ABSTRACT
Access to safe drinking water is now recognized as a human right by the United Nations. In developed countries like Canada, access to clean water is generally not a matter of concern. However, one in every five First Nations reserves is under a drinking water advisory, often due to unacceptable microbiological quality. In this study, we analyzed source and potable water from a First Nations community for the presence of coliform bacteria as well as various antibiotic resistance genes. Samples, including those from drinking water sources, were found to be positive for various antibiotic resistance genes, namely, ampC, tet(A), mecA, β-lactamase genes (SHV-type, TEM-type, CTX-M-type, OXA-1, and CMY-2-type), and carbapenemase genes (KPC, IMP, VIM, NDM, GES, and OXA-48 genes). Not surprisingly, substantial numbers of total coliforms, including Escherichia coli, were recovered from these samples, and this result was also confirmed using Illumina sequencing of the 16S rRNA gene. These findings deserve further attention, as the presence of coliforms and antibiotic resistance genes potentially puts the health of the community members at risk.
IMPORTANCE In this study, we highlight the poor microbiological quality of drinking water in a First Nations community in Canada. We examined the coliform load as well as the presence of antibiotic resistance genes in these samples. This study examined the presence of antibiotic-resistant genes in drinking water samples from a First Nations Community in Canada. We believe that our findings are of considerable significance, since the issue of poor water quality in First Nations communities in Canada is often ignored, and our findings will help shed some light on this important issue.
INTRODUCTION
Antibiotic resistance in bacteria has been recognized as one of the greatest threats to human health by the World Health Organization (1). Overuse and misuse of antibiotics contribute to the buildup of selective pressure aiding the proliferation of antibiotic-resistant bacteria (2, 3). While hospital environments are notorious for selecting for antibiotic-resistant bacteria, it is now becoming increasingly evident that overuse and misuse of antibiotics are also creating a selective pressure outside hospital settings. Studies over the last few years have shown the presence of antibiotics and of antibiotic-resistant bacteria in the broader environment, including water supplies and soil samples (4). This is indeed alarming as the high number of antibiotic-resistant bacteria in communities makes the treatment of community-acquired infections increasingly challenging (5, 6).
Not surprisingly, water samples from communities that lack access to clean water contain high numbers of bacteria (7–9). While a high bacterial count in the water supply itself poses an increased health risk (10), the presence of antibiotic-resistant bacteria makes this risk even more serious. Lack of access to clean and safe water is a problem that is generally associated with developing countries; however, this is a reality as well for many First Nations communities in Canada. For example, it has been reported that between December 2015 and February 2016, there were 157 drinking water advisories in effect in 110 First Nations communities in Canada (11), which amounts to about 20% of all First Nations reserves in Canada. A national analysis has cited “unacceptable microbiological quality” as the reason in 43% of these advisories (12).
In this study, we examined the bacterial load and diversity as well as the presence of various antibiotic resistance genes in water samples from a northern Manitoba First Nations community. Our work shows high prevalences of Escherichia coli and coliform bacteria and also of antibiotic resistance genes in these water samples. This study reports the presence of antibiotic resistance genes in drinking water in a First Nations community in Canada.
MATERIALS AND METHODS
Community profile.
Water samples were collected from a First Nations community in the Island Lake Region of Manitoba, which is located about 500 km from Winnipeg, Manitoba, Canada. The community has an on-reserve population of approximately 4,000 and a total registered population of about 4,500, with a median age of ∼20 years. This is a fly-in community, which is accessible from Winnipeg by two flights per day during summer (with no road access during summer) and by ice road during winter. The community has a water treatment plant, and just over 300 homes in the community are served by piped tap water. The majority of the houses that do not have piped tap water from the water treatment plant get their water delivered by a water truck, which fills up at the water treatment plant. During the time of sample collection for this study, there was only one operational water truck for the community. Water is stored in cisterns (water storage tanks used in approximately 150 homes) with most cisterns located underground. Families in homes without running water use small plastic buckets to obtain their drinking water from a community standpipe after which the water is stored (often in open buckets lined with a garbage bag) in the home. It is also quite common to use the lake water for washing and cleaning purposes. In addition, families sometimes utilize lake water as drinking water or collect rain water for general washing and cleaning purposes.
Sample collection and bacteriological and chlorine analysis.
Water samples were collected between 21 and 24 July 2014 from various sites within the community (Table 1) using standard methods (SM) (13): SM 9060A (sample bottle pretreatment) and SM 9060B (preservation and storage). The samples were collected over a period of only 3 days due to the challenges associated with the access to the community; however, we tried to overcome this limitation by collecting samples from multiple households and sources. During water collection, the Hach Chlorine Pocket Colorimeter II (VWR, Mississauga, ON, Canada) was used to determine free residual and total residual chlorine following the adapted U.S. EPA DPD Method 8021 (14). For all other analyses, samples were transported in coolers to Winnipeg by air on the same day of collection when possible or on the next morning after being stored overnight in a refrigerator. In addition, water collected from a tap located on the University of Manitoba grounds and a sample collected from the Red River flowing through the University of Manitoba grounds were used as control samples. Total coliform and E. coli counts were immediately processed upon receipt of the samples in our laboratories in Winnipeg using the “Standard Methods for the Examination of Water and Wastewater” of the American Public Health Association/American Water Works Association/Water Environment Federation as outlined in SM 9222 (13). Briefly, 100 ml of water sample was filtered through a sterile polyethersulfone membrane (0.22-μm pore size, 47-nm diameter; Mo Bio Laboratories, Carlsbad, CA, USA), and the filter paper was placed on agar plates containing Brilliance E. coli and coliform medium (Fisher Scientific, Ottawa, ON, Canada) and incubated at 35°C for 24 h. Some samples required dilution as the bacterial counts were too high. All samples were plated within 24 h of collection, and a control sample was included every time samples were plated. Bacterial counts in water samples were calculated as follows: E. coli CFU/milliliter = number of purple colonies/volume of filtered sample (100 ml) × dilution factor; total coliform bacteria CFU/milliliter = number of purple + pink colonies/volume of filtered sample (100 ml) × dilution factor. We were unable to plate biological replicates for the samples due to the limited number of samples we were able to collect and bring to our laboratories in Winnipeg in a timely fashion.
TABLE 1.
Description of water samples used in the study
| Sample designation | Description |
|---|---|
| T | Tap water samples from homes with running water. T1, T2, T3, and T4 represent samples from four different households. |
| C | Water samples from underground cisterns. C3 was filled 7 days prior to the sampling day, while C4 was filled 1 day prior to the sampling day. The last fill day for C1 and C2 was not known. |
| P | Water samples from the tap in the water treatment plant. P1 and P2 represent samples collected on two different days. |
| B | Water samples from plastic storage containers at home. B1 is a sample taken from an open plastic bucket that was sitting on the floor in a kitchen. Water in B1 is from a community fountain. B2 is a sample taken from a plastic container that had a lid that was filled with water obtained from the truck. B3 was taken from a relatively large white plastic storage container that had a small tap. B4 was a sample taken from an open bucket that had a garbage bag in it. |
| BS | BS1 and BS2 are samples taken from the community fountain. |
| LBS | LBS1 is a lake sample used as drinking water. |
| LPS | These represent plant source water (from the lake). LPS1, LPS2, LPS3 and LPS4 are samples collected on different days. |
| LBC | LBC1, LBC2, and LBC3 are from the lake at a location where kids frequently swim. |
| RBC | RBC1 is rainwater collected from a large drum; the rainwater is used to clean the house. |
| FBO | Control samples. Two controls were collected on each day, one consisted of a bottle with ultrapure wate, and this bottle was kept closed during the day. The second control also consisted of ultrapure water, but the cap was opened for 10 s and hence the water was exposed to air. |
| RR | Red River water samples from Winnipeg used for comparative analysis. |
| WPg TW | Tap water collected from Winnipeg used as a control. |
Extraction of DNA.
DNA was extracted from water samples immediately upon arrival in our laboratories in Winnipeg by filtering 100 ml of water samples (R. Li, H. M. Tun, M. Jahan, A. Farenhorst, A. Kumar, W. G. D. Fernando, and E. Khafipour, submitted for publication) through sterile polyethersulfone membranes (0.22-μm pore size; Mo Bio Laboratories, Carlsbad, CA, USA). Filters were subjected to DNA extraction using the PowerWater DNA isolation kit (Mo Bio Laboratories), following the manufacturer's instructions. The DNA concentration was quantified using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The DNA samples were normalized, and quality was verified by PCR amplification of the 16S rRNA gene using universal primers (Table 2) as described previously (15). Amplicons were verified by agarose gel electrophoresis. DNA samples were stored at −80°C until further analyses. Sterile water was used as an extraction control. The yield of DNA by this method ranged from 5 ng/μl to 275 ng/μl (100 μl in total), which was sufficient for the library construction as well as the quantitative PCR (qPCR) analysis described below.
TABLE 2.
List of primers used in this study
| Primer name | Sequence | Target gene (amplicon size, bp) | Reference or source |
|---|---|---|---|
| 27F | GAAGAGTTTGATCATGGCTCAG | 16S rRNA universal (variable) | 15 |
| 342R | CTGCTGCCTCCCGTAG | ||
| ampC_F1 | TGAGTTAGGTTCGGTCAGCA | ampC (98) | This study |
| ampC_R1 | AGTATTTTGTTGCGGGATCG | ||
| vanA_F1 | AtAAAGCGCTCGGCTGTAGA | vanA (98) | This study |
| vanA_R1 | GAAACCGGGCAGAGTATTGA | ||
| tetA_F1 | AGGTGGATGAGGAACGTCAG | tet(A) (96) | This study |
| tetA_R1 | AGATCGCCGTGAAGAGGCG | ||
| mecA_F1 | CTGATGGTATGCAACAAGTCG | mecA (97) | This study |
| mecA_R1 | TGAGTTCTGCAGTACCGGATT | ||
| SHV-UP | CGCCGGGTTATTCTTATTTGTCGC | blaSHV (1,016) | 54 |
| SHV-LO | TCTTTCCGATGCCGCCGCCAGTCA | ||
| TEM-G | TTGCTCACCCAGAAACGCTGGTG | blaTEM (708) | 54 |
| TEM-H | TACGATACGGGAGGGCTTACC | ||
| CTX-U1 | ATGTGCAGYACCAGTAARGTKATGGC | blaCTX-M (593) | 54 |
| CTX-U2 | TGGGTRAARTARGTSACCAGAAYCAGCGG | ||
| OXA1-F | CGCAAATGGCACCAGATTCAAC | blaOXA-1 (464) | 54 |
| OXA1-R | TCCTGCACCAGTTTTCCCATACAG | ||
| CMY2-A | TGATGCAGGAGCAGGCTATTCC | blaCMY-2 (323) | 54 |
| CMY2-B | CTAACGTCATCGGGGATCTGC | ||
| KPC-1 | ATGTCACTGTATCGCCGTC | blaKPC (863) | 54 |
| KPC-2 | AATCCCTCGAGCGCGAGT | ||
| IMP1 | CCWGATTTAAAAATYGARAAGCTTG | blaIMP (522) | 54 |
| IMP2 | TGGCCAHGCTTCWAHATTTGCRTC | ||
| VIM1 | GTTTGGTCGCATATCGCAAC | blaVIM (382) | 54 |
| VIM2 | AATGCGCAGCACCAGGATAGAA | ||
| NDM-F | GGTGCATGCCCGGTGAAATC | blaNDM (660) | 54 |
| NDM-R | ATGCTGGCCTTGGGGAACG | ||
| GES-2 | ATCAGCCACCTCTCAATGG | blaGES (302) | 50 |
| GES-3 | TAGCATCGGGACACATGAC | ||
| OXA-48A | TTGGTGGCATCGATTATCGG | blaOXA-48 (744) | 55 |
| OXA-48B | GAGCACTTCTTTTGTGATGGC |
Library construction and Illumina sequencing.
The microbial compositions of the water samples were determined by PCR amplification of the V4 region of the 16S rRNA gene using modified F515/R806 primers (16) as described before (17). For each sample, the PCR was performed in duplicate and contained 1.0 μl of prenormalized DNA, 1.0 μl of each forward and reverse primer (10 μM), 12 μl of high-performance liquid chromatography (HPLC)-grade water (Fisher Scientific, Ottawa, ON, Canada), and 10 μl of 5 Prime HotMasterMix (5 Prime, Inc., Gaithersburg, MD, USA). Reactions consisted of an initial denaturing step at 94°C for 3 min, followed by 35 amplification cycles at 94°C for 45 s, 50°C for 60 s, and 72°C for 90 s, with a final extension step at 72°C for 10 min in an Eppendorf Mastercycler pro (Eppendorf, Hamburg, Germany). PCR products were purified using a ZR-96 DNA cleanup kit (Zymo Research, Irvine, CA, USA) to remove primers, deoxynucleoside triphosphates (dNTPs), and reaction components. The V4 library was then generated by pooling 200 ng of each sample, quantified by PicoGreen double-stranded DNA (dsDNA) (Invitrogen, Burlington, ON, Canada). This was followed by multiple dilution steps using prechilled hybridization buffer (HT1; Illumina, San Diego, CA, USA) to bring the pooled amplicons to a final concentration of 5 pM, as determined with a Qubit 2.0 Fluorometer (Life Technologies, Burlington, ON, Canada). Finally, 15% of the PhiX control library was spiked into the amplicon pool to improve the unbalanced and biased base composition, a common characteristic of low-diversity 16S rRNA libraries. Customized sequencing primers for read1 (5′-TATGGTAATTGTGTGCCAGCMGCCGCGGTAA-3′), read2 (5′-AGTCAGTCAGCCGGACTACHVGGGTWTCTAAT-3′), and the index read (5′-ATTAGAWACCCBDGTAGTCCGGCTGACTGACT-3′) were synthesized and purified by polyacrylamide gel electrophoresis (Integrated DNA Technologies, Coralville, IA, USA) and added to the MiSeq reagent kit v2 (300-cycle; Illumina). The 150 paired-end sequencing reaction was performed on a MiSeq Illumina platform at the Gut Microbiome Laboratory (Department of Animal Science, University of Manitoba, Winnipeg, MB, Canada).
Bioinformatic analysis.
The FLASH assembler (18) was used to merge overlapping paired-end Illumina fastq files, and all sequences with mismatches in the overlapping region were discarded. The output of the fastq file was then analyzed by downstream computational QIIME pipelines (19). Assembled reads were demultiplexed according to the barcode sequences and exposed to additional quality filters so that reads with ambiguous calls and those with Phred quality scores (Q scores) of <20 were discarded. Chimeric reads were filtered using UCHIME (20), and sequences were assigned to operational taxonomic units (OTU) using the QIIME implementation of UCLUST (21) at a 97% pairwise identity threshold. Taxonomies were classified to the representative sequence of each OTU using the Ribosomal Database Project (RDP) classifier (22) and aligned with the Greengenes Core reference database (23) using PyNAST algorithms (24).
qPCR.
Absolute quantification of resistance genes in water samples was carried out for four different genes, namely, ampC, vanA, tet(A), and mecA, using the StepOnePlus real-time PCR system (Life Technologies Inc., Burlington, ON, Canada), following a previously described method with slight modifications (25). DNA samples were diluted to a concentration of 0.724 ng/μl, and 2.68 μl of the diluted DNA (to a total of 1.94 ng of DNA per reaction) was used in a total reaction volume of 8 μl which contained 9 μM respective primers and 2× SsoFast EvaGreen Supermix (Bio-Rad Canada, Mississauga, ON, Canada). Primers used in the study are listed in Table 2. Reactions were carried out in triplicate. The copy number of each gene per nanogram of DNA was calculated by creating a standard curve for each gene. Briefly, DNA was isolated from bacterial cells that contained the target genes using the DNA extraction kit (Biobasic, Markham, ON, Canada). The bacterial strains used were clinical isolates E. coli 99270 and E. coli ER2925 for ampC and tet(A), respectively, a methicillin-resistant Staphylococcus aureus clinical isolate HA-MRSA 100697 for mecA, and Enterococcus faecium ATCC 13048 for vanA. For the creation of the standard curve, we first calculated the copy number of each gene per nanogram of DNA in bacterial strains used as positive controls with the following formula (http://www6.appliedbiosystems.com/support/tutorials/pdf/quant_pcr.pdf): m = n/1.069 × 10−21 (g/bp), where n is genome size in base pairs and m is mass in grams.
Genome sizes used for each of the strains are as follows: 4,640,000 bp for E. coli; 2,839,469 bp for methicillin-resistant S. aureus; and 3,218,031 bp for E. faecium. The sizes of the genomes represent published sequences of these organisms, and while they may not represent the exact sizes of the genomes of the clinical isolates used in our study, we suspect that the sizes are fairly comparable to those used in our calculations. The standard curve was created by using a 10-fold dilution series for the target gene copy number starting with 200,000 copies to 20 copies. The copy number per nanogram of DNA for each sample was then calculated using the slope of the standard curve. Although there are other known methods that either calculate the relative quantification of the 16S rRNA gene copy number or absolute quantification of the target gene per volume of the water sample (26–28), we used calibration curves derived from different antibiotic resistance reference bacteria for absolute quantification. We prefer this method, which has been used previously (25), because our samples were likely to contain uncharacterized bacteria and also contain bacteria with different genetic backgrounds (e.g., multicopy plasmids), and, therefore, we believe our method of quantifying resistance genes normalizing against the amount of DNA circumvents the possible problems associated with mismatches in the 16S qPCR primers.
Multiplex PCR detection of β-lactamase and carbapenemase genes.
A multiplex PCR was carried out for the detection of five different β-lactamase genes, namely, SHV-type, TEM-type, CTX-M-type, OXA-1, and CMY-2-type. A list of primers used in the study is provided in Table 2. PCR was performed with Q5 high-fidelity DNA polymerase (New England BioLabs, Whitby, ON, Canada) using the amplification cycle that consisted of the following steps: 1 cycle at 95°C (15 min); 30 cycles at 94°C for 30 s, 63.5°C for 90 s, and 72°C for 90 s; and a final extension step at 72°C for 7 min. Final concentrations of the primers used were as follows: SHV and CMY-2, 0.3 μM each; TEM, 0.1 μM; CTX, 0.4 μM; and OXA-1, 0.2 μM. PCRs were validated using the following strains as positive controls: Klebsiella pneumoniae N09-00080 for SHV, TEM, CTX, OXA-1, and CMY-2; K. pneumoniae N09-0431 for KPC-2; E. coli 12-123T for OXA-48; E. coli A44413 for GES-5; E. coli 10469T for NDM; Pseudomonas aeruginosa C-10 for VIM-2; and A. baumannii C-3 for IMP-4.
Detection of six different carbapenemase-encoding genes, namely, KPC, IMP, VIM, NDM, GES-5, and OXA-48, was carried out using separate PCRs. All genes, except IMP, were detected using a multiplex reaction, while IMP detection was carried out in a separate reaction. Primers for this reaction are listed in Table 2. Final concentrations of the primers used in the reaction were 0.2 μM for each except for OXA-48 (0.1 μM). The PCR consisted for the following steps: 1 amplification cycle at 95°C (15 min); 30 cycles at 94°C for 30 s, 60°C for 90 s, and 72°C for 60 s; and a final extension step at 72°C for 7 min. PCR products were resolved on a 1.3% agarose gel.
Accession number(s).
The sequencing data were deposited into the Sequence Read Archive (SRA) of NCBI (http://www.ncbi.nlm.nih.gov/sra) and can be accessed via accession number SRR3189861.
RESULTS
Bacteriological and chlorine analysis.
The results are summarized in Table 3. While we did not find any coliforms (including E. coli) in samples collected from the water treatment plant, we detected both total coliforms and E. coli from all other samples with the exception of one tap water sample (T3). Health Canada's “Guidelines for Canadian Drinking Water Quality” require a total coliform and E. coli count of 0/100 ml for treated water to be deemed safe for drinking (www.healthcanada.gc.ca/waterquality). Substantial counts (150 to ∼700 CFU/ml) were observed for various samples collected from the lake, including the area near the inlet of the water treatment plant (LBS, LPS, and LBC). We also found high numbers of total coliforms (420 to 5,000 CFU/ml) and E. coli (390 to 2,000 CFU/ml) in samples from the community fountain. Water samples collected from various sources within houses (tap, cisterns, and buckets) also showed the presence of both total coliforms and E. coli. Two of the bucket samples collected from homes without running water, B1 and B2, showed extremely high numbers of total coliforms (14,600 and 1,100 CFU/ml, respectively) and E. coli (2,300 and 1,090 CFU/ml, respectively), and piped water samples from households showed the presence of 1 to 2 CFU/ml bacteria. Field blanks did not show the presence of any bacteria.
TABLE 3.
Total coliform and E. coli counts and total chlorine residual and free chlorine residual in various water samples
| Sample | Total coliforms (CFU/100 ml) | E. coli (CFU/100 ml) | Total chlorine residual (ppm) | Free chlorine residual (ppm) |
|---|---|---|---|---|
| P1 | 0 | 0 | 0.94 | 0.67 |
| P2 | 0 | 0 | 0.94 | 0.67 |
| T1 | 2 | 1 | 0.16 | 0.04 |
| T2 | 2 | 1 | 0.16 | 0.07 |
| T3 | 0 | 0 | 0.15 | 0.04 |
| T4 | 1 | 1 | 0.14 | 0.03 |
| C1 | 11 | 5 | 0.03 | 0.03 |
| C2 | 3 | 3 | 0.12 | 0.03 |
| C3 | 220 | 20 | 0.02 | 0 |
| C4 | 180 | 10 | 0.11 | 0.04 |
| B1 | 14,600 | 1,100 | 0.04 | 0.01 |
| B2 | 2,300 | 1,090 | 0.23 | 0.05 |
| B3 | 20 | 20 | 0.21 | 0.18 |
| B4 | 50 | 40 | 0.29 | 0.16 |
| BS1 | 420 | 390 | 0.25 | 0.07 |
| BS2 | 5,000 | 2,000 | 0.25 | 0.07 |
| LBS1 | 150 | 100 | 0.13 | 0.12 |
| LPS1 | 230 | 180 | 0.18 | 0.1 |
| LPS2 | 90 | 60 | 0.18 | 0.1 |
| LPS3 | 170 | 120 | 0.18 | 0.1 |
| LPS4 | 110 | 80 | 0.18 | 0.1 |
| LBC1 | 690 | 0 | 0.12 | 0.08 |
| LBC2 | 230 | 110 | 0.12 | 0.08 |
| LBC3 | 450 | 330 | 0.12 | 0.08 |
| Wpg TW1 | 0 | 0 | 0.93 | 0.72 |
None of the water samples from the community had more than 0.2 ppm of residual free chlorine (minimum concentration recommended by the World Health Organization) except for the posttreatment water directly collected in the treatment plant (P1 and P2) (Table 3). Similarly, total chlorine levels were the highest in the samples collected from the plant.
Determination of microbial diversity.
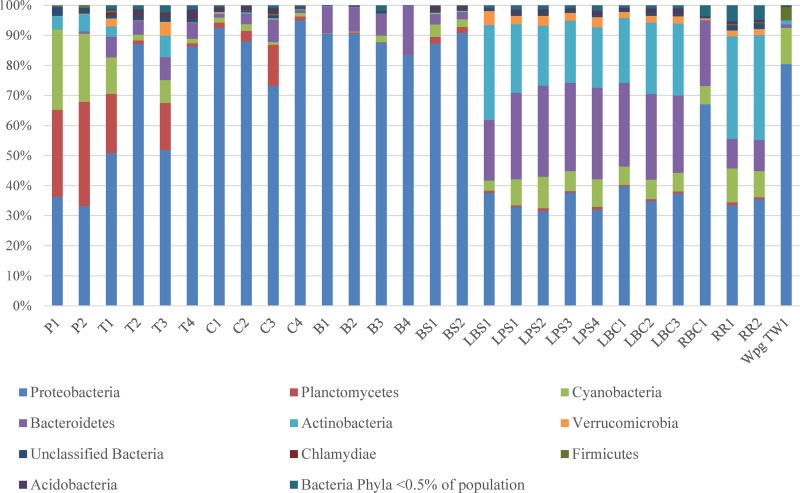
Taxonomic classification of clustered OTU revealed the presence of 46 bacterial phyla. Abundant phyla (>1% of the population) included Proteobacteria, Bacteroidetes, Actinobacteria, Cyanobacteria, Planctomycetes, Firmicutes, Verrucomicrobia, and Acidobacteria, whereas Armatimonadetes, TM6, Chlorobi, Chloroflexi, Chlamydiae, and Gemmatimonadetes were in medium abundance (between 0.1 and 1% of the population), and the remaining 32 phyla were in low abundance (<0.1% of the population) (Fig. 1). The results for the community analysis showed that samples from the lake, irrespective of the location they were collected from, had a very similar community profile. The same was true for the two posttreatment samples from the treatment plant. Water samples collected from households or from fountains showed a profile that was distinct from that for the posttreatment samples from the treatment plant. Interestingly, we observed a significantly large proportion of Proteobacteria in household/fountain samples compared to that in the samples from the treatment plant (Fig. 1).
FIG 1.
Microbial community composition in water samples determined by amplification of V4 region of 16S rRNA and MiSeq Illumina sequencing. Sample descriptions are provided in Table 1.
Detection of antibiotic resistance genes.
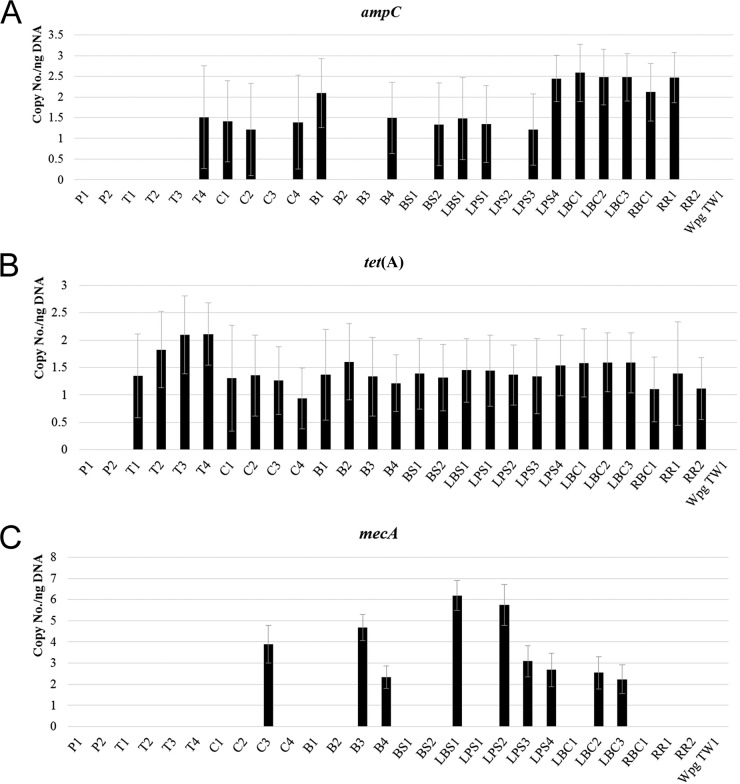
Results for the presence of antibiotic resistance genes detected using qPCR are shown in Fig. 2. ampC was detected in one tap water sample (T4), three cisterns (C1, C2, and C4), two bucket samples (B1 and B4), one community fountain sample (BS2), and all lake samples except LPS2 (Fig. 2A). The most abundant resistance gene in water samples was tet(A), which was present in all samples, including the tap water samples, except for the posttreatment samples collected from the water treatment plant (Fig. 2B). mecA was detected in six different lake water samples (LBS1, LPS2, LPS3, LPS4, LBC2, and LBC3), two bucket samples (B3 and B4), and one cistern sample (C3) (Fig. 2C). We did not detect vanA in any of our samples.
FIG 2.
Quantification of ampC (A), tet(A) (B), and mecA (C) genes in water samples. Copy number/ng of total DNA was determined using qPCR and calculated using a standard curve created using control bacterial strains. Sample description is provided in Table 1.
Results for the detection of β-lactamase and carbapenemase genes using PCR are shown in Table 4. Among the β-lactamase genes, we detected the presence of TEM in most of the samples except T2 and T3 (tap water), C3 and C4 (cisterns), B1 and B2 (buckets), and LPS1 and LPS2 (plant source lake water). The SHV gene was found only in RBC1, and CTX-M was found only in two bucket samples (B3 and B4). We did not detect the OXA-1 or CMY-2 genes. As for the carbapenemase genes, we found the VIM-like carbapenemase gene in 9 out of 25 water samples tested from the community, including all eight lake water samples tested: OXA-48 and GES were found in four water samples each (OXA-48 in C1, C2, LBC3, and RBC1 and GES in C1, B2, B4, and LBC3) and NDM in two (B2 and B4) water samples. It is worth mentioning that in this study, we detected the presence of antibiotic resistance genes and not their expression.
TABLE 4.
Detection of β-lactamase and carbapenemase genes in various water samples
| Sample | β-Lactamase genes |
Carbapenemase genes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SHV | TEM | CTX-M | OXA-1 | CMY-2 | KPC | OXA-48 | NDM | VIM | GES | IPM | |
| P1 | − | + | − | − | − | − | − | − | − | − | − |
| P2 | − | + | − | − | − | − | − | − | − | − | − |
| T1 | − | + | − | − | − | − | − | − | − | − | − |
| T2 | − | − | − | − | − | − | − | − | − | − | − |
| T3 | − | − | − | − | − | − | − | − | − | − | − |
| T4 | − | + | − | − | − | − | − | − | − | − | − |
| C1 | − | + | − | − | − | − | + | − | − | + | − |
| C2 | − | + | − | − | − | − | + | − | + | − | − |
| C3 | − | − | − | − | − | − | − | − | − | − | − |
| C4 | − | − | − | − | − | − | − | − | − | − | − |
| B1 | − | − | − | − | − | − | − | − | − | − | − |
| B2 | − | − | − | − | − | − | − | + | − | + | − |
| B3 | − | + | + | − | − | − | − | − | − | − | − |
| B4 | − | + | + | − | − | − | − | + | − | + | − |
| BS1 | − | + | − | − | − | − | − | − | − | − | − |
| BS2 | − | + | − | − | − | − | − | − | − | − | − |
| LBS1 | − | + | − | − | − | − | − | − | + | − | − |
| LPS1 | − | − | − | − | − | − | − | − | + | − | − |
| LPS2 | − | − | − | − | − | − | − | − | + | − | − |
| LPS3 | − | + | − | − | − | − | − | − | + | − | − |
| LPS4 | − | + | − | − | − | − | − | − | + | − | − |
| LBC1 | − | + | − | − | − | + | − | − | + | − | − |
| LBC2 | − | + | − | − | − | − | − | − | + | − | − |
| LBC3 | − | + | − | − | − | + | + | − | + | + | − |
| RBC1 | + | + | − | − | − | + | + | − | − | − | − |
| RR1 | − | + | − | − | − | − | − | − | − | − | − |
| RR2 | − | − | − | − | − | − | − | − | − | − | − |
DISCUSSION
Access to clean running drinking water is among the most important socioeconomic determinants of health in a community and is considered one of the defining features of a developed country (10). However, it has been recognized that First Nations communities on reserves in Canada do not have the same security of access to safe drinking water sources as do most other Canadians (30). There are approximately 600 First Nations reserves in Canada, and in the past decade, the number of communities under drinking water advisories show an increase, from about 100 communities in 2003 (12) to 127 in 2015 (11). Consumption of unsafe water has a negative impact on the health of a community. Therefore, it is not surprising that due to a lack of access to safe water, there is a high prevalence of infections like bacterial gastroenteritis and impetigo in First Nations communities (31, 32).
In this study, we were interested in investigating the prevalence of antibiotic resistance genes in water samples from one such First Nations community. To this end, we first carried out analysis for total coliforms and E. coli and found substantial numbers of these organisms in all samples collected from homes or from lakes (Table 3). This was done by using Brilliance agar, which distinguishes E. coli from other coliforms on the basis of β-d-glucuronidase production by E. coli. Even though this medium is unable to identify hemorrhagic E. coli that do not produce the enzyme or strains of E. coli that produce small amounts of the enzyme (33), it is widely used for the detection of coliforms and E. coli. In accordance with the presence of coliforms and E. coli in our samples, we found significantly lower-than-recommended concentrations of chlorine (0.2 ppm) (34) in samples from homes. This indicates a problem with the water distribution and storage systems, including dissipation of chlorine in the water pipes that are connected to the home or community fountain and in the water tanker that delivers water to the cisterns where the water is stored. Therefore, it is not surprising that samples from households that collect their water from the community fountain and then store it in buckets have high numbers of coliforms and E. coli. As listed in Table 1, the practices for cleaning buckets and changing water can vary a lot from household to household.
We carried out microbial community analysis of our samples in order to get a better sense of how different samples compared with each other with respect to the diversity of bacteria (Fig. 1). Our results show that all the lake water samples, regardless of the site of sampling, have very similar microbial community structure. The interesting pattern we observed was the difference in the microbial community profiles of posttreatment water samples from the water treatment plant and samples collected from households. Specifically, we found a higher proportion of Proteobacteria in the various household samples than in the treated water sampled in the water treatment plant (Fig. 1). The higher proportion of Proteobacteria in water samples from buckets indicates contamination from human sources (Fig. 1). The higher proportions of Proteobacteria in the tap or cistern water samples also suggest that once water leaves the treatment plant, there is likely to be introduction of Proteobacteria at different sources, which correlates well with our data on total coliforms and E. coli in these samples. Although the sources of contamination were not investigated, they may include (i) leaks in water pipes, allowing surface and subsurface water contaminated with animal fecal matter to seep into pipes, (ii) risk of the water truck hose becoming contaminated with animal fecal matter when it is unintentionally exposed to land surfaces during the process of water delivery to cisterns, and (iii) unlocked cistern lids, increasing the potential for contamination.
In the absence of access to any data on the type or rate of antibiotic prescription in the community, we decided to look for a subset of genes that have previously been shown to be commonly found in various aquatic environments (35, 36). A two-pronged approach to detect various antibiotic resistance genes, a qPCR method and a multiplex PCR method, was used. qPCR was used to detect ampC (β-lactam resistance), tet(A) (tetracycline resistance), mecA (methicillin resistance), and vanA (vancomycin resistance). β-Lactams are common antibiotics used to treat infections in both humans and animals, and their use has resulted in the emergence of resistance by β-lactamases like AmpC (37). ampC-encoded β-lactamases have been detected in wastewater as well as drinking water (38), and we also detected ampC in most of the lake water samples and in some of the samples from different households (Fig. 2A). Tetracycline-resistant bacteria are common in the environment, particularly due to the subtherapeutic use of this antibiotic in livestock (39), and tet(A) (a tetracycline efflux protein-encoding gene) has previously been shown to be present in both the hospitals and aquatic environments (40, 41). Tet genes have been associated with anthropogenic impacts, and a number of these genes have been found in various pollution sources (42). While tet(A) was the most common resistance gene in our samples, as it was found in all but two posttreatment samples collected from the treatment plant (Fig. 2B), at present it is not clear to us what constitutes the selection pressure for tetracycline resistance in these samples. Determining the selection pressure will be our goal in future studies.
We also found the mecA gene in some of the samples (Fig. 2C). The presence of mecA is indicative of methicillin-resistant Staphylococcus aureus (MRSA), which has been shown to be present in various aquatic environments (25, 41), although it has also been shown to be present in nonstaphylococcal pathogens (43). mecA was primarily detected in the lake water and bucket samples and in one cistern sample. One possible explanation for finding mecA in bucket samples is the practice of individuals dipping their hands in these buckets for various purposes. Also, some of the lake sites are frequented largely by children for play/recreational activities on a daily basis in the summertime, which could lead to an increased risk of exposure to methicillin-resistant pathogens (44). To what extent, if any, the high numbers of resistant organisms in the water environment pose a health risk to the community members remains to be quantified.
In addition to the above genes, we investigated the presence of five different β-lactamase and six different carbapenemase genes (Table 4). Some recent studies have reported the presence of carbapenemase genes in wastewater; for example VIM-2 has been isolated from hospital wastewater (45), OXA-type from sewage (46), and NDM-1 from patients' feces (which can make its way into the water supply) (47). These genes are responsible for resistance to carbapenems, which are often used as the antibiotics of last resort for treating antibiotic-resistant infections. Thus, infections associated with carbapenem-resistant bacteria are associated with high mortality (48). All of the genes tested except OXA-1 and CMY-2 β-lactamase and IPM carbapenemase genes were detected in at least one of the samples (Table 4). Of interest is the detection of blaNDM in two of the bucket samples. NDM-1 is a fairly recently discovered carbapenemase that has disseminated quite rapidly from its origin in India to the rest of the world (47, 49). While NDM has been detected in Canada (50), we are not aware of any NDM-positive cases from First Nations reserves in Canada, and, therefore, detection of NDM in the samples we tested is a matter of concern. Furthermore, the fact the most of the resistance genes detected in this study are found on plasmids (51, 52) is an additional cause for worry because these resistance determinants can potentially be passed to other susceptible organisms. Overall, we did not observe a clear correlation between high coliform numbers and the presence of antibiotic resistance genes. For example, sample C2 was positive for TEM, OXA-48, VIM (Table 4), ampC, and tet(A) (Fig. 2A and B) but had a coliform count of 3 (Table 3); conversely, sample BS2 had a coliform count of 5,000, but the amounts of ampC and tet(A) in this sample were no higher than those in the other positive samples. These data suggest a possible role of noncoliform organisms in housing a number of resistance genes. However, as stated earlier, in the absence of data on the usage of these antibiotics in the community, it is not clear what may be contributing to the selection pressure for these genes in the organisms.
This study investigated the prevalence of antibiotic-resistant genes in water samples from a First Nations community in Canada. This work also highlights the critical nature of the poor water quality in the First Nations community in our study even when the community members, albeit not all, have access to running tap water. However, it is important to reiterate that our data show that the water from the water treatment plant is safe to drink, and microorganisms appear to get introduced during the process of distribution and storage. The presence of such a large number of bacteria along with antibiotic resistance genes puts the health of community members at a risk. Coincidently, a recent report by Statistics Canada points out that First Nations adults are more likely to die from infectious diseases than the rest of Canadians (53), although the importance of the role of drinking water in this remains to be investigated. Therefore, despite some limitations in our study, such as sampling at one time point (due to the isolated nature of this fly-in community, which also hampered our ability to collect biological replicates) and lack of access to the antibiotic prescription data for the community, it highlights certain critical problems associated with water safety in some communities in Canada.
ACKNOWLEDGMENTS
We acknowledge the help and cooperation of the members of the First Nations community in our study without whom this study would not have been possible. We are grateful to George Zhanel (Department of Medical Microbiology, University of Manitoba) for clinical isolates used as positive controls and to Michael Mulvey (National Microbiology Laboratory, Winnipeg, Canada) for providing the protocol as well as the positive controls for multiplex PCRs. We also thank Kari Kumar (Division of Extended Education, University of Manitoba) for the critical reading of the manuscript.
This work was supported by Discovery (A.K., W.G.D.F.) and CreateH2O (A.F.) grants funded by the Natural Science and Engineering Research Council of Canada as well as a University of Manitoba Start-up grant (E.K.). D.M.F. was funded by a Canada Research Chair grant to Peter Loewen, Department of Microbiology, University of Manitoba. R.P. is funded by a postdoctoral fellowship from the Government of Madhya Pradesh, India.
REFERENCES
- 1.World Health Organization. 2014. Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland: http://www.who.int/drugresistance/documents/surveillancereport/en/. [Google Scholar]
- 2.Levy SB, O'Brien TF, Alliance for the Prudent Use of Antibiotics. 2005. Global antimicrobial resistance alerts and implications. Clin Infect Dis 41:S219–S220. doi: 10.1086/432443. [DOI] [PubMed] [Google Scholar]
- 3.Davies J, Davies D. 2010. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarmah AK, Meyer MT, Boxall ABA. 2006. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Doern GV, Brown SD. 2004. Antimicrobial susceptibility among community-acquired respiratory tract pathogens in the USA: data from PROTEKT US 2000−01. J Infect 48:56–65. doi: 10.1016/S0163-4453(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 6.Woerther P-L, Angebault C, Lescat M, Ruppé E, Skurnik D, El Mniai A, Clermont O, Jacquier H, Da Costa A, Renard M, Bettinger RM, Epelboin L, Dupont C, Guillemot D, Rousset F, Arlet G, Denamur E, Djossou F, Andremont A. 2010. Emergence and dissemination of extended-spectrum β-lactamase-producing Escherichia coli in the community: lessons from the study of a remote and controlled population. J Infect Dis 202:515–523. doi: 10.1086/654883. [DOI] [PubMed] [Google Scholar]
- 7.Brown KD, Kulis J, Thomson B, Chapman TH, Mawhinney DB. 2006. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci Total Environ 366:772–783. doi: 10.1016/j.scitotenv.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Marrs CF, Simon C, Xi C. 2009. Wastewater treatment contributes to selective increase of antibiotic resistance among Acinetobacter spp. Sci Total Environ 407:3702–3706. doi: 10.1016/j.scitotenv.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Middleton JH, Salierno JD. 2013. Antibiotic resistance in triclosan tolerant fecal coliforms isolated from surface waters near wastewater treatment plant outflows (Morris County, NJ, USA). Ecotoxicol Environ Safety 88:79–88. doi: 10.1016/j.ecoenv.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Ashbolt NJ. 2015. Microbial contamination of drinking water and human health from community water systems. Curr Environ Health Rep 2:95–106. doi: 10.1007/s40572-014-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Health Canada. 2015. Drinking water advisories in First Nations Communities. Health Canada, Ottawa, ON, Canada: http://www.hc-sc.gc.ca/fniah-spnia/promotion/public-publique/water-dwa-eau-aqep-eng.php#a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health Canada. 2009. Drinking water advisories in First Nations communities in Canada: A national overview 1995−2007. Health Canada, Ottawa, ON, Canada: http://www.hc-sc.gc.ca/fniah-spnia/alt_formats/pdf/pubs/promotion/environ/2009_water-qualit-eau-canada/2009_water-qualit-eau-canada-eng.pdf. [Google Scholar]
- 13.Rice EW, Baird RB, Eaton AB, Clesceri LS. 2012. Standard methods for the examination of water and wastewater, 22nd ed American Public Health Association, American Water Works Association, Water Environment Federation. [Google Scholar]
- 14.Hach Company. 2002. Water analysis handbook. Hach Company, Loveland, CO. [Google Scholar]
- 15.Khafipour E, Li S, Plaizier JC, Krause DO. 2009. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl Environ Microbiol 75:7115–7124. doi: 10.1128/AEM.00739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derakhshani H, Tun HM, Khafipour E. 2016. An extended single-index multiplexed 16S rRNA sequencing for microbial community analysis on MiSeq Illumina platforms. J Basic Microbiol 56:321–326. doi: 10.1002/jobm.201500420. [DOI] [PubMed] [Google Scholar]
- 18.Magoč T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander J, Bollmann A, Seitz W, Schwartz T. 2015. Microbiological characterization of aquatic microbiomes targeting taxonomical marker genes and antibiotic resistance genes of opportunistic bacteria. Sci Total Environ 512–513:316–325. doi: 10.1016/j.scitotenv.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 26.Shanks OC, Kelty CA, Oshiro R, Haugland RA, Madi T, Brooks L, Field KG, Sivaganesan M. 2016. Data acceptance criteria for standardized human-associated fecal source identification quantitative real-time PCR methods. Appl Environ Microbiol 82:2773−2782. doi: 10.1128/AEM.03661-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aydin S, Ince B, Ince O. 2015. Application of real-time PCR to determination of combined effect of antibiotics on Bacteria, Methanogenic Archaea, Archaea in anaerobic sequencing batch reactors. Water Res 76:88–98. doi: 10.1016/j.watres.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 28.Makowska N, Koczura R, Mokracka J. 2016. Class 1 integrase, sulfonamide and tetracycline resistance genes in wastewater treatment plant and surface water. Chemosphere 144:1665–1673. doi: 10.1016/j.chemosphere.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30.Auditor General of Canada. 2011. Status report to the House of Commons: programs for First Nations on reserves. Office of the Auditor General of Canada, Ottawa, ON, Canada: http://www.oag-bvg.gc.ca/internet/docs/parl_oag_201106_04_e.pdf. [Google Scholar]
- 31.Murdocca C. 2010. “There is something in that water”: race, nationalism, and legal violence. Law Soc Inquiry 35:369–402. doi: 10.1111/j.1747-4469.2010.01189.x. [DOI] [Google Scholar]
- 32.National Advisory Committee on Immunization. 2010. An Advisory Committee statement: Canada communicable disease report. Public Health Agency of Canada, Ottawa, ON, Canada: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/10vol36/acs-14/index-eng.php. [Google Scholar]
- 33.Fricker CR, Warden PS, Eldred BJ. 2010. Understanding the cause of false negative β-d-glucuronidase reactions in culture media containing fermentable carbohydrate. Lett Appl Microbiol 50:547–551. doi: 10.1111/j.1472-765X.2010.02834.x. [DOI] [PubMed] [Google Scholar]
- 34.Health Canada. 2009. Guidelines for Canadian drinking water quality: guideline technical document. Health Canada, Ottawa, ON, Canada: http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/water-eau/chlorine-chlore/tech_doc_chlor-eng.pdf. [Google Scholar]
- 35.Xu L, Ouyang W, Qian Y, Su C, Su J, Chen H. 2016. High-throughput profiling of antibiotic resistance genes in drinking water treatment plants and distribution systems. Environ Pollut 213:119–126. doi: 10.1016/j.envpol.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Karkman A, Johnson TA, Lyra C, Stedtfeld RD, Tamminen M, Tiedje JM, Virta M. 2016. High-throughput quantification of antibiotic resistance genes from an urban wastewater treatment plant. FEMS Microbiol Ecol 92:fiw014 doi: 10.1093/femsec/fiw014.. [DOI] [PubMed] [Google Scholar]
- 37.Voolaid V, Tenson T, Kisand V. 2013. Aeromonas and Pseudomonas species carriers of ampC FOX genes in aquatic environments. Appl Environ Microbiol 79:1055–1057. doi: 10.1128/AEM.03171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz T, Kohnen W, Jansen B, Obst U. 2003. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol Ecol 43:325–335. doi: 10.1111/j.1574-6941.2003.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 39.Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. 2010. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol 8:251–259. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 40.Rhodes G, Huys G, Swings J, McGann P, Hiney M, Smith P, Pickup RW. 2000. Distribution of oxytetracycline resistance plasmids between aeromonads in hospital and aquaculture environments: implication of Tn1721 in dissemination of the tetracycline resistance determinant TetA. Appl Environ Microbiol 66:3883–3890. doi: 10.1128/AEM.66.9.3883-3890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naquin A, Shrestha A, Sherpa M, Nathaniel R, Boopathy R. 2015. Presence of antibiotic resistance genes in a sewage treatment plant in Thibodaux, Louisiana, USA. Bioresour Technol 188:79–83. doi: 10.1016/j.biortech.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 42.Chen B, Liang X, Huang X, Zhang T, Li X. 2013. Differentiating anthropogenic impacts on ARGs in the Pearl River Estuary by using suitable gene indicators. Water Res 47:2811–2820. doi: 10.1016/j.watres.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 43.Kassem II, Esseili MA, Sigler V. 2008. Occurrence of mecA in nonstaphylococcal pathogens in surface waters. J Clin Microbiol 46:3868–3869. doi: 10.1128/JCM.01035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huijbers PMC, Blaak H, de Jong MCM, Graat EAM, Vandenbroucke-Grauls CMJE, de Roda Husman AM. 2015. Role of the environment in the transmission of antimicrobial resistance to humans: a review. Environ Sci Technol 49:11993–12004. doi: 10.1021/acs.est.5b02566. [DOI] [PubMed] [Google Scholar]
- 45.Quinteira S, Ferreira H, Peixe L. 2005. First isolation of blaVIM-2 in an environmental isolate of Pseudomonas pseudoalcaligenes. Antimicrob Agents Chemother 49:2140–2141. doi: 10.1128/AAC.49.5.2140-2141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muniesa M, Garcia A, Miro E, Mirelis B, Prats G, Jofre J, Navarro F. 2004. Bacteriophages and diffusion of beta-lactamase genes. Emerg Infect Dis 10:1134–1137. doi: 10.3201/eid1006.030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. 2009. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G. 2012. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 18:54–60. doi: 10.1111/j.1469-0691.2012.03857.x. [DOI] [PubMed] [Google Scholar]
- 49.Bushnell G, Mitrani-Gold F, Mundy LM. 2013. Emergence of New Delhi metallo-β-lactamase type 1-producing Enterobacteriaceae and non-Enterobacteriaceae: global case detection and bacterial surveillance. Int J Infect Dis 17:e325–e333. doi: 10.1016/j.ijid.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 50.Mataseje LF, Bryce E, Roscoe D, Boyd DA, Embree J, Gravel D, Katz K, Kibsey P, Kuhn M, Mounchili A, Simor A, Taylor G, Thomas E, Turgeon N, Mulvey MR. 2012. Carbapenem-resistant Gram-negative bacilli in Canada 2009−10: results from the Canadian Nosocomial Infection Surveillance Program (CNISP). J Antimicrob Chemother 67:1359–1367. doi: 10.1093/jac/dks046. [DOI] [PubMed] [Google Scholar]
- 51.Livermore DM. 1995. β-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev 8:557–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Queenan AM, Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park J, Tjepkema M, Goedhuis N, Pennock J. 2015. Avoidable mortality among First Nations adults in Canada: a cohort analysis. Health Rep 26:10−16 http://www.statcan.gc.ca/pub/82-003-x/2015008/article/14216-eng.pdf. [PubMed] [Google Scholar]
- 54.Mulvey MR, Grant JM, Plewes K, Roscoe D, Boyd DA. 2011. New Delhi metallo-beta-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg Infect Dis 17:103–106. doi: 10.3201/eid1701.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brink AJ, Coetzee J, Corcoran C, Clay CG, Hari-Makkan D, Jacobson RK, Richards GA, Feldman C, Nutt L, van Greune J, Deetlefs JD, Swart K, Devenish L, Poirel L, Nordmann P. 2013. Emergence of OXA-48 and OXA-181 carbapenemases among Enterobacteriaceae in South Africa and evidence of in vivo selection of colistin resistance as a consequence of selective decontamination of the gastrointestinal tract. J Clin Microbiol 51:369–372. doi: 10.1128/JCM.02234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]