Abstract
Immune checkpoint blockade has shown significant therapeutic efficacy in melanoma and other solid tumors, but results in ovarian cancer have been limited. With evidence that tumor immunogenicity modulates the response to checkpoint blockade, and data indicating that BRCA-deficient ovarian cancers express higher levels of immune response genes, we hypothesized that BRCA ovarian tumors would be vulnerable to checkpoint blockade. To test this hypothesis, we used an immunocompetent BRCA1-deficient murine ovarian cancer model to compare treatment with CTLA-4 or PD-1/PD-L1 antibodies alone or combined with targeted cytotoxic therapy using a PARP inhibitor. Correlative studies were performed in vitro using human BRCA1 cells. We found that CTLA-4 antibody, but not PD-1/PD-L1 blockade, synergized therapeutically with the PARP inhibitor, resulting in immune-mediated tumor clearance and long-term survival in a majority of animals (P < 0.0001). The survival benefit of this combination was T-cell mediated and dependent on increases in local IFNγ production in the peritoneal tumor environment. Evidence of protective immune memory was observed more than 60 days after completion of therapy. Similar increases in the cytotoxic effect of PARP inhibition in the presence of elevated levels of IFNγ in human BRCA1 cancer cells support the translational potential of this treatment protocol. These results demonstrate that CTLA-4 blockade combined with PARP inhibition induces protective antitumor immunity and significant survival benefit in the BRCA1 tumor model, and support clinical testing of this regimen to improve outcomes for women with hereditary ovarian cancer.
Introduction
Recent advances in the development of immunotherapeutics have focused on T-cell checkpoint blockade to promote the induction and maintenance of an antitumor effector response (1). To date, significant therapeutic benefit has been realized with antibodies to cytotoxic T-lymphocyte antigen-4 (CTLA-4, CD152) or programmed cell death protein-1 (PD-1, CD279) in melanoma and other solid tumors (2). The rationale for this approach is based on evidence that T-cell activity is locally suppressed in the tumor microenvironment of many cancers, and that release of these inhibitory signals permits immunologic clearance of tumor cells (1). With phase III studies documenting long-term survival in as many as 40% of patients with advanced melanoma, current efforts are focused on identifying patients who are likely to respond and developing combination strategies to extend the benefit of checkpoint blockade to a majority of patients with cancer (2).
Ovarian cancer has been identified as a rational target for immune therapy; however, these tumors have been considered relatively resistant to checkpoint blockade (3, 4). This is based on studies in murine models and clinical trials that showed limited response of ovarian tumors to CTLA-4 antibodies (5–7). Although 2 patients included in an early clinical trial of CTLA-4 blockade experienced a transient decrease in serum tumor markers, clinical disease regression has not been demonstrated (6, 7). Because of the poor prognosis associated with ovarian cancer and the clear need for new treatment options, identifying strategies to enhance the efficacy of immunomodulatory regimens for the treatment of this disease remains a priority.
A recent study demonstrating that patients responding to CTLA-4 inhibition for the treatment of melanoma were more likely to have genetically heterogeneous tumors that expressed a panel of antigenic peptides indicates that tumor immunogenicity modulates the efficacy of checkpoint blockade (8). On the basis of this, and other studies indicating that enhanced tumor antigenicity sensitizes cancers to checkpoint blockade therapy, combinatorial treatment regimens using cytotoxic agents together with checkpoint inhibitors have been proposed to optimize clinical outcomes (4). With evidence that a subset of ovarian cancers associated with germline mutations in BRCA1/2 genes may be more immunogenic (9–11), we hypothesized that BRCA1− tumors would be particularly vulnerable to checkpoint blockade.
Approximately 10% to 20% of ovarian cancer cases are attributed to hereditary syndromes, most commonly germline mutations in BRCA-1/2 genes that regulate double-stranded DNA repair (12, 13). Targeted therapy of BRCA-deficient (BRCA−) cancers has been achieved using poly(ADP-ribose) polymerase (PARP) inhibitors, which block BRCA-independent DNA repair and induce selective lethality in BRCA1− cancer cells (14, 15). Although PARP inhibitors significantly improve progression-free survival in patients with germline BRCA mutations, to date this strategy has not demonstrated an improvement in cancer-specific mortality (16–18). With evidence that immune priming is required for successful anti–CTLA-4 therapy, we tested whether targeted cytotoxic therapy with a PARP inhibitor would sensitize ovarian tumors to immune checkpoint blockade and optimize survival in a hereditary cancer model.
Here, we demonstrate that combined treatment using a PARP inhibitor together with CTLA-4 blockade induces long-term survival in a BRCA1-deficient ovarian tumor model. The efficacy of this regimen is mediated by the local induction of antitumor immunity and the production of increased levels of interferon-g (IFNγ) in the peritoneal tumor environment. A similar response by human BRCA1− cancer cells to PARP inhibition in the presence of high levels of IFNγ supports the translational relevance of this strategy for the treatment of women with hereditary ovarian cancer.
Materials and Methods
Ovarian cancer cell lines and murine tumor models
The BRCA1-deficient (BR5-Akt, BRCA1−) and sufficient (T22) epithelial ovarian cancer cell lines were generated on an FVB background as previously described (19) and were a kind gift from Dr. Sandra Orsulic (Cedars-Sinai). The ID8 cancer cell line was generated from C57BL/6 ovarian epithelial cells (20). Murine cell lines were maintained in DMEM (Hyclone) supplemented with 10% Fetalclone III (Hyclone), 10 mmol/L HEPES (Corning), 1 mmol/L sodium pyruvate (Hyclone), 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco; DMEM-Complete). The human ovarian cancer cell lines were purchased from the ATCC and cultured according to the ATCC protocol: UWB1.289 WT (BRCA-1 deficient) was established from a papillary serous ovarian tumor from a patient with a germline BRCA1 mutation within exon 11 and a deletion of the wild-type allele, and UWB1.289+BRCA1 cells were transfected to express wild-type BRCA1 using a pcDNA3 plasmid carrying wild-type BRCA1 (21).
Mice were purchased from Charles River Laboratories and maintained in a specific pathogen-free facility. All animal proto- cols were approved by the Institutional Animal Care and Use Committee at the University of New Mexico (Albuquerque, NM). BRCA1− cells (2×105) or ID8 cells (5×106) in PBS (Corning) were injected intraperitoneally in 6- to 8-week old mice. The number of tumor cells inoculated was selected such that mice reliably developed tumors and ascites within 40 to 60 days after tumor challenge. Mice were weighed at least twice weekly and were observed daily for ascites development. For survival studies, mice were sacrificed with any evidence of tumor morbidity or at a weight of 30 g due to ascites accumulation. Alternatively, 2×105 BRCA1− cells in 20 mL were surgically implanted orthotopically under the left ovarian bursa, as previously described (22). At 21 days, mice were euthanized and the ovaries were removed, photographed, and weighed.
Antibodies and reagents
The PARP inhibitor ABT-888 (Veliparib) was purchased from Active Biochem and diluted in PBS from frozen stock for experiments. For in vivo experiments, the PARP inhibitor was administered orally at indicated concentrations in a 20 mL volume using a pipette.
The CTLA-4 hybridoma (clone UC10-4F10-1) and IFNγ hybridoma (clone R4-6A2) were purchased from the ATCC. PD-1 (clone C1-G4) and PD-L1 (B7-H1, clone 10B5) hybridomas were kindly provided by Dr. Lieping Chen (Yale University). Hybridomas were grown, and monoclonal antibodies (mAb) were purified as previously described (23). For in vivo experiments, 100 mg of CTLA-4, 300 mg of PD-1, or PD-L1 mAbs diluted in 300 mL of PBS was injected intraperitoneally at the time points indicated.
Purified tumor necrosis factor-α (TNFα) neutralizing mAb (clone XT3.11) was purchased from BioXcell. Neutralizing anti- bodies to IFNγ or TNFα (0.5 mg) were injected intraperitoneally every 4 days beginning on day 7 after tumor challenge, as previously described (24, 25). Mouse recombinant TNFα was purchased from eBioscience. Recombinant human TNFα, recombinant mouse IFNγ, and recombinant human IFNγ were purchased from BioLegend.
Characterization of leukocyte populations
For ex vivo analysis, T cells were collected from peritoneal washings taken from mice after CO2 euthanization, as previously described (26). Splenocytes were isolated from whole spleens removed following peritoneal washings; cells were disaggregated with the frosted portion of glass slides, passed through a 70-µm mesh screen, and centrifuged at 1,500 rpm for 5 minutes. Red blood cells were lysed with ACK buffer, centrifuged, resuspended in DMEM-C, and passed through a second 70-µm screen. Viable cells were counted by Trypan blue exclusion and used for analyses.
In vitro tumor cell death assays
For in vitro analysis of PARP inhibitor and cytokine-mediated tumor cell lethality, 1 104 murine tumor cells per well were plated in 24-well plates and exposed to increasing concentrations of the PARP inhibitor alone or with IFNγ or TNFα with a final volume of 1 mL/well. Human ovarian cancer cell lines UWB1.289wt and UWB1.289þBRCA1 were plated at 1×105 cells per well due to their slower growth rate. After 72 hours, samples were retrieved using a 1:1 mix of cellstripper (Corning) and trypsin–EDTA (Corning) to release adherent cells, and centrifuged for 3 minutes at 1,500 rpm. Cells were then washed in PBS, stained with the fixable viability dye ZombieNIR (BioLegend) for 10 minutes, and fixed for analysis. For cells stained intracellularly for active caspase-3, the manufacturer's instructions were followed (active Caspase-3 apoptosis kit; BD Biosciences).
Tumor colony assay
To quantitatively compare peritoneal tumor burden, an ex vivo tumor colony assay was adapted from Shoemaker and colleagues (27). Briefly, peritoneal cells were plated in 10-fold serial dilutions starting at 1×106 cells/mL in 48-well plates (Costar). After 72 hours of incubation, colonies of growing tumor cells were distinguished from adherent non-tumor cells by circular colony formation of 10 or more cells (i.e., non-tumor cells were not observed to proliferate or form circular colonies). Colonies were counted at the dilution in which colonies were easily distinguishable and separated from other colonies. This value was multiplied by the dilution value for the number of tumor cells/ million cells in the peritoneal cavity.
T-cell functional assays
For analysis of cytokine production by T cells, 5 106 total peritoneal cells or splenocytes were restimulated in 24-well plates with cell stimulation cocktail [1.34 µmol/L ionomycin, 81 nmol/L phorbol 12-myristate 13-acetate (PMA), 10.6 µmol/L brefeldin A and 2 µmol/L monensin; eBioscience]. After 5 hours, cells were harvested, fixed, and permeabilized before staining for intracellular cytokine production (eBioscience FoxP3 staining buffer set).
For T-cell receptor (TCR)–specific restimulation of T cells, 5×106 cells were plated in 24-well plates coated with 10 µg/ mL anti-CD3 antibody (no azide/low endotoxin; clone 145- 2C11; BD Biosciences). After 18 hours, samples were harvested, pooled, and filtered through a 0.45˜µm syringe filter. Supernatant was assayed for IFNγ (eBioscience) or TNFα (BioLegend) by enzyme-linked immunosorbant assays (ELISA). To test whether cytokines produced by T cells contributed to a tumor cell cytotoxicity when combined with the PARP inhibitor, 250 mL of cell-free supernatant was added to 24-well plates containing 1 104 murine tumor cells and the PARP inhibitor in a final volume of 1 mL (1:4 dilution of supernatant). After 72 hours, cells were assayed for viability as described above.
Flow cytometry and analysis
Flow cytometry was performed using a BD Biosciences LSRFortessa. For direct ex vivo staining of lymphocytes, 1×106 to 2×106 cells were resuspended in 100 mL of FACS buffer (PBS þ 1% Fetalclone III), and Fc receptors were blocked for 10 to 15 minutes at 4 C with 1 µg/sample of 2.4G2 mAb (CD16 and CD32 blockade) and 1 µg/sample of mouse IgG (ThermoPierce). Cells were fluorescently labeled with antibodies for 30 minutes at 4 C, washed, and resuspended in fixation buffer (2% formaldehyde in PBS), or intracellularly stained according to the manufacturer's protocol (eBiosciences). The following fluorescently labeled anti- mouse mAbs were used for flow cytometry: IFNγ -FITC, IFNγ-PE, CD44-PerCP-Cy5.5, CD4-APC, CD8a-APC (BioLegend); CD62L- FITC, FoxP3-PE, TNFα-PerCP-eFluor 710, CD45-eFluor 450, CD4-eFluor450, CD62L-eFluor 450, CD4-APC-eFluor 780, and CD8a-APC-eFluor 780 (eBioscience); active caspase-3-PE (BD Biosciences). Flow cytometry data were analyzed with FCS Express 4 (De Novo Software).
Histologic analysis
Omenta were retrieved from experimental mice and fixed in 10% formalin vials (Evergreen Scientific). Tissue samples were sectioned (5 µm), stained with hematoxylin (Sigma-Aldrich) and eosin (RICCA Chemical Company), and imaged at 20 magnification with a Nikon DS-Fi1 camera mounted on a Nikon Eclipse E400 microscope running NIS-Elements software.
Statistical methods
Statistical analysis was done using Excel (Microsoft) and Graph-Pad Prism 6 (Graphpad Software, Inc.). All data are presented as mean with error bars representing the SEM. Statistical comparisons between experimental groups were analyzed by one-way and two- way analysis of variance followed by the Tukey procedure for multiple comparisons. Pairwise comparisons of two groups were made using the Student t test. The Kaplan–Meier survival curve were computed, and the log-rank statistic was used to evaluate differences among groups. A P value of <0.05 was taken to indicate statistical significance. In all figures, P values are denoted as follows: 0000, P < 0.0001; 000, P < 0.005; 00, P < 0.025; 0, P < 0.05.
Results
IFNγ enhances the cytotoxic effect of PARP inhibition in a BRCA1 ovarian cancer model
Early studies of CTLA-4 blockade demonstrated that in vivo treatment induces the expansion of memory CD8þ T-cell populations capable of producing IFNγ and TNFα (28, 29). IFNγ and TNFα have been shown to inhibit proliferation and promote apoptosis of tumor cells in many tumor models (30–32). As a preliminary step to evaluating combined checkpoint blockade and PARP inhibition, we tested whether IFNγ or TNFα would similarly amplify the cytotoxic effect of PARP inhibition in a BRCA1 cancer model in vitro.
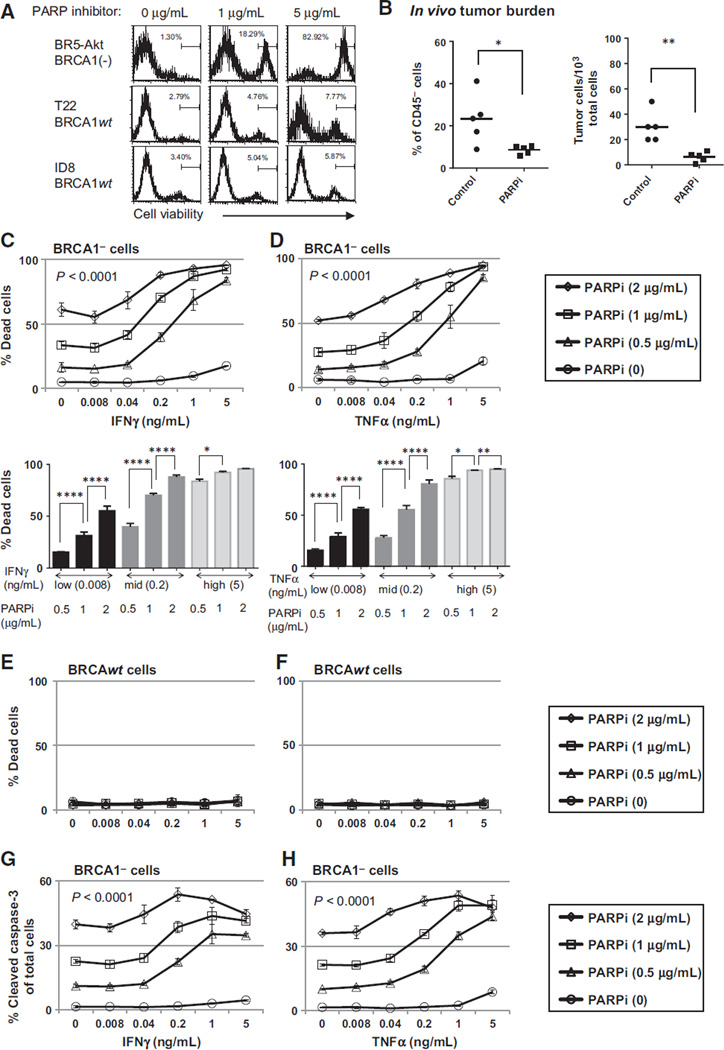
The BR5 ovarian cancer cell lines are currently the only available BRCA-deficient murine ovarian cancer model that can be used in immunocompetent mice (19). BR5-Akt overexpress Akt (hereafter referred to as BRCA1 cells), and intraperitoneal inoculation of this cell line results in the rapid accumulation of peritoneal tumors with high-grade serous histology and abdominal ascites, mimicking the patterns observed in patients. We confirmed the presence of the inactivating insertion in BRCA1 in these cells with PCR (not shown), and the "BRCAness" phenotype by selective sensitivity to PARP inhibition in vitro compared with syngeneic cell lines with wild-type BRCA function (Fig. 1A). In vivo cytotoxicity was also demonstrated in response to PARP inhibition in this model, with a reduction in tumor burden observed in treated mice as early as day 7 after intraperitoneal inoculation with BRCA1 tumor cells (Fig. 1B; n 1/4 5 mice per group treated with 40 mg/kg/day of the PARP inhibitor beginning on day 3 after tumor challenge).
Figure 1.
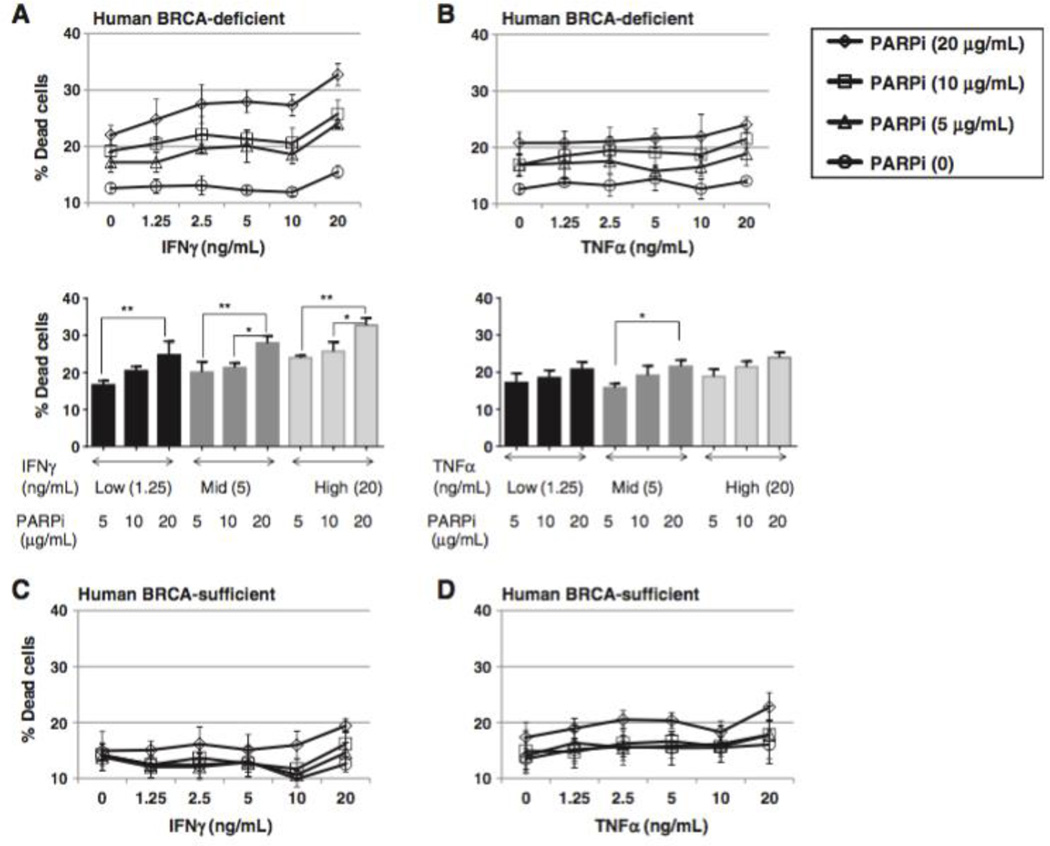
Th1-type cytokines enhance the cytotoxic effect of PARP inhibition in BRCA1 ovarian cancer cells. A, BRCA1 (BR5-Akt) and BRCAwt (T22, ID8) cell lines were cultured in 24-well plates at a starting concentration of 1 104 cells per well with or without the PARP inhibitor (PARPi) at the indicated dose, and analyzed at 72 hours for cell viability by flow cytometry. B, peritoneal cells were harvested by PBS wash on day 7 from BRCA1 tumor–bearing mice treated with the PARPi (40 mg/kg/day; days 3–6) and analyzed by flow cytometry for the percentage of CD45 tumor cells (left). In addition, total ascites cells were plated at serial dilutions for 72 hours, and the number of tumor colonies among total ascites cells was counted as an estimate of viable tumor burden (right). (Left, P 1/4 0.0271; right, P 1/4 0.0032, Student t test.) C and D, BRCA1 cells were cultured in 24-well plates for 72 hours in the presence of 0, 0.5, 1, or 2 µg/mL of PARPi and either IFNγ (C, top, PARPi dose effect, P < 0.0001; IFNγ dose effect, P < 0.0001; interaction, P < 0.0001) or TNFα (D, top, PARPi dose effect, P < 0.0001; TNFα dose effect, P < 0.0001, interaction, P < 0.0001) at the concentrations indicated. Cells were then stained with a fixable cell viability dye and analyzed by flow cytometry for the percentage of dead cells. Top dose-response curves show the interaction dose effect between cytokine concentration and PARP inhibitor treatment. Bottom bar graphs illustrate the dose effect independent of interaction. E and F, BRCAwt (T22) cells were assayed for viability as in C and D and show no treatment effect with increasing concentrations of cytokines or the PARP inhibitor. G and H, the fixable viability dye allowed for analysis of intracellular staining for active caspase-3. BRCA cells treated with PARPi and IFNγ (G) or TNFα (H) were intracellularly stained and cells positive for active caspase-3 are presented as a percentage of total cells (PARPi dose effect, P < 0.0001; IFNγ dose effect, P < 0.0001; interaction, P < 0.0001). Assays were repeated a minimum of three times. 0, P < 0.05; 00, P < 0.025; 000, P < 0.005; 0000, P < 0.0001 by ANOVA and Tukey procedure for multiple comparisons.
We next evaluated the cytotoxic efficacy of increasing concentrations of IFNγ or TNFα alone or combined with the PARP inhibitor in both the BRCA1 and BRCAwt cell lines. When tumor cells were exposed to IFNγ or TNFα at concentrations approximating physiologic conditions, a minimal cytotoxic effect was observed in both models. However, when IFNγ or TNFα was combined with the PARP inhibitor, a significantly higher rate of cell death was induced when compared with PARP inhibition alone. This effect was not seen in BRCAwt tumor cells, in keeping with the selective sensitivity of BRCA1 cells to PARP inhibition (Fig. 1C–F). Cell death in BRCA1 cells exposed to PARP inhibition and TNFα or IFNγ was also associated with increases in activated caspase-3, indicating that treatment induced tumor cell apoptosis (Fig. 1G and H), while no change in caspase-3 was noted in BRCAwt cells (data not shown). However, as the entire population of dead/dying cells did not express active caspase-3, the possible contribution of other cell death pathways could not be excluded. These results demonstrate an interaction between TNFα, IFNγ, and the PARP inhibitor in the BRCA1 model, and indicate that increases in effector cytokines in the tumor environment may enhance tumor cell death following targeted therapy.
Checkpoint blockade with CTLA-4 antibody combined with PARP inhibition enhances T-cell effector function in the peritoneal tumor environment
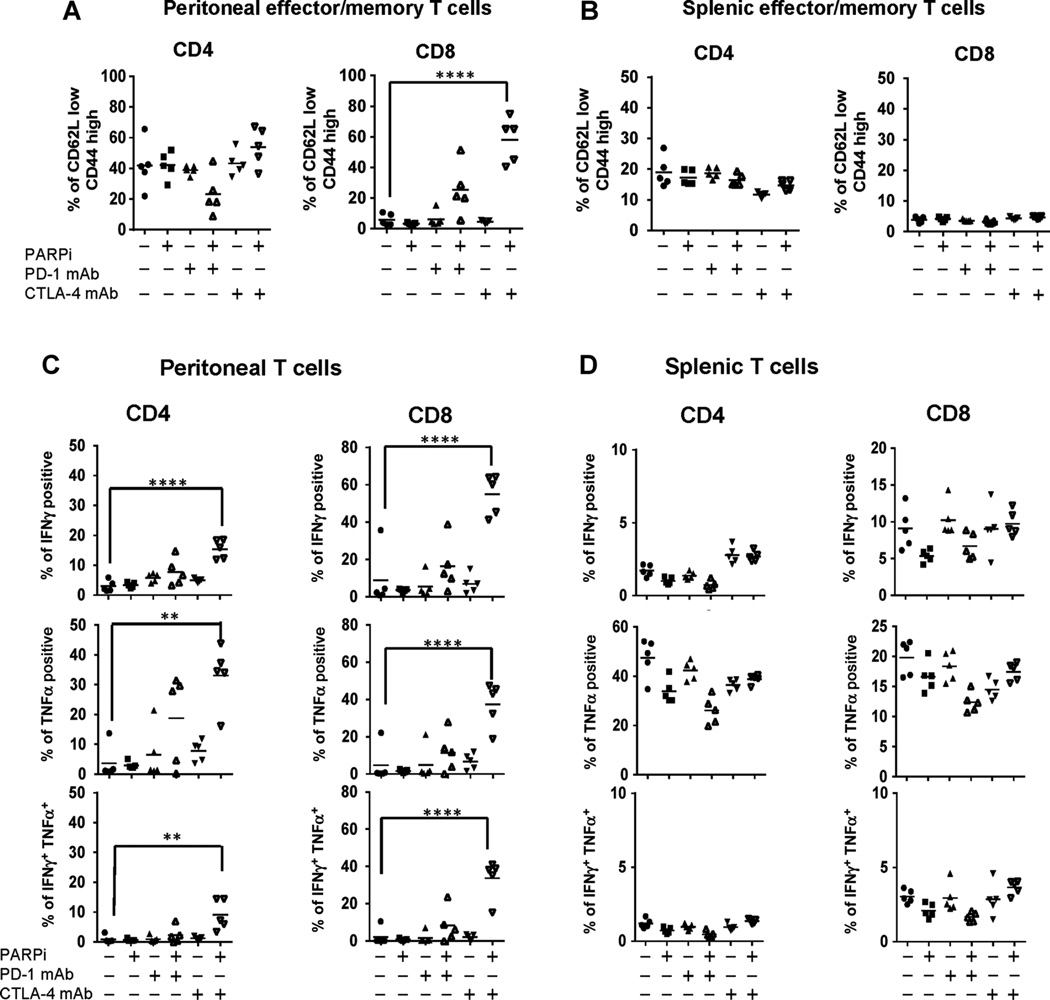
In addition to increasing the frequency of T cells producing IFNγ, CTLA-4 antibody treatment has been shown to enhance the proliferative response of effector T cells (29, 33). To evaluate whether checkpoint blockade modified the phenotype or functional status of T cells in the BRCA1 tumor environment in vivo, we subjected peritoneal T cells from treated mice to flow-cytometric analysis. In these experiments, BRCA1 tumor– bearing animals were treated with antibodies to CTLA-4 or PD-1 as monotherapy or combined with PARP inhibition (20 mg/kg/day). CTLA-4 or PD-1 antibody was administered on day 4 after tumor challenge. In addition, a second dose of PD-1 was administered on day 11 because PD-1 signaling is thought to affect peripheral T-cell exhaustion, a late event in the establishment of antitumor immunity. Results from these experiments demonstrated a marked increase in the proportion of CD8þ cells with an effector/memory phenotype in mice receiving CTLA-4 antibody together with the PARP inhibitor (Fig. 2A). This effect was specifically observed among T cells in the peritoneal tumor environment, and not in the spleen (Fig. 2B). In contrast, despite evidence that ovarian tumors express PD-L1 (34, 35), and that tumor-infiltrating lymphocytes upregulate PD-1, blockade of the PD-1–PD-L1 pathway did not significantly increase the proportion of effector/memory cells in this model (Fig. 2A and B).
Figure 2.
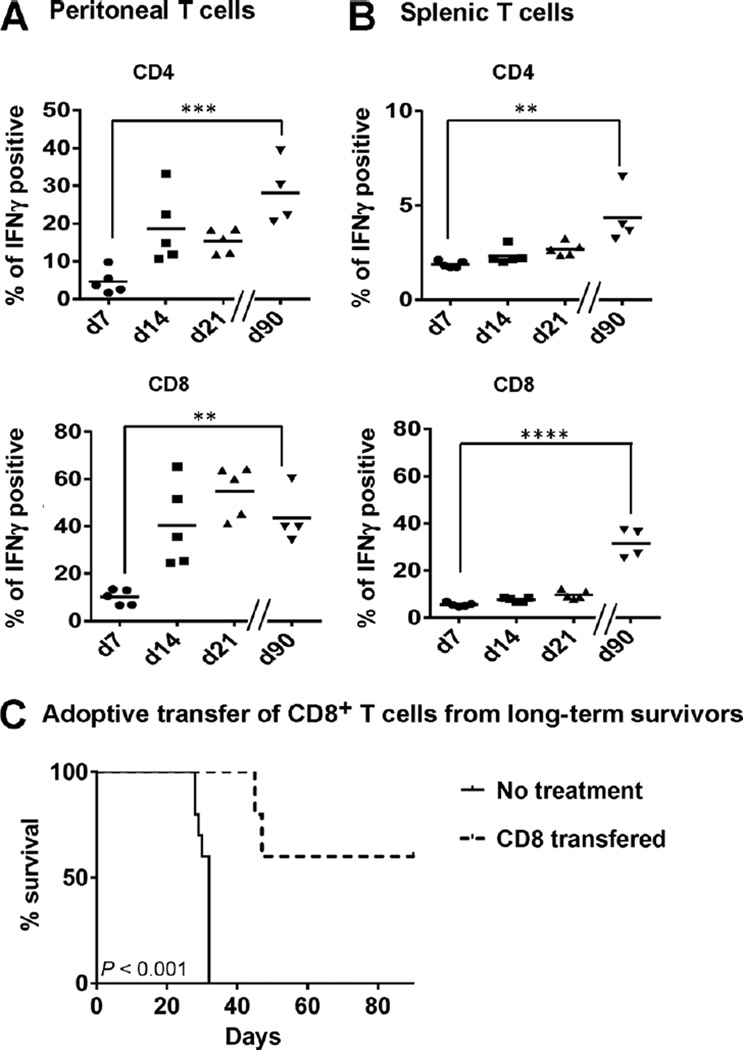
Checkpoint blockade with CTLA-4 antibody combined with PARP inhibition enhances T-cell effector function in the peritoneal tumor environment. Using the protocol depicted in Supplementary Fig. S1, BRCA1 tumor–bearing mice were sacrificed on day 21 for analysis (n 1/4 5/group). Peritoneal (A) and splenic (B) CD4+ and CD8+ T cells were analyzed for CD44 and CD62L expression, and percentage of CD62Llow/CD44hi cells was gated to determine the percentage of effector/memory CD4+ and CD8+ T cells of total CD4+ or CD8+ T cells, respectively. Peritoneal (C) cells and splenocytes (D) were restimulated in 24- well plates with PMA and ionomycin for 5 hours in the presence of Golgi transport inhibitors. CD4+ and CD8+ T cells were then analyzed by flow cytometry for intracellular cytokine expression. The percentages of T cells from treated animals producing IFNγ (top), TNFα (middle), or both (bottom) were compared with untreated controls. Data are representative of two experiments: * , P < 0.05; ** , P < 0.025; **** , P < 0.0001 by ANOVA and Tukey procedure for multiple comparisons.
To determine whether changes in T-cell phenotype following checkpoint blockade in combination with PARP inhibition were associated with increases in local levels of IFNγ or TNFα, we evaluated peritoneal T cells retrieved on days 7, 14, and 21 for intracellular cytokine production by flow cytometry. Cells were restimulated ex vivo for 5 hours with PMA and ionomycin in the presence of Golgi transport inhibitors, and then CD4+ and CD8+ T cells were analyzed for IFNγ, and TNFα cytokine production. In these experiments, neither monotherapy with CTLA-4 or PD-1 blockade alone nor PD-1 blockade together with PARP inhibition significantly increased cytokine production by CD4+ or CD8+ T cells in peritoneal samples (Fig. 2C). In contrast, combination therapy with the PARP inhibitor and CTLA-4 antibody did significantly boost both IFNγ and TNFα production in CD4þ and CD8þ T cells in the peritoneal tumor environment on day 21. The percentage of polyfunctional peritoneal CD8þ T cells producing both IFNγ and TNFα was also significantly increased in mice receiving CTLA-4 antibody and PARP inhibitor combined therapy. Like the increase in effector/memory cells, these changes in cytokine production were evident in the peritoneal tumor environment, but similar effects were not seen among splenic T cells (Fig. 2D). From these data, we conclude that CTLA-4 blockade, but not inhibition of PD-1 signaling, induces a Th1 effector phenotype among T cells in the peritoneal tumor environment when combined with PARP inhibition in a BRCA1 ovarian cancer model.
Increases in IFNγ production in response to combined CTLA-4 blockade and PARP inhibition in vivo are sufficient to enhance tumor cell cytotoxicity
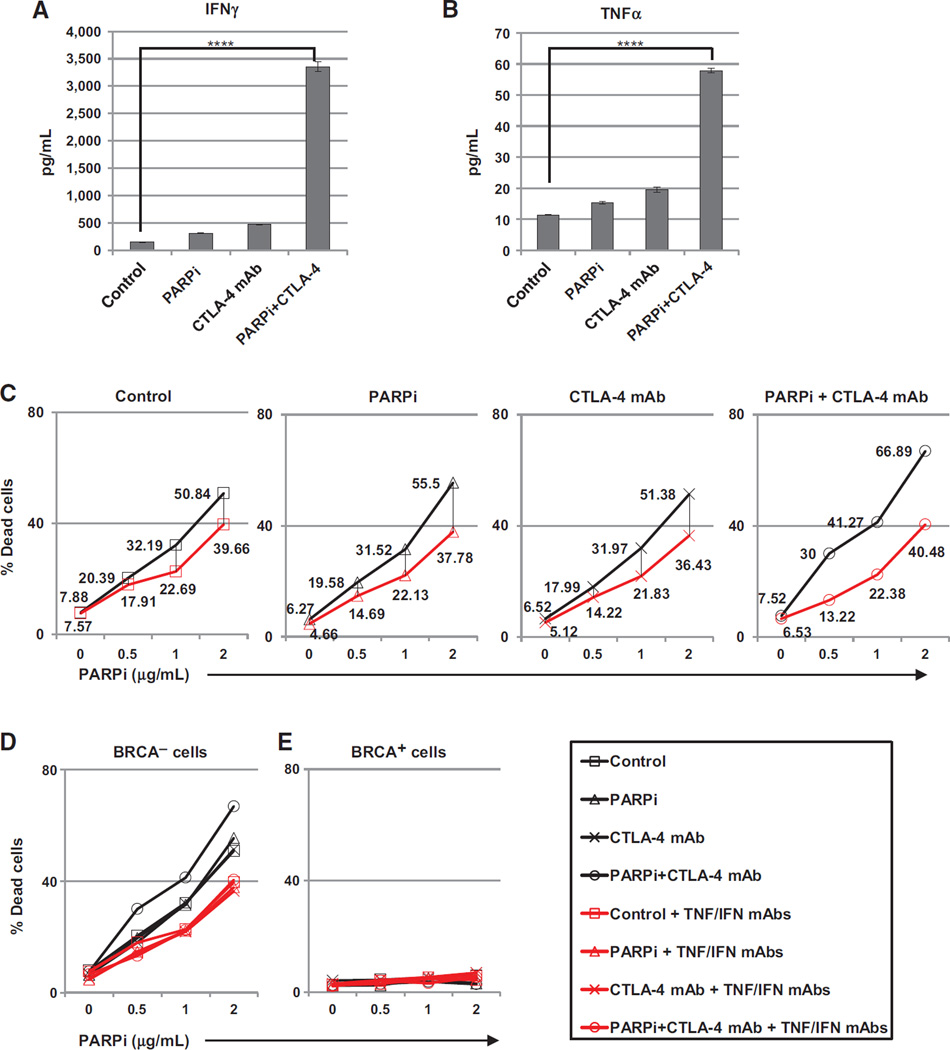
Our results demonstrate that combined therapy with the PARP inhibitor and CTLA-4 antibody enhances IFNγ and TNFα production by peritoneal T cells in response to TCR-independent stimulation with PMA and ionomycin. In order to quantify production of these cytokines specifically in response to TCR engagement by peritoneal T cells, we cultured day 21 peritoneal cells with anti-CD3 antibody for 18 hours in culture. In this manner, only T cells were stimulated to express effector cytokines, allowing direct quantification of IFNγ and TNFα (35). The results of this experiment demonstrated that both IFNγ and TNFα levels were highest following anti-CD3 treatment of peritoneal cells from mice receiving combined treatment with the PARP inhibitor and CTLA-4 antibody, with a greater than 20-fold increase in IFNγ and an approximately 5-fold increase in TNFα compared with controls (Fig. 3A and B).
Figure 3.
Increases in IFNγ production in response to combined CTLA-4 blockade and PARP inhibition in vivo are sufficient to enhance tumor cell cytotoxicity. Mice were treated as in Supplementary Fig. S1 and euthanized on day 21 followed by retrieval of peritoneal cells (n 1/4 5/group). A total of 5 106 peritoneal cells were restimulated ex vivo with 10 µg/mL of anti-CD3 for 18 hours. Cell-free supernatants were harvested and pooled by treatment group, and levels of IFNγ (A) and TNFα (B) were determined by ELISA. C–E, supernatants were added to BRCA cells at a 4 dilution with DMEM-C in the presence of PARPi at indicated concentrations and cultured 72 hours in the absence (black lines) or presence (red lines) of IFNγ and TNFα neutralizing mAbs (10 µg/mL). C, cells were analyzed for viability by flow cytometry, and values shown are the percentages of dead cells with or without neutralizing antibody treatment. D, overlay of data in C. E, the same as D using BRCAwt cells (T22). *, P < 0.05; **, P < 0.025; ***, P < 0.005; ****, P < 0.0001 by ANOVA and Tukey procedure for multiple comparisons.
To determine whether high cytokine levels in the peritoneal tumor environment could induce a cytotoxic effect similar to that seen using recombinant cytokines in vitro (Fig. 1), tumor cells were cultured with cell-free supernatant retrieved from the T-cell stimulation experiments described above in the presence of increasing concentrations of the PARP inhibitor. As expected, the supernatant from restimulated T cells harvested from BRCA1 tumor–bearing mice receiving combination therapy had the greatest effect on BRCA1 tumor cell death when combined with the PARP inhibitor in vitro (Fig. 3C and D, black lines). Supporting a role for IFNγ and TNFα in this effect, cell death in response to supernatant from T cells retrieved from mice receiving combination therapy was attenuated when neutralizing antibodies to IFNγ or TNFα were added to cell cultures (Fig. 3C and D, red lines). As seen with recombinant cytokines in vitro, this effect was restricted to BRCA1 tumor cells; BRCAwt cells cultured under the same conditions showed no evidence of cytotoxicity (Fig. 3E). Together, these data indicate that combination therapy with the PARP inhibitor and CLTA-4 antibody promotes a strong Th1 T-cell response in the peritoneal tumor environment that results in local increases in effector cytokines that are sufficient to enhance BRCA1 tumor clearance in vivo.
Combined treatment with a PARP inhibitor and CTLA-4 antibody promotes IFNγ-mediated tumor rejection in a BRCA1− tumor model
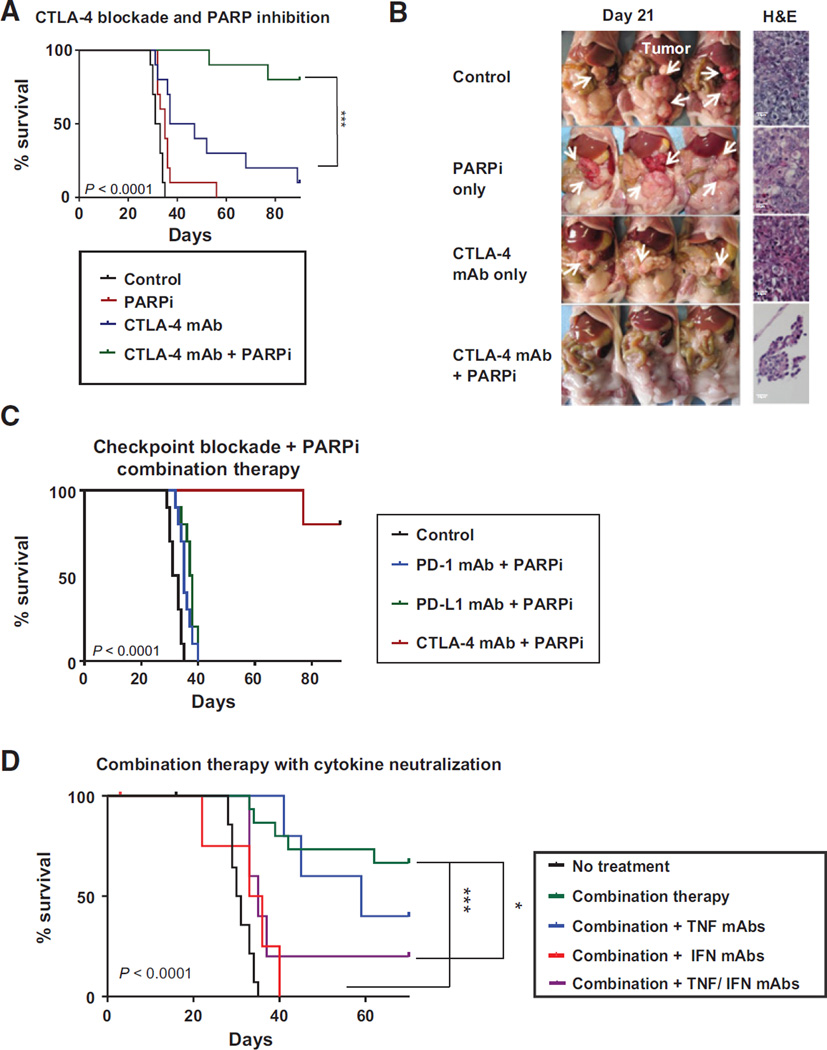
With evidence that IFNγ enhances the cytotoxic effect of PARP inhibition in vitro, and that PARP inhibition combined with CTLA-4 blockade increased IFNγ production and an effector phenotype among peritoneal T cells in vivo, we evaluated the impact of this regimen on survival in the BRCA1 tumor model. To do this, animals inoculated with BRCA1 cancer cells were treated with checkpoint blockade monotherapy or combined treatment with the PARP inhibitor (40 mg/kg/day), and survival was measured from the day of tumor challenge until animals reached a weight of 30 g due to ascites accumulation (Supplementary Fig. S1). Results from these experiments demonstrated limited benefit from CTLA-4 monotherapy, in keeping with prior reports (4, 5); however, treatment with the CTLA-4 antibody together with PARP inhibition resulted in a synergistic therapeutic effect with long-term tumor-free survival in a majority of animals (Fig. 4A). As early as day 21, all control mice and animals receiving monotherapy had bulky solid tumors in the abdominal cavity; however, among mice receiving combination therapy, only 1 mouse had visible tumor and 2 additional mice had evidence of microscopic omental implants (Fig. 4B). When experimental animals receiving combined CTLA-4 blockade and the PARP inhibitor were examined by necropsy on day 90, no gross tumor was evident in surviving mice. A similar effect was observed when tumors were inoculated orthotopically under the ovarian bursa and solid tumor growth was examined on day 21 (Supplementary Fig. S2). In contrast with CTLA-4, no survival benefit was observed when PARP inhibition was combined with PD-1 or PD-L1 blockade (Fig. 4C). This is in agreement with prior data (36) and with our T-cell functional analyses, indicating PD-1/PD-L1 blockade in combination with PARP inhibition had limited effects on T- cell activation and cytokine induction (Fig. 2). Animals inoculated with BRCAwt tumors showed that no significant survival benefit was observed following treatment with the PARP inhibitor alone or in combination with CTLA-4 antibody, which was associated with a lack of any effect on peritoneal T-cell populations in these mice (Supplementary Fig. S3).
Figure 4.
Combined treatment with a PARP inhibitor and CTLA-4 antibody promotes IFNγ-mediated tumor rejection in a BRCA1 tumor model. A, mice were treated as depicted in Supplementary Fig. S1 (40 mg/kg/day PARPi), and survival was compared with that of untreated controls (n 1/4 10/group). B, on day 21, the peritoneal cavity was exposed for evaluation of macroscopic solid tumor burden, and histologic sections of omentum were examined for microscopic tumor implants. Representative samples are shown. C, combination therapy with PARP inhibition (40 mg/kg/day) and antibody blockade of CTLA-4, PD-1, or PD-L1 as in Supplementary Fig. S1 (n 1/4 5–10 per group). D, survival of mice treated with PARPi (20 mg/kg/day) and CTLA-4 mAb combination therapy and neutralizing antibodies to TNFα , IFNγ, or both every 4 days beginning on day 7 (n 1/4 5–15/group); survival comparisons are the Kaplan–Meier curves. Differences among groups were determined with the log-rank (Mantel–Cox) test: *, P < 0.05;***, P < 0.005. Each experiment was repeated at least twice, with representative data shown.
With evidence that combined therapy with the PARP inhibitor and CTLA-4 mAb enhances IFNγ and TNFα production by local T cells in BRCA1 tumor–bearing mice, we next tested whether the therapeutic efficacy of PARP inhibition together with CTLA-4 blockade was dependent on elevations in effector cytokines in vivo. To do this, mice were treated with combination therapy using the PARP inhibitor (20 mg/kg/day) and CTLA-4 antibody together with neutralizing antibodies to IFNγ, TNFα, or both, and monitored for survival. This experiment demonstrated that neutralization of TNFα had a minimal effect on outcomes; however, IFNγ was required for the survival effect of combined treatment in vivo (Fig. 4D). Thus, CTLA-4 checkpoint blockade and targeted cytotoxicity with PARP inhibition promoted immune- mediated rejection of high-grade BRCA1 tumors, resulting in long-term tumor-free survival.
The survival benefit of combined treatment with the PARP- inhibitor and CTLA-4 antibody is due to lasting effects on peritoneal T cells
One of the most remarkable effects of checkpoint blockade has been the lasting therapeutic effects exhibited among patients who respond to treatment (4). Using sarcoma tumor models, Gubin and colleagues (37) have demonstrated that CTLA-4 blockade can induce protective immunologic memory. With evidence that tumor clearance following combined CTLA-4 blockade and PARP inhibition is dependent on local changes in T-cell phenotype and cytokine production, and that it can produce a lasting treatment benefit, we evaluated peritoneal T cells from long-term survivors for evidence of a memory response. To do this, peritoneal and splenic T cells were harvested from mice surviving past day 90 after treatment with CTLA-4 antibody and the PAPR inhibitor and analyzed for cytokine production. Following ex vivo stimulation with PMA and ionomycin, very high levels of IFNγ production were observed in both peritoneal and splenic CD4+ and CD8+ T cells from combination treated mice (Fig. 5A and B). Thus, although initial changes in T-cell phenotype and functional status were specifically seen in the peritoneal tumor environment, treated animals did develop evidence of a systemic memory response, which was associated with long-term survival following combined exposure to the CTLA-4 antibody and the PARP inhibitor.
Figure 5.
Combined treatment with the PARP inhibitor and CTLA-4 antibody induces protective immunity. Peritoneal cells (A) and splenocytes (B) were retrieved on days 7, 14, and 21 from combination therapy— treated mice, and from long-term survivors on day 90, and restimulated ex vivo with PMA and ionomycin for analysis by flow cytometry for intracellular IFNγ production by CD4+ and CD8+ T cells. * , P < 0.05; ** , P < 0.025, Tukey multiple comparisons test. C, adoptive transfer of CD8+ T cells from long-term survivors protects recipients from tumor development. CD8+ T cells were isolated by MACS-negative selection from long-term combination therapy survivors, pooled, and 2×105 cells were adoptively transferred to recipient mice 12 hours prior to challenge with 2×105 BRCA- tumor cells. Mice were monitored for survival (n 1/4 5/group) log-rank (Mantel–Cox) test.
To confirm that the extended therapeutic benefit of combined treatment with the PARP inhibitor and CTLA-4 antibody was T-cell mediated, adoptive transfer experiments were performed. To do this, 2 × 105 CD8+ splenocytes retrieved from mice surviving greater than 90 days following combined treatment were transferred to recipient animals, followed by intraperitoneal tumor challenge after 12 hours. In this experiment, a majority of mice receiving CD8 cells pooled form three long-term survivors were themselves protected from intraperitoneal tumor challenge: All experimental animals survived longer than the control group, and 3 of 5 had no evidence of tumor at day 90 (Fig. 5C). We interpret these results as confirmation that combined therapy using CTLA-4 blockade and PARP inhibition induced a population of effector T cells that increased local levels of IFNγ and promoted the establishment of protective immunologic memory.
IFNγ enhances cytotoxicity in human BRCA1 tumor cells exposed to a PARP-inhibitor in vitro To test the translational potential of this treatment protocol, we examined the effect of PARP inhibition with increasing doses of recombinant IFNγ or TNFα on human ovarian cancer cells in vitro. For these studies, we used the BRCA1 UWB1.289 cell line and UWB1.289 transfected to express wild-type BRCA1 (BRCA1wt), as previously described (21). The results demonstrated that IFNγ, but not TNFα, increased cytotoxicity in BRCA1 cells treated with the PARP inhibitor in vitro (Fig. 6A and B). As in the murine experiments, this effect was not observed in BRCA1wt cells (Fig. 6C and D). These data suggest that PARP inhibition in combination with CTLA-4 blockade may have similar therapeutic benefit in patients with BRCA1 ovarian cancers and support further study of this combination.
Figure 6.
IFNγ enhances cytotoxicity in human BRCA1 tumor cells exposed to a PARP inhibitor in vitro. BRCA1 UWB1.289 cells or UWB1.289 cells transfected with competent BRCA1 were cultured with titrated doses of the PARP inhibitor in the presence of recombinant human IFNγ or TNFα as indicated. After 72 hours, cells were analyzed for viability by flow cytometry. A, BRCA1 UWB1.289 cells exposed to IFNγ and PARP inhibition (top, PARPi dose effect, P < 0.0001; IFNγ dose effect, P < 0.0001; interaction, P 1/4 0.3872 based on two-way ANOVA; bottom, * , P < 0.05; **, P < 0.025 by Tukey multiple comparisons test). B, BRCA1 UWB1.289 cells exposed to TNFα and PARP inhibition (top, TNFα dose effect, P 1/4 0.0499; interaction, P 1/4 0.9655 by two-way ANOVA; bottom, *, P < 0.05, Tukey multiple comparisons test). C and D, BRCA1- transfected UWB1.289 cells exposed to IFNγ or TNFα and PARP inhibition (no statistically significant dose effect for IFNγ or TNFα).
Discussion
Here, we demonstrate that CTLA-4 antibody combined with targeted therapy using a PARP inhibitor promotes long-term survival in a BRCA1 ovarian cancer model, and that this effect is mediated by local increases in IFNγ production by T cells in the tumor environment. Combination therapy rapidly increases T-cell recruitment, activation, and cytokine production in the peritoneal cavity, and this is followed by the induction of lasting systemic effector/memory T-cell immunity. Our finding of a similar trend in human BRCA1 cells treated with the PARP inhibitor in the presence of elevated levels of IFNγ, and evidence that ascites T cells from women with ovarian cancer express CTLA-4, support the translational potential of this treatment strategy.
An important finding from these experiments is the effect of combined treatment on the peritoneal tumor environment. Prior studies using checkpoint inhibition antibodies have demonstrated a discrepancy between receptor occupancy among peripheral T cells and tumor-infiltrating T cells (36). As checkpoint inhibition is a locally mediated effect, any therapeutic benefit of checkpoint blockade requires engagement of lymphocytes in the tumor microenvironment (4). Our results demonstrate that effector/ memory T cells were specifically increased in the peritoneal cavity in response to combination therapy, indicating that this regimen can effectively modulate T cells in the ovarian tumor environment. Because the ovarian tumor environment is considered tolerogenic (3), these changes indicate a significant reversal in the local immune conditions that is associated with long-term survival benefit. Furthermore, we expect that the localized increase in IFNγ following combined treatment may prove clinically advantageous by limiting systemic toxicity (38). It is notable that despite expression of PD-1 on ascites T cells and PD-L1 on ovarian tumor cells, inhibition of this pathway had no significant impact on survival in the BRCA1 model. This finding was unexpected in light of a recent report describing PD-1 pathway blockade as a strategy to enhance the efficacy of tumor vaccines in an ovarian cancer model (33). Despite differences in the dose and schedule of PD-1 antibody administration in this study, the effect of PD-1 blockade on T-cell function was similar to our results, with approximately 2% of CD8þ T cells from treated animals producing IFNγ, which in both cases was not significantly different from controls. We interpret the selective efficacy of CTLA-4 blockade we observed as evidence that activation of new lymphocyte clones, rather than reversal of T-cell exhaustion, was responsible for immune-mediated tumor clearance and long-term survival in the BRCA1 model (1, 36, 39).
Strengths of this study include our use of an immunocompetent model that reliably develops high-grade serous tumors that replace the omentum and implant on serosal surfaces of the bowel and peritoneal cavity, mimicking the most common pattern found in patients. The fact that parallel results were demonstrated with BRCA1 human tumor cells supports further testing of this combination for the treatment of clinical disease. In addition, the long-term survival we observed has not been demonstrated previously in other studies using this model (40). Although our study is focused on ovarian cancer, we expect that our results can be extended to other BRCA cancers, such as breast, prostate, or pancreatic tumors, that are vulnerable to the targeted effect of PARP inhibition. We are also currently investigating strategies to extend similar benefits to patients with sporadic ovarian cancer, using metronomic chemotherapy to sensitize tumors to check- point inhibition (41).
Finally, these results document a novel mechanism for BRCA1 tumor cell death driven by an interaction between the PARP inhibitor and IFNγ in vitro. As a highly conserved, ubiquitous molecule required for posttranslational protein modification, PARP is involved in many cellular processes in addition to DNA repair, including roles in apoptotic cell death and metabolic pathways (42–45). The observation that only a portion of tumor cell death in vitro was due to apoptosis, as shown by caspase-3 cleavage, indicates that additional cytotoxic pathways are engaged in response to IFNγ and the PARP inhibitor. Accumulating reports describe a role for PARP inhibitors in the prevention of catalytic function and PARP trapping, and it is postulated that different forms of cell death may be induced by specific mechanisms of PARP inhibition (46–48). Therefore, it will be important to evaluate specific PARP inhibitors in combination with immune checkpoint blockade to better parse the mechanisms of cell death and to optimize treatment protocols for patients.
On the basis of these data, we propose a two-phase model to describe distinct roles for the PARP inhibitor in promoting tumor regression when combined with CTLA-4 blockade. In phase I, PARP inhibition directly induces tumor cell damage, which primes and diversifies an antitumor T-cell response, a process that is amplified by CTLA-4 blockade (49). In phase II, local T cells activated in the presence of CTLA-4 blockade produce increased levels of IFNγ above a threshold required to enhance the cytotoxic efficacy of PARP inhibition, resulting in additional therapeutic benefit through cell-intrinsic pathways. This model indicates that the therapeutic benefit of PARP inhibition can be significantly amplified by inclusion in immunotherapeutic protocols.
In summary, these data show that long-term survival can be achieved in a BRCA1 ovarian tumor model using a PARP inhibitor combined with CTLA-4 checkpoint blockade. The fact that both PARP inhibitors and CTLA-4 antibodies have been well tolerated as monotherapy in women with ovarian cancer, together with our in vitro data using human BRCA1 cancer cells, supports the rapid translation of this treatment protocol for clinical testing. In addition, our results add to prior work suggesting that BRCA mutation status can be used to identify candidates for immuno-therapeutic protocols, improving our ability to identify a treatment effect for selected patients (50). Finally, we anticipate that further evaluation of PARP inhibitors in combination with specific immune checkpoint pathways will uncover optimal pairings that will expand the clinical utility of this strategy for the treatment of patients with other BRCA cancers, or with tumors deficient in alternate DNA-repair pathways.
Supplementary Material
Acknowledgments
Grant Support
This study was supported by an Ovarian Cancer Research Fund Liz Tilberis Early Career Award.
Footnotes
Note: Supplementary data for this article are available at Cancer Immunology Research Online (http://cancerimmunolres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest.
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: T. Higuchi, D.B. Flies, L. Ronner, S.F. Adams Development of methodology: T. Higuchi, D.B. Flies, G. Mantia-Smaldone, S.F. Adams
Acquisition of data (provided animals, acquired and managed patients, pro- vided facilities, etc.): T. Higuchi, D.B. Flies, N.A. Marjon, G. Mantia-Smaldone, S.F. Adams
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): T. Higuchi, D.B. Flies, N.A. Marjon, P.A. Gimotty, S.F. Adams
Writing, review, and/or revision of the manuscript: T. Higuchi, D.B. Flies, N.A. Marjon, G. Mantia-Smaldone, L. Ronner, P.A. Gimotty, S.F. Adams Study supervision: S.F. Adams
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immuno-therapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavou eV, Th edrez A, Lev^eque J, Foucher F, Henno S, Jauffret V, et al. Immunity of human epithelial ovarian carcinoma: the paradigm of immune suppression in cancer. J Transl Med. 2013;11:147. doi: 10.1186/1479-5876-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ai M, Curran MA. Immune checkpoint combinations from mouse to man. Cancer Immunol Immunother. 2015;64:885–892. doi: 10.1007/s00262-014-1650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang YF, Zou JP, Mu J, Wijesuriya R, Ono S, Walunas T, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–4041. [PubMed] [Google Scholar]
- 6.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 anti- body blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8þ tumor-infiltrating lymphocytes and a high CD8þ/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAlpine JN, Porter H, Kobel M, Nelson BH, Prentice LM, Kalloger SE, et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol. 2012;25:740–750. doi: 10.1038/modpathol.2011.211. [DOI] [PubMed] [Google Scholar]
- 11.Clarke B, Tinker AV, Lee CH, Subramanian S, van de Rijn M, Turbin D, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009;22:393–402. doi: 10.1038/modpathol.2008.191. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi H, Ohno S, Sasaki Y, Matsuura M. Hereditary breast and ovarian cancer susceptibility genes (review) Oncol Rep. 2013;30:1019–1029. doi: 10.3892/or.2013.2541. [DOI] [PubMed] [Google Scholar]
- 13.Russo A, Calo V, Bruno L, Rizzo S, Bazan V, Di Fede G. Hereditary ovarian cancer. Crit Rev Oncol Hematol. 2009;69:28–44. doi: 10.1016/j.critrevonc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 15.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2- deficient tumours with inhibitors of poly(ADP- ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 16.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 17.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 18.Kummar S, Fleming GF, Oza AM, Sullivan DM, Gandara DR, Naughton M, et al. Randomized trial of oral cyclophosphamide and veliparib in high- grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin Cancer Res. 2015;21:1574–1582. doi: 10.1158/1078-0432.CCR-14-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing D, Orsulic S. A mouse model for the molecular characterization of brca1-associated ovarian carcinoma. Cancer Res. 2006;66:8949–8953. doi: 10.1158/0008-5472.CAN-06-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 21.DelloRusso C, Welcsh PL, Wang W, Garcia RL, King MC, Swisher EM. Functional characterization of a novel BRCA1-null ovarian cancer cell line in response to ionizing radiation. Mol Cancer Res. 2007;5:35–45. doi: 10.1158/1541-7786.MCR-06-0234. [DOI] [PubMed] [Google Scholar]
- 22.Cordero AB, Kwon Y, Hua X, Godwin AK. In vivo imaging and therapeutic treatments in an orthotopic mouse model of ovarian cancer. J Vis Exp. 2010;42:e2125. doi: 10.3791/2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flies DB, Wang S, Xu H, Chen L. Cutting edge: a monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol. 2011;187:1537–1541. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner JG, Rakhmilevich AL, Burdelya L, Neal Z, Imboden M, Sondel PM, et al. Anti-CD40 antibody induces antitumor and antimetastatic effects: the role of NK cells. J Immunol. 2001;166:89–94. doi: 10.4049/jimmunol.166.1.89. [DOI] [PubMed] [Google Scholar]
- 25.Grinberg-Bleyer Y, Saadoun D, Baeyens A, Billiard F, Goldstein JD, Gregoire S, et al. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J Clin Invest. 2010;120:4558–4568. doi: 10.1172/JCI42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008 doi: 10.1002/0471142735.im1401s83. Chapter 14:Unit 14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoemaker RH, Wolpert-DeFilippes MK, Kern DH, Lieber MM, Makuch RW, Melnick NR, et al. Application of a human tumor colony-forming assay to new drug screening. Cancer Res. 1985;45:2145–2153. [PubMed] [Google Scholar]
- 28.Paradis TJ, Floyd E, Burkwit J, Cole SH, Brunson B, Elliott E, et al. The anti-tumor activity of anti-CTLA-4 is mediated through its induction of IFN gamma. Cancer Immunol Immunother. 2001;50:125–133. doi: 10.1007/s002620100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8þ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci U S A. 2011;108:266–271. doi: 10.1073/pnas.1016791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita H, Hosoi A, Ueha S, Abe J, Fujieda N, Tomura M, et al. Cytotoxic T lymphocytes block tumor growth both by lytic activity and IFNγ dependent cell-cycle arrest. Cancer Immunol Res. 2015;3:26–36. doi: 10.1158/2326-6066.CIR-14-0098. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF- dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest. 2008;118:1398–1404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall L, Burke F, Barton C, Smyth J, Balkwill F. IFN-gamma induces apoptosis in ovarian cancer cells in vivo and in vitro. Clin Cancer Res. 2003;9:2487–2496. [PubMed] [Google Scholar]
- 33.Khan S, Burt DJ, Ralph C, Thistlethwaite FC, Hawkins RE, Elkord E. Tremelimumab (anti-CTLA4) mediates immune responses mainly by direct activation of T effector cells rather than by affecting T regulatory cells. Clin Immunol. 2011;138:85–96. doi: 10.1016/j.clim.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maine CJ, Aziz NH, Chatterjee J, Hayford C, Brewig N, Whilding L, et al. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63:215–224. doi: 10.1007/s00262-013-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller CH, Maher SG, Young HA. Clinical use of interferon-gamma. Ann N Y Acad Sci. 2009;1182:69–79. doi: 10.1111/j.1749-6632.2009.05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg MS, Xing D, Ren Y, Orsulic S, Bhatia SN, Sharp PA. Nanoparticle- mediated delivery of siRNA targeting Parp1 extends survival of mice bearing tumors derived from Brca1-deficient ovarian cancer cells. Proc Natl Acad Sci U S A. 2011;108:745–750. doi: 10.1073/pnas.1016538108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Sullivan CC, Moon DH, Kohn EC, Lee JM. Beyond breast and ovarian cancers: PARP inhibitors for BRCA mutation-associated and BRCA-like solid tumors. Front Oncol. 2014;4:42. doi: 10.3389/fonc.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkle A, Virag L. Poly(ADP-ribose): PARadigms and PARadoxes. Mol Aspects Med. 2013;34:1046–1065. doi: 10.1016/j.mam.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Bai P, Virag L. Role of poly(ADP-ribose) polymerases in the regulation of inflammatory processes. FEBS Lett. 2012;586:3771–3777. doi: 10.1016/j.febslet.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Aredia F, Scovassi AI. Poly(ADP-ribose): a signaling molecule in different paradigms of cell death. Biochem Pharmacol. 2014;92:157–163. doi: 10.1016/j.bcp.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 45.Andrabi SA, Umanah GK, Chang C, Stevens DA, Karuppagounder SS, Gagne JP, et al. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. Proc Natl Acad Sci U S A. 2014;111:10209–10214. doi: 10.1073/pnas.1405158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steffen JD, Brody JR, Armen RS, Pascal JM. Structural implications for selective targeting of PARPs. Front Oncol. 2013;3:301. doi: 10.3389/fonc.2013.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Lorenzo SB, Patel AG, Hurley RM, Kaufmann SH. The elephant and the blind men: making sense of PARP inhibitors in homologous recombination deficient tumor cells. Front Oncol. 2013;3:228. doi: 10.3389/fonc.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. 2014;20:2424–2432. doi: 10.1158/1078-0432.CCR-13-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantia-Smaldone G, Ronner L, Blair A, Gamerman V, Morse C, Orsulic S, et al. The immunomodulatory effects of pegylated liposomal doxorubicin are amplified in BRCA1–deficient ovarian tumors and can be exploited to improve treatment response in a mouse model. Gynecol Oncol. 2014;133:584–590. doi: 10.1016/j.ygyno.2014.03.565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.