Abstract
Introduction
Two thirds of newly diagnosed lung cancer patients in the U.S. would not meet the current USPSTF (United States Preventive Services Task Force) screening criteria, suggesting a need for amendment to the high-risk definition. To provide evidence of additional high-risk subpopulations and estimated gains and losses, we conducted a two-step study using three cohorts.
Methods
Two prospective cohorts are comprised of 5988 primary lung cancer patients diagnosed between 1997-2011 (Hospital) and 850 defined-community residents (Community); the retrospective cohort is the Olmsted County population (Minnesota, USA) followed for 28 years (1984-2011). Subgroups of lung cancer patients who might have been identified using additional determinates were estimated and compared between Community and Hospital cohorts. Findings were supported by indirect comparative projections of two ratios: benefit to harm and cost to effectiveness.
Results
Former cigarette smokers with 30 or more pack-years and 15-30 quit-years age 55-80 formed the largest subgroup not meeting the current screening criteria, constituting 12% of the hospital cohort and 17% of community cohort. Using the expanded criteria suggested by our study may add 19% more CT examinations for detecting 16% more cases when comparing to the USPSTF criteria. Meanwhile, the increases of false positive, over-diagnosed and radiation-related lung cancer death are 0.6%, 0.1%, and 4.0%, respectively.
Conclusions
Current USPSTF screening criteria exclude many patients at high risk for lung cancer. Individuals who are under 81 years, had 30 or more pack-year smoking history, and had quit for 15-30 years may significantly increase the number of non-over diagnosis screen-detected lung cancers, does not significantly add false positive cases, saves more lives, yet with an acceptable amount of elevated scan exposure and cost.
Keywords: lung cancer, low dose CT, screening, smoking cessation, former smokers
INDRODUCTION
With the declining percentage of the United States population who smoke, lung cancer incidence and mortality have been decreasing among men in the past three decades, and only recently, has shown decrease among women.1 Meanwhile, former cigarette smokers remain at a high risk for lung cancer although at lower risk than they would have been had they continued smoking2. As a consequence, more people with lung cancer are now diagnosed in former smokers rather than in current smokers.3 Specifically, less than 18% of United States adults are current smokers and more than 30% are former smokers.3, 4 As of 2014, use of low-dose computed tomography (LDCT) screening for lung cancer was recommended by the United States Preventive Services Task Force (USPSTF), i.e., to annually screen people aged 55-80 years of age who have smoked 30 or more pack-years of cigarettes and are either current smokers or have quit within 15 years.5, 6 This recommendation was based on the entry criteria of the National Lung Screening Trial (NLST) but with an extension of the upper age limit of 74.7 However, our recent report showed that approximately two thirds of newly diagnosed lung cancer patients would not have met the current USPSTF high-risk criteria for LDCT screening.8 Particularly, we found a 24% fall-off in meeting screening-eligibility criteria (from 57% in 1984-1990 to 43% in 2005-2011) which exceeded the 17% decline in incidence of lung cancer (from 53 to 44/100,000) over the same time intervals. Herein we have conducted further investigations to delineate the high-risk subpopulations based on evidence from two prospective lung cancer patient cohorts and a retrospective community cohort. Our goal was to improve the identification of individuals at high-risk for lung cancer by (1) demonstrating the chronological patterns of patients who would have been the beneficiaries or missed-outs under USPSTF criteria for lung cancer screening in two contrasting cohorts, and (2) providing indirect evidence of a new subpopulation that should be considered in the definition of high risk and the potential benefit versus harm as well as projected cost versus effectiveness.
MATERIALS AND METHODS
Study population
This study included two steps: description and validation. Step I utilized two prospectively-followed lung cancer cohorts, one based on Mayo Clinic referral patients (i.e., the Hospital Cohort, N=5988) and another from Olmsted County (Minnesota USA) residents (i.e., the Community Cohort, N=850). The Hospital Cohort included patients diagnosed with pathologically confirmed primary lung cancer at Mayo Clinic in Minnesota during a 15-year period (between January 1, 1997 and December 31, 2011)9 who were not Olmsted County residents. The Community Cohort was matched to the same 15 years of diagnosis as the Hospital Cohort.8 All cases were identified using the Rochester Epidemiology Project database, which has maintained a comprehensive medical record linkage system for over 60 years of almost all persons residing in Olmsted County.10, 11 This population comprises ~140,000 persons of whom 83% are non-Hispanic white, is socioeconomically similar to the US white population, and a representative of the Midwestern US population. More details were published previously.8, 12 This study was approved by the Institutional Review Boards of Mayo Clinic and Olmsted County Medical Center.
Step II was to provide indirect evidence supporting the findings in Step I. We have derived comparative benefit-to-harm and cost-to-effectiveness among three sets of criteria: NLST, USPSTF, and the expanded criteria suggested by our study (Our Study in brief), based on the information provided in the models by de Koning et al.6 Although hypothetical and indirect, the comprehensive models built by de Koning and colleagues are very helpful in initial evaluation of the impact (positive and negative) of a potential high-risk subpopulation given the lack of individual-level smoking history data or up-to-date and accurate smoking history information for entire populations of interest. Briefly, the modeling groups standardized input data on smoking histories and non–lung cancer mortality to simulate life histories of the U.S. cohort born in 1950, which uses an updated version of the National Cancer Institute's Smoking History Generator. Their models assumed 100% screening adherence; the data derived from trials with short duration (e.g., 4-9 years) were extrapolated to lifetime follow-up, and smoking history data from 1-2 decade ago were assumed current.6, 13-16
Specifically, we have adapted and integrated the following 11 items selected from the Tables 1 and 2 in the article by de Konig and colleagues:6 1. total CT examinations, including screening, 2. screening detected cases, 3. reduction in lung cancer mortality, 4. total cases detected at an early stage, 5. average screening exam nations per person screened, 6. screening examinations per lung cancer death averted, 7. screening examinations per life year gained, 8. average false-positive results per person screened, 9. over-diagnosis, 10. over-diagnosis percent of screening-detected cases, and 11.radiation-related lung cancer deaths.
Table 1.
Demographical and clinical features of Community and Hospital Lung Cancer Cohorts, Diagnosed 1997-2011
| Characteristics* | Prospectively Enrolled and Followed 6838 Lung Cancer Patients |
P value | |
|---|---|---|---|
| Community Cohort N=850 | Hospital Cohort N=5988 | ||
| Age at diagnosis, year | <0.0001 | ||
| Mean ± SD | 68.8 (11.0) | 65.0 (11.1) | |
| Median (Q1, Q3) | 70.0 (62.0, 77.0) | 66.0 (58.0, 73.0) | |
| Gender | 0.2602 | ||
| Male | 444 (52.2%) | 3251 (54.3%) | |
| Mean age ± SD | 68.9 (10.6) | 66.4 (10.9) | |
| Median age (Q1, Q3) | 70.0 (63.0, 77.0) | 68.0 (60.0, 74.0) | |
| Female | 406 (47.8%) | 2737 (45.7%) | |
| Mean age ± SD | 68.7 (11.4) | 63.4 (11.2) | |
| Median age (Q1, Q3) | 70.0 (61.0, 77.0) | 65.0 (56.0, 71.0) | |
| Race | 0.0335 | ||
| White | 811 (95.4%) | 5577 (93.5%) | |
| Others | 39 (4.6%) | 386 (6.5%) | |
| Cigarette smoking status | <0.0001 | ||
| Never smoker | 77 (9.0%) | 1008 (16.8%) | |
| Former smoker | 422 (49.8%) | 3056 (51.0%) | |
| Current smoker | 350 (41.2%) | 1924 (32.1%) | |
| Smoking pack-years (for smokers) | 0.0025 | ||
| 1-19 | 88 (11.4%) | 781 (15.8%) | |
| 20-29 | 82 (10.6%) | 583 (11.8%) | |
| ≥30 | 601 (78.0%) | 3580 (72.4%) | |
| Quit-years (for former smokers) | 0.0001 | ||
| <15 years | 241 (57.1%) | 1443 (47.2%) | |
| ≥15 years | 181 (42.9%) | 1613 (52.8%) | |
Missing data on “Race” of 13 patients (0.2%) in Hospital Cohort, and on “Smoking pack-year” of 1 (0.1%) patient in Community Cohort and of 27 patients (0.5%) in Hospital Cohort, respectively.
Data are presented as mean ± SD or No (percentage), unless otherwise indicated.
Table 2.
Comparative Benefit-to-harm and Cost-to-effectiveness of 11 Selected Items* among Three Programs: National Lung Screening Trial (NLST), U.S. Preventive Services Task Force (USPSTF), and the Our Study**
| Setting of LDCT annual screening entry criteria and comparisons | 1. Total CT Examinations, Including Screening, setting NLST at 100% as reference | 2. Screening Detected Cases, setting NLST at 100% as reference | 3. Reduction in Lung Cancer mortality in % | 4. Total Cases Detected at an Early Stage, % | 5. Average Screening Examinations per Person Screened, N | 6. Screening Examinations per Lung Cancer Death Averted, N | 7. Screening Examinations per Life-Year Gained, N | 8. Average False-Positive Results per Person Screened, N | 9. Over-diagnosis % of all cases | 10. Over-diagnosis % of screening-detected cases | 11.Radiation-Related Lung Cancer Deaths, N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NLST | 100 | 100 | 12.3 | 48.4 | 13.8 | 577 | 49 | 3.2 | 2.7 | 8.7 | 24 |
| USPSTF vs. NLST | + 8.2 | +19.7 | +1.7 | +2.1 | +1.1 | +3 | +3 | +0.4 | +1.0 | +1.2 | +0 |
| Our study* vs. NLST | +29.0 | +39.0 | +3.5 | +3.7 | +3.1 | +6 | +5 | +0.7 | +1.6 | +1.3 | +1 |
| Our study* vs. USPSTF | +19.3 | +16.1 | +1.8 | +1.6 | +2.0 | +3 | +2 | +0.5 | +0.6 | +0.1 | +1 |
Derived and adapted from Tables 1-2 of de Koning et al, Annals of Internal Medicine, 2014.
Used a screening program most similar to A-55-80-30-25 in de Koning et al, Annals of Internal Medicine, 2014 (i.e., A-55-80-30-30):
Their comprehensive models standardized input data on smoking histories and non–lung cancer mortality to simulate life histories of the U.S. 1950 birth cohort.15-18 We used a screening program “A-55-80-30-25” in the models of de Koning et al, meaning annual LDCT start age at 55-years, stop age (80-year), ≥30 pack-years smoked, and ≤25 years since quitting; this model is most similar to our findings as supported by our previous work.2, 8
Data collection
For each patient, medical records were reviewed and abstracted for information including demographics (age, gender, and race); occupational exposure history; tobacco exposure history; lung cancer histology, staging, treatment modality; family history of lung cancer and other co-morbidity conditions. For the hospital cohort, information was also obtained from an interview and/or a follow-up questionnaire. The patient interview and/or annual follow-up questionnaire obtained detailed information on tobacco history, occupational exposure history, and family cancer history. Tobacco history information included current or former use, duration, the average amount of cigarettes smoked per day, and the number of years since quitting smoking. Current smokers were defined as those actively smoking and included those who had stopped smoking within one year prior to their lung cancer diagnosis. Former smoker were defined as having quit smoking for ≥1 or more years before diagnosis. Never smokers were defined as those who had smoked fewer than 100 cigarettes during his or her lifetime. Pack-years are calculated by multiplying the number of packs smoked daily by the number of years smoked. The history of chronic obstructive pulmonary disease (COPD) was determined based on explicit diagnosis documented in the medical history with pulmonary function tests in the medical record. Family history of lung cancer was defined as having at least one first-degree relative (parent, sibling or offspring) with lung cancer. Positive exposure to asbestos was based on self-reported direct contact with the asbestos-containing material for at least a year and corroborated by the occupation history (job titles and tasks) at least 5 years prior to lung cancer diagnosis.
Statistical Analysis
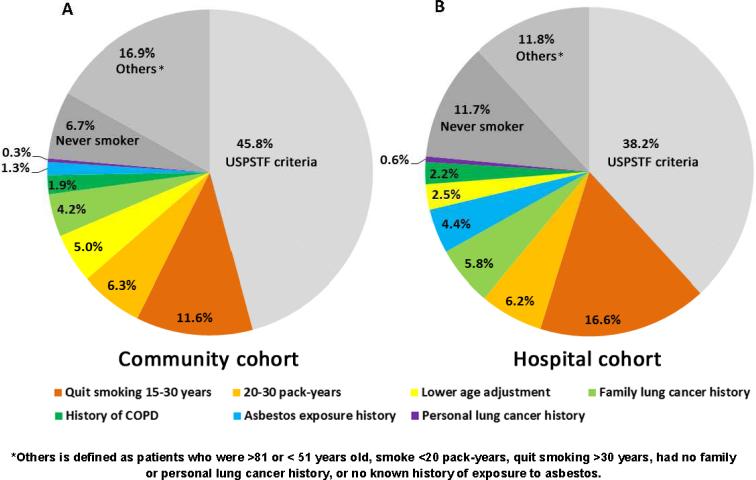
In Step I, we did three descriptive analyses: (1) Calculating and sorting the frequencies of selected key characteristics as follows: age groups (50-54, 55-80, >80); smoking status (ever vs. never smokers), cigarettes: pack-years (pack-years, <20, 20-30, >30), and quitting smoking years (quit-years <15, 15-30, >30); prior histories of COPD, lung cancer, and asbestos exposure; and family history of lung cancer in first-degree relatives; (2) illustrating by pie charts of subgroups by frequency in those not meeting USPSTF criteria; (3) calculating and illustrating distribution of pack-years and quit-years in patients not meeting USPSTF Criteria.
In Step II, we have made three levels of hypothetical comparisons: first is the relative gain of USPSTF versus NLST, second is our study versus NLST, and the third is the relative gain of our study versus USPSTF. For clarity and simplicity in comparisons, we set the estimates of NLST as the standardized reference values at 100 or 100% when involving the actual numbers, e.g., total CT examinations and screening detected cases. For parameters of common knowledge, e.g., reduction in lung cancer mortality and total cases detected at an early stage, the original model-based percentage for NLST was used as the comparator, with the predicted change (in %) by USPSTF and Our Study. For the estimated means, e.g., average screening examinations per person screened per life year gained, average false-positive results per person screened, and over-diagnosis of screening-detected cases, the original model-based average for NLST was used as the comparator, with the predicted change (in %) by USPSTF and Our Study.
RESULTS
Patients’ Characteristics of the Two Prospective Lung Cancer Cohorts
Table 1 presents basic information and comparisons of 5988 hospital and 850 community lung cancer cohorts, diagnosed between 1997 and 2011. Patients’ characteristics differed between the two cohorts, reflecting the typical referral bias in a tertiary medical center. All variables but sex ratio are significantly different; specifically, compared to the community cohort, the hospital cohort is younger, higher representation of never smokers, lighter smokers, and long-term quitters. Despite these differences, the two cohorts showed remarkably consistent results in following three aspects:
1. Subgroups Outside of USPSTF Screening Criteria
Figure 1 illustrates the relative proportions of the screening eligible and ineligible for the two patient cohorts, by the order of subgroup frequency. For individuals under more than one variable, they were grouped within the larger subgroup. The frequencies of the selected risk factors in each cohort are summarized in Supplementary Table 1. Using the Community Cohort as a standard reference group (Figure 1A), 46% of cases were found to meet the USPSTF criteria, whereas only 38% were found for the Hospital Cohort. Outside of the portion under the USPSTF screening criteria, we compared eight factors of the patients in terms of additional intensity categories of tobacco smoking exposure, age 50-54 years at the time of diagnosis, family and personal history of lung cancer, prior history of COPD and asbestos exposure. The two most frequently occurring characteristics in both cohorts are “quit smoking 15-30 years” (12% and 17%) and “smoked 20-30 pack-years” (6.3% and 6.2%). In both cohorts, “history of COPD” (1.9% vs. 2.2%) and “personal lung cancer history” (0.3% vs. 0.6%) were the lowest in frequency among all presented factors.
Figure 1.
Subgroup distribution by frequency of known risk factors in lung cancer patients who were <81 years old, diagnosed 1997-2011
(A) Community Cohort;
(B) Hospital Cohort.
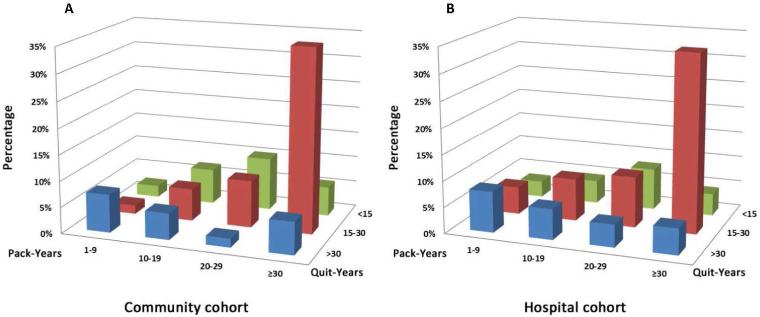
2. Distribution of Pack-years and Quit-years in Patients Ineligible under USPSTF Criteria
We examined the distribution of pack-years and quit-years in those less than 81 years old and ineligible by the USPSTF screening criteria in both cohorts, as depicted in Figure 2 (A-B); clearly standing out is the subgroup of those with ≥30 pack-years and having quit smoking for more than 15 years. Therefore, we propose an addition to the current USPSTF screening criteria by adding former smokers who quit smoking 15-30 years and had at least a 30 pack-year history.
Figure 2.
Distribution of Pack-Years and Quit-Years in patients who <81 years old ineligible by NLST and USPSTF criteria, 1997-2011.
(A) Community Cohort;
(B) Hospital Cohort
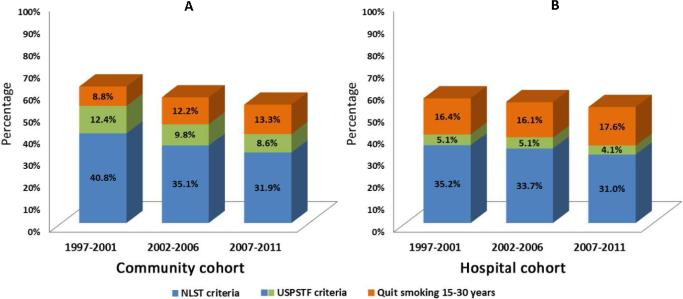
3. Temporal Pattern Change by NLST and USPSTF Screening Criteria
In the Community cohort, 35.7% of the total patients would have met the entry criteria of the NLST, and 45.8% met the USPSTF screening criteria. In the Hospital cohort, only 33.4% and 38.2% would have met the respective criteria. Figure 3 (A-B) illustrates the relative proportion of alternative criteria over three 5-year intervals of both cohorts (i.e., 1997-2001, 2002-2006, and 2007-2011). The bottom proportion for each bar presents the NLST criteria (55–74 years, smoked at least 30 pack-years, and former smokers quitting 15 years or fewer); the middle proportion is for age group 75-80 (extended by USPSTF); and the top proportion shows our proposed addition of former smokers with 30+ pack years and 15-30 quit years. More specifically, in the Community cohort (Figure 3A), the proportion of patients who were NLST-eligible decreased from 40.8% to 31.9% (P=0.017), and the USPSTF-eligible decreased from 53.2% to 40.5% (P=0.002) over these 15 years. On the other hand, the proportion of the proposed criteria increased; particularly noted is coverage of patients meeting the proposed criteria at the most recent time interval was at 52%.
Figure 3.
Temporal pattern of coverage proportion by NLST and USPSTF screening criteria, 1997-2011.
(A) Community Cohort;
(B) Hospital Cohort
The same decreasing eligibility trends were observed in the Hospital cohort (Figure 3B): the NLST-eligible from 35.2% to 31.0% (P=0.003) and the USPSTF-eligible from 40.3% to 35.1% (P<0.001). More strikingly in the most recent time interval, the proportion of lung cancer cases added by the quit-years 15-30 subgroup (17.6%), is over 4-times larger than the addition of 75-80 year-of-age by USPSTF (4.1%) to the NLST criteria.
Potential Benefit versus Harm and Projected Cost versus Effectiveness
Table 2 provides hypothetical projections of benefit versus harm and cost versus effectiveness through eleven itemized comparisons (labeled columns 1-11), reflecting meaningful relative gains and losses of the three sets of criteria: NLST, USPSTF, and Our Study, purposefully not involving actual expense and productivity measures. Five illustrative points of pros and cons are listed below.
Balance of total computed tomography (CT) screening examinations and screening detected lung cancer cases (columns 1 and 2): comparing to NLST estimates at 100 as a reference, the USPSTF would add 8.2% more total CT examinations for 19.7% gain in screening-detected lung cancer cases; whereas our study suggested criteria (“Our Study” in brief) may add 29% more total CT examinations for 39% gain in screening-detected lung cancer cases. Our Study versus USPSTF: 19% more CT examinations as a trade of 16% more lung cancer cases detected.
In the models where NLST reduces lung cancer mortality at 12.3%, a greater reduction in mortality may be achieved as suggested by our study than the predicted reduction by USPSTF (column 3, i.e., 15.8% [12.3+3.5] vs. 14% [12.3+1.7]). Our Study versus USPSTF: a 1.8% deeper reduction in lung cancer mortality.
In the models where 48.4% of the total detected cases in NLST were in early stage, a higher proportion of early-stage lung cancer may be achieved as suggested by our study than the predicted increase by USPSTF (column 4, i.e., 52.1% [48.4+3.7] vs. 50.5% [48.4+2.1]). Our Study versus USPSTF: a 1.6% increase in detecting lung cancer at an early stage.
Weighing the average screening examinations per person screened (column 5), per lung cancer death averted (column 6), and per life year gained (column 7), the reference of NLST yielded 13.3, 577, and 49, respectively. Compared to the NLST, USPSTF gained 1.1, 3 and 3; Our Study gained 3.1, 6, and 5, respectively. Our Study versus USPSTF: an increase in average screening examinations by 2 per person screened, by 3 per lung cancer death averted, and by 2 per life-year gained by 2, respectively.
Concerns of false positivity (column 8), over-diagnosed cases (columns 9-10), and radiation-related lung cancer death (column 11): The increases of false positive, over-diagnosed and radiation-related lung cancer death are minimal by all comparisons.
DISCUSSION
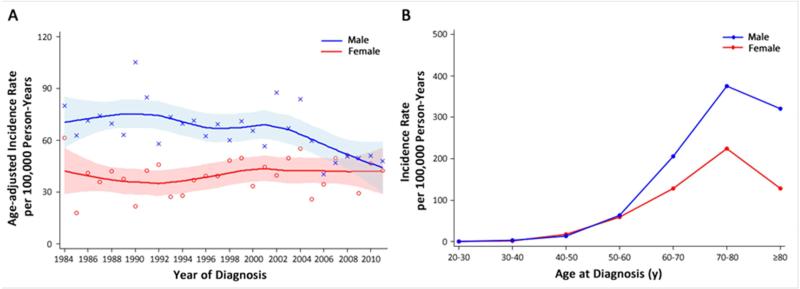
Based on the latest data from Surveillance Epidemiology and End Results (SEER) and National Program of Cancer Registries (NPCR), lung cancer incidence in the United States peaked in 1984 among men while continuing to increase among women until 2006.19 We found a similar trend in Olmsted County population (Figure 4), supporting the generalizability and validity of our data to be representative of the United States general population. During 2005-2012, the proportion of heavy smokers who smoked ≥30 cigarettes per day in the United States declined significantly, from 12.6% to 7.0%.3 Our recent report revealed that less than 40% of subjects diagnosed with lung cancer would meet the USPSTF screening criteria,8 confirming the need to expand the current criteria if the desire is to target the population at high risk.20, 21
Figure 4.
Trends in the incidence of lung cancer, Olmsted County, Minnesota, USA 1984-2011.
(A) By calendar year of diagnosis and adjusted by age.
(B) By age.
Since 2002, former smokers have outnumbered current smokers; and from 1998 to 2012, the proportion of US adults who were current cigarette smokers declined from 24.1% to 18.1%.3, 4 Former smokers remain at greatly elevated risk relative to never smokers even though lower than if they had continued to smoke.2, 14-15 The fact that majority of lung cancer cases diagnosed in the United States today are former smokers reflects success in smoking cessation efforts, which also reflects continued high-risk status of former smokers.22-25 We specifically evaluated quitting smoking 15-30 years because our previous study showed the risk of lung adenocarcinoma remained elevated up to 30 years beyond smoking cessation for both former heavy and light smokers.2 We also have reported a trend that the proportion of lung cancer patients who smoked ≥30 pack-years was decreasing and former smokers who quit smoking ≥15 years were increasing over time in Olmsted County.8 A striking observation from the current study is the distribution of pack-years and quit-years in those ineligible under USPSTF criteria, as in Figure 2, where we found that, compared to other risk categories, “quit smoking 15-30 years” accounted for the greatest percentage of those with lung cancer.
Our results also showed that, compared to the NLST entry criteria, lung cancer cases in Community and Hospital cohorts who met the USPSTF criteria only increased by 4.1%-8.6% (Figure 3, 2007-2011). In contrast, when adding former smokers with 15-30 quit-years, the increase in those meeting criteria that actually got lung cancer was 13.3%-17.6%. Therefore, assuming screening could reduce mortality and be cost-effective in the same magnitude as expected without increasing harm, high-risk subpopulations outside of USPSTF criteria need to be reconsidered, especially those who have sustained smoking cessation beyond 15 years. Taking advantage of the patients from the Olmsted County population, we were able to compare the local and referral patients whose lung cancer was diagnosed during the same time period (Figure 4).
We acknowledge several limitations. First, data from Olmsted County may not be generalizable to the entire United States in terms of racial distribution, disease patterns, and access to care. Individuals of Caucasian race, known to have lower incidence of lung cancer, are overrepresented in Olmsted County. Second, although we found that “quit smoking 15-30 years” and “smoked 20-30 pack-years” are the two highest percentages of patient subgroups in both cohorts, the proportions of patients older than 81 years, never smokers, history of COPD, and history of asbestos exposure in the hospital cohort differ from the community cohort, likely due to the referral practice and patients’ self-preference. Third, we were unable to develop a prediction model for individualized lung cancer risk assessment, as reported by other studies,26-31 which require complete data on all known risk factors in the entire Olmsted County population; such data is not currently available. However, based on the modeling data provided by de Koning and colleagues,6 we were able to indirectly project the impact of extending the screened population to include former smokers of >15 years cessation, which may significantly increase the number of non-over diagnosis screen-detected lung cancers, saves more lives, have an acceptable amount of increased scan exposure and cost, yet unlikely add significantly false positive cases.
Nonetheless, our two prospective patient cohorts differed significantly in many aspects but provided consistent study results, from the case-detection perspective, i.e., the relative proportions of diagnosed lung cancer patients with known risk factors. This is one of the efficient designs to capture, in a timely manner, whether the end results of a disease under screening reasonably reflects the pre-defined high-risk population, although the most definitive answer to justify a change in high-risk definition being cost effectiveness requires directly calculating risks, having the denominator to know the number needed to screen, and how screening is implemented in the real world.32
CONCLUSIONS
In both Community and Hospital Cohorts, the trend in percentage of lung cancer patients who met the USPSTF screening inclusion criteria decreased between 1997 and 2011. The decreasing trend of screening-eligibility in both cohorts exceeded the decline in incidence over time, which demonstrates that the current lung cancer screening entry criteria did not identify those who actually got lung cancer. Inclusion of identifiable high-risk subpopulations should be reconsidered to improve current screening criteria. Our current and previous studies provide evidence that former smokers with 15-30 quit-years remain at high risk and should be considered as eligible for LDCT screening for lung cancer. The current USPSTF recommendation to stop screening after 15 years of smoking cessation is not reflective of continued high risk, although participation of the expanded population in screening setting needs to be further evaluated.
ACKNOWLEDGMENTS
We thank Barbara A Abbott, Jennifer S Sauver, Walter A Rocca, and Barbara P Yawn, for their Rochester Epidemiology Project resource made available to this study. The authors appreciate Connie Edwards for her technical assistance with the manuscript.
Funding/Support: This study was funded by grants R03 CA77118, R01 CA80127 and R01 CA84354 from the U.S. National Institutes of Health; Rochester Epidemiology Project R01 AG034676 from the National Institute on Aging; and Mayo Clinic Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: all authors had financial support from the NIH. Mayo Clinic Foundation, and Rochester Epidemiology Project for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Contributor Information
Ping Yang, Division of Epidemiology, Department of Health Sciences Research, Mayo Clinic, 200 First St. SW, Rochester, MN, USA 55905.
Yi Wang, School of Environmental Science and Public Health, Wenzhou Medical University, Chashan University Town, Wenzhou, Zhejiang Province 325035.
Jason A Wampfler, Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, Mayo Clinic, Rochester, MN USA 55905.
Dong Xie, Department of Thoracic Surgery, Shanghai Pulmonary Hospital, 1239 SiPing Road, Yangpu District, Shanghai, P.R. China.
Shawn M Stoddard, Registered Nurse Division of Epidemiology, Department of Health Sciences Research, Mayo Clinic, 200 First St. SW, Rochester, MN, USA 55905.
Jun She, Shanghai Respiratory Institute, Fudan University, No. 83 Wanghangdu Road, Jing'an District, Shanghai, P.R. China.
David E Midthun, Division of Pulmonary and Critical Care Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN, USA 55905.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ebbert JO, Yang P, Vachon CM, et al. Lung Cancer Risk Reduction After Smoking Cessation: Observations From a Prospective Cohort of Women. J Clin Oncol. 2003;21(5):921–926. doi: 10.1200/JCO.2003.05.085. [DOI] [PubMed] [Google Scholar]
- 3.Agaku IT, King BA, Dube SR. Current cigarette smoking among adults - United States, 2005-2012. MMWR. Morbidity and mortality weekly report. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults--United States, 2007. MMWR. Morbidity and mortality weekly report. 2008;57:1221–6. [PubMed] [Google Scholar]
- 5.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–8. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 6.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:311–20. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Midthun DE, Wampfler JA, et al. Trends in the proportion of patients with lung cancer meeting screening criteria. JAMA. 2015;313:853–5. doi: 10.1001/jama.2015.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang P, Allen MS, Aubry MC, et al. Clinical Features of 5,628 Primary Lung Cancer Patients: Experience at Mayo Clinic from 1997-2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 10.Beard CM, Jedd MB, Woolner LB, et al. Fifty-year trend in incidence rates of bronchogenic carcinoma by cell type in Olmsted County, Minnesota. J Natl Cancer Inst. 1988;80:1404–1407. doi: 10.1093/jnci/80.17.1404. [DOI] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–24. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–53. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865–73. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 15.Anderson CM, Burns DM, Dodd KW, et al. Chapter 2: Birth-cohort-specific estimates of smoking behaviors for the U.S. population. Risk analysis : an official publication of the Society for Risk Analysis. 2012;32(Suppl 1):S14–24. doi: 10.1111/j.1539-6924.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg MA, Feuer EJ, Yu B, et al. Chapter 3: Cohort life tables by smoking status, removing lung cancer as a cause of death. Risk analysis : an official publication of the Society for Risk Analysis. 2012;32(Suppl 1):S25–38. doi: 10.1111/j.1539-6924.2011.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort-specific smoking histories, 1965-2009. American Journal of Preventive Medicine. 2014;46:e31–7. doi: 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon J, Meza R, Krapcho M, et al. Chapter 5: Actual and counterfactual smoking prevalence rates in the U.S. population via microsimulation. Risk analysis : an official publication of the Society for Risk Analysis. 2012;32(Suppl 1):S51–68. doi: 10.1111/j.1539-6924.2011.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Lung Cancer Screening. Version 1.2012. 2012 [Google Scholar]
- 21.Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–8. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 22.Yang P, Cerhan JR, Vierkant RA, et al. Adenocarcinoma of the Lung is Strongly Associated with Cigarette Smoking: Further Evidence From a Prospective Study of Women. Am J Epidemiol. 2002;156(12):1114–1122. doi: 10.1093/aje/kwf153. [DOI] [PubMed] [Google Scholar]
- 23.Ashraf H, Saghir Z, Dirksen A, et al. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low-dose CT: final results after a 5-year screening programme. Thorax. 2014;69:574–9. doi: 10.1136/thoraxjnl-2013-203849. [DOI] [PubMed] [Google Scholar]
- 24.Fry JS, Lee PN, Forey BA, et al. How rapidly does the excess risk of lung cancer decline following quitting smoking? A quantitative review using the negative exponential model. Regulatory toxicology and pharmacology : RTP. 2013;67:13–26. doi: 10.1016/j.yrtph.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Tammemagi MC, Berg CD, Riley TL, et al. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106:dju084. doi: 10.1093/jnci/dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tammemagi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLOS Medicine. 2014;11:e1001764. doi: 10.1371/journal.pmed.1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKee BJ, Hashim JA, French RJ, et al. Experience with a CT screening program for individuals at high risk for developing lung cancer. Journal of the American College of Radiology : JACR. 2015;12:192–7. doi: 10.1016/j.jacr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 2008;98:270–6. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raji OY, Duffy SW, Agbaje OF, et al. Predictive accuracy of the Liverpool Lung Project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med. 2012;157:242–50. doi: 10.7326/0003-4819-157-4-201208210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99:715–26. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- 31.Bach PB, Kattan MW, Thornquist MD, et al. Variations in Lung Cancer Risk Among Smokers. J Natl Cancer Inst. 2003;95(6):470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 32.Black WC, Gareen IF, Soneji SS, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014;371:1793–802. doi: 10.1056/NEJMoa1312547. [DOI] [PMC free article] [PubMed] [Google Scholar]