTD-SE: n = 12, t(11) = 3.47, P < 0.01; shamLTD-EE: n = 9, t(8) = 2.87, P = 0.02; LTD-EE: n = 11, t(10) = 1.44, P = 0.18; paired t test]. (J–L) Summarized results showing that saline or cocaine self-administration training of rats used for A, B, F, and G and Fig. 2 E and H (J); C–E and H–J (K); and Fig. 2 C–H (L).
Subsequent behavioral results (Fig. 2I and Fig. S2I) are consistent with the interpretation of the above cellular findings. Specifically, 7-d SE after in vivo LTD did not affect the recovery of incubated cocaine craving, tested right after SE exposure (on withdrawal day 53). Whereas 7-d EE following sham LTD did not affect incubation of cocaine craving, the same EE applied after in vivo LTD prevented the recovery of incubated cocaine craving. Thus, BLA-to-NAc LTD appears to re-silence cocaine-generated silent synapses in this projection, and this priming process unleashes the anti-incubation effects of EE.
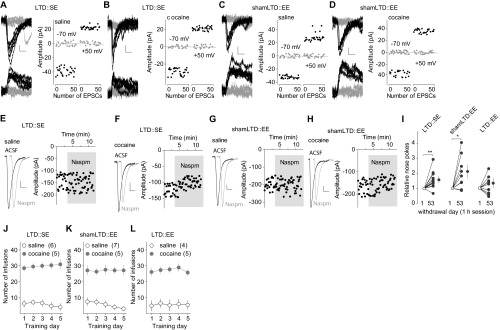
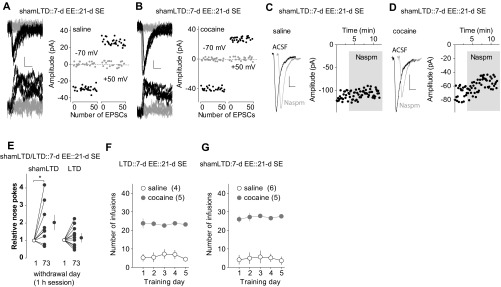
To determine whether the anti-incubation effects of the combined LTD and EE treatment are long-lasting, we applied BLA-to-NAc LTD 45 d after cocaine self-administration, followed by 7-d EE, and then housed these rats in regular SE cages for additional 21 d (Fig. 3A). Cocaine-exposed rats with sham LTD and 7-d EE were used as controls. By the end of the additional 21-d SE (on overall withdrawal day 73), BLA-to-NAc silent synapses remained at basal (control) levels in EE rats with sham LTD, accompanied by high Naspm sensitivity of BLA-to-NAc synapses (Fig. 3 B–G and Fig. S3 A–D, F, and G) as well as incubated cocaine craving (Fig. 3H and Fig. S3E). In contrast, in EE rats with in vivo LTD and additional 21-d SE housing, although BLA-to-NAc silent synapses returned to basal (control) levels, the Naspm sensitivity of BLA-to-NAc synapses remained low (Fig. 3 B–G and Fig. S3 A–D), and these rats (LTD-EE) did not redevelop incubation of cocaine craving (Fig. 3H and Fig. S3E). Thus, EE combined with BLA-to-NAc LTD achieved a long-lasting anti-incubation effect.
Fig. 3.
Long-lasting anti-incubation effects by LTD-EE combination. (A) Time line showing the time points of manipulations after withdrawal from saline or cocaine self-administration. (B and C) EPSCs evoked at −70 or +50 mV by optogenetic minimal stimulations (Left) over 100 trials (Right) from example recordings in saline-exposed (B) or cocaine-exposed (C) rats with 21-d SE after BLA-to-NAc LTD on withdrawal day 45 and subsequent 7-d EE. (D) Summarized results showing a low the percentage of silent synapses in BLA-to-NAc projections in all manipulation groups [saline-shamLTD, 7.6 ± 1.5; cocaine-shamLTD, 7.8 ± 2.4; saline-LTD, 13.3 ± 3.8; cocaine-LTD, 8.6 ± 2.9; F(1,13) = 0.9, P = 0.37, two-way ANOVA]. (E and F) Example EPSCs (Left) and time course (Right) before and during perfusion of Naspm in saline-exposed (E) or cocaine-exposed (F) rats with 21-d SE after BLA-to-NAc LTD on withdrawal day 45 and subsequent 7-d EE. (G) Summarized results showing that LTD-EE–induced abolishment of Naspm sensitivity of BLA-to-NAc synapses in cocaine-exposed rats persisted after an additional 21-d SE housing [F(3,13) = 3.5, P < 0.05, two-way ANOVA with repeated measures; P < 0.05, before vs. during Naspm in cocaine rats with shamLTD, Bonferroni posttest]. (H) Summarized result showing that LTD-EE-induced reduction in cocaine incubation also persisted over an additional 21-d SE [F(1,18) = 5.3, P < 0.05, two-way ANOVA with repeated measures; P < 0.01, withdrawal day 1 vs. day 73 in rats with shamLTD; P < 0.01, shamLTD vs. LTD on withdrawal day 73, Bonferroni posttest]. (I) Schemes showing the hypothesized dynamics of cocaine-generated silent synapses in the BLA-to-NAc projection after cocaine withdrawal, upon in vivo LTD and EE effects after LTD.
Fig. S3.
Additional results associated with Fig. 3. (A and B) EPSCs evoked at −70 or +50 mV by optogenetic minimal stimulations (Left) over 100 trials (Right) in example recordings from saline-exposed (A) and cocaine-exposed (B) rats with in vivo shamLTD on withdrawal day 45, subsequent 7-d EE, and additional 21-d SE. (C and D) Example EPSCs (Left) and the time course (Right) before and during perfusion of Naspm in saline-exposed (C) and cocaine-exposed (D) rats with shamLTD on withdrawal day 45, subsequent 7-d EE, and additional 21-d SE. (E) Normalized results showing that incubation of cocaine craving was intact in cocaine-exposed rats with in vivo shamLTD on withdrawal day 45, subsequent 7-d EE, and additional 21-d SE but was abolished in rats with in vivo LTD on withdrawal day 45, subsequent 7-d EE, and additional 21-d SE [shamLTD: n = 9, t(8) = 2.43, P = 0.04; LTD: n = 11, t(10) = 1.05, P = 0.32]. (F and G) Summarized results showing saline or cocaine self-administration training of rats used for A–D and Fig. 3 D and H (F) and Fig. 3 B–G (G).
Discussion
Our current results support a linear cellular cascade at BLA-to-NAc excitatory synapses following cocaine self-administration, withdrawal, in vivo LTD, and EE. Specifically, our findings suggest that: (i) cocaine self-administration generates silent synapses in the BLA-to-NAc projection; (ii) during withdrawal, silent synapses mature in this projection by recruiting CP-AMPARs; (iii) LTD re-silences these matured silent synapses by internalizing CP-AMPARs; and (iv) EE promotes insertion of nonCP-AMPARs to those regenerated silent synapses (Fig. 3I). The result is a sustained synaptic change in the BLA-to-NAc projection and a sustained reduction in cocaine seeking. Whereas this model is the most parsimonious explanation of the present data, other possibilities exist. For example, instead of insertion of nonCP-AMPARs, it is possible that EE promotes elimination of re-silenced synapses. Along this scenario, an earlier study shows that EE facilitates both LTP and LTD at excitatory synapses, indicating bidirectional effects of EE (36). In addition, EE activates microglia (37), which may promote synapse elimination. Although these possibilities cannot be ruled out, the extensive and coherent effects of EE on promoting circuitry formation and strengthening synapses (4) indicate that insertion of AMPARs is likely the predominant cellular effect of EE in cocaine-exposed rats after LTD.
If the switch from CP-AMPARs to nonCP-AMPARs is the major cellular consequence of LTD-EE treatment that mediates its anti-incubation effect, how does this switch change NAc neuronal function and associated behaviors? Answers to this question may lie in the unique properties of CP-AMPARs. One important feature of CP-AMPARs is their higher single-channel conductance compared with nonCP-AMPARs (22 vs. 8.8 pS) (38). Thus, newly matured silent synapses and potentially new BLA-to-NAc branches/projections, although a minority by number, are functionally predominant. As such, replacing CP-AMPARs with nonCP-AMPARs can sharply dilute the behavioral influence of these new, silent synapse-created circuits. Another feature of CP-AMPARs is their Ca2+ permeability. In CP-AMPAR–containing spines, [Ca2+] remains relatively high upon continuous synaptic activity in vivo. Ca2+-dependent signals that regulate postsynaptic properties, such as sensitization–desensitization of synaptic receptors, receptor conductance, and subcellular location of receptors, are therefore tonically activated, resulting in different forms of synaptic transmission and plasticity. In addition, many types of ion channels (e.g., Ca2+-activated K+ channels) are either directly activated by Ca2+ or regulated indirectly by Ca2+ signaling. Tonic activation/regulation of these ion channels may influence the propagation of synaptic responses from these CP-AMPAR–containing spines to soma. Thus, switching CP-AMPARs with nonCP-AMPARs in matured silent synapses, even without structural changes, may effectively alter the information flow through these synapses.
Why does EE selectively insert nonCP-AMPARs after the matured silent synapses were “reopened” by LTD in cocaine-exposed rats? In the hippocampus, developmentally generated silent synapses are prone to recruit GluA1-homomeric AMPARs (i.e., CP-AMPARs) via an experience-dependent process involving activation of NMDARs and CaMKII signaling (39, 40). Similarly, within the BLA and infralimbic projections to NAc, cocaine-generated silent synapses mature by recruiting CP-AMPARs, also likely associated with NMDAR/CaMKII-dependent processes (16, 27). After LTD induction, CP-AMPARs are likely to be transiently internalized (Fig. 1), and because of the high tonic activity of CaMKII and other signaling cascades (41), CP-AMPARs are reinserted shortly after LTD as a default route. In contrast, the synapse-promoting effects of EE are largely mediated by more long-lasting forms of structural and even transcriptional regulation, involving PSD-95, neurotrophins, and glial signals that promote synaptic maturation (4). These multidimensional synapse-nurturing effects of EE may compete and eventually predominate after in vivo LTD in cocaine-exposed rats, resulting in insertion of nonCP-AMPARs and attenuation of cocaine craving.
By detecting the dynamic evolution of cocaine-generated silent synapses, our present study reveals that priming the synaptic state in key NAc afferent pathways is essential for EE to achieve robust and sustained anti-relapse effects. These results provide a mechanistic basis to understand EE and related behavioral therapies for treating drug addiction.
Materials and Methods
Detailed experimental procedures are provided in SI Materials and Methods.
Subjects.
Male Sprague–Dawley rats (Harlan) at postnatal days 28–30 were used at the beginning of the experiments. The rats were used in all experiments in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh or Mount Sinai School of Medicine.
SE and EE housing.
In the SE housing condition, rats were singly housed in typical rodent cages (38 cm long × 26 cm wide × 18 cm high). In the EE housing condition, which was modified from previously established EE cages (42), rats were housed in a large plastic cage (93 cm long × 53.3 cm wide × 49.5 cm high), which contained objects including toys, tunnels of different shapes, running wheels, and a wooden ladder attached to a metal platform.
Behavioral Studies.
Drugs.
Cocaine HCl [Provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program] was dissolved in 0.9% NaCl saline. Ketamine and xylazine were mixed for anesthesia.
Viral delivery.
A 26-gauge injection needle was used to bilaterally inject 1 µL (0.2 µL/min) of the AAV2-ChR2-YFP solution via Hamilton syringe into the BLA [anterior and posterior (AP): –2.50 mm; medial and lateral (ML): ±4.80 mm; dorsal and ventral (DV): –8.50 mm].
i.v. self-administration training.
Cocaine self-administration training began 5–14 d after surgery. On day 1, rats were placed in the self-administration chamber for an overnight training session on a fixed-ratio 1 (FR1) reinforcement schedule. A nose poke into the active hole resulted in a cocaine infusion (0.75 mg/kg in 0.10 mL over 6 s) and illumination of a conditioned stimulus (CS) light inside the nose poke hole. The CS light remained on for 6 s, whereas the house light was illuminated for 20 s, during which nose pokes to the active hole were counted but resulted in no cocaine infusions. After the 20 s, the house light was turned off, and the next nose poke in the active hole resulted in a cocaine infusion again. Nose pokes in the inactive hole had no reinforcement consequences but were recorded. Rats that received at least 40 cocaine infusions during the overnight session were moved on into the 5-d self-administer procedure, in which, ∼24 h after the overnight training, rats were allow to self-administer cocaine 2 h/d for 5 consecutive days on a FR1 reinforcement schedule. Same or similar cocaine self-administration procedures and standards were used in our previous studies (43–45). Rats that did not meet the overnight number of infusions criterion (n = 5) were removed from the study.
Measurement of cue-induced cocaine seeking after withdrawal.
We assessed incubation of cue-induced cocaine craving in an extinction test (1 h) conducted after 1 or 45 d of withdrawal from cocaine self-administration. During the test, active nose pokes resulted in contingent delivery of the CS but not cocaine. For behavioral assays without electrophysiology, we used within-subject assessment (46, 47); the same rats were tested for cocaine seeking on withdrawal days 1 and 45, 46, 53, or 73. For electrophysiology experiments, we used between-subject assessments; different groups of rats were killed on withdrawal day 1, 45, 46, 53, or 73 without the extinction test.
Optogenetic Procedures.
For in vivo optical control of the BLA-to-NAc projection, two 105-µm core optic fibers were modified for the attachment to an internal cannula, creating the optical neural interface (ONI). The ONI was attached with a fiber channel/physical contact (FC/PC) adaptor to a 473-nm blue laser diode (IkeCool), and light pulses were generated through a stimulator (A-M Systems). The light intensity through the optical fiber, which was measured by a light sensor (S130A; Thor Labs), was adjusted to ∼10 mW. Before attaching to the ONI, the rat was briefly sedated with isoflurane. Once the rat was fully awake, an optogenetic LTD protocol was administered at 5 Hz × 3 min × 3 times, with a 5-min interval (laser pulse duration, 1.0 ms). Once this 19-min LTD protocol was concluded, the rat was placed in a testing chamber. Control rats were also briefly sedated with isoflurane, and a sham optic fiber was attached to the head-mounted guide cannula. After fully awake, the rats remained in their home cages for 19 min and were then placed into a testing chamber.
Data Acquisition and Analysis.
In all electrophysiology experiments, the data were coded such that the experimenters were not aware of the treatments of the animals when performing data analysis. Data were decoded after data analysis for final presentation. All results are shown as means ± SEM. Each experiment was replicated in at least three to four rats (one to three cells were recorded from each rat) for electrophysiological analysis and eight rats for behavioral tests. No data points were excluded unless specified in the experimental procedure. All results were analyzed using animal-based statistics. For experiments in which the end points were from individual cells, such as EPSCs, the percentage of silent synapses, and Naspm sensitivity (Figs. 1 D and F, 2 E and H, and 3 D and G), we used the averaged value of a parameter from all cells recorded from an animal to represent the parameter of this animal.
SI Materials and Methods
Subjects.
Male Sprague–Dawley rats (Harlan) at 28–30 d old were used at the beginning of the experiments. Rats were singly housed under a 12-h light/dark cycle (on at 0700 hours; off at 1900 hours). Temperature (22 ± 1°C) and humidity (60 ± 5%) were controlled. Behavioral experiments were performed during the daytime.
SE and EE housing.
In the SE housing condition, rats were singly housed in a typical rodent cage (38 cm long × 26 cm wide × 18 cm high), containing regular bedding without additional objects except two pieces of Kimwipe paper. Food and water were provided ad libitum. SE is the standard housing condition of the “home cages” in this study. In the EE housing condition, which was modified from previously established EE cages (42), rats were housed in a large plastic cage (93 cm long × 53.3 cm wide × 49.5 high), which contained objects including toys, tunnels of different shapes, running wheels, and a wooden ladder attached to a metal platform (Fig. 2A). The floor was covered with regular bedding. Food and water were hung on the lateral wall and available ad libitum.
Time points of measurements.
There were several key time points when we obtained experimental measurements and used abbreviations in the paper. (i) A 5-d self-administration procedure following an “overnight training”: in this procedure, rats were placed in the operant chambers overnight (∼12 h) and then returned to their home cages (SE) for 24 h, followed by the 5-d procedure; (ii) “withdrawal day 1”: we operationally defined that the time point 20–24 h after the last cocaine self-administration session was the withdrawal day 1; (iii) “withdrawal day 45”: we operationally defined that 43–47 d after the 5-d cocaine self-administration procedure was the withdrawal day 45; (iv) “right after in vivo LTD or shamLTD”: for behavioral experiments, rats were placed to the operant chambers immediately after LTD induction and disconnection of the optical fibers. The time elapsed from the end of the LTD induction to the onset of behavioral measurement was within 2 min. For electrophysiological measurements, rats were anesthetized and decapitated immediately after LTD induction for slice preparation. The time elapsed from the end of in vivo LTD to anesthesia was within 1 min. Brain slices were recorded 0.5–7 h after slice preparation; (v) “24 h after LTD”: immediately after in vivo LTD induction and disconnection of optical fibers, rats were placed to SE or EE cages for 23–25 h before the behavioral tests or slice preparation; and (vi) “after 7-d EE/SE”: right after in vivo LTD or shamLTD, rats were placed in EE/SE chambers for 7 d. After this, rats were divided into three groups. Group 1 was transferred to SE cages and housed there for another 21 d (Fig. 3). Group 2 was placed into operant chambers for behavioral tests (Fig. 2). Group 3 was used for slice preparation and electrophysiology (Fig. 2).
Viral Vectors.
Recombinant adeno-associated vectors (AAV2) expressing venus-tagged ChR2 H134R were pseudotyped with AAV1/2 capsid proteins. HEK293T cells were cotransfected with the plasmids pF6 (adenoviral helper plasmid), pRVI (cap and rep genes for AAV serotype 2), pH21 (cap gene for AAV serotype 1 and rep gene for serotype 2), and the AAV plasmid, using linear polyethylenimine-assisted transfection (45). The helper plasmids were kindly provided by M. Schwarz, Max-Planck Institute, Heidelberg (48). Cultures grown in DMEM (Biochrom) with 10% (vol/vol) substituted FBS (no. S0115; Biochrom) were harvested from 15 cm × 15 cm dishes after 48 h. AAVs were isolated and purified as previously described (48). Briefly, HEK293T cells were lysed with sodium desoxycholate and repeated freeze-thaw cycles in the presence of Benzonase-Nuclease HC (Novagen). From the supernatant, AAVs were isolated by iodixanol gradient centrifugation from the 40% and 54% interphase. AAVs were then desalted by ultrafiltration, filtered through 0.2-μm Millex-GV filter units (Millipore), and stored at 4 °C in 500 µL of PBS with 10 mM MgCl2 and 25 mM KCl. The ChR2 gene was packaged with an YFP gene into a recombinant, replication-defective form of AAV.
Behavioral Studies.
Drugs.
Cocaine HCl (Provided by the NIDA Drug Supply Program) was dissolved in 0.9% NaCl saline. Ketamine and xylazine were mixed for anesthesia (purchased from Drug Enforcement Administration-designated vendor at the University of Pittsburgh).
Viral delivery.
A 26-gauge injection needle was used to bilaterally inject 1 µL (0.2 µL/min) of the AAV2-ChR2-YFP solution via Hamilton syringe into the BLA (AP: –2.50 mm; ML: ±4.80 mm; DV: –8.50 mm), using a Thermo Orion M365 pump. Injection needles were left in place for 5 min following injection. ChR2-YFP was given 1–2 wk before any experimental manipulation to ensure maximal infection; therefore, electrophysiological analyses were conducted on withdrawal day 1 or 45 after the last section of cocaine/saline self-administration.
Catheter implantation.
Self-administration surgery was as described in refs. 43 and 49. Briefly, a silastic catheter was inserted into the right auricle through the external jugular vein, and the distal end was led s.c. to the back between the scapulas. Catheters are constructed from silastic tubing (∼5 cm; inner diameter, 0.020 inch; outer diameter , 0.037 inch) connected to a Quick Connect Harness (SAI Infusion). Rats were allowed to recover for 5–14 d. During recovery, the catheter was flushed daily with 0.1 mL of heparin (10 U/mL) and gentamicin (5 mg/mL) in sterile saline to help protect against infection and catheter occlusion.
Self-administration apparatus.
Experiments were conducted in operant-conditioning chambers enclosed within the sound-attenuating cabinets (Med Associates). Each chamber contains an active and inactive nose poke holes, a food dispenser, a CS light in each nose poke hole, and a house light. No food or water was provided in the chamber during the training sessions.
i.v. self-administration training.
Cocaine self-administration training began 5–14 d after surgery. On day 1, rats were placed in the self-administration chamber for an overnight training session on a FR1 reinforcement schedule. A nose poke into the active hole resulted in a cocaine infusion (0.75 mg/kg in 0.10 mL over 6 s) and illumination of a CS light inside the nose poke hole. The CS light remained on for 6 s, whereas the house light was illuminated for 20 s, during which nose pokes to the active hole were counted but resulted in no cocaine infusions. After the 20 s, the house light was turned off, and the next nose poke in the active hole resulted in a cocaine infusion again. Nose pokes in the inactive hole had no reinforcement consequences but were recorded. Rats that received at least 40 cocaine infusions during the overnight session were moved on into the 5-d self-administer procedure, in which, ∼24 h after the overnight training, rats were allow to self-administer cocaine 2 h/d for 5 consecutive days on a FR1 reinforcement schedule. Same or similar cocaine self-administration procedures and standards were used in our previous studies (43–45). Rats that did not meet the overnight number of infusions criterion (n = 5) were removed from the study.
Measurement of cue-induced cocaine seeking after withdrawal.
We assessed incubation of cue-induced cocaine craving in an extinction test (1 h) conducted after 1 or 45 d of withdrawal from cocaine self-administration. During the test, active nose pokes resulted in contingent delivery of the CS but not cocaine. For behavioral assays without electrophysiology, we used within-subject assessment (46, 47); the same rats were tested for cocaine seeking on withdrawal days 1 and 45. For electrophysiology experiments, we used between-subject assessments; different groups of rats were killed on either withdrawal days 1 or 45, without the extinction test.
Optogenetic Procedures.
Construction of ONI.
For in vivo optical control of the BLA-to-NAc projection, two 105-µm core optic fibers were modified for the attachment to an internal cannula, creating the ONI. When the ONI was secured in vivo to the guide cannula, the stripped fiber extended 1.0 mm past the tip of the cannula. This experimental setup was based on a previously verified setup (50) with slight modifications. The ONI was secured in vivo to the cannula head-mount only during stimulation.
In vivo stimulation.
The ONI was attached with an FC/PC adaptor to a 473-nm blue laser diode (IkeCool), and light pulses were generated through a stimulator (A-M Systems). The light intensity through the optical fiber, which was measured by a light sensor (S130A; Thor Labs), was adjusted to ∼10 mW. Before attaching to the ONI, the rat was briefly sedated with isoflurane. Once the rat was awake, an optogenetic LTD protocol was administered at 5 Hz × 3 min × 3 times, with a 5-min interval (laser pulse duration, 1.0 ms). Once this 19-min LTD protocol was concluded, the rat was placed in a testing chamber. Control rats were also briefly sedated with isoflurane, and a sham optic fiber was attached to the head-mounted guide cannula. After awake, the rats remained in their home cages for 19 min and were then placed into a testing chamber.
In vitro stimulation.
All evoked responses were delivered using an IkeCool laser at 473 nM, ∼10 mW, through the microscope’s 40× objective. The duration (0.01–1 ms) of the light pulse was decreased until an optimally evoked response was achieved.
Electrophysiological Studies.
Slice preparation.
The rat was first anesthetized with isoflurane and subsequently transcardially perfused with 4 °C cutting solution (in mM: 135 N-methyl-d-glucamine, 1 KCl, 1.2 KH2PO4, 0.5 CaCl2, 1.5 MgCl2, 20 choline-HCO3, 11 glucose; pH adjusted to 7.4 with HCl; and saturated with 95% O2/5% CO2). The rat was then decapitated, and the brain was removed and glued to a block before being sliced using a Leica VT1200s vibratome in a 4 °C cutting solution. Coronal slices (300-µm thick) were cut such that the preparation contained the signature anatomical landmarks (e.g., the anterior commissure) that clearly delineated the NAc subregions. After allowing 1–2 h for recovery, one slice was transferred from a holding chamber to a submerged recording chamber, where the slice was continuously perfused with oxygenated artificial cerebral spinal fluid (aCSF), and was maintained at 30 ± 1 °C.
Drugs.
d-Aminophosphonovaleric acid (APV) was used at a concentration of 50 µM to inhibit NMDAR-mediated responses. Picrotoxin (100 µM) was used to inhibit GABAAR-mediated responses. 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) (5 µM) was used to inhibit AMPAR-mediated responses. Naspm (200 µM) was used to selectively inhibit GluA2-lacking AMPARs. APV, NBQX, and Naspm were purchased from R&D Systems, and all other chemicals were purchased from Sigma-Aldrich.
Whole-cell recordings.
Standard whole-cell voltage-clamp recordings were used with a MultiClamp 700B amplifier (Molecular Devices). During recording, slices were superfused with aCSF that was heated to 30 ± 1 °C by passing the solution through a feedback-controlled in-line heater (Warner Instruments) before entering the chamber. Recordings were made under visual guidance (40×, differential interference contrast optics) with electrodes (3–5 MΩ). Expression of ChR2-YFP in neurons or processes was verified using an Olympus BX51WI fluorescent/DIC microscope before recordings. For all recordings, the series resistance was 8–14 MΩ and was left uncompensated. The series resistance was monitored continuously during all recordings, and a change beyond 15% resulted in exclusion of the cell from data analyses. The decay of the test pulse-induced capacitance currents, the kinetics of EPSCs, leak currents, and stimulation artifacts were also simultaneously monitored, abnormalities of which precluded the subsequent use of these recordings. Synaptic currents were recorded with a MultiClamp 700B amplifier (Molecular Devices), filtered at 3 kHz, amplified five times, and then digitized at 20 kHz with a Digidata 1440A analog-to-digital converter (Molecular Devices).
Silent synapse analysis.
Neurons in the NAc shell were randomly selected for recording. Minimal stimulation experiments were performed as previously described (20, 24, 51). After obtaining a small (<50 pA) EPSC at –70 mV, the duration of the light pulse was reduced by small increments to the point that failures vs. successes of synaptically evoked events could be clearly distinguished visually. Pulse duration and frequency were then kept constant for the rest of the experiment. For each cell, 50–100 traces were recorded at –70 mV, and 50–100 traces were recorded at +50 mV. Recordings were then repeated at –70 and +50 mV for another one or two rounds. Each cell was recorded >two rounds. Only cells with relatively constant failure rates (changes <10%) between rounds were included for calculating the percentage of silent synapses. The percentage of silent synapses was assessed using the minimal stimulation assay (20). This assay is based on two theoretical assumptions: the presynaptic release sites are independent, and the release probability (P) across all synapses, including silent synapses, is identical. As such, the total number (t) of recorded synapses is equal to the sum of the number of silent (s) and nonsilent synapses (n), t = n + s. At −70 mV, the failure rate of this set of synapses (F−70) is determined by n and P: F−70 = (1 − P)n (Eq. 1). At +50 mV, the failure rate (F+50) is determined by n + s and P: F+50 = (1 − P)(n + s) (Eq. 2). These two equations can be transferred to (Eq. 1′) Ln (F−70) = nLn (1 − P); and (Eq. 2′) Ln (F+50) = (n + s)Ln (1 − P). Thus (Eq. 3), n = Ln (F−70)/Ln (1 − P); and (Eq. 4) s + n = Ln (F+50)/Ln(1 − P). Solving Eqs. 3 and 4 for Eq. 5: s = 1 − Ln(F−70)/Ln(F+50). Eq. 5 was thus used to estimate the percentage of silent synapses (20).
Data Acquisition and Analysis.
In all electrophysiology experiments, the data were coded such that the experimenters were not aware of the treatments of the animals when performing data analysis. Data were decoded after data analysis for final presentation. All results are shown as means ± SEM. Each experiment was replicated in at least three to four rats (one to three cells were recorded from each rat) for electrophysiological analysis and eight rats for behavioral tests. No data points were excluded unless specified in the experimental procedure. All data were analyzed using animal-based statistics. For experiments in which the endpoints were from individual cells, such as EPSCs, the percentage of silent synapses, and Naspm sensitivity (Figs. 1 D and F, 2 E and H, and 3 D and G), we used the averaged value of a parameter from all cells recorded from an animal to represent the parameter of this animal. Note that in experiments involving Naspm perfusion, we observed decreases, no change, and increased in the amplitudes of EPSCs from individual NAc MSNs during Naspm perfusion. In all presented recordings, cells with different directions of pharmacological responses exhibited stable baselines and minimal changes in the access resistance, leak current, kinetics of EPSCs, or stimulation artifacts throughout the recording processes. These cells were included in data analyses and statistical evaluations in a fully unbiased manner.
A total of 257 rats were used, including 120 for recordings and 137 for behavioral test. A total of 56 rats were excluded because of catheter fallouts or unsuccessful acquisition of cocaine self-administration (n = 8), no/low viral expression (n = 17), inaccurate cannulations (n = 18), damaged optical fibers (n = 9), or overdosed isoflurane (n = 4).
Acknowledgments
This research was supported by National Institutes of Health (NIH) Extramural Grants DA035805 (to Y.H.), MH101147 (to Y.H.), DA008227 (to E.J.N.), DA014133 (to E.J.N.), DA023206 (to Y.D.), DA034856 (to Y.D.), and DA040620 (to E.J.N. and Y.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524739113/-/DCSupplemental.
References
- 1.Renner MJ, Rosenzweig MR. The golden-mantled ground squirrel (Spermophilus lateralis) as a model for the effects of environmental enrichment in solitary animals. Dev Psychobiol. 1987;20(1):19–24. doi: 10.1002/dev.420200106. [DOI] [PubMed] [Google Scholar]
- 2.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 3.Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92(3):377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7(9):697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 5.Smith JK, Neill JC, Costall B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology (Berl) 1997;131(1):23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- 6.Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 2011;214(2):557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofford RS, Prendergast MA, Bardo MT. Pharmacological manipulation of glucocorticoid receptors differentially affects cocaine self-administration in environmentally enriched and isolated rats. Behav Brain Res. 2015;283:196–202. doi: 10.1016/j.bbr.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neisewander JL, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20(2):798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiel KJ, et al. Environmental enrichment counters cocaine abstinence-induced stress and brain reactivity to cocaine cues but fails to prevent the incubation effect. Addict Biol. 2012;17(2):365–377. doi: 10.1111/j.1369-1600.2011.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiel KJ, Pentkowski NS, Peartree NA, Painter MR, Neisewander JL. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression. Neuroscience. 2010;171(4):1187–1196. doi: 10.1016/j.neuroscience.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauvet C, Goldberg SR, Jaber M, Solinas M. Effects of environmental enrichment on the incubation of cocaine craving. Neuropharmacology. 2012;63(4):635–641. doi: 10.1016/j.neuropharm.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34(13):2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: When, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BR, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16(11):1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behav Brain Res. 2009;199(1):89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: Recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229(3):453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: Implications for the expression of LTP. Neuron. 1995;15(2):427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375(6530):400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 21.Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381(6577):71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 22.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9(11):813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanse E, Seth H, Riebe I. AMPA-silent synapses in brain development and pathology. Nat Rev Neurosci. 2013;14(12):839–850. doi: 10.1038/nrn3642. [DOI] [PubMed] [Google Scholar]
- 24.Huang YH, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63(1):40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koya E, et al. Silent synapses in selectively activated nucleus accumbens neurons following cocaine sensitization. Nat Neurosci. 2012;15(11):1556–1562. doi: 10.1038/nn.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci. 2014;35(8):374–383. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma YY, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83(6):1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 29.Foster TC, Gagne J, Massicotte G. Mechanism of altered synaptic strength due to experience: Relation to long-term potentiation. Brain Res. 1996;736(1-2):243–250. doi: 10.1016/0006-8993(96)00707-x. [DOI] [PubMed] [Google Scholar]
- 30.Green EJ, Greenough WT. Altered synaptic transmission in dentate gyrus of rats reared in complex environments: Evidence from hippocampal slices maintained in vitro. J Neurophysiol. 1986;55(4):739–750. doi: 10.1152/jn.1986.55.4.739. [DOI] [PubMed] [Google Scholar]
- 31.Naka F, Narita N, Okado N, Narita M. Modification of AMPA receptor properties following environmental enrichment. Brain Dev. 2005;27(4):275–278. doi: 10.1016/j.braindev.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Lichti CF, et al. Environmental enrichment alters protein expression as well as the proteomic response to cocaine in rat nucleus accumbens. Front Behav Neurosci. 2014;8:246. doi: 10.3389/fnbeh.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung CK, Herms J. Structural dynamics of dendritic spines are influenced by an environmental enrichment: An in vivo imaging study. Cereb Cortex. 2014;24(2):377–384. doi: 10.1093/cercor/bhs317. [DOI] [PubMed] [Google Scholar]
- 34.Bednarek E, Caroni P. β-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron. 2011;69(6):1132–1146. doi: 10.1016/j.neuron.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 35.Loweth JA, Tseng KY, Wolf ME. 2014. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology 76 Pt B:287–300.
- 36.Artola A, et al. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci. 2006;23(1):261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 37.Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 38.Ozawa S, Iino M, Tsuzuki K. Two types of kainate response in cultured rat hippocampal neurons. J Neurophysiol. 1991;66(1):2–11. doi: 10.1152/jn.1991.66.1.2. [DOI] [PubMed] [Google Scholar]
- 39.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105(3):331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 40.Zhu JJ, Malinow R. Acute versus chronic NMDA receptor blockade and synaptic AMPA receptor delivery. Nat Neurosci. 2002;5(6):513–514. doi: 10.1038/nn0602-850. [DOI] [PubMed] [Google Scholar]
- 41.Anderson SM, et al. CaMKII: A biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11(3):344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 42.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 43.Mu P, et al. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30(10):3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otaka M, et al. Exposure to cocaine regulates inhibitory synaptic transmission in the nucleus accumbens. J Neurosci. 2013;33(16):6753–6758. doi: 10.1523/JNEUROSCI.4577-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suska A, Lee BR, Huang YH, Dong Y, Schlüter OM. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proc Natl Acad Sci USA. 2013;110(2):713–718. doi: 10.1073/pnas.1206287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: A review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 47.Lu L, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66(2):137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilpel N, Landeck N, Klugmann M, Seeburg PH, Schwarz MK. Rapid, reproducible transduction of select forebrain regions by targeted recombinant virus injection into the neonatal mouse brain. J Neurosci Methods. 2009;182(1):55–63. doi: 10.1016/j.jneumeth.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 49.Brown TE, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31(22):8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330(6002):385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18(2):269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]