Abstract
Background
One of the most common deficits in patients with schizophrenia (SZ) is in working memory (WM), which has wide-reaching impacts across cognition. However, prior approaches to studying WM in SZ have employed tasks that require multiple cognitive-control processes, making it difficult to determine which specific cognitive and neural processes underlie the WM impairment.
Methods
We used fMRI to investigate component processes of WM in SZ. Eighteen healthy controls (HCs) and 18 patients with SZ performed an item-recognition task that permitted separate neural assessments of 1) WM maintenance, 2) inhibition, and 3) interference-control in response to recognition probes.
Results
Prior to inhibitory demands, posterior ventrolateral prefrontal cortex (VLPFC), an area involved in WM maintenance, was activated to a similar degree in both HCs and patients, indicating preserved maintenance operations in SZ. When cued to inhibit items from WM, HCs showed reduced activation in posterior VLPFC, commensurate with appropriately inhibiting items from WM. However, these inhibition-related reductions were absent in patients. When later probed with items that should have been inhibited, patients showed reduced behavioral performance and increased activation in mid-VLPFC, an area implicated in interference-control. A mediation analysis indicated that impaired inhibition led to increased reliance on interference-control and reduced behavioral performance.
Conclusions
In SZ, impaired control over memory, manifested through proactive inhibitory deficits, leads to increased reliance on reactive interference-control processes. The strain on interference-control processes results in reduced behavioral performance. Thus, inhibitory deficits in SZ may underlie widespread impairments in WM and cognition.
Keywords: fMRI, Prefrontal cortex, Executive Function, Inhibition, Retrieval, Interference Control, Schizophreina
Introduction
Schizophrenia (SZ) is a psychiatric illness characterized by a diverse range of clinical symptoms and disturbances in behavior. Although diagnosis is made based upon the occurrence of positive and negative symptoms (1), it is the cognitive symptoms that are most strongly associated with patients’ functional outcome (2). Among the most frequently noted of the cognitive symptoms in SZ are deficits in working memory (WM) (3–6). WM is a complex construct comprised of component processes that enable the short-term retention of information in order to guide goal-directed behavior (7, 8). WM supports a variety of cognitive abilities, including learning, reasoning, verbal comprehension and academic success (9), and might even be a better predictor of academic success than IQ (10). Due to its centrality in cognition, it is not surprising that WM deficits in SZ are associated with impairments in social and occupational functioning (11, 12). Hence, understanding breakdowns in the component processes in WM in SZ is fundamental to understanding not only cognitive function in the disorder, but also the disorder itself.
Previous studies have provided the groundwork for addressing behavioral WM deficits in SZ. Deficits have been attributed to a failure in cognitive-control over the inhibition of irrelevant information in WM or the selection of responses at retrieval (e.g., 13–18). However, prior approaches to studying WM in SZ have employed tasks requiring multiple cognitive-control processes that are challenging to disentangle, making it difficult to determine which specific component processes are impaired (16, 19–25).
To help address this issue, we previously examined patients with SZ and healthy controls (HCs) in a task that dissociated two forms of cognitive control over WM (26). We compared the filtering of irrelevant distractors before items entered WM and the inhibition of irrelevant distractors after information had entered WM (27, 28). We found that patients with SZ were unimpaired when they had to filter items before they entered WM, indicating intact encoding processes and ruling out a general WM deficit. Yet, the same patients were impaired when they had to inhibit irrelevant distractors after information had entered WM. These data support the idea that WM deficits are not global in SZ. However, since the behavioral data could not examine ongoing processing, and only reflected the response to the probe, we could not determine whether the deficit was due to a failure to inhibit irrelevant information in WM or to interference at retrieval.
In the current study, we used functional magnetic resonance imaging (fMRI) to examine patients with SZ and HCs during a single cognitive task that allowed us to separate the neural mechanisms contributing to 1) the maintenance of information in WM prior to inhibitory demands, 2) the inhibition of irrelevant items from WM, and 3) the resolution of interference of irrelevant information at the time of retrieval. Thus, the design separated various phases of WM to investigate the precise subcomponents of WM that are impaired in patients with SZ, which ultimately lead to difficulties during retrieval.
Participants were first presented with a memory-set consisting of two red and two blue words (see Supplement: Figure S1). Thereafter, the four words were removed from view. This constituted the PreCue, maintenance-only phase. Participants were instructed, via an instruction-cue, to retain only two of the words, corresponding to the instruction-cue’s color (e.g. blue), and to consider only these words when responding to the test probe. The PostCue phase measured inhibitory-control of items in WM. Here, participants should reduce their WM load by inhibiting memory representations of the two irrelevant (e.g. red) words, retaining representation of only the two relevant (e.g. blue) words. Finally, in the third and final phase of the task, participants retrieved information from WM. A test probe required a positive response if it matched one of the two target words (Valid; e.g., either of the blue words) and a negative response if it either matched a word that should have been inhibited (Lure; e.g. either of the red words), or if it had not been presented in the trial (Control).
We hypothesized that patients with SZ would be specifically impaired at inhibiting information in WM, reflected in the second phase of the task. The hypothesis makes the following predictions: in the maintenance-only portion of the task prior to the cue (PreCue), patients and HCs should show equivalent neural activations in areas involved in WM maintenance, particularly the posterior areas of the left ventrolateral prefrontal cortex (VLPFC, BA 44), thought to reflect phonological rehearsal (29, 30). After the instruction-cue (PostCue), HCs were predicted to show a reduction in maintenance-related activation in posterior VLPFC, which has been shown to vary linearly based on the verbal WM load (29), due to the inhibition of irrelevant items. By contrast, activation in this area was predicted to remain elevated in patients commensurate with their inability to inhibit items from WM. These inhibitory failures in patients were expected to lead to increased interference during the third, retrieval-phase of the task. Here, we predicted that patients would have more difficulty in distinguishing Lures from Valid items. If Lure word representations were successfully inhibited from WM, performance on Lure probes should be equivalent to performance on Control probes. However, if items were not appropriately inhibited, then Lure probes would require additional interference-control processes to be distinguished from Valid probes. This difficulty was expected to be reflected in increased activation in the left mid-VLPFC (BA 45), an area associated with the resolution of WM-based conflict (31). Hence, a deficit in inhibiting information in WM was predicted to lead a dynamic pattern of neural differences between patients with SZ and HCs.
Methods
Participants
Data from 18 HCs and 18 patients with SZ are reported. Demographics are shown in Table 1 along with clinical ratings for the patients. Another 4 patients and 4 HCs were tested but not included in the analysis because they did not respond on more than 20% of trials or had an error rate of 2 standard deviations above the respective group average in one of the probe-type conditions. See Supplemental Methods for a complete description of the participants.
Table 1.
Demographic, Clinical, and Neuropsychological Characteristics
| Variable | SZ | HC |
|---|---|---|
| N | 18 | 18 |
| Age (in years) | 37.9 (9.3) | 36.3 (8.2) |
| Sex (f/m) | 7/11 | 5/13 |
| Handedness | ||
| Right | 17 | 17 |
| Left | 1 | 1 |
| Ambidextrous | 0 | 0 |
| Education | 14.3 (2.4) | 15.6 (2.0) |
| Negative sympoms (SANS) | ||
| Affective flattening | 2.00 (1.14)/5 | |
| Alogia | 0.83 (1.42)/5 | |
| Avolition/apathy | 1.71 (1.40)/5 | |
| Asociality/anhedonia | 2.39 (1.42)/5 | |
| Attention | 1.41 (1.33)/5 | |
| Positive symptoms (SAPS) | ||
| Hallucinations | 1.17 (1.76)/5 | |
| Delusions | 1.22 (1.40)/5 | |
| Bizarre Behavior | 1.06 (1.30)/5 | |
| Thought Disorder | 0.72 (1.23)/5 | |
| Depression (Calgary) | 2.61 (2.28)/9 | |
Materials
The stimuli consisted of 80 four-letter nouns that have been used in previous studies with this paradigm (26–28).
Procedure
Participants were presented with a 4-word memory-set for 4000ms and were instructed to retain the items in memory (Supplement: Figure S1). Two of the words were presented in red and the other 2 were in blue. The PreCue phase consisted of a 6000–8000ms retention-interval following the memory-set presentation. Thereafter, a cue, presented for 2000ms, instructed participants to retain in memory only the words of one color (red on half the trials, blue on the other half). The PostCue WM retention-interval was 6000–8000ms. Then, participants were presented with a probe word requiring an affirmative response if the probe matched either of the words that should have been retained in WM (e.g. POOL and TILL), and negative response otherwise. All responses were made using the non-dominant hand with a middle finger press indicating a negative response and an index finger press indicating a positive response. On 50% of the trials the probe matched one of the words that should be in WM (Valid probes); on 25% of the trials the probe matched one of the words that should have been inhibited from WM (Lure probes), and on the remaining 25% of the trials the probe did not match any word presented on that trial (Control probes). Control probes were restricted to stimuli that had not appeared for at least 3 subsequent trials in order to minimize effects of proactive interference. Participants completed 8 blocks of 12 trials each. Feedback was given on practice trials, completed outside of the scanner, but not on experimental trials.
See Supplemental Methods for additional details regarding the procedure and fMRI methods.
fMRI Analysis
Univariate analyses were conducted using the general linear model implemented in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). Regressors-of-interest included the WM maintenance period prior to the cue (PreCue), the WM maintenance period following the cue (PostCue), and the retrieval probe. Maintenance-related regressors spanned the length of the maintenance interval while probe-related regressors were modeled as an impulse. Separate probe-related regressors were included for each probe type (Valid, Lure, Control). Events from trials in which an error occurred were modeled separately and were excluded from subsequent analyses. Two 16 second fixation periods were included in each run that were modeled with a separate regressor that served as a measure of baseline activation. Additional nuisance regressors were included to capture activation related to encoding and the cue. All of the regressors described above were convolved with a canonical hemodynamic response function.
Two contrasts of interest were estimated for each participant. The first examined WM maintenance-related activation by contrasting PreCue – PostCue. The second examined interference-control processes at retrieval by contrasting Lure – Control trials. Contrast images for each participant were submitted to second-level 2-sample t-tests. Additional analyses separately examining Encoding and PreCue are reported in Supplemental Results.
To identify regions involved in WM-maintenance and interference-control, we performed whole-brain analyses collapsing across group. These analyses were thresholded at p < 0.001 at the voxel-level with a 74 voxel cluster extent providing correction for multiple comparisons (p < 0.05 family-wise error corrected) according to simulations with AlphaSim. Areas demonstrating significant activation were subsequently tested for group differences. Since these regions were identified through analyses that collapsed across group, these areas provide unbiased estimates for examining group differences. Given previous demonstrations of the role of the left posterior VLPFC in WM maintenance (29) and left mid-VLPFC in interference-control (27, 28–33), we focused on voxels showing significant activation within these regions (see Supplemental Methods for region-of-interest definitions). For completeness, we also report whole-brain analyses separately for each group, as well as whole-brain group differences (Supplement: Tables S1–S3).
Brain-brain and brain-behavior relationships were estimated in the left posterior VLPFC and mid-VLPFC (see Supplemental Methods). We used the Lure – Control difference in error rate as a behavioral metric of interference-control. Neural effects assessed the PreCue – PostCue and Lure – Control contrast. Relationships were tested using robust regression, which is more robust to outliers than other correlation methods (34). Spearman correlations are also reported for completeness. To examine the relationship between mid-VLPFC, posterior-VLPFC, and behavioral performance, we performed mediation analysis using the mediation toolbox implemented in SPM (35, 36). Paths were estimated with robust regression and significance was assessed using a permutation test with 10,000 samples.
Results
Behavioral Performance
The data of interest were the mean error rates and the reaction times for correct trials (Valid, Lure, and Control). For each participant, trials on which reaction times were ±2.5 standard deviations their individual mean in each probe-type condition were excluded from the analysis (mean 2.25 trials for HCs; 2.43 for patients).
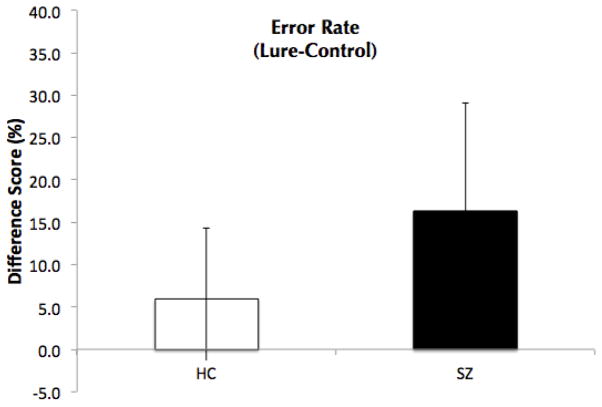
Separate repeated measures ANOVAs with Probe-Type as a within-subjects variable, and Group (HC or SZ) as a between-subjects variable were computed for error rates and reaction times. For error rate, there was a significant main effect of Probe-Type (F(2, 68)=27.1, p<.001) and a significant main effect of Group (F(1, 34)=15.3, p<.001), such that the patients made more errors than the HCs. Critically, there was a significant interaction between Probe-Type and Group (F(2, 68)=5.7, p=.005). While patients made more errors than HCs in all three Probe-Types [Valid (t(34)=3.46, p=.001; 18.1 (13.5) vs. 5.9 (6.6); Lure (t(34)=3.76, p=.001, 18.15 (13.54) vs. 5.88 (6.58); and Control (t(34)=2.27, p=.03; 5.19 (8.2) vs. 0.72 (1.64)], patients made significantly more errors to Lure probes compared to Control probes relative to HCs (t(34)=−2.9, p=.007; Figure 1). This replicates our previous finding that patients with SZ demonstrate a deficit in the Lure condition (26). Patients also made more errors on Valid probes compared to Control probes relative to HCs (t(34)=−2.6, p=0.02), suggesting that patients had difficulty distinguishing Valid from Lure probes.
Figure 1.
Error rate difference-scores (Lure-Control) for Healthy Controls (HC) and patients with schizophrenia (SZ).
For reaction time, there was a significant main effect of Probe-Type (F(2, 68)=62.2, p<.001) and a significant main effect of Group (F(1, 34)=20.82, p<.001). Patients were significantly slower than HCs for all 3 probes types (Valid: 1067.15 (273.8) vs. 709.4 (183.67); Lure: 1270.41 (295.79) vs. 886.71 (250.74); Control: 1043.3 (272.1) vs. 717.19 (156.67)). However, the Probe-Type X Group interaction was not significant (F(2, 68)=.96, ns). Planned between-group comparison of the Lure vs. Control reaction times difference was not significant (t(34)=−1.28, ns), although a trend towards patient’s exhibiting a greater difference score was evident.
fMRI Results
Maintenance and Inhibitory Control of WM
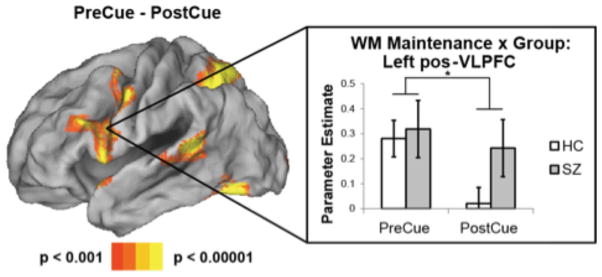
To isolate specific patient deficits, we began by identifying areas involved in maintaining information in WM by contrasting PreCue and PostCue activations (Figure 2). This contrast measured a load effect (i.e. four items PreCue, two items PostCue) predicated on appropriate use of the cue to inhibit items from WM. Previous research has indicated that this assumption is valid in healthy young and older participants (37). As anticipated, the contrast revealed significant differences in the left posterior VLPFC consistent with known WM load-related effects in this region (29); (see Supplement: Table S1 for additional areas).
Figure 2.
Maintenance and inhibition-related activations. Left: contrast of PreCue – PostCue maintenance activations collapsing across groups. Right: parameter estimates extracted from the left posterior ventrolateral prefrontal cortex (pos-VLPFC) averaged across a 5 mm sphere centered around −50 8 22. In this region, healthy controls (HC) and patients with schizophrenia (SZ) demonstrated equivalent PreCue activation. While HC’s demonstrated reduced activation PostCue, patients with SZ did not. These results indicate inhibition-related reductions in pos-VLPFC activation in HC’s, but not patients with SZ. * - p < 0.05.
To examine whether patients demonstrated impaired control over WM, we compared left posterior VLPFC activation between HCs and patients. Failure to inhibit irrelevant content from WM would be expected to reduce the difference between PreCue and PostCue activation in patients due to elevated PostCue activation. Averaging across activation in the entire left posterior VLPFC cluster revealed by the whole-brain analysis above, HCs demonstrated a robust PreCue > PostCue difference (t(17) = 6.90, p < 0.00001). Patients showed a similar, albeit muted effect (t(17) = 3.05, p < 0.01). Direct comparison between the groups revealed a significant interaction with a greater PreCue > PostCue difference in HCs compared to patients (t(34) = 2.39, p < 0.05). To further interrogate this difference, we separately examined ROIs centered around each left posterior VLPFC peak revealed by the whole-brain analysis (see Supplemental Methods). While all portions of the left posterior VLPFC demonstrated a numerical trend for a reduced PreCue > PostCue difference in patients relative to HCs, a significant group difference (t(34) = 2.48, p < 0.05) was found in only a single region (center: −50 8 22; Table 2). In this region, whereas HCs showed a significant PreCue > PostCue difference (t(17) = 5.90, p < 0.0001), patients did not (t(17) = 1.29, p > 0.2), resulting in a group difference (t(34) = 2.48, p < 0.05). While HCs and patients showed similar PreCue activation (t(34) = 0.28, p > 0.75), patients showed significantly increased activation PostCue relative to HCs (t(34) = 1.72, p < 0.05 one-tailed). This is consistent with the behavioral findings that patients with SZ demonstrate a failure to inhibit irrelevant items from WM (Figure 2).
Table 2.
Results in Ventrolateral Prefrontal Cortex
| PreCue-PostCue
| |||
|---|---|---|---|
| peak | HC | SZ | HC vs SZ |
| −52 10 6 | 6.49*** | 2.03† | 1.23 |
| −54 8 16 | 4.75*** | 1.91† | 1.25 |
| −50 8 22 | 5.90*** | 1.29 | 2.48* |
| −52 16 22 | 4.71*** | 2.09† | 1.71† |
| −50 12 28 | 4.49*** | 2.40* | 1.69 |
| Lure-Control
| |||
|---|---|---|---|
| peak | HC | SZ | HC vs SZ |
| −48 24 22 | 1.71 | 4.42*** | −2.28* |
| −40 32 22 | 0.31 | 4.45*** | −3.12** |
| −38 16 22 | 2.22* | 3.04* | −1.18 |
Numbers represent t-statistics for each respective contrast.
p < 0.1;
p < 0.05;
p < 0.005;
p < 0.0005.
Interference-Control at Retrieval
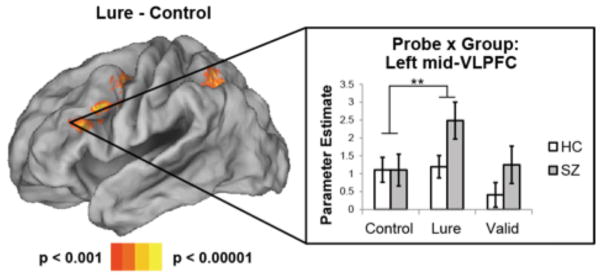
Next, we examined regions involved in interference-control, comparing probe-related activations for Lure probes to Control probes. Here, when collapsing across group, significantly greater activations were observed in the left mid-VLPFC for Lure probes relative to Control probes (Figure 3), consistent with the hypothesized role of this region in interference-control (27, 31, 33, 38; see Supplement: Table S2 for additional areas).
Figure 3.
Interference-control related activations. Left: contrast of Lure – Control probe activations collapsing across groups. Right: parameter estimates extracted from the left mid ventrolateral prefrontal cortex (mid-VLPFC) averaged across a 5 mm sphere centered around −40 32 22. In this region, healthy controls (HC) and patients with schizophrenia (SZ) demonstrated equivalent activation to Control probes. However, activation was significantly elevated for Lure probes in patients with SZ, but not HC’s. These results indicate increased demands on interference-control to Lure probes in patients with SZ. ** - p < 0.005.
In the analyses of maintenance epochs, we observed that patients showed impairments in inhibiting irrelevant content from WM. Such impairments should lead to increased demands on interference-control when responding to Lure probes. Thus, we predicted that patients would show increased Lure > Control activation than HCs. Averaging across the left mid-VLPFC cluster revealed by the whole-brain analysis described above, patients indeed demonstrated strongly increased activation for Lure probes relative to Control probes (t(17) = 4.86, p < 0.0005). By contrast, HCs showed a weaker effect (t(17) = 1.87, p < 0.05 one-tailed). Direct comparisons between groups revealed a significant difference as patients showed a stronger Lure > Control effect than HCs (t(34) = 2.38, p < 0.05). To further explore this difference, we separately examined ROIs centered around each left mid-VLPFC peak revealed by the whole-brain analysis. While no group difference was found in the posterior-most peak (−38 16 22: t(34) = 1.18, p > 0.2), group differences were progressively stronger as activations proceeded anteriorly (−48 24 22: t(34) = 2.28, p < 0.05; −40 32 22: t(24) = 3.12, p < 0.005). As depicted in Figure 3, in the anterior-most mid-VLPFC peak, HCs and patients showed nearly identical activation to Control probes (t(34) = 0.01, p > 0.99), while patients showed significantly increased activation to Lure probes (t(34) = 2.18, p < 0.05). These results suggest that patients require increased interference-control to Lure probes relative to HCs, but are identical to HCs when no interference-control is required.
Relationship Between Inhibition, Interference-Control, and Behavior
The data demonstrate differences between HCs and patients with SZ in neural measures of inhibition and interference-control, and behavioral measures of performance. These measures are likely to be inter-related: impaired control over memory (manifested through inhibitory deficits PostCue) leads to the increased reliance on interference-control processes at the time of the probe. The strain on interference-control processes results in increased behavioral errors at retrieval. To explore this hypothesis, we examined the relationship between maintenance-related activations in the posterior VLPFC, interference-control related activations in the mid-VLPFC, and behavioral performance. To maximize power, the groups were pooled. Neural measures of the posterior VLPFC were drawn from the ROI demonstrating a significant group effect (−50 8 22) and neural measures of the mid-VLPFC were drawn from the anterior-most ROI (−40 32 22) that was maximally distant from the posterior VLPFC. The latter choice minimized overlap in the activation clusters that might occur due to spatial smoothing.
Starting with the posterior-VLPFC, we tested the relationship between inhibition-related reductions in maintenance (PreCue – PostCue activation) and behavioral performance (Lure – Control error-rate). Robust regression indicated a significant negative relationship (t(34) = −3.22, p < 0.005; Spearman’s ρ = −0.5753, p < 0.0005), such that participants who appropriately inhibited irrelevant items from WM showed reduced behavioral errors. Next, we examined the relationship between interference-control related activations in the mid-VLPFC (Lure – Control activation) and behavioral performance. Robust regression indicated a significant positive relationship (t(34) = 2.88, p < 0.01; Spearman’s ρ = 0.4135, p < 0.05): participants that demonstrated the greatest difficulty with the Lure probes behaviorally also demonstrated the greatest interference-control related activations in the mid-VLPFC. Finally, we examined the relationship between inhibition, as indexed by PostCue reductions in maintenance-related activation in the posterior VLPFC, and interference-control, as indexed by Lure > Control activations in the mid-VLPFC. Robust regression indicated a significant negative relationship (t(34) = −3.04, p < 0.005; Spearman’s ρ = −0.4829, p < 0.005). Repeating the above analyses with a co-variate for group led to similar results (posterior-VLPFC and behavior: t(33) = −2.34, p < 0.05; mid-VLPFC and behavior: t(33) = 1.87, p = 0.07; posterior-VLPFC and mid-VLPFC: t(33) = −2.48, p < 0.05). Together, these results indicate a strong inter-relationship between the posterior VLPFC, mid-VLPFC, and behavioral performance.
Thus far we have speculated that impaired inhibitory control over WM in patients leads to increased reliance on interference-control at the probe and subsequent behavioral impairments. Such an account predicts that inhibitory control over WM mediates the relationship between activations in the mid-VLPFC related to interference-control and behavioral interference. To examine this possibility, we performed mediation analysis including the PreCue – PostCue contrast in posterior VLPFC (inhibition), the Lure – Control contrast in mid-VLPFC (interference-control), and the Lure – Control difference in error-rate (behavioral performance). Confirming the centrality of inhibitory control over WM, a significant mediation effect was found (z = 2.08, p < 0.05; Figure 4). When accounting for the mediating effect of inhibitory control over WM, the relationship between interference-control in the mid-VLPFC and behavioral performance was no longer significant (z = 1.30, p > 0.15). All other paths were significant (all z > 2.40, p < 0.05). To determine the selectivity of this effect, we calculated an alternative model using the interference-control related activations in the mid-VLPFC as a mediator between the posterior VLPFC (inhibitory control) and behavioral performance. In this model, the mediation effect was not significant (z = 0.61, p > 0.5).
Figure 4.
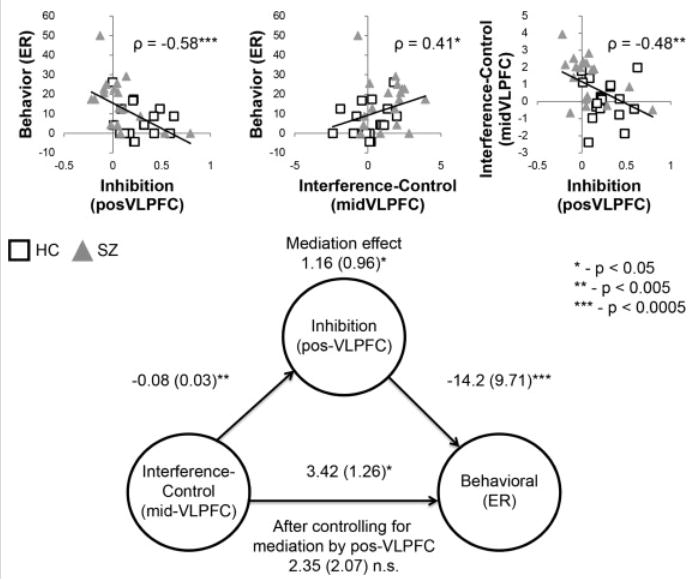
Correlations and mediation analysis. Top: correlations between measures of interest. Behavioral performance reflects the behavioral difference in error rate (ER) between Lure and Control probes. Inhibition reflects the neural difference in activation in the posterior ventrolateral prefrontal cortex (pos-VLPFC) between PreCue and PostCue. Interference control reflects the neural difference in activation in the mid-VLPFC between Lure and Control probes. Bottom: mediation analysis results depicting the mediating effect of inhibition on the relationship between neural measures of interference control and behavioral performance. After controlling for the mediating effect of inhibition, the relationship between interference control and behavior was no longer significant. HC, healthy controls; SZ, patients with schizophrenia.
For completeness, we calculated all other possible models by fully rotating all measures. No other significant mediation effects were found (Table 3). Hence, the hypothesized model – inhibition mediates the relationship between interference-control and behavior – was the only model that yielded significant results. Taken together, these results provide strong evidence that control over WM is the crux that links interference-control and behavioral WM impairments in SZ.
Table 3.
Mediation Results
| X-M-Y | a | b | c′ | c | ab | |
|---|---|---|---|---|---|---|
| midVLPFC-posVLPFC-ER | paths (s.e.) | −0.08 (0.03) | −14.2 (9.71) | 2.35 (2.07) | 3.42 (1.26) | 1.16 (0.96) |
| z | −3.03** | −3.81*** | 1.22 | 2.40* | 2.08* | |
| ER-posVLPFC-midVLPFC | paths (s.e.) | −0.01 (0.00) | −2.42 (1.54) | 0.02 (0.04) | 0.06 (0.03) | 0.03 (0.02) |
| z | −3.57*** | −1.64 | 0.33 | 1.51 | 1.74† | |
| ER-midVLPFC-posVLPFC | paths (s.e.) | 0.06 (0.03) | −0.06 (0.03) | −0.01 (0.00) | −0.01 (0.00) | −0.00 (0.00) |
| z | 1.54 | −2.19* | −1.95† | −3.57*** | −1.23 | |
| midVLPFC-ER-posVLPFC | paths (s.e.) | 3.53 (1.25) | −0.01 (0.00) | −0.06 (0.03) | −0.08 (0.03) | −0.03 (0.01) |
| z | 2.38* | −1.94† | −2.17* | −2.89** | −1.73† | |
| posVLPFC-ER-midVLPFC | paths (s.e.) | −23.3 (6.20) | 0.02 (0.04) | −2.42 (1.55) | −2.81 (1.15) | −0.41 (0.90) |
| z | −3.21** | 0.30 | −1.67† | −2.80* | −0.61 | |
| posVLPFC-midVLPFC-ER | paths (s.e.) | −2.81 (1.15) | 2.35 (2.09) | −14.2 (9.80) | −23.33 (6.22) | −6.59 (6.53) |
| z | −2.78* | 1.20 | −3.89*** | −3.13** | −0.45 |
Results from mediation analysis from all 6 possible models of mid-ventrolateral prefrontal cortex (midVLPFC), posterior ventrolateral prefrontal cortex (posVLPFC), and error-rate (ER) interactions. The hypothesized model is in bold. X-M-Y denotes the relationship between the predictor (X), mediator (M), and outcome (Y).
p < 0.1;
p < 0.05;
p < 0.005;
p < 0.0005.
Additional analyses are reported in the Supplemental Material.
Discussion
This study investigated the component processes that underlie WM deficits in SZ. Our results indicate WM impairments in SZ in three specific ways. First, patients demonstrated impaired inhibition of irrelevant content in WM evidenced by reduced PreCue to PostCue activation difference in the posterior VLPFC. Second, patients exhibited a failure to overcome familiarity-induced interference of Lure probes demonstrated by increased behavioral error-rates to Lure probes relative to Control probes. Finally, patients exhibited increased reliance on interference-control at the time of retrieval, evidenced by selectively increased activation to Lure probes relative to Control probes in the mid-VLPFC. These data indicate that impaired inhibitory control in WM has downstream consequences that adversely impact behavior.
Our data also indicated that patients made more errors on Valid probes than HCs, even after controlling for performance on Control probes. This result may also stem from inappropriate inhibitory control. When irrelevant items are appropriately removed from WM, Valid probes are distinguished from Lure probes as a function of memory strength. However, if irrelevant items are not inhibited, Valid and Lure items are more difficult to distinguish. Hence, impaired inhibitory control can simultaneously lead to erroneous endorsements of Lure probes and rejections of Valid probes.
A recent review (39) suggested SZ-related deficits in a variety of cognitive domains including WM could be explained by impairments in proactive control, which allows for goal-relevant information to be activated and irrelevant information to be inhibited in anticipation of cognitive demands that require use of the information (40, 41). The present results fit well within such a framework. While HCs appropriately inhibited irrelevant items from WM in anticipation of the probe, SZs failed to do so, consistent with impaired proactive control. The engagement of proactive control is flexible and may be useful during different phases of tasks depending on demands (40). Previous research has demonstrated impairments during WM encoding and maintenance in patients with SZ (42–45), a pattern that contrasts with the present results. In those studies, encoding and maintenance demands were likely increased due to the use of abstract stimuli (42, 43) or increased loads (44, 45). In such cases, HCs may enlist proactive control processes to facilitate encoding and maintenance, whereas SZs do not. This may take the form of chunking or re-coding strategies to ease demands on maintenance processes. Our data indicate that with verbal material and manageable load, patients with SZ exhibit largely intact encoding and maintenance, but are impaired in proactively inhibiting items from WM in preparation for future responding. These data suggests that patients with SZ have preserved basic maintenance processes, but impaired cognitive control over maintained information.
Our results corroborate a growing body of research in HCs that links the mid-VLPFC to resisting interference and appropriate selection of information at the time of retrieval (8, 46). The mid-VLPFC (BA 45) is thought to select goal-relevant information when multiple competing representations are active in memory (47). The left mid-VLPFC has also been implicated in the resolution of proactive interference, in which memory of a past experience interferes with processing of a subsequent experience (31–33, 38, 47). A common selection mechanism may account for both forms of control (48).
Similar impairments in inhibitory control may underlie cognitive deficits in other psychiatric disorders such as depression, obsessive compulsive disorder, or attention deficit hyperactivity disorder (49–53). In a similar task to that used here, patients with depression demonstrated a specific deficit in inhibiting negatively, but not positively valenced content from WM (51, 54). This deficit was hypothesized to underlie the rumination of negative information in depression. Thus, examining inhibition and its correlates is an important endeavor to pinpoint cognitive impairments in psychiatric populations in general.
Supplementary Material
Acknowledgments
Funding for this study was also provided by the Columbia University Department of Psychiatry (E. S). The Columbia University Department of Psychiatry had no further role in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. This research was also supported in part by R21 MH098271 (C.M.) and F32 NS082069 (D.N.).
We thank Dr. Ragy Girgis, Dr. Joshua Kantrowitz, Dr. Jacob Ballon, Dr. Roberto Gil, Dr. Winnie Leung and Nicole Gaine for their help recruiting patients for the study. We would also like to thank Dr. Kevin Ochsner, Dr. Daphna Shohamy and Dr. G. Elliott Wimmer for their close reading and helpful comments on the manuscript.
Footnotes
This manuscript is dedicated to Edward E. Smith.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part I: Description. Journal of Personality Disorders. 1995;9:83–91. [Google Scholar]
- 2.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- 4.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 5.Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Arch Gen Psychiatry. 2002;59:146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- 6.Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, Lieberman JA. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Arch Gen Psychiatry. 2003;60:238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- 7.Baddeley A, Logie R, Bressi S, Della Sala S, Spinnler H. Dementia and working memory. Q J Exp Psychol A. 1986;38:603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- 8.Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 10.Alloway TP, Alloway RG. Investigating the predictive roles of working memoryand IQ in academic attainment. J Exp Child Psychol. 2010;106:20–29. doi: 10.1016/j.jecp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Bowen L, Wallace CJ, Glynn SM, Nuechterlein KH, Lutzker JR, Kuehnel TG. Schizophrenic individuals’ cognitive functioning and performance in interpersonal interactions and skills training procedures. J Psychiatr Res. 1994;28:289–301. doi: 10.1016/0022-3956(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 12.Corrigan PW, Green MF, Toomey R. Cognitive correlates to social cue perception in schizophrenia. Psychiatry Res. 1994;53(2):141–151. doi: 10.1016/0165-1781(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 13.Barch DM, Carter CS, Perlstein W, Baird J, Cohen JD, Schooler N. Increased Stroop facilitation effects in schizophrenia are not due to increased automatic spreading activation. Schizophr Res. 1999;39:51–64. doi: 10.1016/s0920-9964(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 14.Barch DM, Carter CS, Cohen JD. Factors influencing Stroop performance in schizophrenia. Neuropsychology. 2004;18(3):477–484. doi: 10.1037/0894-4105.18.3.477. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald AW, III, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 16.Cohen JD, Dunbar KO, Barch DM, Braver TS. Issues concerning relative speed of processing hypotheses, schizophrenic performance deficits, and prefrontal function: comment on Schooler et al. (1997) J Exp Psychol Gen. 1997;126(1):37–41. doi: 10.1037//0096-3445.126.1.37. [DOI] [PubMed] [Google Scholar]
- 17.Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102(24):8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 19.Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res. 2001;12(3):467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- 20.Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: An event-related fMRI study of the Stroop Task. Cogn Brain Res. 2003;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 21.Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magneticresonance imaging. J Neurosci. 2001;21(19):7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonides J, Nee DE. Assessing dysfunction using refined cognitive methods. Schizophr Bull. 2005;31(4):823–829. doi: 10.1093/schbul/sbi053. [DOI] [PubMed] [Google Scholar]
- 23.Fleming K, Goldberg TE, Gold JM, Weinberger DR. Verbal working memory dysfunction in schizophrenia: use of a Brown-Peterson paradigm. Psychiatry Res. 1995;56(2):155–161. doi: 10.1016/0165-1781(95)02589-3. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg TE, Weinberger DR, Pliskin NH, Berman KF, Podd MH. Recall memory deficit in schizophrenia: A possible manifestation of prefrontal dysfunction. Schizophr Res. 1989;2(3):251–257. doi: 10.1016/0920-9964(89)90001-7. [DOI] [PubMed] [Google Scholar]
- 25.Randolph C, Gold JM, Carpenter CJ, Goldberg TE, Weinberger DR. Release from proactive interference: Determinants of performance and neuropsychological correlates. J Clin Exp Neuropsych. 1992;14(5):785–800. doi: 10.1080/01688639208402863. [DOI] [PubMed] [Google Scholar]
- 26.Smith EE, Eich TS, Cebenoyan D, Malapani C. Intact and impaired cognitive-control processes in schizophrenia. Schizophr Res. 2011;126:132–137. doi: 10.1016/j.schres.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Nee DE, Jonides J. Dissociable interference-control processes in perception and memory. Psychol Sci. 2008;19:490–500. doi: 10.1111/j.1467-9280.2008.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nee DE, Jonides J. Common and distinct neural correlates of perceptual and memorial selection. Neuroimage. 2009;45:963–975. doi: 10.1016/j.neuroimage.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith EE, Jonides J. Neuroimaging analyses of human working memory. ProcNatl Acad Sci U S A. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: evidence from PET. Psychol Sci. 1996;7(1):25–31. [Google Scholar]
- 31.Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 32.Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci U S A. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nee DE, Jonides J, Berman MG. Neural mechanisms of proactive interference-resolution. Neuroimage. 2007;38(4):740–751. doi: 10.1016/j.neuroimage.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47(3):821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberauer K. Removing irrelevant information from working memory. A cognitive aging study with the modified Sternberg task. JEP: LMC. 2001;27:948–957. [PubMed] [Google Scholar]
- 38.Zhang JX, Leung HC, Johnson MK. Frontal activations associated with accessing and evaluating information in working memory: an fMRI study. Neuroimage. 2003;20:1531–1539. doi: 10.1016/j.neuroimage.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Conway ARA, Jarrold C, Kane MJ, Miyake A, Towse JN, editors. Variation in Working Memory. Oxford University Press; 2007. pp. 76–106. [Google Scholar]
- 41.Bunge SA, Wallis JD, Parker A, Brass M, Crone EA, Hoshi E, Sakai K. Neural circuitry underlying rule use in humans and nonhuman primates. J Neurosci. 2005;25(45):10347–10350. doi: 10.1523/JNEUROSCI.2937-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anticevic A, Repovs G, Barch DM. Working Memory Encoding and Maintenance Deficits in Schizophrenia: Neural Evidence for Activation and Deactivation Abnormalities. Schizophr Bull. 2011;39(1):168–78. doi: 10.1093/schbul/sbr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anticevic A, Repovs G, Corlett PR, Barch DM. Negative and Non-emotional Interference with Visual Working Memory in Schizophrenia. Biol Psychiatry. 2011;70(12):1159–68. doi: 10.1016/j.biopsych.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MR, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, Pearlson GD. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry. 2006;60(1):11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Driesen NR, Leung H-C, Calhoun VD, Constable RT, Gueorguieva R, Hoffman R, et al. Impairment during Working Memory Maintenance and Response in Schizophrenia: Functional Magnetic Resonance Imaging Evidence. Biol Psychiatry. 2008;64(12):1026–34. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranganath C. Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277– 289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 47.Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cereb Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- 48.Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J. A Meta-analysis of Executive Components of Working Memory. Cereb Cortex. 2012;23(2):264–82. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harkin B, Miellet S, Kessler K. What checkers actually check: an eye tracking study of inhibitory control and working memory. PLoS One. 2012;7(9):e44689. doi: 10.1371/journal.pone.0044689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. J Abnorm Psychol. 2008;117(1):182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- 51.Joormann J, Nee DE, Berman MG, Jonides J, Gotlib IH. Interference resolution in major depression. Cogn Affect Behav Neurosci. 2010;10(1):21–33. doi: 10.3758/CABN.10.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakao T, Nakagawa A, Nakatani E, Nabeyama M, Sanematsu H, Yoshiura T, et al. Working memory dysfunction in obsessive-compulsive disorder: a neuropsychological and functional MRI study. J Psychiatr Res. 2009;43(8):784–791. doi: 10.1016/j.jpsychires.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 53.Schecklmann M, Ehlis AC, Plichta MM, Dresler T, Heine M, Boreatti-Hummer A, et al. Working Memory and Response Inhibition as One Integral Phenotype of Adult ADHD? A Behavioral and Imaging Correlational Investigation. J Atten Disord. 2012 doi: 10.1177/1087054711429702. [DOI] [PubMed] [Google Scholar]
- 54.Berman MG, Nee DE, Casement M, Kim HS, Deldin P, Kross E, et al. Neural and behavioral effects of interference resolution in depression and rumination. Cogn Affect Behav Neurosci. 2011;11(1):85–96. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.