Abstract
Perivascular epithelioid tumour (PEComas) of the gynaecological tract are rare tumours which were first recognised and diagnosed within the last twenty years. They represent a unique diagnostic challenge with regard to their accurate and reproducible distinction from more common entities such as smooth muscle tumours of the uterine corpus. In this review article we trace the development of the concept of the PEComa tumour family, highlight what is known about extra-gynaecological tract PEComa at an immunohistochemical, molecular and therapeutic level and then present a summary of all reported cases of gynaecological tract PEComa to date. In the summary, we highlight rare subtypes of gynaecological tract PEComa, and compare the performances of extant prognostic classification systems for malignancy in these tumours.
Demonstration of the expression of the melanocytic marker HMB-45 in angiomyolipoma (AML)1, 2 and clear cell “sugar” tumours (CCST) of lung3 led to the concept of PEComa as a family of tumours occurring at many sites and characterised by the presence of an epithelioid cell of mixed myo-melanocytic immunophenotype. This family included previously recognised entities such as AML, CCST, lymphangioleiomyomatosis (LAM) and clear cell myomelanocytic tumour of the falciform ligament/ligamentum teres (CCMT). Subsequently, sporadic HMB-45-positive clear cell tumours of other sites such as pancreas4 and uterus5 were described.
The cell-of-origin of PEComas has not been unequivocally established. Early reports speculated tumour origin from vessel walls,6 or from a “peculiar muscle cell” based on morphology and expression of myomelanocytic markers.3 Embryological and in vitro studies have provided evidence for origin from a neural-crest stem cell that is capable of myoid and melanocytic differentiation and which can occur in embryologic development and tissue repair.7–11
Tuberous Sclerosis Complex (TSC) is characterised by the development of tumours at various sites, including brain, heart and kidney. Genetically, TSC is associated with mutations in TSC1 or TSC2 (located on 9q 34 and 16q13 respectively), leading to impaired production of the proteins hamartin and tuberin respectively. TSC1 and TSC2 interact as heterodimers that inhibit the mTOR pathway; their inactivation leads to increased cell growth and proliferation.12 The prototypical PEComa associated with TSC is renal AML. While ~80% of patients with TSC have AML, <50% of all renal AMLs and <10% of extrarenal AML occur in patients with TSC.13 The majority of cases of PEComatosis (widespread multifocal macroscopic and microscopic nodules of PEComa cells involving multiple sites in the gynaecological tract and pelvis) occurred in TSC patients,14–18 and the uterus of a single TSC patient harboured a subserosal AML, a sclerosing PEComa of lower uterine segment and diffuse LAM of the uterine corpus.19 Thus, TSC-associated loss of function of TSC1/TSC2 can lead to a phenotypic spectrum (classical AML-like, classical PEComa, sclerosing PEComa, LAM) that is also recognised in sporadic PEComas.
Histologically, PEComa is characterised by the presence of predominantly epithelioid cells with clear, granular or eosinophilic cytoplasm, arranged in nests or sheets, with little intervening stroma. Many PEComas have a low mitotic rate of ≤1 per 50 high power fields (HPFs). Areas of necrosis may be seen. PEComas demonstrate varying levels of nuclear pleomorphism, including multinucleate giant cells and “spider cells”, analogous to those seen in cardiac rhabdomyoma.20 Other features include macronucleoli and intranuclear pseudoinclusions.20 While most reported PEComas have been epithelioid, they show a cytological spectrum from purely spindled to purely epithelioid (and combinations of the two). Rare features include sex-cord-like features21 and prolactin secretion.22, 23
Sclerosing PEComa occurs predominantly in the retroperitoneum of women, and rarely in the uterus and pelvis. They show cords of epithelioid cells within densely sclerotic stroma. Areas of intimate association between tumour cells and blood vessels are often identified.24
PEComas are defined by the immunohistochemical (IHC) co-expression of myoid markers (SMA, desmin, caldesmon) and melanocytic markers (HMB-45, Melan-A, MiTF). Expression varies with morphology: tumours with predominant spindle cell morphology show strong expression of muscle markers and limited expression of melanocytic markers; predominantly epithelioid tumours may strongly express melanocytic markers with limited muscle marker expression. Recently, cathepsin K, a transcriptional target of the MiTF family, emerged as a sensitive marker for PEComa.25–27 However, cathepsin K is not specific for PEComa, and is commonly positive in melanoma, alveolar soft part sarcoma, and mesenchymal tumours, including leiomyosarcoma.28
Renal and extrarenal AMLs/PEComas exhibit true melanocytic differentiation in the form of melanosomes at various stages of development,29, 30 and positive Masson-Fontana staining. Grossly and microscopically pigmented examples of PEComa have been reported.31–34
Limited genomic studies have suggested that loss of heterozygosity (LOH) at the TSC2 locus may play an important role in sporadic PEComa tumourigenesis, similar to its role in TSC-related and sporadic renal AML35–37 and sporadic pulmonary LAM.38 Comparative genomic hybridization (CGH) on 9 PEComas (including one uterine case) showed multiple chromosomal imbalances, with 16p loss in 6 cases and X-chromosomal gains in 6 cases.39 Malinowska et al40 demonstrated loss of immunohistochemical expression of tuberin in 4 PEComas and LOH or allelic loss of at least one TSC2 microsatellite marker in two of those four cases. Kenerson et al41 reported immunohistochemical evidence of mTOR pathway activation (increased levels of phospho-p70S6K, reduced levels of phospho-AKT and loss of tuberin expression) in sporadic AMLs and extrarenal PEComas. Based on these limited studies, several clinical trials of mTOR pathway inhibitors in malignant PEComa have been initiated.
PEComa of the gynaecological tract
Uterine Corpus
To date, 78 cases of uterine corpus PEComas have been reported in the English language literature (Table 1).5, 16–20, 24, 39, 42–72
TABLE 1.
Summary of clinico-pathological features of PEComas of the gynaecological tract
| Uterine corpus | Cervix | Vagina | Adnexa | Broad ligament | Vulva | |
|---|---|---|---|---|---|---|
|
| ||||||
| N | 78 | 11 | 7 | 6 | 5 | 1 |
|
| ||||||
| Age at diagnosis median (range) [years] | 47.5 (9–79) | 46 (25–61) | 28 (6–57) | 49 (33–63) | 25 (24–57) | 20 |
|
| ||||||
| Associations | ||||||
| TSC | 5 (6%) | 1 (9%) | - | 2 (33%) | - | |
| Other PEComa family tumors | LAM (n=3) | - | - | - | - | |
| PEComatosis | 4 (5%) | 1 (9%) | - | - | - | |
|
| ||||||
| Tumour size – median (range) [cm] | 5cm (0.2–30cm) (n=67) | 3.9 (1–12) (n=9) | 3 (1.5–9) (n=5) | 4.2 (2.5–15) (n=6) | 11.5 (4–17) (n=5) | 2 |
|
| ||||||
| Morphology | ||||||
| Sclerosing PEComa | 9 (12%) | 0 | 0 | 2 (33%) | 1 (20%) | |
| Cell shape | - | |||||
| Epithelioid | 43/74 (58%) | 6/10 (60%) | 6/7 (86%) | 3/6 (50%) | 2/4 (50%) | |
| Spindle | 1/74(1%) | 4/10 (40%) | - | 1/4 (25%) | ||
| Epithelioid + spindle | 30/74 (41%) | 1/7 (14%) | 3/6 (50%) | 1/4 (25%) | ||
|
| ||||||
| Necrosis | 31/74 (42%) | 4/10 (40%) | 1/5 (20%) | 2/6 (33%) | 3/4 (75%) | - |
|
| ||||||
| Nuclear atypia | Significant, severe or extensive in 25/62 (40%) | Moderate to severe in 7/10 (70%) | Severe atypia not seen (5/5, 0%) | “Severe”/“significant” atypia in 4/6 (67%) | Varying from none to severe | None |
|
| ||||||
| Mitotic activity | <=1 (38, 52%) Rare (7, 10%) 2–222 per 50HPF (28, 38%) (n=73) | “zero”, “rare” or ≤1/50HPF (8/9, 89%) | Absent or “rare” 4/5 (80%)* | Variable: ≤1 (3/6) to 97/50HPF | Rare | |
|
| ||||||
| IHC | (see also Table 2) | |||||
| HMB-45 | 71/72 (99%) | 8/8 (100%)## | 6/6 (100%)# | 6/6 (100%) | 4/4 (100%) | 1/1 (100%) |
| Melan-A | 21/46 (46%) | 4/5 (80%) | 1/4 (25%) | 3/3 (100%)@ | 1/3 (33%) | |
| MiTF | 14/21 (67%) | 0/1 (0%) | 1/1 (100%)$ | |||
| SMA | 53/68 (80%) | 5/8 (63%) | 2/4 (50%) | 4/5 (80%) | 4/4 (100%)^ | |
| Desmin | 39/62 (63%) | 3/5 (60%) | 0/2 (0%) | 4/6 (67%) | 1/2 (50%)^^ | 0/1 (0%) |
| Caldesmon | 17/22 (77%) | 2/2 (100%) | ||||
| Cytokeratin | 2/43 (5%) | |||||
| ER | 10/19 (53%) | |||||
| PR | 11/13 (85%) | |||||
| PAX8 | 0 | |||||
| CD10 | 4/28 (14%) | |||||
| CD34 | 0 | |||||
| Vimentin | 11/18 (61%) | |||||
| Inhibin | 1/20 (5%) | |||||
|
| ||||||
| Follow-up | ||||||
| Duration median (range) [months] | 20 (1.5–168) | 28 (9–42) | 14.5 (3–54) | 9 (4–72) | 13.5 (11–18) | 48 |
| Died of disease | 10/63 (16%) | 1/9 (11%)78 | - | 1/4 (25%) | - | - |
| No evidence of disease | 44/63 (70%) | 8/9 (89%) | 6/7 (86%) | 3/4 (75%) | 2/4 (50%) | 1/1 (100%) |
| Alive with disease | 9/63 (14%) | - | 1/7 (14%) | 2/4 (50%) | - | |
A single TFE3-translocation-associated case had a mitotic count of 5 per 50HPF
Strong or diffuse in 3/3 cases;
Strong or diffuse in 6/6 cases
focally weakly positive in 2 of 3 cases, and was strongly positive in 1 of 3 cases
weakly positive in less than half of tumour cells
focally positive in 2 tumours;
focally positive
Tumour morphology was described in 74 cases (Table 1). A summary of the reported IHC findings is presented in Table 2. A mixed myo-melanocytic phenotype, with positivity for at least one melanocytic and one muscle marker, was confirmed in 66/73 (90%) cases. The remaining tumours44, 45, 48, 71 (including 3 TFE3-translocation-associated PEComas71) were HMB45-positive but negative for multiple muscle markers.71 On ultrastructural examination, 7/11 (64%) tumours5, 42, 48, 52, 59, 70 showed evidence of melanocytic differentiation (presence of premelanosomes or melanosomes).
TABLE 2.
Expression of myoid and melanocytic immunohistochemical markers in uterine corpus PEComas
| IMMUNOHISTOCHEMISTRY RESULT | EXTENT OF POSITIVITY | ||||
|---|---|---|---|---|---|
| IHC | n | POSITIVE (%) | n | <50% OF CELLS | ≥50% OF CELLS |
| HMB45 | 72 | 71/72 (99%) | 57 | 31/57 (54%) | 26/57 (46%) |
| Melan-A | 46 | 21/46 (46%) | 20 | 13/20 (65%) | 7/20 (35%) |
| MiTF | 21 | 14/21 (66%) | 12 | 5/12 (42%) | 7/12 (58%) |
| S100 | 48 | 5/48 (10%) | |||
| Tyrosinase | 3 | 0/3 (0%) | |||
| SMA | 68 | 53/68 (80%) | 39 | 16/39 (41%) | 23/39 (59%) |
| Desmin | 62 | 39/62 (63%) | 18 | 7/18(39%) | 11/18 (61%) |
| Caldesmon | 22 | 17/22 (77%) | 5 | 3/5 (60%) | 2/5 (40%) |
| Cathepsin | 12 | 12/12 (100%) | |||
| Myosin/MSA | 4 | 2/4 (50%) | |||
Reported chromosomal copy number alterations include trisomy X (n=1)61 and multiple gains and losses including 16p11.1-p13.3, which contains the TSC2 locus (n=1).39 Conversely, no evidence of LOH at the TSC1 or TSC2 loci were identified in another PEComa.52
Twelve patients had evidence of tumour metastasis at the time of diagnosis.20, 42, 44, 54, 55, 58, 60, 69 Median survival for those who died of disease was 20 months (Table 1).
Cervix
Eleven cervical PEComas have been reported.28, 33, 53, 73–79 One tumour demonstrated X polysomy and rearrangement of TFE3,33 while another tumour showed bi-allelic somatic deletion of TSC1.78 One patient (11%) recurred locally within 4 months, but had no evidence of disease at 19 months post-diagnosis (Table).79
Vagina
Seven vaginal PEComa have been reported20, 53, 67, 71, 80–82 (Table 1). Three patients were aged less than 18 years.80–82
Adnexa
6 adnexal PEComas have been reported to date.20, 32, 83–85 (Table 1). A mixed myo-melanocytic immunophenotype was demonstrated in 5 cases.20, 84, 85 TFE3 rearrangement was identified in one tumour.32 A 63-year-old patient who died of disease at four months had a 15cm sclerosing PEComa.84 While the presence of severe atypia was noted, the presence of necrosis and mitotic rate were not recorded.
Broad Ligament
Five PEComas of the broad ligament have been reported27, 31, 34, 53, 86 (Table 1). A mixed myo-melanocytic immunophenotype was demonstrated in all 4 tested tumours.27, 31, 34, 86 Ultrastructural studies in a single tumour showed pre-melanosomes, consistent with melanocytic differentiation.31
Vulva
There is a single case report of a vulval/perineal PEComa (Table 1).87 Ultrastructural studies were negative for pre-melanosomes.
Subtypes of PEComa in the Gynaecological Tract
PEComatosis
Six cases of PEComatosis have been described in the gynaecological tract.16–18, 66, 76, 88 Median patient age was 43.5 (range 29–70) years. Four (67%) patients had TSC.16–18, 66 The dominant tumour mass was located in the uterine corpus (n=4),16–18, 66 vaginal remnant post-hysterectomy (n=1)88 and cervix (n=1).76 Other sites involved by PEComatosis included ovary (n=4), lymph nodes, broad ligament, omentum, peritoneum and small bowel wall. Dominant tumour size ranged from 0.8 to 6 cm (mean 2.9cm).
The dominant tumours showed mixed epithelioid and spindled morphology (3/4 tumours), while one tumour was wholly spindled.16 Necrosis was present in 1/5 cases,18 and mitoses ranged from “rare” and ≤1 to 20 per 50 HPF. Atypia was noted in four tumours (moderate in 3). HMB-45 was positive in 5/5 cases (“strongly” positive in 3/4 where extent/intensity was reported), while Melan-A was strongly positive in 3/3 cases. SMA was positive in 6/6 cases (3/3 strongly positive), while desmin was positive in 3/4 cases. No ultrastructural studies have been reported in PEComatosis. One case showed balanced chromosomal studies.76
Follow-up information was reported in 4/6 cases;16, 66, 76, 88 3/4 patients had no evidence of disease after 12 to 168 months of follow-up. One patient was alive with disease at 12 months.88
Sclerosing PEComa
Reported case involved the uterine corpus (n=9),16, 19, 24, 43, 64 adnexa (n=2)84, 85 and broad ligament (n=1).27 Two patients had TSC,16, 19 and one patient had PEComatosis.16 Median patient age (n=11) at diagnosis was 46 (range 29–63) years. Mean tumour size (n=10) was 3.75cm (range 0.8–15cm). Four tumours were purely epithelioid, 1 was spindled and 1 was mixed epithelioid-spindled. No necrosis or atypia were identified (n=6) and no mitotic activity was seen (n=5).
IHC for HMB-45 was positive in 9/9 tumours (“strong” or “diffuse” positivity in 6/8). Melan-A was negative in 5/6 cases while MiTF was negative in 5/5 cases. SMA was strongly and/or diffusely positive in 8/8 tested tumours, while 7/7 tested tumours were positive for desmin (6/7 “strong” or “diffuse”). No ultrastructural evidence of melanocytic differentiation was seen in a single tumour.43
Nine patients had no disease recurrence with a median follow-up of 19.5 months (range 12–168 months).16, 19, 24, 43, 64, 84 One patient died of her disease four months after diagnosis.84
TFE3 translocation-associated PEComa
Transcription factor E3 (TFE3) is a member of the microphthalmia-associated family of transcription factors (MiTF). Translocations involving the TFE3 locus at Xp11.2 have been reported in epithelioid clear cell tumours such as alveolar soft part sarcoma and Xp11.2 translocation-associated renal cell carcinoma. In recent years, 18 cases of TFE3 translocation-associated PEComa have been reported in kidney,25, 89 bladder,90 colon,91, 92 pelvic soft tissue,25 ovary,32 vagina,71 and uterus.58, 71, 91 These tumours appear to be characterised by predominantly epithelioid morphology without pleomorphism, and immunohistochemical positivity for HMB-45, TFE3 and Cathepsin K, and negativity for MiTF,91, 92 SMA and desmin.25, 32, 71, 91, 92 This suggests that TFE3-positive PEComa may represent a distinct subgroup within the PEComa family.71 Activation of the mTOR pathway may not necessarily play a role in these tumours, which has implications with respect to patient entry into clinical trials of mTOR pathway inhibitors.40
PEComas associated with TFE3 translocations are immunoreactive for TFE3, but the converse is not necessarily true; for example, 3 gynaecological tract PEComas which were TFE3-positive on IHC were negative for TFE3 translocation on FISH.71 Since TFE3 is ubiquitously expressed at a low level in many cell types, the use of sensitive immunohistochemical techniques may yield positive IHC results in tumours which lack TFE3 translocations.91 Therefore, weak IHC staining for TFE3 on should be interpreted with caution. At present, our approach to TFE3-immunoreactive tumours is to perform FISH to confirm the presence of the translocation.
Assessment of Malignancy in Uterine PEComa
Folpe et al's53 prognostic system, based on retrospective analysis of 26 PEComas of multiple sites, divided PEComas into benign, uncertain malignant potential (UMP) and malignant groups based on histologic criteria (Table 3). Subsequently, some deficiencies in this system became apparent. While the categorization of cases with no worrisome features (benign) or two or more worrisome features (malignant) is straightforward, it is unclear how to categorize those PEComas with a single worrisome feature such as elevated mitotic count, necrosis or infiltrative growth pattern.
TABLE 3.
Folpe criteria for prognosis in PEComa (from Folpe et al53)
| Criteria | Percentage Fulfilling Criteria With Aggressive Behavior | Comment | |
|---|---|---|---|
| Benign | No worrisome features (<5 cm, non-infiltrative, non-high nuclear grade and cellularity, mitotic rate ≤1/50HPF, no necrosis, no vascular invasion) | 0 of 22 (0%) | |
|
| |||
| Uncertain malignant potential | Nuclear pleomorphism/multinucleated giant cells only or | 0 of 6 (0%) | “Symplastic” PEComa probably benign, but few reported cases |
| Size >5 cm only | 2 of 17 (12%) | Large tumors should be extensively sampled to exclude areas with other worrisome features | |
|
| |||
| Malignant | Two or more worrisome features (>5 cm, infiltrative, high nuclear grade and cellularity, mitotic rate ≥1/50HPF, necrosis, vascular invasion) | 12 of 17 (71%) | |
Recently, Schoolmeester et al20 applied the Folpe criteria to 16 gynaecological tract PEComas and proposed a revised system (Table 4) which set a higher threshold for malignancy (≥4 worrisome features) and yielded greater specificity and positive and negative predictive values for subsequent malignant behaviour without sacrificing sensitivity. In addition, they combined the benign and UMP categories into one group, in which no malignant behaviour was observed during the limited follow-up period of the study.
TABLE 4.
Schoolmeester criteria for prognosis in PEComa (from Schoolmeester et al20)
| Criteria | Cases with known metastasis meeting criteria | Cases without known metastasis meeting criteria | |
|---|---|---|---|
| Benign or uncertain malignant potential | Tumors with <4 features: gross size ≥5cm, high-grade nuclear features, necrosis, vascular invasion, or a mitotic rate ≥1/50HPF | 0 of 9 (0%) | 7 of 7 (100%) |
|
| |||
| Malignant | Tumors with 4 or more features | 9 of 9 (100%) | 0 of 7 (0%) |
We extracted all reported histologic and outcome data from 78 uterine PEComas in order to compare the Folpe and Schoolmeester systems. In an attempt to rectify the deficiencies in the Folpe system outlined above, we tested a modification of the Folpe criteria (Folpe-modified), wherein tumours with a single “worrisome” features such as maximum dimension of ≥5 cm, infiltrative edge or mitotic count >1/10HPF are considered benign. It was not possible to define an upper limit to tumour size or mitotic count due to the small number of reported tumours with a single worrisome feature, but pending data from additional cases, clinicians may use their judgment and move the tumour to the UMP category if they consider the mitotic count or tumour size worryingly high (criteria arbitrarily used in this analysis were tumour size >10cm or mitoses >3/10 HPF). Tumours with isolated marked atypia, maximum dimension >10cm or mitotic count >3/10 HPF in the absence of other worrisome criteria should be considered UMP tumours due to the lack of available data.
For our analysis, it was necessary to discard “cellularity” as a criterion as it is poorly defined and is not assessed in most reports. Furthermore, features such as “infiltrative edge” and “lymphovascular invasion” (LVI) were not explicitly reported in many papers. We assumed that LVI was not identified if it was not explicitly stated as being present. Tumours in which the nature of the advancing edge was not reported were considered non-assessable using the Folpe and Schoolmeester criteria (unless the tumour reached thresholds for malignancy based on other worrisome features).
Using both Folpe and, no benign or UMP tumour behaved in a malignant fashion, although the Folpe-modified criteria allowed more tumours to be categorised and recognised as benign (Table 5). The Schoolmeester criteria showed superior specificity and positive predictive value (with all sixteen tumours categorised as “malignant” showing metastasis or local recurrence) than the Folpe and Folpe-modified systems, but seven of 47 tumours (15%) classified as benign/UMP behaved in a malignant fashion (Table 5).
TABLE 5.
Comparative analysis of PEComa classifications
| Classification | Benign | UMP | Malignant | Not assessable | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cases | Malignant behaviour | Cases | Malignant behaviour | Cases | Malignant behaviour | Cases | |
| N | N (%) | N | N (%) | N | N (%) | N | |
| Folpe | 10 | 0 (0) | 5 | 0 (0) | 42 | 29 (69) | 21* |
|
| |||||||
| Folpe-modified | 24 | 0 (0) | 5 | 0 (0) | 42 | 29 (69) | 7 |
|
| |||||||
| Schoolmeester | 51 | 7 (14) | 17 | 17 (100) | 10 | ||
14 due to Folpe criteria deficits
The Folpe and Folpe-modified criteria show greater sensitivities and negative predictive values than the Schoolmeester criteria. However, tumours meeting the more stringent Schoolmeester definition of malignancy frequently recurred early. Until data from larger numbers of tumours are available, the Folpe-modified criteria may be used first to categorize tumours, with subsequent application of the Schoolmeester criteria to help identify malignant tumours at high risk of early recurrence.
Challenges in Diagnosis of gynaecological tract PEComa – the PEComa/Smooth Muscle Tumour (SMT) Morphologic Spectrum
The differential diagnosis of uterine PEComa versus LMS is an area of diagnostic controversy. The diagnosis of uterine PEComa is particularly challenging because of the relative frequency of smooth muscle tumours (SMTs). By middle age, up to 80% of women are believed to have uterine leiomyomas (LM).93, 94 PEComas express a myoid immunophenotype and may have spindled morphology and therefore, it is unsurprising that they share many features with common SMTs such as LM and leiomyosarcoma (LMS).
While evidence of a myo-melanocytic immunophenotype was initially considered a discriminatory feature in favour of PEComa, at least focal positivity for melanocytic markers such as HMB-45, Melan-A and MiTF has been demonstrated in uterine SMTs.95–97 In 5 LMS with spindled and epithelioid morphology, the majority of HMB45-positive areas exhibited clear cell morphology, but some HMB45 positivity was also demonstrable in spindled areas.96 Evidence of the phenotypic plasticity of LMS is illustrated by a report of the development of a clear cell, diffusely HMB45-positive, predominantly SMA-negative metastasis from a HMB45-negative primary epithelioid LMS.95 Cathepsin K was recently promoted as a useful IHC marker for the differential diagnosis between PEComa and LMS, but further study has demonstrated cathepsin K expression in a proportion of LMS.98
Although the uterus is now the most commonly reported extrarenal site for PEComa,14, 15 there has been some controversy as to whether uterine PEComa is a distinct entity.14, 15, 21, 95–97, 99 Evidence from four TSC patients with uterine PEComa, in whom the uterine tumour developed in the context of PEComatosis involving multiple gynaecological sites16–18, 88 appears to confirm the existence of uterine PEComa. However, it is unclear whether a subset of tumours currently diagnosed as sporadic uterine PEComas actually represent uterine SMTs with variant histological and/or immunohistochemical features.
There are several reasons for caution when considering a diagnosis of uterine PEComa. Firstly, there is overlap between the histological and IHC features of PEComas and uterine SMTs. While features such as epithelioid appearance, vascular architecture and the presence of spider cells, multinucleate giant cells and macronucleoli are proposed as being characteristic of PEComa,20 their utility in distinguishing PEComa from uterine SMTs has not been validated. While classical spindled uterine LMS is a straightforward diagnosis in most cases, purely epithelioid LMS (rare) and, more commonly, mixed spindled and epithelioid LMS frequently pose diagnostic dilemmas due to their histopathological overlap with PEComas. As a result, there is variation between pathologists in the diagnosis of PEComa.
Secondly, criteria for malignancy in uterine LMS are well established, but much less so for PEComa. As discussed, the prognostic system for PEComa is in evolution as it is based on retrospective analyses of small tumour cohorts.20, 53 A uterine tumour on the morphologic spectrum between SMT and PEComa which demonstrates features such as infiltrative margin and mitotic count >2/10 HPF may be labelled as benign or malignant, according to the classification of the tumor as a SMT or PEComa, respectively. This has major implications for the patient in terms of the risk of overtreatment, inappropriate treatment (eg mTOR pathway inhibition) and psychological morbidity.
Thirdly, there is little available molecular data. While several small studies of both TSC-associated and sporadic renal AML have confirmed the importance of TSC2 loss-of-function, it has been shown in only 3 uterine PEComas.39, 40
Four patients with gynaecological PEComa have been reported to have received mTOR inhibitors (sirolimus and temsirolimus);69, 78, 100 follow up is available on 2 patients, who died of their disease, at 9 and 10 months respectively. In none of these four cases was LOH or mutation of TSC2 demonstrated, nor was activation of the mTOR pathway confirmed. In our opinion, due to inter-pathologist variation and uncertainty in the diagnosis of uterine PEComa, all tumours accepted into clinical trials of mTOR pathway inhibitors should have molecular analysis to confirm loss of TSC2 and/or loss-of-function of tuberin with mTOR pathway activation.
What is the practicing pathologist to do when faced with a mixed spindled/epithelioid gynaecological tract tumour with mixed myoid and melanocytic marker expression? Fadare99 proposed that morphologically-conventional SMTs be labelled as such, even if they focally express melanocytic markers (this should be noted in the report), while classical PEComa should also be diagnosed as such. He proposes that non-classical epithelioid mesenchymal tumours should be labeled as epithelioid tumours of uncertain malignant potential (UMP). In our opinion, it would be helpful to include a note in the pathology report explaining the basis for diagnostic uncertainty when using this term. For PEComas, the use of the Folpe-modified and Schoolmeester criteria may provide additional prognostic information to aid patient management. While these guidelines may help in the majority of cases, the ultimate question remains as to what constitutes a PEComa of the gynaecological tract outside of the setting of TSC, and how it can be reproducibly differentiated from SMTs. It is likely that molecular genetic studies will give us greater insight into the nature of gynaecological tract PEComa and its relationship to uterine SMTs, and help resolve this ongoing diagnostic dilemma.
Figure 1.
Morphologic features of uterine PEComa. A: Sheets of epithelioid cells without intervening stroma. B: Cells with abundant eosinophilic, fibrillary cytoplasm, prominent nucleoli and occasional nuclear pseudoinclusions. C & D: Perivascular cuffing by tumor cells.
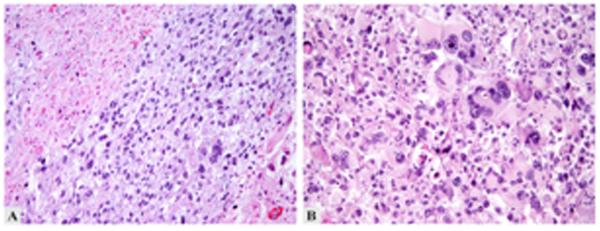
Figure 2.
Malignant PEComa of uterus. A: Pleomorphic tumor with necrosis (upper left). B: Multinucleate tumor cells with macronucleoli.
REFERENCES
- 1.Pea M, Bonetti F, Zamboni G, et al. Melanocyte-marker-HMB-45 is regularly expressed in angiomyolipoma of the kidney. Pathology. 1991;23(3):185–8. doi: 10.3109/00313029109063563. [DOI] [PubMed] [Google Scholar]
- 2.Ashfaq R, Weinberg AG, Albores-Saavedra J. Renal angiomyolipomas and HMB-45 reactivity. Cancer. 1993;71(10):3091–7. doi: 10.1002/1097-0142(19930515)71:10<3091::aid-cncr2820711032>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti F, Pea M, Martignoni G, et al. Clear cell (“sugar”) tumor of the lung is a lesion strictly related to angiomyolipoma--the concept of a family of lesions characterized by the presence of the perivascular epithelioid cells (PEC) Pathology. 1994;26(3):230–6. doi: 10.1080/00313029400169561. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni G, Pea M, Martignoni G, et al. Clear cell “sugar” tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996;20(6):722–30. doi: 10.1097/00000478-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Pea M, Martignoni G, Zamboni G, et al. Perivascular epithelioid cell. Am J Surg Pathol. 1996;20(9):1149–53. doi: 10.1097/00000478-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Bonetti F, Pea M, Martignoni G, et al. PEC and sugar. Am J Surg Pathol. 1992;16(3):307–8. doi: 10.1097/00000478-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Flores A. Evidence on the neural crest origin of PEComas. Rom J Morphol Embryol. 2011;52(1):7–13. [PubMed] [Google Scholar]
- 8.Hirschi KK, Majesky MW. Smooth muscle stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276(1):22–33. doi: 10.1002/ar.a.10128. [DOI] [PubMed] [Google Scholar]
- 9.Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23(6):727–37. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 10.Reiman HM, Goellner JR, Woods JE, et al. Desmoplastic melanoma of the head and neck. Cancer. 1987;60(9):2269–74. doi: 10.1002/1097-0142(19871101)60:9<2269::aid-cncr2820600928>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee SS, Bishop PW, Nicholson CM, et al. Malignant melanoma showing smooth muscle differentiation. J Clin Pathol. 1996;49(11):950–1. doi: 10.1136/jcp.49.11.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355(13):1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 13.Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48(1):75–82. doi: 10.1111/j.1365-2559.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 14.Fadare O. Perivascular Epithelioid Cell Tumors (PEComas) and Smooth Muscle Tumors of the Uterus. Am J Surg Pathol. 2007;31(9):1454–5. doi: 10.1097/PAS.0b013e318039b218. author reply 1455–6. [DOI] [PubMed] [Google Scholar]
- 15.Fadare O. Perivascular epithelioid cell tumor (PEComa) of the uterus: an outcome-based clinicopathologic analysis of 41 reported cases. Adv Anat Pathol. 2008;15(2):63–75. doi: 10.1097/PAP.0b013e31816613b0. [DOI] [PubMed] [Google Scholar]
- 16.Fang CL, Lin YH, Chen WY. Microscopic endometrial perivascular epithelioid cell nodules: a case report with the earliest presentation of a uterine perivascular epithelioid cell tumor. Diagn Pathol. 2012;7:117. doi: 10.1186/1746-1596-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froio E, Piana S, Cavazza A, et al. Multifocal PEComa (PEComatosis) of the female genital tract associated with endometriosis, diffuse adenomyosis, and endometrial atypical hyperplasia. Int J Surg Pathol. 2008;16(4):443–6. doi: 10.1177/1066896908316067. [DOI] [PubMed] [Google Scholar]
- 18.Liang SX, Pearl M, Liu J, et al. “Malignant” uterine perivascular epithelioid cell tumor, pelvic lymph node lymphangioleiomyomatosis, and gynecological pecomatosis in a patient with tuberous sclerosis: a case report and review of the literature. Int J Gynecol Pathol. 2008;27(1):86–90. doi: 10.1097/pgp.0b013e318150df37. [DOI] [PubMed] [Google Scholar]
- 19.Lim GS, Oliva E. The morphologic spectrum of uterine PEC-cell associated tumors in a patient with tuberous sclerosis. Int J Gynecol Pathol. 2011;30(2):121–8. doi: 10.1097/PGP.0b013e3181fa5a99. [DOI] [PubMed] [Google Scholar]
- 20.Schoolmeester JK, Howitt BE, Hirsch MS, et al. Perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: clinicopathologic and immunohistochemical characterization of 16 cases. Am J Surg Pathol. 2014;38(2):176–88. doi: 10.1097/PAS.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 21.Carvalho FM, Carvalho JP, Maluf FC, et al. A new morphological variant of uterine PEComas with sex-cord-like pattern and WT1 expression: more doubts about the existence of uterine PEComas. Ann Diagn Pathol. 2010;14(2):129–32. doi: 10.1016/j.anndiagpath.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Korytnaya E, Liu J, Camelo-Piragua S, et al. Ectopic prolactin secretion from a perivascular epithelioid cell tumor (PEComa) J Clin Endocrinol Metab. 2014;99(11):3960–4. doi: 10.1210/jc.2014-2623. [DOI] [PubMed] [Google Scholar]
- 23.Proust-Lemoine E, Mitchell V, Deruelle P, et al. Ectopic hyperprolactinaemia in a woman with a mesocolic perivascular epithelioid cell tumor (“PEComa”) Ann Endocrinol (Paris) 2008;69(3):240–3. doi: 10.1016/j.ando.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Hornick JL, Fletcher CD. Sclerosing PEComa: clinicopathologic analysis of a distinctive variant with a predilection for the retroperitoneum. Am J Surg Pathol. 2008;32(4):493–501. doi: 10.1097/PAS.0b013e318161dc34. [DOI] [PubMed] [Google Scholar]
- 25.Shen Q, Rao Q, Xia QY, et al. Perivascular epithelioid cell tumor (PEComa) with TFE3 gene rearrangement: clinicopathological, immunohistochemical, and molecular features. Virchows Arch. 2014;465(5):607–13. doi: 10.1007/s00428-014-1655-x. [DOI] [PubMed] [Google Scholar]
- 26.Martignoni G, Bonetti F, Chilosi M, et al. Cathepsin K expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney. Mod Pathol. 2012;25(1):100–11. doi: 10.1038/modpathol.2011.136. [DOI] [PubMed] [Google Scholar]
- 27.Rao Q, Cheng L, Xia QY, et al. Cathepsin K expression in a wide spectrum of perivascular epithelioid cell neoplasms (PEComas): a clinicopathological study emphasizing extrarenal PEComas. Histopathology. 2013;62(4):642–50. doi: 10.1111/his.12059. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Pan F, Qiao J, et al. Perivascular epithelioid cell tumor of the cervix with malignant potential. Int J Gynaecol Obstet. 2013;123(1):72–3. doi: 10.1016/j.ijgo.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Gaffey MJ, Mills SE, Zarbo RJ, et al. Clear cell tumor of the lung. Immunohistochemical and ultrastructural evidence of melanogenesis. Am J Surg Pathol. 1991;15(7):644–53. doi: 10.1097/00000478-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Kaiserling E, Krober S, Xiao JC, et al. Angiomyolipoma of the kidney. Immunoreactivity with HMB-45. Light- and electron-microscopic findings. Histopathology. 1994;25(1):41–8. doi: 10.1111/j.1365-2559.1994.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Lim SJ, Choi H, et al. Malignant clear-cell myomelanocytic tumor of broad ligament--a case report. Virchows Arch. 2006;448(6):867–70. doi: 10.1007/s00428-006-0161-1. [DOI] [PubMed] [Google Scholar]
- 32.Lee SE, Choi YL, Cho J, et al. Ovarian perivascular epithelioid cell tumor not otherwise specified with transcription factor E3 gene rearrangement: a case report and review of the literature. Hum Pathol. 2012;43(7):1126–30. doi: 10.1016/j.humpath.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Zhang R, Wang ZY, et al. Malignant perivascular epithelioid cell tumor (PEComa) of cervix with TFE3 gene rearrangement: a case report. Int J Clin Exp Pathol. 2014;7(9):6409–14. [PMC free article] [PubMed] [Google Scholar]
- 34.Rys J, Karolewski K, Pudelek J, et al. Perivascular epithelioid tumor (PEComa) of the falciform/broad ligament. Pol J Pathol. 2008;59(4):211–5. [PubMed] [Google Scholar]
- 35.Henske EP, Neumann HP, Scheithauer BW, et al. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer. 1995;13(4):295–8. doi: 10.1002/gcc.2870130411. [DOI] [PubMed] [Google Scholar]
- 36.Smolarek TA, Wessner LL, McCormack FX, et al. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62(4):810–5. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wullich B, Henn W, Siemer S, et al. Clonal chromosome aberrations in three of five sporadic angiomyolipomas of the kidney. Cancer Genet Cytogenet. 1997;96(1):42–5. doi: 10.1016/s0165-4608(96)00267-1. [DOI] [PubMed] [Google Scholar]
- 38.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97(11):6085–90. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan CC, Jong YJ, Chai CY, et al. Comparative genomic hybridization study of perivascular epithelioid cell tumor: molecular genetic evidence of perivascular epithelioid cell tumor as a distinctive neoplasm. Hum Pathol. 2006;37(5):606–12. doi: 10.1016/j.humpath.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Malinowska I, Kwiatkowski DJ, Weiss S, et al. Perivascular epithelioid cell tumors (PEComas) harboring TFE3 gene rearrangements lack the TSC2 alterations characteristic of conventional PEComas: further evidence for a biological distinction. Am J Surg Pathol. 2012;36(5):783–4. doi: 10.1097/PAS.0b013e31824a8a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenerson H, Folpe AL, Takayama TK, et al. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol. 2007;38(9):1361–71. doi: 10.1016/j.humpath.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruco LP, Pilozzi E, Wedard BM, et al. Epithelioid lymphangioleiomyomatosis-like tumour of the uterus in a patient without tuberous sclerosis: a lesion mimicking epithelioid leiomyosarcoma. Histopathology. 1998;33(1):91–3. doi: 10.1046/j.1365-2559.1998.0415g.x. [DOI] [PubMed] [Google Scholar]
- 43.Michal M, Zamecnik M. Hyalinized Uterine Mesenchymal Neoplasms with HMB-45-Positive Epithelioid Cells: Epithelioid Leiomyomas or Angiomyolipomas? Report of Four Cases. Int J Surg Pathol. 2000;8(4):323–328. doi: 10.1177/106689690000800411. [DOI] [PubMed] [Google Scholar]
- 44.Bonetti F, Martignoni G, Colato C, et al. Abdominopelvic sarcoma of perivascular epithelioid cells. Report of four cases in young women, one with tuberous sclerosis. Mod Pathol. 2001;14(6):563–8. doi: 10.1038/modpathol.3880351. [DOI] [PubMed] [Google Scholar]
- 45.Vang R, Kempson RL. Perivascular epithelioid cell tumor ('PEComa') of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol. 2002;26(1):1–13. doi: 10.1097/00000478-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Dimmler A, Seitz G, Hohenberger W, et al. Late pulmonary metastasis in uterine PEComa. J Clin Pathol. 2003;56(8):627–8. doi: 10.1136/jcp.56.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greene LA, Mount SL, Schned AR, et al. Recurrent perivascular epithelioid cell tumor of the uterus (PEComa): an immunohistochemical study and review of the literature. Gynecol Oncol. 2003;90(3):677–81. doi: 10.1016/s0090-8258(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 48.Park SH, Ro JY, Kim HS, et al. Perivascular epithelioid cell tumor of the uterus: immunohistochemical, ultrastructural and molecular study. Pathol Int. 2003;53(11):800–5. doi: 10.1046/j.1440-1827.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- 49.Darai E, Bazot M, Barranger E, et al. Epithelioid angiomyolipoma of the uterus: a case report. J Reprod Med. 2004;49(7):578–81. [PubMed] [Google Scholar]
- 50.Gao Z, Bhuiya T, Anderson A. Perivascular epithelioid cell tumour (PEComa) of the uterus associated with malignant neoplasm of the female genital tract. J Obstet Gynaecol. 2004;24(5):600–4. doi: 10.1080/01443610410001722905. [DOI] [PubMed] [Google Scholar]
- 51.Bernardo Bega R, Camino FB, Fernandez MDC, et al. The PEComa tumor: could it be considered an independent neoplastic entity. J Gynecol Surg. 2005;21:161–166. [Google Scholar]
- 52.Bosincu L, Rocca PC, Martignoni G, et al. Perivascular epithelioid cell (PEC) tumors of the uterus: a clinicopathologic study of two cases with aggressive features. Mod Pathol. 2005;18(10):1336–42. doi: 10.1038/modpathol.3800433. [DOI] [PubMed] [Google Scholar]
- 53.Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29(12):1558–75. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 54.Fukunaga M. Perivascular epithelioid cell tumor of the uterus: report of four cases. Int J Gynecol Pathol. 2005;24(4):341–6. doi: 10.1097/01.pgp.0000168515.83557.89. [DOI] [PubMed] [Google Scholar]
- 55.Jeon IS, Lee SM. Multimodal treatment using surgery, radiotherapy, and chemotherapy in a patient with a perivascular epithelioid cell tumor of the uterus. J Pediatr Hematol Oncol. 2005;27(12):681–4. doi: 10.1097/01.mph.0000193475.06870.d5. [DOI] [PubMed] [Google Scholar]
- 56.Rammeh Rommani S, Trabelsi A, Attia L, et al. Perivascular epithelioid cell tumor of the uterus: a case report. Pathologica. 2006;98(6):649–51. [PubMed] [Google Scholar]
- 57.Gan MF, Yu CK, Jin M, et al. Perivascular epithelioid cell tumor of the uterus: report of three cases. Chin Med J (Engl) 2007;120(6):526–8. [PubMed] [Google Scholar]
- 58.Cho HY, Chung DH, Khurana H, et al. The role of TFE3 in PEComa. Histopathology. 2008;53(2):236–49. doi: 10.1111/j.1365-2559.2008.03057.x. [DOI] [PubMed] [Google Scholar]
- 59.Yavuz E, Cakr C, Tuzlal S, et al. Uterine perivascular epithelioid cell tumor coexisting with pulmonary lymphangioleiomyomatosis and renal angiomyolipoma: a case report. Appl Immunohistochem Mol Morphol. 2008;16(4):405–9. doi: 10.1097/PAI.0b013e318137a9c2. [DOI] [PubMed] [Google Scholar]
- 60.Liu JL, Lin YM, Lin MC, et al. Perivascular epithelioid cell tumor (PEComa) of the uterus with aggressive behavior at presentation. Hematol Oncol Stem Cell Ther. 2009;2(3):426–30. doi: 10.1016/s1658-3876(09)50013-1. [DOI] [PubMed] [Google Scholar]
- 61.Yamagata Y, Kawauchi S, Tamura H, et al. A case of HMB45-negative perivascular epithelioid cell tumor (PEComa) of the uterine corpus: a possible diagnostic application of molecular-cytogenetic analysis. Eur J Gynaecol Oncol. 2009;30(2):216–9. [PubMed] [Google Scholar]
- 62.Clay MR, Gibson P, Lowell J, et al. Microscopic uterine lymphangioleiomyomatosis perivascular epithelioid cell neoplasm: a case report with the earliest manifestation of this enigmatic neoplasm. Int J Gynecol Pathol. 2011;30(1):71–5. doi: 10.1097/PGP.0b013e3181efe08d. [DOI] [PubMed] [Google Scholar]
- 63.Horn LC, Einenkel J. Uterine perivascular epitheloid cell tumor (PEComa) with CD117 and PNL2 positivity and entrapped endometriotic glands, mimicking sex-cordlike differentiation. Ann Diagn Pathol. 2011;15(3):216–8. doi: 10.1016/j.anndiagpath.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Yamada Y, Yamamoto H, Ohishi Y, et al. Sclerosing variant of perivascular epithelioid cell tumor in the female genital organs. Pathol Int. 2011;61(12):768–72. doi: 10.1111/j.1440-1827.2011.02737.x. [DOI] [PubMed] [Google Scholar]
- 65.Bleeker JS, Quevedo JF, Folpe AL. Malignant perivascular epithelioid cell tumor of the uterus. Rare Tumors. 2012;4(1):e14. doi: 10.4081/rt.2012.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang W, Li G, Wei-qiang Z. Multifocal PEComa (PEComatosis) of the female genital tract and pelvis: a case report and review of the literature. Diagn Pathol. 2012;7:23. doi: 10.1186/1746-1596-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye HY, Chen JG, Luo DL, et al. Perivascular epithelioid cell tumor (PEComa) of gynecologic origin: a clinicopathological study of three cases. Eur J Gynaecol Oncol. 2012;33(1):105–8. [PubMed] [Google Scholar]
- 68.Cossu A, Paliogiannis P, Tanda F, et al. Uterine perivascular epithelioid cell neoplasms (PEComas): report of two cases and literature review. Eur J Gynaecol Oncol. 2014;35(3):309–12. [PubMed] [Google Scholar]
- 69.Ghosh I, Arun I, Sen S, et al. Metastatic perivascular epithelioid cell tumor responding to mammalian target of rapamycin inhibition. Indian J Med Paediatr Oncol. 2014;35(1):99–102. doi: 10.4103/0971-5851.133733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okada H, Terado Y, Fujiwara M, et al. Perivascular epithelioid cell tumor of the uterus. Pathol Int. 2014;64(3):151–3. doi: 10.1111/pin.12137. [DOI] [PubMed] [Google Scholar]
- 71.Schoolmeester JK, Dao LN, Sukov WR, et al. TFE3 Translocation-associated Perivascular Epithelioid Cell Neoplasm (PEComa) of the Gynecologic Tract: Morphology, Immunophenotype, Differential Diagnosis. Am J Surg Pathol. 2014 doi: 10.1097/PAS.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu Y, Shi HY, Huang HF. Uterine perivascular epithelioid cell tumour. J Obstet Gynaecol. 2014;34(6):519–22. doi: 10.3109/01443615.2014.914475. [DOI] [PubMed] [Google Scholar]
- 73.Azad NS, Aziz AB, Pervez S, et al. Uterine perivascular epithelioid cell tumour presenting as a cervical mass. J Pak Med Assoc. 2006;56(2):83–4. [PubMed] [Google Scholar]
- 74.Bradshaw MJ, Folpe AL, Croghan GA. Perivascular epithelioid cell neoplasm of the uterine cervix: an unusual tumor in an unusual location. Rare Tumors. 2010;2(4):e56. doi: 10.4081/rt.2010.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Celik H, Kefeli M, Cetinkaya M, et al. Perivascular Epithelioid Cell Tumor (PEComa) of the Uterine Cervix in a Patient with Tuberous Sclerosis Complex: A Literature Review. Turk Patoloji Derg. 2014:1–5. doi: 10.5146/tjpath.2014.01274. [DOI] [PubMed] [Google Scholar]
- 76.Fadare O, Parkash V, Yilmaz Y, et al. Perivascular epithelioid cell tumor (PEComa) of the uterine cervix associated with intraabdominal “PEComatosis”: A clinicopathological study with comparative genomic hybridization analysis. World J Surg Oncol. 2004;2:35. doi: 10.1186/1477-7819-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Natella V, Merolla F, Giampaolino P, et al. A huge malignant perivascular epithelioid cell tumor (PEComa) of the uterine cervix and vagina. Pathol Res Pract. 2014;210(3):186–8. doi: 10.1016/j.prp.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28(5):835–40. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamamoto E, Ino K, Sakurai M, et al. Fertility-sparing operation for recurrence of uterine cervical perivascular epithelioid cell tumor. Rare Tumors. 2010;2(2):e26. doi: 10.4081/rt.2010.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalyanasundaram K, Parameswaran A, Mani R. Perivascular epithelioid tumor of urinary bladder and vagina. Ann Diagn Pathol. 2005;9(5):275–8. doi: 10.1016/j.anndiagpath.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Ong LY, Hwang WS, Wong A, et al. Perivascular epithelioid cell tumour of the vagina in an 8 year old girl. J Pediatr Surg. 2007;42(3):564–6. doi: 10.1016/j.jpedsurg.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 82.Cho HJ, Lee MK, Kang BM, et al. A 6-year-old girl with vaginal spotting who was diagnosed with perivascular epithelioid cell neoplasm after vaginoscopic resection. Obstet Gynecol Sci. 2014;57(5):409–11. doi: 10.5468/ogs.2014.57.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson AE, Yang X, Young RH. Epithelioid angiomyolipoma of the ovary: a case report and literature review. Int J Gynecol Pathol. 2002;21(1):69–73. doi: 10.1097/00004347-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 84.Ramaiah S, Ganesan R, Mangham DC, et al. Malignant variant of sclerosing perivascular epithelioid cell tumor arising in the adnexa. Int J Gynecol Pathol. 2009;28(6):589–93. doi: 10.1097/PGP.0b013e3181a3a4de. [DOI] [PubMed] [Google Scholar]
- 85.Surico D, Codeca C, Boldorini R, et al. Sclerosing PEComa: a case report and review of the literature. Minerva Chir. 2013;68(1):125–8. [PubMed] [Google Scholar]
- 86.Fink D, Marsden DE, Edwards L, et al. Malignant perivascular epithelioid cell tumor (PEComa) arising in the broad ligament. Int J Gynecol Cancer. 2004;14(5):1036–9. doi: 10.1111/j.1048-891X.2004.014549.x. [DOI] [PubMed] [Google Scholar]
- 87.Tazelaar HD, Batts KP, Srigley JR. Primary extrapulmonary sugar tumor (PEST): a report of four cases. Mod Pathol. 2001;14(6):615–22. doi: 10.1038/modpathol.3880360. [DOI] [PubMed] [Google Scholar]
- 88.Salviato T, Altavilla G, Busatto G, et al. Diffuse intra-abdominal clear cell myomelanocytic tumor: report of an unusual presentation of “PEComatosis” simulating peritoneal mesothelioma. Ann Diagn Pathol. 2006;10(6):352–6. doi: 10.1016/j.anndiagpath.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 89.Ohe C, Kuroda N, Hes O, et al. A renal epithelioid angiomyolipoma/perivascular epithelioid cell tumor with TFE3 gene break visualized by FISH. Med Mol Morphol. 2012;45(4):234–7. doi: 10.1007/s00795-012-0584-5. [DOI] [PubMed] [Google Scholar]
- 90.Williamson SR, Bunde PJ, Montironi R, et al. Malignant perivascular epithelioid cell neoplasm (PEComa) of the urinary bladder with TFE3 gene rearrangement: clinicopathologic, immunohistochemical, and molecular features. Am J Surg Pathol. 2013;37(10):1619–26. doi: 10.1097/PAS.0b013e318293729d. [DOI] [PubMed] [Google Scholar]
- 91.Argani P, Aulmann S, Illei PB, et al. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010;34(10):1395–406. doi: 10.1097/PAS.0b013e3181f17ac0. [DOI] [PubMed] [Google Scholar]
- 92.Tanaka M, Kato K, Gomi K, et al. Perivascular epithelioid cell tumor with SFPQ/PSF-TFE3 gene fusion in a patient with advanced neuroblastoma. Am J Surg Pathol. 2009;33(9):1416–20. doi: 10.1097/PAS.0b013e3181a9cd6c. [DOI] [PubMed] [Google Scholar]
- 93.Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 94.Brooks SE, Zhan M, Cote T, et al. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol Oncol. 2004;93(1):204–8. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 95.Silva EG, Bodurka DC, Scouros MA, et al. A uterine leiomyosarcoma that became positive for HMB45 in the metastasis. Ann Diagn Pathol. 2005;9(1):43–5. doi: 10.1053/j.anndiagpath.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 96.Silva EG, Deavers MT, Bodurka DC, et al. Uterine epithelioid leiomyosarcomas with clear cells: reactivity with HMB-45 and the concept of PEComa. Am J Surg Pathol. 2004;28(2):244–9. doi: 10.1097/00000478-200402000-00013. [DOI] [PubMed] [Google Scholar]
- 97.Simpson KW, Albores-Saavedra J. HMB-45 reactivity in conventional uterine leiomyosarcomas. Am J Surg Pathol. 2007;31(1):95–8. doi: 10.1097/01.pas.0000213346.57391.70. [DOI] [PubMed] [Google Scholar]
- 98.Zheng G, Martignoni G, Antonescu C, et al. A broad survey of cathepsin K immunoreactivity in human neoplasms. Am J Clin Pathol. 2013;139(2):151–9. doi: 10.1309/AJCPDTRTO2Z4UEXD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fadare O. Uterine PEComa: appraisal of a controversial and increasingly reported mesenchymal neoplasm. Int Semin Surg Oncol. 2008;5:7. doi: 10.1186/1477-7800-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Benson C, Vitfell-Rasmussen J, Maruzzo M, et al. A retrospective study of patients with malignant PEComa receiving treatment with sirolimus or temsirolimus: the Royal Marsden Hospital experience. Anticancer Res. 2014;34(7):3663–8. [PubMed] [Google Scholar]