ABSTRACT
The Agrobacterium tumefaciens C58 genome harbors an operon containing the dmeR (Atu0890) and dmeF (Atu0891) genes, which encode a transcriptional regulatory protein belonging to the RcnR/CsoR family and a metal efflux protein belonging to the cation diffusion facilitator (CDF) family, respectively. The dmeRF operon is specifically induced by cobalt and nickel, with cobalt being the more potent inducer. Promoter-lacZ transcriptional fusion, an electrophoretic mobility shift assay, and DNase I footprinting assays revealed that DmeR represses dmeRF transcription through direct binding to the promoter region upstream of dmeR. A strain lacking dmeF showed increased accumulation of intracellular cobalt and nickel and exhibited hypersensitivity to these metals; however, this strain displayed full virulence, comparable to that of the wild-type strain, when infecting a Nicotiana benthamiana plant model under the tested conditions. Cobalt, but not nickel, increased the expression of many iron-responsive genes and reduced the induction of the SoxR-regulated gene sodBII. Furthermore, control of iron homeostasis via RirA is important for the ability of A. tumefaciens to cope with cobalt and nickel toxicity.
IMPORTANCE The molecular mechanism of the regulation of dmeRF transcription by DmeR was demonstrated. This work provides evidence of a direct interaction of apo-DmeR with the corresponding DNA operator site in vitro. The recognition site for apo-DmeR consists of 10-bp AT-rich inverted repeats separated by six C bases (5′-ATATAGTATACCCCCCTATAGTATAT-3′). Cobalt and nickel cause DmeR to dissociate from the dmeRF promoter, which leads to expression of the metal efflux gene dmeF. This work also revealed a connection between iron homeostasis and cobalt/nickel resistance in A. tumefaciens.
INTRODUCTION
Cobalt is required by coenzyme B12-dependent enzymes and several proteins (1, 2). However, cobalt overload can cause cellular toxicity by catalyzing the generation of reactive oxygen species (3, 4), which leads to iron and glutathione depletion, and thus disturbing iron homeostasis (4–6). Cobalt competes with iron in heme proteins (7) and inhibits the activity of iron-sulfur (Fe-S) proteins as shown in Escherichia coli (5) and Salmonella enterica (6). To avoid cobalt toxicity, levels of intracellular cobalt must be properly controlled. Cobalt trafficking systems in the cell, including uptake systems, efflux systems, and metallochaperones, help maintain cobalt at levels suitable for growth (8).
To prevent intracellular cobalt overload-mediated toxicity, excessive amounts of cobalt are eliminated by efflux systems involving components such as the major facilitator superfamily (MFS), P1B-4-ATPase, resistance nodulation cell division (RND), cation/proton antiporter, and cation diffusion facilitator (CDF). The E. coli RcnA (resistance to cobalt and nickel) efflux pump belongs to a unique family that is responsible for the detoxification of cobalt and nickel (9). The expression of rcnA is negatively regulated by RcnR (10). CoaT is a P1B-4-ATPase that is responsible for cobalt export in Synechocystis (11). Induction of coaT expression is mediated by the MerR-like transcriptional activator CoaR in the presence of cobalt, while the vitamin B12 pathway represses coaT transcription (11). Mycobacterium smegmatis CtpD (12) and Mycobacterium tuberculosis CtpJ (13) are also cobalt-exporting P1B-4-ATPases that have been shown to play an important role in controlling cobalt homeostasis. Cupriavidus metallidurans CH34 extrudes cobalt using two RND systems (the cobalt and nickel resistance system [CnrCBA] [14] and the cobalt, zinc, and cadmium resistance system [CzcABC] [15]), the CDF protein CzcD (16), and the P1B-4-ATPase CzcP (17). The CzcD protein negatively regulates CzcCBA by extruding the inducing cations (16), while CzcP functions as a metal-resistant enhancer (17). In addition to CzcD, C. metallidurans CH34 contains another CDF protein, DmeF (divalent metal efflux), which plays a role in controlling cobalt homeostasis (18). Disruption of the dmeF gene causes the CnrCBA and CzcCBA RND systems to become ineffective in cobalt transport. This suggests that cobalt is exported from the cytoplasm to the periplasm via the CDF proteins and that cobalt is then transported by the RND systems from the periplasm to outside the cell (18).
Agrobacterium tumefaciens is a soilborne plant pathogen that causes crown gall tumor disease. Cobalt transporters have not been characterized in A. tumefaciens. Rhizobium leguminosarum, a close relative of A. tumefaciens, belongs to the Alphaproteobacteria and contains the dmeRF operon that is responsible for exporting cobalt and nickel (19). R. leguminosarum DmeR represses dmeRF transcription. Expression of dmeRF is inducible in response to increased levels of cobalt and nickel. The R. leguminosarum dmeRF mutant is hypersensitive to cobalt and nickel. The dmeRF genes have been shown to play an important role in R. leguminosarum during free-living and symbiotic interactions with legume plants under high-cobalt and high-nickel conditions (19). Cobalt stress and iron homeostasis are linked. Induction of the ferric uptake regulator (Fur) regulon has been observed in response to cobalt stress, which may provide iron to out-compete cobalt (5, 6). Unlike E. coli, in which iron regulation is mediated by Fur, iron regulation in A. tumefaciens is controlled by the iron response regulator (Irr) and the rhizobial iron regulator (RirA) under low-iron and high-iron conditions, respectively (20, 21). In contrast to Irr, RirA is assumed to require the Fe-S cluster as its cofactor (22, 23). Disruption of an Fe-S cluster synthesis gene (sufS2) in the suf operon impairs RirA repression activity in A. tumefaciens (24). The A. tumefaciens genome contains genes that are homologous to dmeR (Atu0890) and dmeF (Atu0891) (25). Here, the molecular mechanism of the regulation of A. tumefaciens dmeRF and its role in controlling intracellular metal levels were determined. Furthermore, the effect of cobalt toxicity on the activities of Fe-S proteins, including RirA and SoxR, was investigated. A link between the control of iron homeostasis via RirA and cobalt/nickel tolerance was revealed.
MATERIALS AND METHODS
Bacterial strains, culture conditions, plasmids, and primers.
The bacterial strains and plasmids used in this work are listed in Table 1. E. coli and A. tumefaciens were aerobically grown in Luria broth (LB) at 37οC and 28οC, respectively. LA refers to LB medium containing 1.5% agar. The growth conditions and antibiotic concentrations used were the same as those previously reported (24). Induction broth with a pH of 5.5 (IB 5.5; the vir-inducing condition) was used in the virulence assay (26). Primers are listed in Table S1 in the supplemental material. All mutant strains were confirmed by Southern blotting. The cloned DNA region was confirmed by sequencing (Macrogen). Plasmids were transferred into A. tumefaciens by electroporation (26). General molecular techniques were performed according to standard protocols (27).
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Characteristic(s)a or genotype | Reference or source |
|---|---|---|
| A. tumefaciens strains | ||
| NTL4 | Wild-type strain, a Ti plasmid-cured derivative of strain C58 | 48 |
| DF156 | dmeF::pKNOCK-Km, Kmr | This study |
| DR151 | dmeR::pKNOCK-Gm, Gmr | This study |
| PN094 | rirA::pKNOCK-Km, Kmr | 20 |
| WK074 | irr::pKNOCK-Gm, Gmr | 37 |
| E. coli strains | ||
| BW20767 | Host for plasmids pKNOCK-Gm and pKNOCK-Km | 49 |
| DH5α | Host for general DNA cloning | 50 |
| Plasmids for gene inactivation | ||
| pKNOCK-Gm | Suicide vector, Gmr | 51 |
| pKNOCK-Km | Suicide vector, Kmr | 51 |
| pKNOCKmDMEF | Internal coding region of dmeF cloned into pKNOCK-Km, Kmr | This study |
| pKNOCKDMER | Internal coding region of dmeR cloned into pKNOCK-Gm, Gmr | This study |
| Plasmids for complementation | ||
| pBBR1MCS-4 | Expression vector, Apr (pBBR) | 30 |
| pDmeF | Full-length dmeF cloned into pBBR1MCS-4, Apr | This study |
| pDmeR | Full-length dmeR cloned into pBBR1MCS-4, Apr | This study |
| pRirA | Full-length rirA cloned into pBBR1MCS-4, Apr | 20 |
| Promoter-lacZ fusions | ||
| pUFR027lacZ | Promoter probe vector, Tcr | 31 |
| pDmeRF-lacZ | The dmeRF promoter fused to lacZ of pPR9TT, Apr | This study |
| Plasmid for the virulence assay | ||
| pCMA1 | pTiC58traM::nptII, Kmr | 52 |
| Plasmids for protein expression and purification | ||
| pASK-IBA7 | Protein expression vector, Apr | IBA |
| pStep-tag-DmeR | Coding region of dmeR cloned into pASK-IBA7, Apr | This study |
Apr, ampicillin resistance; Gmr, gentamicin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance.
RT-PCR.
Total RNA was extracted from log-phase cells of wild-type A. tumefaciens strain NTL4 grown in LB medium and induced with 500 μM CoCl2 for 15 min, and reverse transcription-PCR (RT-PCR) was performed using previously described protocols (20, 28). Primers BT4733 and BT4734 were used to amplify the junction of the dmeR and dmeF genes.
5′ RACE.
The transcriptional start site of dmeR was determined using RNA that was isolated from log-phase NTL4 cells grown in LB and treated with 500 μM CoCl2 for 15 min. The 5′ rapid amplification of cDNA ends (5′ RACE) (Roche) was performed according to the manufacturer's recommendations, using specific primers SP1 (BT4096) and SP2 (BT4619).
qRT-PCR analysis.
RNA isolation, cDNA preparation, and quantitative real-time PCR (qRT-PCR) were performed as previously described (20, 24). The gene-specific primers for dmeF, hmuT, shmR, fbpA, mbfA, hemA, fdx, irpA, sufS2, fssA, and 16S rRNA are listed in Table S1 in the supplemental material. The amount of a specific mRNA target was normalized to the amount of a housekeeping gene 16S rRNA. Fold changes in gene expression are relative to untreated samples from wild-type NTL4 (WT) using the 2−ΔΔCT method (29). The data were reported as the means of biological triplicates plus or minus the standard deviation (SD).
Construction of dmeF and dmeR mutant strains (DF156 and DR151).
A. tumefaciens mutant strains were constructed using an insertional gene inactivation method (20). The internal coding regions of dmeF (BT4569 and BT4570, 222 bp) and dmeR (BT4567 and BT4568, 176 bp) were PCR amplified from genomic wild-type NTL4 with the gene-specific primers. The PCR products were cloned into pKNOCK-Km and pKNOCK-Gm, generating pKNOCKmDMEF and pKNOCKDMER, respectively. The resulting plasmids were individually electroporated into the wild-type NTL4 strain. The mutant strains, DF156 and DR151, were selected on LA plus 30 μg/ml of kanamycin (Km) and LA plus 60 μg/ml of gentamicin (Gm) plates, respectively.
Cloning of functional dmeF and dmeR genes for complementation.
The PCR fragments containing full-length dmeR (BT4618 and BT4619) and dmeF (BT4620 and BT4621) genes were individually cloned into the SmaI site of pBBR1MCS-4 (30), generating the plasmids pDmeR and pDmeF, respectively.
Construction of a dmeRF promoter-lacZ transcriptional fusion and β-galactosidase activity assay.
To construct the pDmeRF-lacZ plasmid, DNA fragments (108 bp) that contained the dmeRF promoter region were amplified from A. tumefaciens NTL4 genomic DNA using PCR with the primers BT4618 and BT4735 and then cloned into the promoter probe vector pUFR027lacZ, a derivative of pUFR027 (31), as previously reported (24). The β-galactosidase (β-Gal) activity assay (32) was performed as previously described (24) using log-phase cells carrying pDmeRF-lacZ grown in LB. The results are presented as specific activity, which was calculated in units per milligram of protein.
Overexpression and purification of rDmeR.
The coding region of dmeR was amplified via PCR using the primers BT5406 and BT5407, which contain a BsaI site. The PCR products were digested with BsaI and cloned into BsaI-digested pASK-IBA7 (IBA, Germany), generating the plasmid pStrep-tag-DmeR, to produce Strep-tag II fused to the N terminus of DmeR (recombinant DmeR protein [rDmeR]).
Log-phase E. coli DH5α cells carrying the pStrep-tag-DmeR plasmid grown in LB at 37°C were induced with 200 ng/ml anhydrotetracycline for 1 h. The cells were then harvested, washed, and resuspended in buffer W (100 mM Tris-HCl pH 8.0, 250 mM NaCl). The cell suspension was sonicated, and cell debris was removed via centrifugation. The clear lysate was allowed to bind to a Strep-Tactin Sepharose column and then washed with buffer W. The rDmeR protein was subsequently eluted with elution buffer (100 mM Tris-HCl pH 8.0, 250 mM NaCl, 2.5 mM desthiobiotin). The eluted fractions were concentrated using Amicon Ultra centrifugal filters, with a molecular weight cutoff (MWCO) of 10,000 (Millipore). The rDmeR protein with the expected molecular size (11.8 kDa) was detected, and protein purity was assessed via SDS-PAGE and Coomassie blue staining (see Fig. S1 in the supplemental material).
Electrophoretic mobility shift assay.
The DNA fragment that contained the dmeRF promoter region (108 bp) was amplified via PCR using the primers BT4618 and BT4735. The DNA fragment (2 pmol) was end labeled with [γ-32P]dATP and T4 polynucleotide kinase. The 32P-labeled DNA probe was then purified using Sephadex G-50. Electrophoretic mobility shift assays were carried out in 20-μl reaction mixtures containing 1× binding buffer (20 mM Tris-HCl pH 7, 50 mM KCl, 1 mM dithiothreitol [DTT], 5% glycerol, 0.5 μg/ml calf thymus DNA, and 0.05 mg/ml bovine serum albumin [BSA]), purified rDmeR protein (0.25, 0.5, 0.75, or 1 μM), and 1 μl of diluted 32P-labeled DNA probe (1:2,000) in the absence or presence of 1 mM EDTA and 200 μM metals (CoCl2, CuSO4, FeCl3, MnCl2, NiCl2, or ZnCl2). Following incubation at room temperature for 30 min, the reaction mixtures were resolved in 8% nondenaturing polyacrylamide gels in 1× TB buffer (89 mM Tris-HCl and 89 mM boric acid) at 4°C at a constant voltage of 120 V for 1 h. After electrophoresis, the gels were dried and then subjected to autoradiography using the Typhoon FLA 7000 phosphor imaging system (GE Healthcare Life Sciences).
DNase I footprinting assay.
The 32P-labeled dmeRF promoter probe (32P-PdmeRF, 108 bp) was generated via PCR using the primers 32P-end-labeled BT4618 and nonlabeled BT4735. The 32P-PdmeRF PCR products were separated on a 1.8% agarose gel, and the 108-bp band was cut out and purified using a Qiagen PCR purification column. The DNase I footprinting experiment was carried out in a 50-μl binding reaction mixture containing 1× binding buffer (20 mM Tris-HCl pH 7, 50 mM KCl, 1 mM DTT, 5% glycerol, 0.5 μg/ml calf thymus DNA, and 0.05 mg/ml BSA), 1 mM EDTA, 32P-PdmeRF probe, and rDmeR. The binding reaction mixture was incubated at room temperature for 30 min before digestion in a total reaction mixture volume of 100 μl with 0.2 units of DNase I (Promega), 5 M CaCl2, 10 mM MgCl2 and 1 μg/ml salmon sperm at 37°C for 35 s. The DNase I digestion reaction was stopped by adding 700 μl of stop solution (650 μl of absolute ethanol, 50 μl of 3 M sodium acetate, and 1 μl of 1 mg/ml yeast tRNA), and then the reaction mixture was incubated at −20°C for 1 h. The digested DNA products were harvested via centrifugation at 10,000 rpm for 15 min. The resultant DNA pellet was washed with 70% ethanol, resuspended in Milli-Q water, and separated on an 8% polyacrylamide and 8 M urea sequencing gel in 1× TBE buffer (89 mM Tris-HCl, 89 mM boric acid, and 2 mM EDTA [pH 8]) at room temperature at a constant voltage of 1,800 V for 1.15 h. Dideoxy DNA sequencing of the 108-bp dmeRF promoter was carried out using the primer BT4618 and was run alongside the DNase I footprint.
Inductively coupled plasma mass spectrometry.
Cells were grown in LB that was individually supplemented with 100 μM CoCl2, CuSO4, FeCl3, MnCl2, NiCl2, or ZnCl2 at 28°C for 24 h. Samples were prepared, and the metals were measured in parts per billion (ppb) as previously described (33).
Virulence assay.
A. tumefaciens strains carrying the plasmid pCMA1 were used to infect young Nicotiana benthamiana plants according to a previously described protocol (28, 34). Tumor formation was observed 3 weeks after inoculation.
RESULTS
The A. tumefaciens dmeRF operon is inducible by cobalt and nickel.
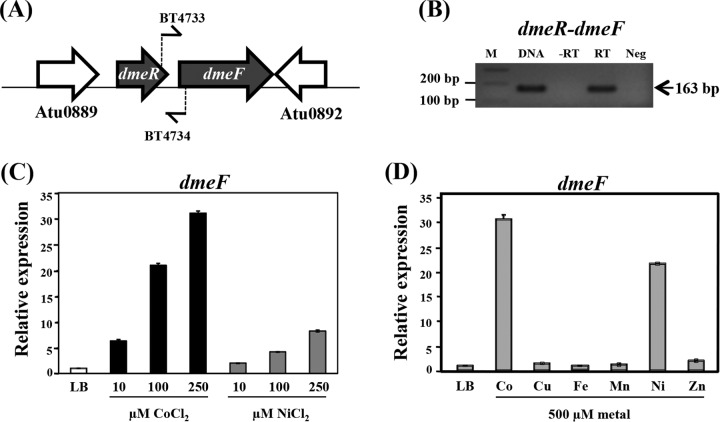
The arrangement of the dmeR (Atu0890) and dmeF (Atu0891) genes is shown in Fig. 1A. A. tumefaciens dmeRF is flanked by the Atu0889 and Atu0892 genes encoding proteins with unknown functions. The dmeR and dmeF genes are cotranscribed as confirmed through RT-PCR analysis (Fig. 1B). The expression of the A. tumefaciens dmeF (dmeFAt) gene was increased by cobalt and nickel treatment (10, 100, and 250 μM) in a dose-dependent manner (Fig. 1C), with cobalt serving as a better inducer. The expression of dmeFAt in response to various metals was determined using qRT-PCR (Fig. 1D). At the high concentration of 500 μM, CoCl2 and NiCl2 caused induction of dmeFAt expression by ∼30-fold and ∼20-fold, respectively, while other metals, including CuSO4, FeCl3, MnCl2, and ZnCl2, caused a less than 2-fold induction.
FIG 1.
(A) Gene organization of A. tumefaciens dmeRF. The dmeR (Atu0890) gene is located upstream of the dmeF (Atu0891) gene and is separated by 78 bp. The Atu0889 and Atu0892 genes encode proteins of unknown function. The locations of the primers BT4733 and BT4734 used for RT-PCR analysis are indicated. (B) RT-PCR analysis. RNA was isolated from log-phase cells of the wild-type NTL4 strain grown in LB medium and induced with 500 μM CoCl2 for 15 min and was then treated with DNase I, which was followed by treatment with reverse transcriptase (RT) or no treatment (−RT, a DNA-contamination control). Chromosomal DNA from NTL4 cells was amplified using the primers BT4733 and BT4734 and was used as a positive control (DNA). Neg indicates a reaction mixture without a template that is used as a negative control. The expected size of the PCR product is indicated. M indicates a 100-bp ladder marker. (C and D) Induction of dmeF by various metals examined through quantitative real-time PCR (qRT-PCR) analysis. Log-phase NTL4 cells grown in LB were either untreated or treated with 500 μM CoCl2, CuSO4, FeCl3, MnCl2, NiCl2, or ZnCl2 for 15 min. The fold changes in dmeF expression are expressed relative to the untreated control (LB, regarded as 1). The experiment was performed in biological triplicate, and the error bars indicate the standard deviations.
The dmeRF promoter is negatively regulated by DmeR.
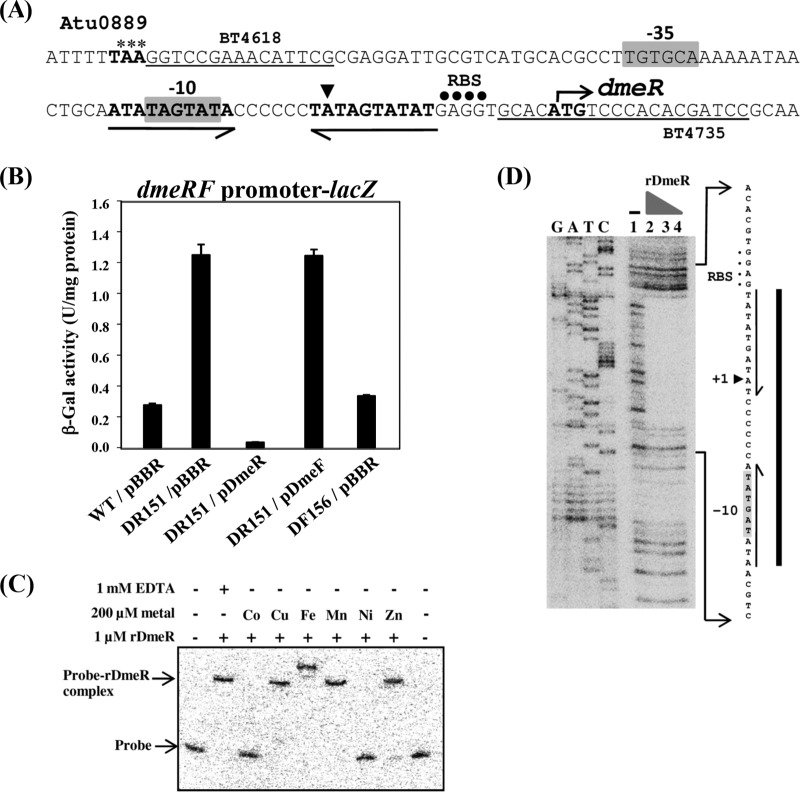
The A. tumefaciens dmeR (dmeRAt) gene encodes a protein that belongs to the RcnR/CsoR transcriptional regulator family (35). The upstream region of the dmeRAt gene contains a predicted regulatory binding site for the RcnR/CsoR family that consists of a C-tract flanked by an AT-rich inverted repeat (5′-ATATAGTATACCCCCCTATAGTATAT-3′) (36), suggesting that the dmeRFAt operon may be regulated by the DmeRAt protein. The features of the dmeRFAt promoter are shown in Fig. 2A. The transcriptional start site of dmeRFAt at the adenine (A) residue was determined via 5′ RACE (Fig. 2A). The potential −35 and −10 sequences (TGTGCA and TAGTAT, respectively) were found with respect to the transcriptional start site.
FIG 2.
(A) The A. tumefaciens dmeRF promoter. The ATG start codon for dmeR is shown in bold with the bent arrow. The putative ribosome-binding site (RBS) is marked with black dots. The potential −35 and −10 sequences are indicated in the gray boxes. The transcriptional start site of dmeR at the adenine (A) residue, determined using 5′ RACE, is indicated with a black triangle. The AT-rich inverted repeat (arrows), separated by 6 bp of C-tract, is a predicted regulatory binding site for the RcnR/CsoR family. The TAA stop codon for Atu0889 is marked with asterisks. The locations of the primers BT4618 and BT4735 used for construction of the pDmeRF-lacZ plasmid (dmeRF promoter-lacZ transcriptional fusion) are underlined. (B) β-Gal activity assay. β-Gal activities were determined from log-phase cells that carry the pDmeRF-lacZ plasmid grown in LB medium. The cells were wild-type NTL4 (WT) and the mutant strain DR151 (dmeR mutation), containing a plasmid vector (pBBR) or functional dmeR and dmeF genes from the multicopy plasmids pDmeR and pDmeF, respectively. The error bars indicate the standard deviations. (C) Electrophoretic mobility shift assay. Effect of Co(II) and Ni(II) on the interaction of rDmeR and the dmeRF promoter. The DNA fragment that contains the dmeRF promoter region (108 bp) amplified by PCR with the primers BT4618 and BT4735 was end labeled with 32P. The 32P-labeled dmeRF promoter (Probe) was incubated with purified rDmeR protein in the absence (−) and presence (+) of 1 mM EDTA or a 200 μM concentration of various metals (CoCl2, CuSO4, FeCl3, MnCl2, NiCl2, or ZnCl2). The free probe and the probe-rDmeR complex are indicated with arrows. (D) DNase I footprint of rDmeR bound to the 32P-end-labeled 108-bp dmeRF promoter. Lanes GATC, dideoxy DNA sequencing of the 108-bp dmeRF promoter using primer 32P-end-labeled BT4618; lane 1, no rDmeR added; lanes 2 to 4, rDmeR added (10, 5, and 1 μM, respectively). The region of the promoter protected by rDmeR from DNase I digestion is marked with a thick line. The predicted −35 and −10 sequences, RBS, and the AT-rich inverted repeat are indicated.
The DR151 (dmeR mutation) and DF156 (dmeF mutation) strains were generated to determine the functions of the transcriptional regulator DmeRAt and the CDF-type DmeFAt transporter. To test whether DmeRAt regulates the expression of dmeRFAt, the pDmeRF-lacZ (dmeRF promoter-lacZ transcriptional fusion) plasmid was constructed and transferred into the wild-type NTL4 (WT), mutant, and complemented strains, and β-galactosidase (β-Gal) activity was measured. The β-Gal activity obtained from the mutant strain DR151 (DR151/pBBR, ∼1.25 U/mg protein) was higher than that from untreated WT cells (WT/pBBR, ∼0.27 U/mg protein) (Fig. 2B) and WT cells treated with 500 μM CoCl2 for 30 min (∼0.59 U/mg protein). The high levels of β-Gal activity observed in DR151 could be suppressed by expressing the functional dmeR gene from the multicopy plasmid pDmeR (DR151/pDmeR, ∼0.03 U/mg protein) but not pDmeF (DR151/pDmeF, ∼1.24 U/mg protein) (Fig. 2B). In contrast to DR151, the level of β-Gal activity produced from DF156 (DF156/pBBR, ∼0.33 U/mg protein) was similar to that from WT. These results demonstrated that DmeRAt is the repressor of the dmeRFAt operon.
DmeR binds to the dmeRF promoter, and the interaction is specifically inhibited by cobalt and nickel.
The electrophoretic mobility shift assay demonstrated that the purified recombinant DmeR protein (rDmeR, Strep-tagged DmeR) bound to the 32P-end-labeled 108-bp dmeRF promoter probe (Fig. 2C; see also Fig. S1B in the supplemental material). A single shifted band (rDmeR-probe complex) was observed with increasing rDmeR concentrations when 1 mM EDTA was present in the reactions (see Fig. S1B). When CoCl2 or NiCl2 (1, 10, 100, 250, 500, or 1,000 μM) was added instead of EDTA, the shifted band was not observed starting at a 100 μM concentration of the metal (see Fig. S1C). At a 200 μM concentration of various metals, the retarded band disappeared in the reaction mixtures containing CoCl2 and NiCl2 but was detected in the reaction mixtures containing CuSO4, FeCl3, MnCl2, and ZnCl2 (Fig. 2C). Among the retarded bands, the band from the reaction mixture containing FeCl3 moved slightly more slowly, which may reflect differences in the conformation of the rDmeR-probe complexes. The results from the electrophoretic mobility shift assay supported the view that DmeR represses the dmeRF operon in the absence of cobalt and nickel by directly binding to its promoter region. A DNase I footprinting assay was performed to determine the rDmeR binding site in the dmeRF promoter. The rDmeR protein protected an approximately 26-bp region (5′-ATATAGTATACCCCCCTATAGTATAT-3′) of the dmeRF promoter, spanning the predicted −10 sequence and the AT-rich inverted repeat (Fig. 2D).
The dmeF mutant strain is hypersensitive to cobalt and nickel and exhibits an increased cellular accumulation of these metals.
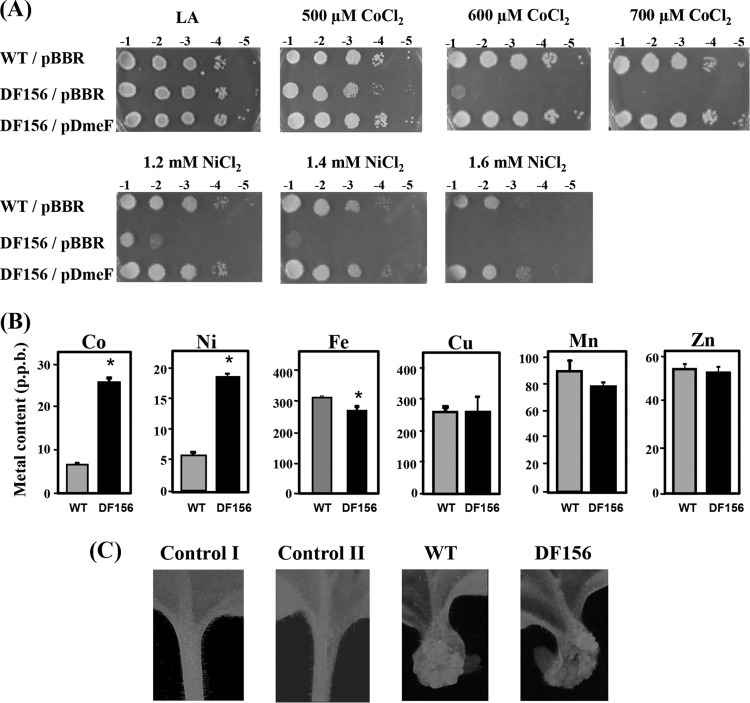
The sensitivities of the WT and DF156 (dmeF mutation) strains to various metals were determined. The DF156 strain was more sensitive to 600 μM CoCl2 (103-fold) and 1.2 mM NiCl2 (103-fold) than the WT strain (Fig. 3A). However, the levels of sensitivity to other metals, including CuSO4, FeCl3, MnCl2, and ZnCl2, were similar in the WT and DF156 strains (data not shown). The hypersensitive phenotype of DF156 can be reversed by complementation with functional dmeF from the multicopy plasmid pDmeF (Fig. 3A). Inductively coupled plasma mass spectrometry analysis was performed to determine the accumulation of metals in WT and DF156. The results showed that DF156 exhibited apparently greater increases in intracellular cobalt (4-fold) and nickel (3-fold) contents than WT (Fig. 3B). The iron content in DF156 was slightly lower than that in WT, whereas the levels of copper, manganese, and zinc were similar in DF156 and WT (Fig. 3B). These results demonstrated that DmeF plays an important role in the detoxification of cobalt and nickel in A. tumefaciens and supported the view that DmeFAt acts as an exporter of cobalt and nickel.
FIG 3.
(A) Sensitivity to cobalt and nickel. Log-phase cells grown in LB were adjusted, serially diluted, and spotted onto LA plates containing CoCl2 (500, 600, or 700 μM) and NiCl2 (1.2, 1.4, or 1.6 mM). WT and DF156 (dmeF mutation) strains contained a plasmid vector (pBBR) or a functional dmeF from the multicopy plasmid pDmeF. Tenfold serial dilutions are indicated. (B) Measurement of intracellular metal contents using inductively coupled plasma mass spectrometry. WT and DF156 (dmeF mutation) cells were grown in LB that was individually supplemented with 100 μM CoCl2, NiCl2, FeCl3,CuSO4, MnCl2, or ZnCl2. Bars marked with an asterisk are significantly different from WT (P < 0.05 in an unpaired Student's t test). The error bars indicate the standard deviations. (C) Virulence assay. The images show tumor formation in N. benthamiana plants that were infected with the WT and DF156 (dmeF mutation) strains carrying the pCMA1 plasmid. Log-phase cells of A. tumefaciens grown in LB and then resuspended in IB 5.5 medium containing 300 μM acetosyringone (AS) were used to inoculate wounded N. benthamiana petioles. Control I, without infection; control II, inoculation of wounded petioles with IB 5.5 plus 300 μM AS. Fifteen petioles were inoculated for each bacterial strain, and representative petioles are shown.
It was found that insertional inactivation of dmeRAt (DR151, dmeR::pKNOCK-Gm) also disrupted the downstream gene dmeFAt. This was supported by the fact that the DR151 strain showed hypersensitivity to cobalt and nickel similar to that shown by DF156 (see Fig. S2 in the supplemental material). Furthermore, the hypersensitive phenotype of DR151 could be completely reversed by pDmeF but not by pDmeR (see Fig. S2).
Disruption of dmeF does not affect the virulence of A. tumefaciens.
The effect of inactivation of dmeF on A. tumefaciens virulence was tested. N. benthamiana plants were infected with log-phase WT and DF156 cells grown in LB medium. Tumor formation results were similar in plants that were infected with WT and DF156 (Fig. 3C), suggesting that the loss of dmeF does not affect the ability of A. tumefaciens to cause disease in the host plant N. benthamiana under the tested conditions.
Cobalt stress causes increased expression of iron-responsive genes.
A. tumefaciens iron homeostasis is regulated by RirA and Irr (20, 21). The promoter regions of hmuT (Atu2460, hemin ABC transporter substrate-binding protein), shmR (Atu2287, hemin receptor), and fbpA (Atu0407, ferric cation ABC transporter substrate-binding protein) contain the iron-responsive operator (IRO) motifs (RirA binding site); therefore, these genes were predicted to be regulated by A. tumefaciens RirA (RirAAt) (22). In contrast, mbfA (Atu0251, iron exporter), hemA (Atu2613, heme biosynthesis), fdx (Atu1350, ferredoxin), and irpA (Atu0288, iron-regulated protein A) were predicted to be regulated by IrrAt due to the presence of the iron control element (ICE) motifs (Irr binding site) in their promoters, while the sufS2 (Atu1825, Fe-S cluster biosynthesis) and fssA (Atu0351, Fe-S scaffold protein) promoters contain both IRO and ICE motifs (22).
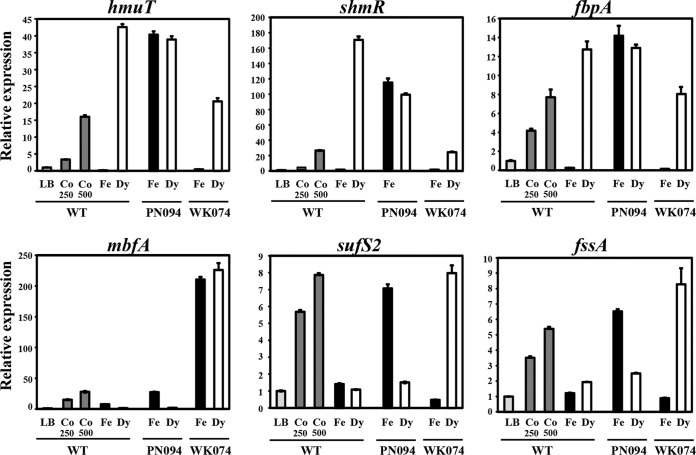
Cobalt toxicity in E. coli and S. enterica has been shown to disturb iron homeostasis (4–6). To investigate whether iron homeostasis in A. tumefaciens may be perturbed by stress from other metals, WT cells were exposed to a 500 μM concentration of various metals (CoCl2, CuSO4, FeCl3, MnCl2, NiCl2, or ZnCl2) for 15 min, and expression of the iron-responsive genes was determined using qRT-PCR. It was found that Co, but not Cu, Mn, Ni, and Zn, induced the expression of iron-responsive genes, including hmuT (14-fold), shmR (30-fold), fbpA (5-fold), mbfA (35-fold), sufS2 (7-fold), and fssA (4-fold) (see Fig. S3 in the supplemental material). These genes were inducible in a concentration-dependent manner, where 500 μM CoCl2 caused greater induction than 250 μM CoCl2 (Fig. 4). In contrast, the expression of the hemA, fdx, and irpA genes was not strikingly inducible by Co (Fig. S3). These results demonstrated that cobalt stress perturbs iron homeostasis in A. tumefaciens.
FIG 4.
Effect of cobalt stress on the expression of iron-responsive genes determined using qRT-PCR. Log-phase cells of the NTL4 (WT), PN094 (rirA mutation), and WK074 (irr mutation) strains grown in LB were either untreated or treated with CoCl2 (250 or 500 μM), FeCl3 (50 μM), or Dy (200 μM) for 15 min. The fold changes in gene expression (hmuT, shmR, fbpA, mbfA, sufS2, and fssA) are expressed relative to the untreated WT sample (LB, regarded as 1). The experiment was performed in biological triplicate, and the error bars indicate the standard deviations.
In WT, the level of induction of the iron-repressed genes hmuT (42-fold), shmR (170-fold), and fbpA (13-fold) by an iron chelator (200 μM 2,2′-dipyridyl [Dy]) was higher than Co induction (Fig. 4). The iron-dependent repression of these genes was lost in the PN094 (rirA mutation) strain, with similar expression levels observed under high-Fe (50 μM FeCl3) and low-Fe (200 μM Dy) conditions (Fig. 4) in accordance with the view that RirAAt is the repressor of these genes under high-Fe conditions. In contrast, the Dy-induced levels of hmuT (20-fold), shmR (24-fold), and fbpA (8-fold) in the WK074 (irr mutation) strain were decreased relative to those from the WT strain treated with Dy, suggesting that A. tumefaciens Irr (IrrAt) is an activator of these genes under low-Fe conditions. The level of shmR activation by 200 μM Dy was also decreased in PN094 (rirA mutation), suggesting the existence of unidentified regulation in addition to IrrAt under low-Fe conditions (Fig. 4).
A. tumefaciens mbfA is negatively regulated by Irr and is inducible in response to high Fe (37). In WT, the expression of mbfA was also inducible by 500 μM CoCl2 (30-fold) to an even greater extent than induction by 50 μM FeCl3 (7-fold) (Fig. 4). The level of mbfA expression in PN094 (rirA mutation) grown in high-Fe conditions (27-fold) was similar to that in the WT strain treated with 500 μM CoCl2. In contrast, WK074 (irr mutation) showed higher constitutive expression of mbfA (210-fold) under high- and low-Fe conditions (Fig. 4).
In WT, the expression of sufS2 and fssA was not markedly responsive to either high-Fe (50 μM FeCl3) or low-Fe (200 μM Dy) conditions, with the observed expression levels lower than those under Co-treated conditions (Fig. 4). The expression of sufS2 and fssA was derepressed in PN094 (rirA mutation) grown in high Fe and in WK074 (irr mutation) grown in low Fe (Fig. 4), which is consistent with the notion that RirAAt and IrrAt are repressors of sufS2 and fssA under high-Fe and low-Fe conditions, respectively.
Cobalt impairs the activation of the SoxR-regulated gene sodBII.
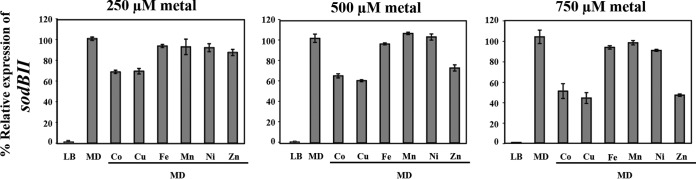
SoxR is a superoxide-sensing transcriptional regulator that requires 2Fe-2S for its activation function (23). A. tumefaciens SoxR (SoxRAt) activates sodBII expression as a defense response to detoxify superoxide anions (38). Cobalt inhibits the activity of Fe-S proteins as shown in E. coli (5) and S. enterica (6). It is possible that Fe-S clusters may be damaged during cobalt stress in A. tumefaciens, as many iron-responsive genes under the control of the Fe-S protein RirA were derepressed under high-cobalt conditions (Fig. 4). To test whether cobalt stress may also affect Fe-S-dependent SoxRAt activity, the induction of sodBII by the superoxide generator menadione (MD; 500 μM) in the absence and presence of CoCl2, CuSO4, FeCl3, MnCl2, NiCl2, or ZnCl2 was measured via qRT-PCR using RNA isolated from the WT cells (Fig. 5). sodBII expression was inducible by ∼1.4 × 103-fold by MD but not by cobalt treatment (250, 500, or 750 μM CoCl2; data not shown). The MD activation of sodBII was reduced by ∼30%, 35%, and 50% in the presence of 250, 500, and 750 μM CoCl2, respectively (Fig. 5). These results demonstrated that Co has a negative effect on the induction of sodBII in response to MD exposure. In addition, Cu and Zn could also inhibit the MD activation of sodBII, while Fe, Mn, and Ni caused a slight effect (Fig. 5).
FIG 5.
qRT-PCR analysis of sodBII induction by menadione in the presence of various metals. Log-phase NTL4 cells grown in LB were either uninduced or induced with 500 μM menadione (MD) in the absence or presence of 250, 500, or 750 μM concentrations of various metals (CoCl2, NiCl2, FeCl3,CuSO4, MnCl2, or ZnCl2) for 15 min. The expression levels are presented as a percentage, relative to those in cells grown in LB induced with 500 μM MD (100%).
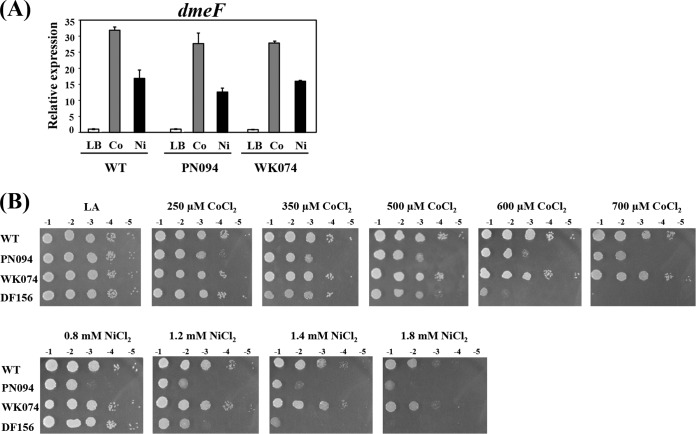
RirA plays a role in coping with cobalt and nickel toxicity.
Iron can protect E. coli from cobalt toxicity (4). An E. coli fur mutant strain exhibits the derepression of iron uptake genes and increases in intracellular iron, leading to increased resistance to cobalt (4). Mutations in A. tumefaciens rirA and irr (rirAAt and irrAt) cause higher and lower levels, respectively, of nonprotein-bound iron (21). Inactivation of either of the iron-sensing regulators rirAAt and irrAt (PN094 and WK074 strains, respectively) has no effect on the metal induction of dmeF (Fig. 6A). However, the loss of RirAAt, but not IrrAt, decreased the ability of A. tumefaciens to cope with cobalt and nickel toxicity (Fig. 6B). The PN094 (rirA mutation) strain was more sensitive to cobalt and nickel than the WT strain (Fig. 6B). These results revealed a link between iron and cobalt/nickel homeostasis in A. tumefaciens via the iron-sensing transcriptional regulator RirA. At lower concentrations of cobalt (250 and 350 μM CoCl2) and nickel (0.8 mM NiCl2), PN094 (rirA mutation) was apparently more sensitive to the metals than DF156 (dmeF mutation) (Fig. 6B). In contrast, at higher concentrations of cobalt (600 and 700 μM CoCl2) and nickel (1.4 and 1.8 mM NiCl2), DF156 was more sensitive to the metals than PN094 (Fig. 6B).
FIG 6.
(A) qRT-PCR analysis of dmeF induction in NTL4 (WT), PN094 (rirA mutation), and WK074 (irr mutation). Log-phase cells grown in LB were either uninduced or induced with 500 μM CoCl2 or NiCl2 for 15 min. The fold changes in dmeF expression are expressed relative to the untreated WT sample (LB, regarded as 1). The experiment was performed in biological triplicate, and the error bars indicate the standard deviations. (B) Sensitivity to cobalt and nickel. Log-phase cells of NTL4 (WT), PN094 (rirA mutation), WK074 (irr mutation), and DF156 (dmeF mutation) grown in LB were adjusted, serially diluted, and spotted onto LA plates containing CoCl2 (250, 350,500, 600, or 700 μM) or NiCl2 (0.8, 1.2, 1.4, or 1.8 mM). Tenfold serial dilutions are indicated.
DISCUSSION
The DmeF protein is a member of the metal exporter CDF family, and very little is known about its function and gene regulation. Thus far, only two DmeF proteins have been studied, from Cupriavidus metallidurans CH34 (DmeFCm) (18) and Rhizobium leguminosarum (DmeFRl) (19). DmeF is classified into the subgroup I Zn-CDFs (39). However, the DmeF proteins have been shown to exhibit broad metal specificity, with the ability to extrude Co(II) and Ni(II) (18, 19). Like DmeFCm and DmeFRl, Agrobacterium tumefaciens DmeF (DmeFAt) exhibits six predicted transmembrane (TM) domains that contain two motifs, which are characteristic of the CDF family (the HX3H motif at TM2 and the HX3D motif at TM5) and are a unique characteristic of the subgroup I CDFs, in the histidine-rich stretch located between TM4 and TM5 (see Fig. S4 in the supplemental material). DmeFAt shares 39% and 65% amino acid identity with DmeFCm and DmeFRl, respectively. Similar to DmeFCm and DmeFRl, DmeFAt was shown to be involved in the detoxification of cobalt and nickel in the present study.
The regulation of dmeF gene expression differs in C. metallidurans CH34 and R. leguminosarum. The dmeFCm gene is constitutively expressed and is not inducible by metals (18), whereas dmeFRl is specifically inducible by cobalt and nickel, with cobalt acting as a more potent inducer (19). dmeFRl is cotranscribed with an upstream gene, dmeR. The transcription of the dmeRFRl operon is negatively controlled by dmeR, which encodes a metal-responsive transcriptional regulatory protein that belongs to the RcnR/CsoR family (19). RcnR senses Co(II) and Ni(II) in the metal-responsive repression of rcnA and encodes a cobalt and nickel efflux protein (10, 40), while CsoR is the Cu(I)-responsive repressor of the copper efflux gene copA (41–43). The predicted DNA operator sites recognized by RcnR/CsoR proteins contain a G or C tract of three to eight bases flanked by AT-rich inverted repeats (36).
The arrangement of the dmeR and dmeF genes and the potential DmeR-binding boxes is conserved in members of Rhizobiaceae, including R. leguminosarum, Sinorhizobium meliloti, and A. tumefaciens (19). Although negative regulation of the dmeRF operon by DmeR has been observed in R. leguminosarum, the molecular mechanism of the regulation has not been previously demonstrated. In the present study, we provide the first evidence of the molecular mechanism by which DmeRAt regulates dmeRF expression. The use of a promoter-lacZ transcriptional fusion, electrophoretic mobility shift assay, and a DNase I footprinting assay revealed that DmeRAt exerts repression of dmeRF transcription through direct binding to the promoter region upstream of dmeR. The apo-DmeRAt form recognizes the 5′-ATATAGTATACCCCCCTATAGTATAT-3′ DNA sequence, while E. coli RcnR (RcnREc) in the apo form binds to 5′-TACTGGGGGGAGTA-3′ (36). The inverted AT repeats are proposed to form sequence-specific recognition sites for the regulators, while the G or C tracts may be important for providing a unique DNA structural feature to facilitate protein-DNA interaction for the RcnR/CsoR family (36). The location of the DmeRAt binding site overlaps that of the predicted −10 sequence of the RNA polymerase recognition site, supporting the view that DmeRAt is a transcriptional repressor. The mechanism of metal-responsive repression of DmeRAt is similar to that for RcnREc in that the regulatory switch occurs upon binding to Co(II) and Ni(II). The binding of these metals may cause conformational changes in DmeRAt, resulting in dissociation from DNA and thus triggering the transcription of the dmeRF operon. DmeRAt shares 43% amino acid identity with RcnREc and exhibits the putative metal-binding residues His3-Cys35-His60-His64, which are the same as those of RcnREc used for Co(II)/Ni(II) coordination (10) (see Fig. S5 in the supplemental material). The metal-binding residues of the Co(II)/Ni(II)-sensing regulators RcnREc and DmeRAt are different from those of CsoR (Cys36-His61-Cys65), a protein from the same family that responds specifically to Cu(I) (41). DmeRAt represses its own transcription, which provides feedback regulation to help rapidly respond to fluctuations in cobalt and nickel concentrations in the environment. In contrast to the dmeRF system, the E. coli rcnR gene is transcribed in the opposite direction relative to rcnA. The expression of the rcnR and rcnA genes is negatively regulated by RcnR (10); moreover, rcnR expression is inducible by Fur in response to iron (44). However, iron has no effect on the induction of the dmeRFAt operon. In some members of the Alphaproteobacteria, including A. tumefaciens, the RirA protein has evolved to carry out the typical Fur function in the regulation of iron-responsive genes (22). Unlike that observed regarding the connection of E. coli Fur and the rcn system, A. tumefaciens RirA (RirAAt) plays no role in the induction of the dmeRF operon.
Cobalt toxicity is caused by direct competition with iron, resulting in impaired Fe-S biogenesis, a defect in Fe-S enzymes, and deregulation of iron homeostasis (4–7). Another aim of this study was to investigate the effect of cobalt stress on the activity of Fe-S proteins (RirA and SoxR) and the expression of iron-responsive genes in A. tumefaciens. RirA and Irr sense iron in the form of Fe-S and heme, respectively, to control iron homeostasis in Alphaproteobacteria (22). Similar to observations made in E. coli (5) and S. enterica (6), cobalt caused increased expression of many iron-responsive genes in A. tumefaciens. Derepression of hmuT, shmR, and fbpA may result from direct damage to the Fe-S regulator RirAAt and/or disruption of the Fe-S supply by cobalt. Impairment of the function of another Fe-S regulator, A. tumefaciens SoxR (SoxRAt), in activating sodBII transcription was also observed at high concentrations of cobalt. In addition to cobalt, copper and zinc were also shown to reduce the activity of SoxRAt. The effects of copper and zinc on SoxRAt are not surprising because these metals have also been reported to damage Fe-S clusters (45, 46). In contrast, copper and zinc had no effect on the expression of hmuT, shmR, and fbpA. One possible explanation is that there are differences in the sensitivities of Fe-S clusters in RirAAt and SoxRAt to be damaged by different metals. Cobalt also caused derepression of the iron exporter gene mbfA, which is under the negative control of A. tumefaciens Irr (IrrAt). Unlike RirAt, IrrAt is not an Fe-S protein. Whether cobalt directly or indirectly damages IrrAt remains unknown. Iron has been shown to induce the oxidation and degradation of Bradyrhizobium japonicum Irr (47). Determination of whether the stability of IrrAt is affected by iron- and cobalt-induced oxidative damage awaits further study. Derepression of sufS2 and fssA under high cobalt conditions may be a consequence of the inactivation of RirAAt and/or IrrAt, as these genes are negatively regulated by both RirAAt and IrrAt.
The upregulation of gene products involved in iron uptake in an E. coli fur mutant strain was found to lead to an increased intracellular iron content, which in turn protects E. coli from cobalt toxicity (4). An A. tumefaciens rirA mutant strain was shown to exhibit derepression of iron uptake genes and higher levels of intracellular iron (21); however, in the present study, this strain was shown to be hypersensitive to cobalt and nickel. These observations reflect the different mechanisms used by E. coli and A. tumefaciens to respond to the toxic effects of metals. Interestingly, the iron regulator RirAAt played a more dominant role in bacterial survival at lower concentrations of cobalt/nickel than the metal efflux protein DmeFAt, whereas DmeFAt became more important than RirAAt in coping with the metals at higher concentrations. These results suggested the existence of differential modes of cellular responses to the toxic effects of cobalt/nickel at low and high concentrations in A. tumefaciens. Furthermore, the control of the homeostasis of a particular metal may also affect the ability of bacteria to resist other metals, although the exact mechanism through which the iron-responsive regulator RirAAt mediates cobalt/nickel resistance is still not known.
One of the common strategies for bacteria to avoid metal toxicity is to pump excess metal ions out of the cell. It was demonstrated in C. metallidurans CH34 that DmeF interacts with the CnrCBA and CzcCBA RND systems (18). Cobalt must be first transferred from the cytoplasm to the periplasm via the CDF protein DmeF, and cobalt can subsequently be transported from the periplasm to the outside of the cell by the RND systems (18). The identification and characterization of the RND system(s) working in cooperation with DmeFAt for metal efflux will be of interest in future studies aimed at obtaining a better understanding of metal transport systems and metal tolerance in A. tumefaciens.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. K. Farrand for the plasmid pCMA1. We also thank P. Srifah Huehne and K. Bhinija for technical assistance with the virulence assay.
This work was supported by the Chulabhorn Research Institute and the Thailand Research Fund grant RSA5880010.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01262-16.
REFERENCES
- 1.Kobayashi M, Shimizu S. 1999. Cobalt proteins. Eur J Biochem 261:1–9. doi: 10.1046/j.1432-1327.1999.00186.x. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee R, Ragsdale SW. 2003. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem 72:209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- 3.Leonard S, Gannett PM, Rojanasakul Y, Schwegler-Berry D, Castranova V, Vallyathan V, Shi X. 1998. Cobalt-mediated generation of reactive oxygen species and its possible mechanism. J Inorg Biochem 70:239–244. doi: 10.1016/S0162-0134(98)10022-3. [DOI] [PubMed] [Google Scholar]
- 4.Fantino JR, Py B, Fontecave M, Barras F. 2010. A genetic analysis of the response of Escherichia coli to cobalt stress. Environ Microbiol 12:2846–2857. [DOI] [PubMed] [Google Scholar]
- 5.Ranquet C, Ollagnier-de-Choudens S, Loiseau L, Barras F, Fontecave M. 2007. Cobalt stress in Escherichia coli. The effect on the iron-sulfur proteins. J Biol Chem 282:30442–30451. [DOI] [PubMed] [Google Scholar]
- 6.Thorgersen MP, Downs DM. 2007. Cobalt targets multiple metabolic processes in Salmonella enterica. J Bacteriol 189:7774–7781. doi: 10.1128/JB.00962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majtan T, Frerman FE, Kraus JP. 2011. Effect of cobalt on Escherichia coli metabolism and metalloporphyrin formation. Biometals 24:335–347. doi: 10.1007/s10534-010-9400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto S, Eltis LD. 2011. The biological occurrence and trafficking of cobalt. Metallomics 3:963–970. doi: 10.1039/c1mt00056j. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigue A, Effantin G, Mandrand-Berthelot MA. 2005. Identification of rcnA (yohM), a nickel and cobalt resistance gene in Escherichia coli. J Bacteriol 187:2912–2916. doi: 10.1128/JB.187.8.2912-2916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwig JS, Leitch S, Herbst RW, Maroney MJ, Chivers PT. 2008. Ni(II) and Co(II) sensing by Escherichia coli RcnR. J Am Chem Soc 130:7592–7606. doi: 10.1021/ja710067d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutherford JC, Cavet JS, Robinson NJ. 1999. Cobalt-dependent transcriptional switching by a dual-effector MerR-like protein regulates a cobalt-exporting variant CPx-type ATPase. J Biol Chem 274:25827–25832. doi: 10.1074/jbc.274.36.25827. [DOI] [PubMed] [Google Scholar]
- 12.Raimunda D, Long JE, Sassetti CM, Argüello JM. 2012. Role in metal homeostasis of CtpD, a Co2+ transporting P1B4-ATPase of Mycobacterium smegmatis. Mol Microbiol 84:1139–1149. doi: 10.1111/j.1365-2958.2012.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raimunda D, Long JE, Padilla-Benavides T, Sassetti CM, Argüello JM. 2014. Differential roles for the Co2+/Ni2+ transporting ATPases, CtpD and CtpJ, in Mycobacterium tuberculosis virulence. Mol Microbiol 91:185–197. doi: 10.1111/mmi.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liesegang H, Lemke K, Siddiqui RA, Schlegel HG. 1993. Characterization of the inducible nickel and cobalt resistance determinant cnr from pMOL28 of Alcaligenes eutrophus CH34. J Bacteriol 175:767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nies DH. 1995. The cobalt, zinc, and cadmium efflux system CzcABC from Alcaligenes eutrophus functions as a cation-proton antiporter in Escherichia coli. J Bacteriol 177:2707–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anton A, Grosse C, Reissmann J, Pribyl T, Nies DH. 1999. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J Bacteriol 181:6876–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherer J, Nies DH. 2009. CzcP is a novel efflux system contributing to transition metal resistance in Cupriavidus metallidurans CH34. Mol Microbiol 73:601–621. doi: 10.1111/j.1365-2958.2009.06792.x. [DOI] [PubMed] [Google Scholar]
- 18.Munkelt D, Grass G, Nies DH. 2004. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J Bacteriol 186:8036–8043. doi: 10.1128/JB.186.23.8036-8043.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubio-Sanz L, Prieto RI, Imperial J, Palacios JM, Brito B. 2013. Functional and expression analysis of the metal-inducible dmeRF system from Rhizobium leguminosarum bv. viciae. Appl Environ Microbiol 79:6414–6422. doi: 10.1128/AEM.01954-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngok-Ngam P, Ruangkiattikul N, Mahavihakanont A, Virgem SS, Sukchawalit R, Mongkolsuk S. 2009. Roles of Agrobacterium tumefaciens RirA in iron regulation, oxidative stress response, and virulence. J Bacteriol 191:2083–2090. doi: 10.1128/JB.01380-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hibbing ME, Fuqua C. 2011. Antiparallel and interlinked control of cellular iron levels by the Irr and RirA regulators of Agrobacterium tumefaciens. J Bacteriol 193:3461–3472. doi: 10.1128/JB.00317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodionov DA, Gelfand MS, Todd JD, Curson AR, Johnston AW. 2006. Computational reconstruction of iron- and manganese-responsive transcriptional networks in Alphaproteobacteria. PLoS Comput Biol 2:e163. doi: 10.1371/journal.pcbi.0020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crack JC, Green J, Thomson AJ, Le Brun NE. 2012. Iron-sulfur cluster sensor-regulators. Curr Opin Chem Biol 16:35–44. doi: 10.1016/j.cbpa.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Bhubhanil S, Niamyim P, Sukchawalit R, Mongkolsuk S. 2014. Cysteine desulphurase-encoding gene sufS2 is required for the repressor function of RirA and oxidative resistance in Agrobacterium tumefaciens. Microbiology 160:79–90. doi: 10.1099/mic.0.068643-0. [DOI] [PubMed] [Google Scholar]
- 25.Wood DW, Setubal JC, Kaul R, Monks DE, Kitajima JP, Okura VK, Zhou Y, Chen L, Wood GE, Almeida NF Jr, Woo L, Chen Y, Paulsen IT, Eisen JA, Karp PD, Bovee D Sr, Chapman P, Clendenning J, Deatherage G, Gillet W, Grant C, Kutyavin T, Levy R, Li MJ, McClelland E, Palmieri A, Raymond C, Rouse G, Saenphimmachak C, Wu Z, Romero P, Gordon D, Zhang S, Yoo H, Tao Y, Biddle P, Jung M, Krespan W, Perry M, Gordon-Kamm B, Liao L, Kim S, Hendrick C, Zhao ZY, Dolan M, Chumley F, Tingey SV, Tomb JF, Gordon MP, Olson MV, Nester EW. 2001. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294:2317–2323. doi: 10.1126/science.1066804. [DOI] [PubMed] [Google Scholar]
- 26.Cangelosi GA, Best EA, Martinetti G, Nester EW. 1991. Genetic analysis of Agrobacterium. Methods Enzymol 204:384–397. doi: 10.1016/0076-6879(91)04020-O. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 28.Bhubhanil S, Sittipo P, Chaoprasid P, Nookabkaew S, Sukchawalit R, Mongkolsuk S. 2014. Control of zinc homeostasis in Agrobacterium tumefaciens via zur and the zinc uptake genes znuABC and zinT. Microbiology 160:2452–2463. doi: 10.1099/mic.0.082446-0. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 31.DeFeyter R, Kado CI, Gabriel DW. 1990. Small, stable shuttle vectors for use in Xanthomonas. Gene 88:65–72. doi: 10.1016/0378-1119(90)90060-5. [DOI] [PubMed] [Google Scholar]
- 32.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 33.Bhubhanil S, Chamsing J, Sittipo P, Chaoprasid P, Sukchawalit R, Mongkolsuk S. 2014. Roles of Agrobacterium tumefaciens membrane-bound ferritin (MbfA) in iron transport and resistance to iron under acidic conditions. Microbiology 160:863–871. doi: 10.1099/mic.0.076802-0. [DOI] [PubMed] [Google Scholar]
- 34.Kamoun S, Hamada W, Huitema E. 2003. Agrosuppression: a bioassay for the hypersensitive response suited to high-throughput screening. Mol Plant Microbe Interact 16:7–13. doi: 10.1094/MPMI.2003.16.1.7. [DOI] [PubMed] [Google Scholar]
- 35.Iwig JS, Rowe JL, Chivers PT. 2006. Nickel homeostasis in Escherichia coli-the rcnR-rcnA efflux pathway and its linkage to NikR function. Mol Microbiol 62:252–262. doi: 10.1111/j.1365-2958.2006.05369.x. [DOI] [PubMed] [Google Scholar]
- 36.Iwig JS, Chivers PT. 2009. DNA recognition and wrapping by Escherichia coli RcnR. J Mol Biol 393:514–526. doi: 10.1016/j.jmb.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 37.Ruangkiattikul N, Bhubhanil S, Chamsing J, Niamyim P, Sukchawalit R, Mongkolsuk S. 2012. Agrobacterium tumefaciens membrane-bound ferritin plays a role in protection against hydrogen peroxide toxicity and is negatively regulated by the iron response regulator. FEMS Microbiol Lett 329:87–92. doi: 10.1111/j.1574-6968.2012.02509.x. [DOI] [PubMed] [Google Scholar]
- 38.Saenkham P, Eiamphungporn W, Farrand SK, Vattanaviboon P, Mongkolsuk S. 2007. Multiple superoxide dismutases in Agrobacterium tumefaciens: functional analysis, gene regulation, and influence on tumorigenesis. J Bacteriol 189:8807–8817. doi: 10.1128/JB.00960-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M. 2007. Phylogenetic and functional analysis of the cation diffusion facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics 8:107. doi: 10.1186/1471-2164-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blaha D, Arous S, Blériot C, Dorel C, Mandrand-Berthelot MA, Rodrigue A. 2011. The Escherichia coli metallo-regulator RcnR represses rcnA and rcnR transcription through binding on a shared operator site: insights into regulatory specificity towards nickel and cobalt. Biochimie 93:434–439. doi: 10.1016/j.biochi.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. 2007. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol 3:60–68. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- 42.Smaldone GT, Helmann JD. 2007. CsoR regulates the copper efflux operon copZA in Bacillus subtilis. Microbiology 153:4123–4128. doi: 10.1099/mic.0.2007/011742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corbett D, Schuler S, Glenn S, Andrew PW, Cavet JS, Roberts IS. 2011. The combined actions of the copper-responsive repressor CsoR and copper-metallochaperone CopZ modulate CopA-mediated copper efflux in the intracellular pathogen Listeria monocytogenes. Mol Microbiol 81:457–472. doi: 10.1111/j.1365-2958.2011.07705.x. [DOI] [PubMed] [Google Scholar]
- 44.Koch D, Nies DH, Grass G. 2007. The RcnRA (YohLM) system of Escherichia coli: a connection between nickel, cobalt and iron homeostasis. Biometals 20:759–771. doi: 10.1007/s10534-006-9039-6. [DOI] [PubMed] [Google Scholar]
- 45.Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A 106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu FF, Imlay JA. 2012. Silver(I), mercury(II), cadmium(II), and zinc(II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl Environ Microbiol 78:3614–3621. doi: 10.1128/AEM.07368-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Panek HR, O'Brian MR. 2006. Oxidative stress promotes degradation of the Irr protein to regulate haem biosynthesis in Bradyrhizobium japonicum. Mol Microbiol 60:209–218. doi: 10.1111/j.1365-2958.2006.05087.x. [DOI] [PubMed] [Google Scholar]
- 48.Luo ZQ, Clemente TE, Farrand SK. 2001. Construction of a derivative of Agrobacterium tumefaciens C58 that does not mutate to tetracycline resistance. Mol Plant Microbe Interact 14:98–103. doi: 10.1094/MPMI.2001.14.1.98. [DOI] [PubMed] [Google Scholar]
- 49.Metcalf WW, Jiang W, Daniels LL, Kim SK, Haldimann A, Wanner BL. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 50.Grant SG, Jessee J, Bloom FR, Hanahan D. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A 87:4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexeyev MF. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques 26:824–826, 828. [DOI] [PubMed] [Google Scholar]
- 52.Hwang I, Cook DM, Farrand SK. 1995. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J Bacteriol 177:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.