ABSTRACT
Phosphorus (P) plays a fundamental role in the physiology and biochemistry of all living things. Recent evidence indicates that organisms in the oceans can break down and use P forms in different oxidation states (e.g., +5, +3, +1, and −3); however, information is lacking for organisms from soil and sediment. The Cuatro Ciénegas Basin (CCB), Mexico, is an oligotrophic ecosystem with acute P limitation, providing a great opportunity to assess the various strategies that bacteria from soil and sediment use to obtain P. We measured the activities in sediment and soil of different exoenzymes involved in P recycling and evaluated 1,163 bacterial isolates (mainly Bacillus spp.) for their ability to use six different P substrates. DNA turned out to be a preferred substrate, comparable to a more bioavailable P source, potassium phosphate. Phosphodiesterase activity, required for DNA degradation, was observed consistently in the sampled-soil and sediment communities. A capability to use phosphite (PO33−) and calcium phosphate was observed mainly in sediment isolates. Phosphonates were used at a lower frequency by both soil and sediment isolates, and phosphonatase activity was detected only in soil communities. Our results revealed that soil and sediment bacteria are able to break down and use P forms in different oxidation states and contribute to ecosystem P cycling. Different strategies for P utilization were distributed between and within the different taxonomic lineages analyzed, suggesting a dynamic movement of P utilization traits among bacteria in microbial communities.
IMPORTANCE Phosphorus (P) is an essential element for life found in molecules, such as DNA, cell walls, and in molecules for energy transfer, such as ATP. The Valley of Cuatro Ciénegas, Coahuila (Mexico), is a unique desert characterized by an extreme limitation of P and a great diversity of microbial life. How do bacteria in this valley manage to obtain P? We measured the availability of P and the enzymatic activity associated with P release in soil and sediment. Our results revealed that soil and sediment bacteria can break down and use P forms in different oxidation states and contribute to ecosystem P cycling. Even genetically related bacterial isolates exhibited different preferences for molecules, such as DNA, calcium phosphate, phosphite, and phosphonates, as substrates to obtain P, evidencing a distribution of roles for P utilization and suggesting a dynamic movement of P utilization traits among bacteria in microbial communities.
INTRODUCTION
Phosphorus (P) is an essential element for the synthesis of many biomolecules, including DNA, RNA, and ATP (1), with no substitute as a building block of life. P is also frequently limiting for a variety of biota, including vascular plants, marine and freshwater phytoplankton, aquatic and terrestrial bacteria, and herbivorous animals (2); thus, understanding how P limitation shapes ecological and evolutionary dynamics is a key step in linking levels of biological organization from genes to ecosystems.
Organisms not only assimilate P in the form of phosphate for their cellular requirements (3) but can also break down and use P forms in different oxidation states (4). Indeed, Van Mooy et al. (5) showed that oceanic P is recycled through a previously unexplored pool of reduced forms of P. This suggests that the P cycle is more complicated than previously thought, in that several P redox states are involved at the global scale; if corroborated, this will change our understanding of global P cycling and ecosystem P limitation, as well as interspecific competition for P. While there is evidence of the importance of reduced P compounds in marine P biogeochemistry (3–5), the importance of reduced P compounds in terrestrial and inland water ecosystems is not well understood, reflecting a lack of information about the presence, abundance, and utilization of reduced P compounds in these ecosystems.
It is well-known that in soils, lakes, and oceans, microorganisms are primarily responsible for P recycling, manipulating the pool of available P through a variety of P transformation processes (e.g., P solubilization, organic matter depolymerization, P mineralization, and P assimilation) (6, 7). P forms in ecosystems include mineral P (e.g., in rocks, soil, and sediment), dissolved and particulate organic P, and dissolved inorganic phosphate (PO43−, here Pi) (8). Pi is the main P source for microorganisms and plants, but its availability in soil, sediment, and water is very low due to its high reactivity with calcium, iron, and aluminum (9). Since primary productivity and growth rates in ecosystems are highly P dependent (10), microbes have evolved numerous mechanisms for uptake and storage of Pi in response to nutrient scarcity (11, 12).
Phosphorus can exist in a range of oxidation states (e.g., +5, +3, +1, and −3) (4, 13). This spectrum of valence states supports a cascade of microbial oxidation-reduction reactions that may have important bioenergetic and ecological consequences analogous to those of the microbial C, N, and S cycles (4). In its most oxidized state (valence +5), P is found as phosphate esters (a mixture of HPO42− and H2PO4−) in many biomolecules, including nucleic acids (DNA and RNA), phospholipids, and phosphoproteins (14). Most phosphate esters are transformed or metabolized by microbes that produce extracellular enzymes (exoenzymes, often called phosphatases in a general form) that cleave organic molecules to liberate Pi for assimilation, as well as C and N in some cases (15). For example, in marine environments, dissolved DNA concentrations can range from 0.2 to 0.88 μg/liter, constituting a significant portion of the organic phosphorus pool (16). Additionally, in marine bacteria, dissolved DNA has been found to sustain 8 to 50% of the bacterial N demand (17).
Among the different classes of phosphatase enzymes, the most frequently studied are phosphomonoesterases (PM, often called acid or alkaline phosphatases, depending on their pH optima), enzymes that mineralize P from inositol phosphates, nucleotides, phosphoproteins, and sugar phosphates (18). However, phosphodiesterases (PD) that mineralize P from nucleic acids, phospholipids, and other diester phosphates (19) are also relevant for microbial P requirements. Among these, phosphate diesters are the main input of organic P (PO) to soils but typically constitute only a small fraction of total soil PO (20).
In a more-reduced state (valence +3), P is also found as phosphite (PO33− [Phi]) and organophosphonates. The organophosphonates are a class of organophosphorus molecules of both biogenic and xenobiotic origin that contain at least one stable carbon-phosphorus (C-P) covalent bond (21). In organophosphonates, this carbon-phosphorus bond replaces one of the four carbon-oxygen-phosphorus bonds of the more common phosphate ester (22). Phosphonates are found largely in unicellular life forms, such as bacteria, archaea, and fungi, occurring either as free molecules or, more usually, in peptide, glycan, or lipid conjugates, such as the 2-aminoethylphosphonic acid (ciliatine) and 2-amino-3-phosphonopropionic acid (phosphonoalanine) components of membrane phosphonolipids (23).
Four different phosphonate-degradative pathways have been reported for various bacterial species: the phosphonopyruvate hydrolase, phosphonoacetate hydrolase, phosphonoacetaldehyde hydrolase (phosphonatase), and the carbon-phosphorus lyase (C-P lyase) pathways (13, 24, 25). Homologous genes for phosphonate degradation pathways are distributed in distantly related bacteria, thus providing strong evidence for lateral gene transfer during evolution (1). Additionally, recent genomics studies have shown that soil and sediment bacteria have a large variety of mechanisms for the transformation, acquisition, and storage of phosphorus when it is both in oxidized and reduced forms (11). These suggest the existence of a terrestrial P redox cycle that might be analogous to the oceanic phosphorus redox cycle described by Van Mooy et al. (5). However, to better understand the global redox phosphorus cycle, it is necessary to identify the environmental controls on C-O-P and C-P compound cycling, the microorganisms involved, and the bioenergetics of the P oxidation and reduction pathways, as suggested by Karl (4), in aquatic and terrestrial energy-limited environments.
Physiological adaptations have been observed in bacteria that have to contend with environments limited in phosphorus. For instance, Bacillus coahuilensis, isolated from the Churince Pond in Cuatro Ciénegas, Coahuila, Mexico, was shown to have unique features compared to most well-studied Bacillus spp., such as having a small genome, lacking teichoic acid (a P-rich cell wall component), and having the capability to synthesize membrane sulfolipids in addition to phospholipids (26).
Here, we report a vast group of bacterial isolates from soil and sediment of a halophile ultraoligotrophic ecosystem which are able to obtain phosphorus from substrates with different oxidation states. We expected that evolution in a P-limited environment would have selected for traits that would allow bacteria to use diverse organic P substrates (C-O-P and C-P) through secretion of exoenzymes for P acquisition. Therefore, the objective of our study was to assess the capabilities of strains to use different P substrates and determine the phylogenetic distribution of these traits. We also wanted to measure the activities of the different exoenzymes in soil and sediment to evaluate the relevance of the different P substrates for microbial communities and their correlation with the presence of specific P utilization traits. We believe that understanding the strategies that microorganisms use to access P in different substrates will help both to understand how P is transformed by bacteria in microbial communities and to aid in elucidating the importance of reduced phosphorus compounds in the terrestrial components of the global phosphorus redox cycle.
MATERIALS AND METHODS
Study site and sampling.
This study was carried out in halophile grassland soil and aquatic sediments in the Cuatro Ciénegas Basin (CCB) in the central region of the Chihuahuan Desert in Coahuila, Mexico. The climate is hot and arid, with temperatures as high as 45°C in July and down to below 0°C in January (27). The mean annual precipitation is 253 mm. On the western side of the basin, Jurassic-era gypsum is the dominant parent material, while on the eastern side, Jurassic-era limestone dominates (28, 29). In both parts of the basin, the grass Sporobolus airoides (Torr.) is the dominant plant species (30).
Three study sites (one for grassland soil sampling and two for sediment sampling) were located within the Churince (CH) drainage on the western side of the CCB. Additionally, one site (for grassland soil sampling) was located within the natural reserve Pozas Azules (PA) on the eastern side of the CCB. At each soil sampling site, a 100- by 50-m plot was demarcated. In total, 10 composite samples were taken at each plot. For sediment sampling, samples were taken from the edge of one of the larger lagoons and from one small fertilized pool (for a more detailed description of the fertilization processes, see reference 31). The distance between each sediment sampling site was approximately 30 m. Soil (10 samples per site) and sediment (five samples from Churince sediment and one sample from Churince fertilized sediment) samples were stored in black plastic bags and refrigerated at 4°C for biogeochemical laboratory analyses; an aliquot of each sample was stored in a Falcon tube and immediately processed for microbial plating (see below).
Biogeochemical analyses.
To allow nutrient concentrations and enzymatic activities to be corrected for soil sample moisture content, a 100-g subsample was oven dried at 75°C to constant weight for soil moisture determination using the gravimetric method.
Dissolved nutrients.
Dissolved and microbial nutrient forms were extracted from moist soil samples with deionized water after shaking for 45 min and then were filtered through a Millipore 0.42-μm-pore-size filter (32). A blank with deionized water was processed in a manner similar to that of the samples to correct the values in case any contamination was present during the procedure. Prior to acid digestion, one aliquot of the filtrate was used to determine the Pi in the deionized-water extract. Total dissolved P (TDP) was determined by acid digestion, followed by colorimetry using a Bran-Luebbe AutoAnalyzer III (Norderstedt, Germany [33]). Total dissolved carbon (TDC) was measured with an autoanalyzer carbon module for liquids (TOC module CM5012; UIC-Coulometrics, Joliet, IL, USA). Dissolved inorganic carbon (DIC) was determined in an acidification module (CM5130). DOC and dissolved organic phosphorus (DOP) were calculated as the difference between total dissolved forms and inorganic dissolved forms (34).
Nutrients within microbial biomass.
C, N, and P within microbial biomass (Cmic, Nmic, and Pmic, respectively) concentrations were determined by the chloroform fumigation-extraction method (34–36). Fumigated and nonfumigated samples were incubated for 24 h at 25°C and under constant moisture. Cmic was extracted from fumigated and nonfumigated samples with 0.5 M K2SO4 and filtered through Whatman no. 42 paper (36). C concentration was measured from each extract as total carbon (TC) and inorganic carbon (IC) by the method described before. The organic C concentration was the difference between TC and IC, and the organic C was used for the Cmic calculations. Cmic was calculated by subtracting the extracted organic carbon in nonfumigated samples from that of fumigated samples and dividing it by a conversion factor, kEC (extractable part of microbial biomass C), of 0.45 (37). Nmic was extracted using the same procedure used for Cmic, but the extract was filtered through Whatman no. 1 paper. The filtrate was acid digested and quantified as TN using the macro-Kjeldahl method (38). Nmic was calculated similarly as Cmic but divided by a kEN factor (extractable part of microbial biomass N after fumigation) of 0.54 (39). Pmic was extracted using 0.5 M NaCO3 at pH 8.5. After this, the fumigation-extraction technique involving chloroform and an acid digestion was performed (40, 41). Pmic was calculated as for Cmic and Nmic but divided by a kP factor (extractable part of microbial biomass P after fumigation) of 0.4 (36, 42). Pmic was determined colorimetrically by the molybdate-ascorbic acid method (35) using a spectrophotometer (Evolution 201; Thermo Scientific, Inc.). Finally, the values of Cmic, Nmic, and Pmic were corrected to dry-soil basis.
Exoenzyme activity analyses.
The activities of three exoenzymes were measured with assay techniques previously reported (43–46). The potential activities of phosphomonoesterase (PM) and phosphodiesterase (PD) were quantified colorimetrically using ρ-nitrophenol (ρNP) substrates, while phosphonatase (PN) activity was determined using (2-aminoethyl)phosphonic acid (2-AEP) as the substrate, and the Pi liberated over time was quantified colorimetrically by the molybdate-ascorbic acid method (35) (Table 1). For all enzymes, we used 2 g of fresh soil and fresh sediment and 30 ml of modified universal buffer (MUB) (pH 9) for exoenzyme extraction. Three replicates and one control (sample without substrate) per sample were prepared. Additionally, three substrate controls (substrate without sample) were included per assay; all were incubated at 30°C. We centrifuged the tubes after the incubation period, and then 750 μl of supernatant was diluted in 2 ml of deionized water. For enzymes with substrates linked to ρNP, we measured the absorbance of ρNP at 410 nm on an Evolution 201 spectrophotometer (Thermo Scientific, Inc.). Finally, exoenzyme activity was expressed in nanomoles of ρNP formed per gram of soil (dry weight) per hour (nmol ρNP [g SDW]−1 h−1) (47). For PN, we measured the absorbance of Pi at 882 nm after ascorbic acid reduction. Finally, PN activity was expressed as parts per million of Pi released per hour (ppm Pi h−1). All enzymatic assays were done at 30°C.
TABLE 1.
Substrates utilized as sole phosphorus source at a final concentration of 0.1 mM and their abbreviations and formulas
| Substrate | Abbreviation | Formula |
|---|---|---|
| Potassium phosphate | PP | KH2PO4 |
| Calcium phosphate | CP | Ca(H2PO4)2H2O |
| (2-Aminoethyl)phosphonic acid | 2-AEP | H2NCH2CH2P(O)(OH)2 |
| Diethyl phosphonoacetaldehyde | 2-PA | (CH3CH2O)2CHCH2P(O)(OCH2CH3)2 |
| Phosphite | Phi | KH2PO3 |
| DNA | DNA | C6H12O6N5Pa |
| P-free control | PFree |
Example of a polymer with a guanosine base.
Isolation of microorganisms.
The isolates in the collection were obtained using an inoculum of sediment and soil for each sample from each of the two main sampling sites (the Churince hydrological system and Pozas Azules). Fresh samples were added to an Eppendorf tube with 800 μl of modified universal buffer (MUB) to achieve a ratio of 1/3 (wt/vol); the resulting suspension was mixed continuously for 60 min. The resultant suspension was used as an inoculum for plating onto petri dishes with modified marine agar medium, a complex medium that includes peptone, yeast extract, and dibasic sodium phosphate (48), and incubated at 37°C for 2 days. Colonies with different morphotypes (i.e., size, shape, and color) were selected. Purification was performed by subculturing on the same medium to ensure that the culture was axenic; all isolates were stored at −80°C in marine medium with 15% (wt/vol) glycerol.
Evaluation of isolates for growth in different phosphorus sources.
A total of 1,163 isolates were obtained (250 from CH soil, 250 from PA soil, 141 from intermediate lagoon sediment of CH, and 512 from the fertilized small-pond sediment of CH). All were isolated and routinely grown on rich medium (marine agar medium). To evaluate preference for different P substrates, we used a defined medium (DM) (49) either with no phosphorus added (DM) or containing different P sources. The base medium (DM) contained (per liter): Tris base, 6.057 g adjusted to pH 8.0; NH4NO3, 0.26 g; MgSO4, 0.48 g; disodium citrate, 1.99 g; ZnCl2, 0.000136 g; NaCl, 5 g; FeCl3, 0.27 g; KCl, 0.1 g; MnCl2, 0.2 g; CaCl, 0.4 g; glucose, 9 g; and amino acid mixture, 0.93 g. Heat-labile substrates (vitamin B complex, biotin, and nicotinic acid) were filter sterilized and added aseptically after autoclaving. Isolates were evaluated for growth in the following six phosphorus sources at a concentration of 1 mM added to the base DM medium: (i) potassium phosphate, (ii) calcium phosphate, (iii) phosphite, (iv) (2-aminoethyl)phosphonic acid, (v) 2-phosphonoacetaldehyde, and (vi) DNA; there was also (vii) a control without P (PFree) (Table 1).
Isolates were grown initially at 37°C for 72 h in DM lacking any P to ensure the depletion of P storage. After that, each isolate was transferred to DM with each one of the six different P sources, along with a control plate lacking P. These were then incubated at 37°C for 72 h. For each isolate that grew on a given P source, we further tested its ability to use that same P source. Only 528 isolates exhibited an ability to grow on DM medium after the 2nd transfer and were selected and used for DNA extraction and 16S rRNA identification.
DNA extraction and 16S rRNA gene amplification by PCR.
Total genomic DNA was extracted from isolates using the phenol-chloroform method (50). The 16S rRNA genes were amplified from the DNA templates by PCR with the universal primers 16S 27F (5′-AGA GTT TGA TCM TGG CTC AG-3′) and 1492R (5′-TAC GGY TAC CTT GTT ACG ACT T-3′) obtained from Sigma (St. Louis, MO, USA). PCRs were run in a thermocycler (Palm-Cycler; Corbett Research) using 30 cycles of 94°C, 55°C, and 70°C. PCR products were sequenced by the Sanger methodology (51) at Cinvestav-Langebio (Irapuato, Mexico). The 16S rRNA sequences were verified with Phred (52), and sequences of at least 500 bp were used for the analysis. Phylogenetic reconstruction was carried out with 528 sequences using the maximum-likelihood method in PhyML (53). The DNA sequences were retrieved from the data set, classified using the Ribosomal Database Project (RDP) Classifier tool (54), and then clustered as operational taxonomic units (OTU) using mothur (55). The tree was edited on the ITOL platform (56).
We analyzed the sequences with AdaptML (57) to define ecologically differentiated populations; the sequences were assigned to the Churince soil, Pozas Azules soil, sediment, and fertilized sediment habitats to identify populations as groups of related strains sharing a common projected habitat.
Statistical analyses.
To compare the abilities of various isolates to grow on the different P sources, a logistic regression model (LRM) with two factors (site and P source) was performed using a generalized linear model in STATISTICA (58). This model is suitable for analyzing data with a binomial distribution (59). Spearman correlations were used to explore relationships among isolates' growth on the different P substrates.
Nucleotide sequence accession numbers.
Sequences from this study for strains from Churince and Pozas Azules soil samples have been deposited in GenBank under the accession numbers KP296259 to KP296570; sequences for strains from Churince sediment have been deposited in GenBank under accession numbers KP340470 to KP340508, KF966199, KF966201, KF966211, KF966216, KF966223, KF966226, KF966228 to KF966230, KF966234, KF966236, KF966247, KF966248, KF966250, KF966253, KF966255, KF966256, KF966264, KF966270, KF966272, KF966276, KF966281, KF966284, KF966289, KF966291, KF966297, KF966304, KF966305, KF966310, KF966311, KF966315, KF966321, KF966324, KF966327, KF966329, KF966332, KF966334, KF966336 to KF966338, KF966343, KF966348, KF966351, KF966355, KF966357, KF966358, KF966367, KF966379, KF966380, KF966386, KF966387, KF966397, KF966410 to KF966419, KF966421 to KF966425, KF966427 to KF966432, KF966434, KF966441, KF966443, KF966447, KF966449, KF966450, KF966452, KF966454, KF966455, KF966467, KF966477, KF966485, KF966486, KF966507, KF966509, KF966515, KF966516, KF966526, and KF966534; and sequences for strains from Churince fertilized sediment have been deposited in GenBank under accession numbers KP296571 to KP296656 (see Table S1 in the supplemental material).
RESULTS
Nutrient and exoenzyme distributions across sites.
To understand the different bacterial strategies for P utilization in the microbial communities from Cuatro Ciénegas ultraoligotrophic ecosystems, we first assessed free and microbe-associated nutrients in soil and sediment, as well as enzymatic activities in the different sampling sites. Our results showed that the sediment samples were characterized by noticeably higher concentrations of dissolved organic carbon (DOC), dissolved organic nitrogen (DON), and dissolved organic phosphorus (DOP) than the soil samples (Table 2). The microbial nitrogen (Nmic) content was also higher in the sediment samples than in the soil samples. Additionally, Cmic/Pmic and Nmic/Pmic ratios were higher in sediment samples than in the soil samples. However, soil samples had higher microbial P (Pmic) concentrations, DOC/DON and DOC/DOP ratios, and Cmic/Nmic ratios (Table 2) than the soil samples.
TABLE 2.
Nutrients quantified in grassland soil and sediment in the Cuatro Ciénegas Basin, Coahuila, Mexico
| Parametera | Soil |
Sediment |
||
|---|---|---|---|---|
| Churince | Pozas Azules | Churince | Fertilized | |
| Nutrient amt [mean (SE) μg g−1] | ||||
| DOC | 172 (9.6) | 99 (5.4) | 333 (160) | 342 |
| DON | 10.39 (1.4) | 5.59 (0.3) | 50.52 (27.8) | 70.24 |
| DOP | 1.54 (0.32) | 0.89 (0.11) | 6.68 (0.25) | 8.35 |
| Cmic | 211 (52.23) | 135 (16.7) | 184 (23.7) | 134 |
| Nmic | 15.48 (3.6) | 11.05 (2.6) | 23.77 (3.4) | 22.54 |
| Pmic | 4.021 (0.03) | 7.769 (0.03) | 2.864 (0.5) | 2.731 |
| Ratios | ||||
| DOC/DON | 16.55 | 17.74 | 6.59 | 4.86 |
| DOC/DOP | 111.5 | 110.5 | 49.82 | 40.93 |
| DON/DOP | 6.73 | 6.23 | 7.56 | 8.42 |
| Cmic/Nmic | 13.26 | 12.19 | 7.75 | 5.95 |
| Cmic/Pmic | 52.37 | 17.34 | 64.38 | 49.13 |
| Nmic/Pmic | 3.85 | 1.42 | 8.29 | 8.25 |
DOC, dissolved organic carbon; DON, dissolved organic nitrogen; DOP, dissolved organic phosphorous; Cmic, carbon within microbial biomass; Nmic, nitrogen within microbial biomass; Pmic, phosphorus within microbial biomass.
We next evaluated patterns of enzymatic expression of different exoenzymes known to be involved in phosphorus scavenging. Table 3 shows that there are strong differences in the amounts and types of exoenzyme activities in the different microbial communities in the study sites. Phosphodiesterase (PD) activity was higher in soil than in sediment samples, whereas the opposite was true for phosphomonoesterases (PM), which were highly active in sediment. Notably, while phosphonatase (PN) activity was detected in soil samples, it was not detectable in sediment samples (Table 3). Notice that a single biochemical condition was used to measure the collective enzymatic activity from either soil or sediment. Since the different enzymes could have differences in the optimal conditions for their assay, possibly correlated with environmental differences at each site, what was measured was the potential enzyme activity.
TABLE 3.
Exoenzyme activities quantified in grassland soil and sediment in the Cuatro Ciénegas Basin, Coahuila, Mexico
| Exoenzyme | Soil |
Sediment |
||
|---|---|---|---|---|
| Churince | Pozas Azules | Churince | Fertilized | |
| Phosphomonoesterase [mean (SE) nmol ρ-NP g−1 SDW h−1] | 105 (19.8) | 24.06 (2.9) | 557 (190) | 597 |
| Phosphodiesterase [mean (SE) nmol ρ-NP g−1 SDW h−1] | 181 (12.2) | 371 (9.8) | 286 (68) | 176 |
| Phosphonatase [mean (SE) ppm Pi h−1] | 63.22 (13.3) | 76.23 (9.76) | 0 | 0 |
Evaluation of isolates' ability to grow on different P sources.
Figure 1 shows the distribution of P-scavenging capabilities among 1,163 isolates from all sampled sites. Unexpectedly, only 80% of the isolates from sediments (both the shallow lagoon and the fertilized small pond) and 60% of the isolates from soil (Churince and Pozas Azules) were capable of growing with potassium phosphate as a sole source of P (Fig. 1). Differences were observed between the four sites when calcium phosphate was used as a sole source of phosphorus. The highest proportion of growth on calcium phosphate was observed for isolates from CH sediment, followed by isolates from soil (CH and PA), and finally by isolates from the P-fertilized sediment sample (Fig. 1).
FIG 1.
Differences in the frequency of utilization of P sources among soil and sediment isolates. The y axis reports the proportion of isolates able to grow under each of the six different phosphorus sources. PP, potassium phosphate; CP, calcium phosphate; Phi, phosphite; 2-PA, 2-phosphonoacetaldehyde; 2-AEP, (2-aminoethyl)phosphonic acid. Isolates tested were recovered from Churince soil (CH), Pozas Azules soil (PA), Churince sediment (S), or fertilized sediment (FS). Since some isolates can grow under more than one P source, the proportion does not add to 1. All three factors were highly significant (P < 0.0001).
Remarkably, 50% of the isolates from CH sediment were capable of growing with phosphite as a sole P source. However, <20% of the isolates from the P-fertilized sediment and <10% of the isolates from soil (CH and PA) were capable of growing with this P source (Fig. 1).
Less than 10% of the isolates from the four sites had the capacity to grow with 2-phosphonoacetaldehyde (2-PA) phosphonate as the sole P source, but ∼20% of the isolates could grow using the P from 2-AEP, the most abundant biogenic phosphonate (13) (Fig. 1).
Ninety percent of the isolates from CH soil, PA soil, and CH sediment were able to grow with DNA as the P source. However, this capacity was less frequently observed (only 30%) among the isolates from the P-fertilized pond sediments (Fig. 1).
All three factors of the study (site [either soil or sediment], P substrate, and the correlation of site and P substrate) were analyzed to determine how these were associated with the occurrence of different P utilization traits. All three were highly significant (P < 0.0001), indicating a strong dependence of P utilization patterns on sample site (sediments versus soil). The strongest Spearman correlation observed was for potassium phosphate and calcium phosphate for soil (0.698 for Churince and 0.523 for Pozas Azules; see Tables S2 and S3 in the supplemental material). For Churince soil, a correlation was found between phosphite and 2-PA (0.402; Table S2) and, in Pozas Azules soil, for DNA and potassium phosphate (0.398; Table S3). Meanwhile, the strongest correlation observed for Churince sediment was between phosphite and 2-AEP (0.447; see Table S4 in the supplemental material) and, for P-fertilized sediment, between DNA and calcium phosphate (0.326; see Table S5 in the supplemental material).
Phylogenetic analysis of bacteria, based on 16S rRNA gene, across all sites and samples.
A total of 528 sequences were analyzed using mothur, yielding 210 operational taxonomic units (OTU) grouped at 99% similarity (121 at 98% and 97 at 97%). The OTU were distributed as follows: 32 OTU from the Churince sediment, 61 OTU from the P-fertilized sediment, 68 OTU from the Churince soil, and 74 OTU from the Pozas Azules soil. The dominant OTU were classified as Firmicutes (approximately 85% were Bacillus and Staphylococcus), followed by Proteobacteria (Aeromonas and Paracoccus) and Actinobacteria. Some bacterial groups were recovered only from some sites, but most groups were recovered at least at low levels from both soil and sediment.
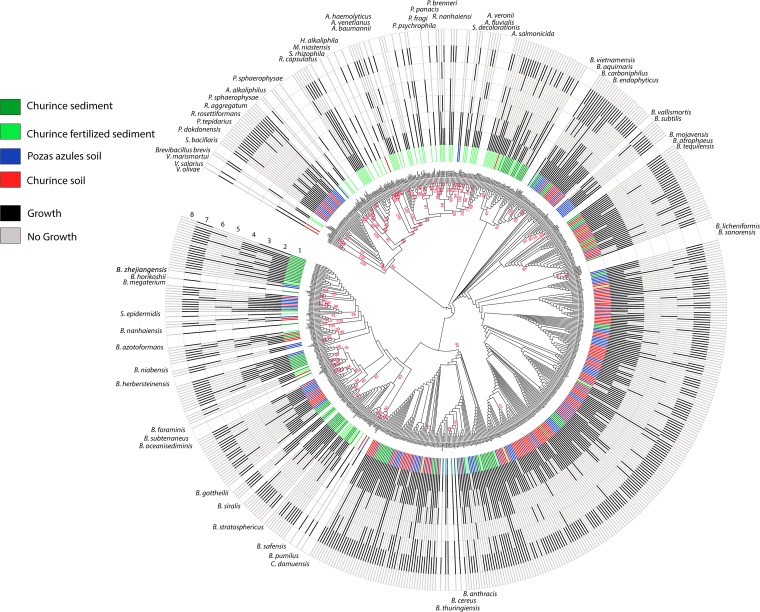
Notably, the distribution of P utilization traits was not associated with particular taxonomic groups (Fig. 2). That is, all clades had members that could use potassium phosphate, calcium phosphate, and/or DNA. There were, however, some clades that lacked members that could grow on phosphite, 2-PA, or 2-AEP. Under close analysis of the genetically cohesive group Bacillus cereus, which was highly represented both in soil and sediment, the number and diversity of traits related to P utilization were higher for members isolated from sediment than from members isolated from soil (Table 4). In general, isolates from sediment had, on average, the capacity to use up to 4 different P substrates, while soil isolates could use only 3. Remarkably, despite the high genetic similarity of the B. cereus group members, the ability to use phosphite seemed to be a trait selected for in isolates from sediment (71.8% of isolates from sediment versus 12% of isolates from soil).
FIG 2.
Distribution of phosphorus utilization capabilities among isolates of different taxonomic groups from soil and sediment communities. Phylogeny based on the 16S rRNA gene. The site of isolation is shown in the innermost circle, labeled 1 with the following colors: blue, Pozas Azules soil; red, Churince soil; dark green, Churince sediment; and light green, Churince fertilized sediment. The outer circles indicate the isolates' ability to grow with a given P source (represented in dark gray); light gray indicates lack of growth of the isolate. The circle numbers refer to P source evaluated: 2, potassium phosphate; 3, calcium phosphate; 4, phosphite; 5, 2-phosphonoacetaldehyde; 6, (2-aminoethyl)phosphonic acid; 7, DNA; and 8, P free.
TABLE 4.
Differences in trait selection for P utilization, notably phosphite, between soil and sediment B. cereus isolates
| Phosphorus source | Isolate source (%) |
|
|---|---|---|
| Soil (n = 55) | Sediment (n = 39) | |
| Potassium phosphate | 93 | 100 |
| Calcium phosphate | 74.5 | 84.6 |
| (2-Aminoethyl)phosphonic acid | 43.6 | 51.2 |
| Diethyl phosphonoacetaldehyde | 10.9 | 20.5 |
| Phosphite | 12.77 | 71.8 |
| DNA | 90.9 | 92.3 |
AdaptML analysis was used to discover ecotypes and determine their preferences for habitats (in this case, soil and sediment, and the different P substrates). Two distinct habitats were uncovered: habitat 1, containing sequences obtained predominantly from soil and fertilized sediment and present in both the basal and the most recent phylogenetic branches; and habitat 2, mostly containing sequences obtained from sediment (Fig. 4). This result suggests that bacteria have different niche adaptations that influence patterns of genotypic differentiation (Fig. 3 and 4). Clearly, soil and sediment act as a filter for different taxonomic groups.
FIG 4.
Assortment of genotypes according to ecological similarity. The analysis was done with AdaptML (51). The 16S rRNA gene sequences are associated with colored bars in the outer ring that represent the sites of isolation (sediment or soil). The predictive model projected two habitats (1 and 2), shown in the inner circles on the phylogenetic branches as dots colored in yellow or purple, which reflect trends in distribution. Taxonomic assignments are shown on the outside based on type strain's sequences included in the analysis. Shown with bars at the left is the distribution of each population among isolation sites for each predicted habitat. Notice that habitat 2 groups most of the sediment isolates (dark green).
FIG 3.
Phosphorus source preferences by bacteria related to B. cereus sensu lato species. This is a closeup of the clade that groups Bacillus cereus, Bacillus thuringiensis, and Bacillus anthracis in Fig. 2. Phylogeny is based on the 16S rRNA gene. The site of isolation is shown in the innermost circle, labeled 1 and indicated by the following colors: blue, Pozas Azules soil; red, Churince soil; dark green, Churince sediment; and light green, Churince fertilized sediment. The outer circles indicate the isolates' ability to grow with a given P source (represented in dark gray); light gray indicates lack of growth of the isolate. The circle numbers refer to P source evaluated: 2, potassium phosphate; 3, calcium phosphate; 4, phosphite; 5, 2-phosphonoacetaldehyde; 6, (2-aminoethyl)phosphonic acid; 7, DNA; 8, P free.
DISCUSSION
Soil and sediment bacteria transform P into different oxidized and reduced forms, just as bacterial communities do in the oceans. Overall, we found that soil and sediment bacterial communities of CCB have a variety of strategies for P scavenging, and they can also use a variety of both oxidized and reduced phosphorus species. Strategies include mineralization of ester phosphates and phosphonates, biosynthesis of different exoenzymes, as well as inorganic P solubilization and phosphite oxidation. Our results indicate that although individual isolates can utilize up to four different P substrates, these capabilities are scattered within and across different taxonomic groups, with diversity in the strategies used between individual isolates. Only 11 of the 1,160 tested isolates were capable of using every substrate evaluated.
Exoenzyme expression was expected to reflect the quality of available organic matter. Our results (Table 3) showed that sediments exhibited the largest phosphomonoesterase activity (often referred to as alkaline phosphatase), suggesting that monoesters (inositol phosphates, sugar phosphates, phosphoproteins, etc.) could be a preferred scavenging option in sediment. In soil sites, in contrast, phosphodiesterase activity seems to be more abundant, suggesting a preference of soil microorganisms for diesters (such as nucleic acids and phospholipids). It is in soils where there is the least amount of DOP (Table 2), so it was expected that all enzymes would be expressed at higher levels in soils than in sediments; that seemed to be the case for phosphonatases and phosphodiesterases (in Pozas Azules). It is noticeable that in Pozas Azules, soil phosphodiesterases played a prominent role. This is in agreement with the number of isolates from this site that exhibited a preference to grow on DNA as a sole source of P (Fig. 1). On the other hand, phosphonatase was detected only in soil. Since isolates from all sites exhibited similar capabilities to use phosphonates, we suggest that in sediments, there were other alternatives for P utilization, and phosphonatases were not being expressed. In fact, it is in sediments that dissolved C, N, and P occurred at a higher concentration (see reference 47 for a detailed analysis of the correlation between nutrients and enzymes in soil and sediments). Phosphorus concentration is known to regulate the expression of numerous genes involved in P uptake and utilization; however, not all P-scavenging enzymes are regulated by P availability. A recent paper from our group showed that in B. coahuilensis alkaline phosphatase activity was regulated by P concentration in one strain, but in another strain, expression was constitutive (60).
Among the P substrates tested in our experiment, potassium phosphate and DNA were the substrates on which the highest percentage of isolates grew (Fig. 1 and 2). The fact that DNA was the preferred P source for isolates from soil and unamended sediment, but not for the isolates from Pi-fertilized sediment, suggests that the addition of Pi enriched for bacteria that are capable of profiting from this easily metabolized and rarely available P substrate. This inference is consistent with differences in the bacterial community composition, as in fertilized sediment, we recovered Actinobacteria and Proteobacteria at high frequencies, while we typically obtained an overrepresentation of Firmicutes (Bacillus) from both soil and sediments.
In ultraoligotrophic environments, DNA released, either by cannibalistic behavior (61), natural cell death, or phages, represents a P-rich resource (62). However, bacterial P acquisition from DNA requires the biosynthesis of phosphodiesterase enzymes. The ability of the microbial community to synthesize phosphodiesterase was documented in all four sites we sampled in CCB (Table 2). DNA is also an N-rich resource (63); thus, microbial investment in DNA mineralization allows the acquisition of both P and N. Our results suggest that this mechanism for P acquisition might be an important way by which microorganisms acquire and recycle P. Although N is also often limiting for growth in this ultraoligotrophic system (46), it has been documented that DNA is not utilized as a source of nitrogen (63).
In contrast to the ubiquity of phosphodiesterases in isolates able to use DNA, only a low percentage of isolates was able to grow in media with phosphonate substrates (2-PA and 2-AEP). However, we did identify isolates with the ability to grow either in 2-PA or 2-AEP in almost all genotypic clusters (Fig. 2). For microorganisms from severely P-limited ecosystems, like CCB, it is an advantage to be able to obtain P from even somewhat unusual forms, such as phosphonates.
Phosphonate degradation has been reported to occur via the phosphonatase pathway in marine bacteria (64), as well as in soil bacteria (65). This pathway is present in diverse bacteria, including representatives of Proteobacteria, Planctomycetes, Cyanobacteria (65), and Firmicutes (11). We observed phosphonatase activity in soil, but we could not detect this activity in sediments. These results are consistent with the idea that microbial degradation of phosphonates is especially favored under conditions of phosphate limitation, as we also observed lower DOP concentrations in soil than in sediments (Table 3). This implies, of course, that phosphonate is available in soil. In general, when P utilization preference is examined, the absolute concentration of a given substrate is meaningful only in relation to the most readily metabolized form, usually phosphate. If phosphate is available, enzymes to obtain alternative P sources are usually not expressed.
Regarding phosphonate utilization, three other phosphonate-degradative pathways have been reported in various bacterial species that rely on different key enzymes: phosphonopyruvate hydrolase, phosphonoacetate hydrolase, and carbon-phosphorus lyase (C-P lyase) (13). The first two pathways are highly substrate dependent, while C-P lyase is not substrate dependent. Our results suggest that the microbial degradation pathway for phosphonates plays out differently in soil (via phosphonatase [Phn]) and sediment (via C-P lyase). These results suggest that different bacteria found in the soil and sediment of CCB can grow under conditions in which the only source of P is 2-PA or 2-AEP.
We also identified isolates that could grow using phosphite as a sole P source. Phosphite (H2PO3− [Phi]) is a reduced form of inorganic phosphate (HPO42− [Pi]), where Phi has a substitution of oxygen by hydrogen; as a result, the two compounds behave quite differently in living organisms (66). Phi cannot enter into the same metabolic pathways as Pi, because most enzymes involved in phosphoryl transfer reactions discriminate between Phi and Pi (67). The oxidation of Phi to Pi in soil is largely due to soil microbial activity (68) by microbes that use Phi for the production of energy and Pi (13). Phosphite and phosphonates have the same valence (+3) (versus +5 for Pi [13]), and the same gene cluster that is required to degrade phosphonates, phn, is also required to oxidize phosphite to phosphate (69). This suggests that there should be close associations between the bacterial taxa that can metabolize Phi and phosphonates. Indeed, our Spearman rank correlation results indicated a significant correlation between Phi and 2-PA and between Phi and 2-AEP (see Tables S2 and S3 in the supplemental material) for CH soil and PA soil, while in sediments, we observed a strong correlation only between Phi and 2-AEP (see Tables S4 and S5 in the supplemental material). Our results support the idea that there is a connection between the use of phosphonates and phosphite, possibly explained by the relationship between the genes involved in phosphonate degradation and phosphite oxidation, as suggested in previous studies (65, 68, 69). However, a diversity of pathways is suggested from this study, as not all strains able to use Phi could use phosphonate; the sources of the Phi are enigmatic. It is suggested that both Phi and phosphonate are produced as antibiotics (4), but there is still work to do to understand how these are produced and why these substrates that are costly to process are preferred by some organisms.
Our description of the different strategies of P acquisition in bacterial isolates from an oligotrophic environment, particularly one limited in Pi, may help in understanding the functioning of microbial communities in Pi-limited environments. The dispersion of traits related to P acquisition within and across taxonomic groups is suggestive of niche-partitioning strategies that also explain how genetic diversity is maintained. Lateral transfer of genes involved in substrate acquisition has been widely described for carbon utilization, including genes that encode enzymatic activities to scavenge for carbon sources and transporters (70).
It has been suggested that phosphate transporter genes, phosphonate utilization genes, and alkaline phosphatase-coding genes have been transferred between bacterial lineages (71–74). It is thus possible that lateral gene transfer during bacterial evolution in this ultraoligotrophic terrestrial environment has played an important role in allowing bacteria to sample and retain diverse genes for P acquisition, resulting in a complex redox cycle for P analogous to that shown for the oceans.
Supplementary Material
ACKNOWLEDGMENTS
We thank Pamela Chavez, Alberto Morón, and Rodrigo Velázquez-Durán for assisting with soil sampling and chemical analysis, and we thank CONANP and Rancho Pozas Azules (PRONATURA) for permission to collect soil.
Y.T.-T., G.O.-Á., and F.G.-O. designed the study; Y.T.-T., M.D.R.-T., and A.I. performed the research; Y.T.-T., M.D.R.-T., and F.G.-O. analyzed the data; Y.T.-T., M.D.R.-T., G.O.-Á., F.G.-O., J.J.E., and V.S. interpreted the data; Y.T.-T. wrote the first draft of the manuscript; and all authors contributed substantially to revisions. All authors edited and approved the final manuscript.
This work was financed by CONACYT grant 220536 (CB-2013-01) to G.O.-Á. and a grant to F.G.-O. from the National Autonomous University of Mexico (PAPIIT DGAPA-UNAM: Análisis de la vulnerabilidad de la dinámica de nutrientes en un ecosistema árido de México, IN204013). V.S. and Y.T.-T. acknowledge support from Alianza WWF-FCS. Y.T.-T. acknowledges the postdoctoral fellowship financed through an IDETEC Sagarpa project to G.O.-Á. and CONACYT scholarship grants to M.D.R.-T.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00160-16.
REFERENCES
- 1.Huang J, Su Z, Xu Y. 2005. The evolution of microbial phosphonates degradative pathways. J Mol Evol 61:682–690. doi: 10.1007/s00239-004-0349-4. [DOI] [PubMed] [Google Scholar]
- 2.Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 3.Benitez-Nelson C. 2015. Ocean chemistry. The missing link in oceanic phosphorus cycling? Science 348:759–760. doi: 10.1126/science.aab2801. [DOI] [PubMed] [Google Scholar]
- 4.Karl DM. 2014. Microbially mediated transformations of phosphorus in the sea: new views of an old cycle. Annu Rev Mar Sci 6:279–337. doi: 10.1146/annurev-marine-010213-135046. [DOI] [PubMed] [Google Scholar]
- 5.Van Mooy BA, Krupke A, Dyhrman ST, Fredricks HF, Frischkorn KR, Ossolinski JE, Repeta DJ, Rouco M, Seewald JD, Sylva SP. 2015. Phosphorus cycling. Major role of planktonic phosphate reduction in the marine phosphorus redox cycle. Science 348:783–785. doi: 10.1126/science.aaa8181. [DOI] [PubMed] [Google Scholar]
- 6.Cotner JB, Biddanda BA. 2002. Small players, large role: microbial influences on biogeochemical processes in pelagic aquatic ecosystems. Ecosystems 5:105–121. doi: 10.1007/s10021-001-0059-3. [DOI] [Google Scholar]
- 7.van der Heijden MGA, Bardgett RD, van Straalen NM. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 8.Steenbergh AK. 2012. The microbial control of phosphorus fluxes in marine sediments. PhD thesis Utrecht University, Utrecht, the Netherlands. [Google Scholar]
- 9.Perroni Y, García-Oliva F, Tapia-Torres Y, Souza V. 2014. Relationship between soil P fractions and microbial biomass in an oligotrophic grassland-desert scrub system. Ecol Res 29:463–472. doi: 10.1007/s11284-014-1138-1. [DOI] [Google Scholar]
- 10.Elser JJ, Watts J, Schampel JH, Farmer J. 2006. Early Cambrian food webs on a trophic knife-edge? A hypothesis and preliminary data from a modern stromatolite-based ecosystem. Ecol Lett 9:295–303. doi: 10.1111/j.1461-0248.2005.00873.x. [DOI] [PubMed] [Google Scholar]
- 11.Alcaraz LD, López-Ramírez V, Moreno-Letelier A, Herrera-Estrella L, Souza V, Olmedo-Álvarez G. 2011. Genomics of bacteria from an ancient marine origin: clues to survival in an oligotrophic environment. In Dar IA, Dar MA (ed), Earth and environmental sciences. InTech, Rijeka, Croatia. [Google Scholar]
- 12.Adams MM, Gomez-Garcia MR, Grossman AR, Bhaya D. 2008. Phosphorus deprivation responses and phosphonate utilization in a thermophilic Synechococcus sp. from microbial mats. J Bacteriol 190:8171–8184. doi: 10.1128/JB.01011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White AK, Metcalf WW. 2007. Microbial metabolism of reduced phosphorus compounds. Annu Rev Microbiol 61:379–400. doi: 10.1146/annurev.micro.61.080706.093357. [DOI] [PubMed] [Google Scholar]
- 14.Martínez A, Osburne MS, Sharma AK, DeLong EF, Chisholm SW. 2012. Phosphite utilization by the marine picocyanobacterium Prochlorococcus MIT9301. Environ Microbiol 14:1363–1377. doi: 10.1111/j.1462-2920.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- 15.Waring BG, Weintraub SR, Sinsabaugh RL. 2014. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 117:101–113. doi: 10.1007/s10533-013-9849-x. [DOI] [Google Scholar]
- 16.Karl DM, Bailiff MD. 1989. The measurement and distribution of dissolved nucleic acids in aquatic environments. Limnol Oceanogr 34:543–558. doi: 10.4319/lo.1989.34.3.0543. [DOI] [Google Scholar]
- 17.Keil RG, Kirchman DL. 1991. Contribution of dissolved free amino acids and ammonium to the nitrogen requirements of heterotrophic bacterioplankton. Mar Ecol Prog Ser 73:1–10. doi: 10.3354/meps073001. [DOI] [Google Scholar]
- 18.Turner BL, Cade-Menun BJ, Condron LM, Newman S. 2005. Extraction of soil organic phosphorus. Talanta 66:294–306. doi: 10.1016/j.talanta.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Turner BL, McKelvie ID, Haygarth PM. 2002. Characterisation of water extractable soil organic phosphorus by phosphatase hydrolysis. Soil Biol Biochem 34:27–35. doi: 10.1016/S0038-0717(01)00144-4. [DOI] [Google Scholar]
- 20.Turrion MB, Lafuente F, Aroca MJ, López O, Mulas R, Ruipérez C. 2010. Characterization of soil phosphorus in a fire-affected forest Cambisol by chemical extractions and 31P-NMR spectroscopy analysis. Sci Total Environ 408:3342–3348. doi: 10.1016/j.scitotenv.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn GM. 1981. Phosphonates as analogues of biological phosphates. Nature 351:515–516. [Google Scholar]
- 22.Wanner BL, Metcalf WW. 1992. Molecular genetic studies of a 10.9-kb operon in E. coli for phosphonate uptake and biodegradation. FEMS Microbiol Lett 100:133–140. doi: 10.1111/j.1574-6968.1992.tb14031.x. [DOI] [PubMed] [Google Scholar]
- 23.Hilderbrand RL. 1983. The role of phosphonates in living systems. CRC Press, Boca Raton, FL. [Google Scholar]
- 24.Kononova SV, Nesmeyanova MA. 2002. Phosphonates and their degradation by microorganisms. Biochemistry 67:184–195. [DOI] [PubMed] [Google Scholar]
- 25.Quinn JP, Kulakova AN, Cooley NA, McGrath JW. 2007. New ways to break an old bond: the bacterial carbon-phosphorus hydrolases and their role in biogeochemical phosphorus cycling. Environ Microbiol 9:2392–2400. doi: 10.1111/j.1462-2920.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- 26.Alcaraz LD, Olmedo G, Bonilla G, Cerritos R, Hernandez G, Cruz A, Ramírez E, Putonti C, Jiménez B, Martínez E, López V, Arvizu JL, Ayala F, Razo F, Caballero J, Siefert J, Eguiarte L, Vielle JP, Martínez O, Souza V, Herrera-Estrella A, Herrera-Estrella L. 2008. The genome of Bacillus coahuilensis reveals adaptations essential for survival in the relic of an ancient marine environment. Proc Natl Acad Sci U S A 105:5803–5808. doi: 10.1073/pnas.0800981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SMN, CONAGUA. 2013. Normales climatológicas por estación. Servicio Meteorológico Nacional, CONAGUA, Mexico City, Mexico. [Google Scholar]
- 28.McKee JW, Jones NW, Long LE. 1990. Stratigraphy and provenance of strata along the San Marcos fault, central Coahuila, Mexico. Geol Soc Am Bull 102:593–614. doi:. [DOI] [Google Scholar]
- 29.IUSS Working Group WRB. 2007. World reference base for soil resources 2006. First update 2007: a framework for international classification, correlation and communication. World Soil Resources Reports No. 103 Food and Agriculture Organization of the United Nations, Rome, Italy: http://www.fao.org/fileadmin/templates/nr/images/resources/pdf_documents/wrb2007_red.pdf. [Google Scholar]
- 30.Perroni Y, García-Oliva F, Souza V. 2014. Plant species identity and soil P forms in an oligotrophic grassland-desert scrub system. J Arid Environ 108:29–37. doi: 10.1016/j.jaridenv.2014.04.009. [DOI] [Google Scholar]
- 31.López-Lozano NE, Eguiarte LE, Bonilla-Rosso G, García-Oliva F, Martínez-Piedragil C, Rooks C, Souza V. 2012. Bacterial communities and the nitrogen cycle in the gypsum soil of Cuatro Ciénegas Basin, Coahuila: a Mars analogue. Astrobiology 12:699–709. doi: 10.1089/ast.2012.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee ZM, Steger L, Corman JR, Neveu M, Poret-Peterson AT, Souza V, Elser JJ. 2015. Response of a stoichiometrically imbalanced ecosystem to manipulation of nutrient supplies and ratios. PLoS One 10:e0123949. doi: 10.1371/journal.pone.0123949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lilienfein J, Qualls RG, Uselman SM, Bidgham SD. 2004. Adsorption of dissolved organic and inorganic phosphorus in soils of weathering chronosequence. Soil Sci Soc Am J 68:620–628. doi: 10.2136/sssaj2004.6200. [DOI] [Google Scholar]
- 34.Jones DL, Willett VB. 2006. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999. doi: 10.1016/j.soilbio.2005.08.012. [DOI] [Google Scholar]
- 35.Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:3136. [Google Scholar]
- 36.Vance ED, Brookes AC, Jenkinson DS. 1987. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. doi: 10.1016/0038-0717(87)90052-6. [DOI] [Google Scholar]
- 37.Joergensen RG. 1996. The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem 28:25–31. doi: 10.1016/0038-0717(95)00102-6. [DOI] [Google Scholar]
- 38.Brookes P, Landman A, Pruden G, Jenkinson D. 1985. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. doi: 10.1016/0038-0717(85)90144-0. [DOI] [Google Scholar]
- 39.Joergensen RG, Mueller T. 1996. The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEN value. Soil Biol Biochem 28:33–37. doi: 10.1016/0038-0717(95)00101-8. [DOI] [Google Scholar]
- 40.Anderson JPE, Domsch KM. 1978. A physiological method for the quantitative measurement of microbial biomass in soil. Soil Biol Biochem 10:215–221. doi: 10.1016/0038-0717(78)90099-8. [DOI] [Google Scholar]
- 41.Coleman DC, Anderson RV, Cole CV, Elliott ET, Woods L, Campion MK. 1978. Trophic interactions in soils as they affect energy and nutrient dynamics. V. Phosphorus transformations. Microb Ecol 4:381–387. [DOI] [PubMed] [Google Scholar]
- 42.Lathja K, Driscoll CT, Jarrell WM, Elliott ET. 1999. Soil phosphorus. Characterization and total element analysis, p 115–142. In Robertson GP, Coleman DC, Bledsoe CS, Sollins P (ed), Standard soil methods for long-term ecological research. Oxford University Press, New York, NY. [Google Scholar]
- 43.Tabatabai MA, Bremner JM. 1969. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307. doi: 10.1016/0038-0717(69)90012-1. [DOI] [Google Scholar]
- 44.Eivazi F, Tabatabai MA. 1977. Phosphatases in soils. Soil Biol Biochem 9:167–172. doi: 10.1016/0038-0717(77)90070-0. [DOI] [Google Scholar]
- 45.Eivazi F, Tabatabai MA. 1988. Glucosidases and galactosidases in soils. Soil Biol Biochem 20:601–606. doi: 10.1016/0038-0717(88)90141-1. [DOI] [Google Scholar]
- 46.Verchot L, Borelli T. 2005. Application of para-nitrophenol (pNP) enzyme assays in degraded tropical soils. Soil Biol Biochem 37:625–633. doi: 10.1016/j.soilbio.2004.09.005. [DOI] [Google Scholar]
- 47.Tapia-Torres Y, Elser JJ, Souza V, García-Oliva F. 2015. Ecoenzymatic stoichiometry at the extremes: how microbes cope in an ultra-oligotrophic desert soil. Soil Biol Biochem 87:34–42. doi: 10.1016/j.soilbio.2015.04.007. [DOI] [Google Scholar]
- 48.Cerritos R, Vinuesa P, Eguiarte LE, Herrera-Estrella L, Alcaraz-Peraza LD, Arvizu-Gomez JL, Olmedo G, Ramirez E, Siefert JL, Souza V. 2008. Bacillus coahuilensis sp. nov, a moderately halophilic species from a desiccation lagoon in the Cuatro Ciénegas Valley in Coahuila, Mexico. Int J Syst Evol Microbiol 58:919–923. doi: 10.1099/ijs.0.64959-0. [DOI] [PubMed] [Google Scholar]
- 49.Hulett FM, Bookstein C, Jensen K. 1990. Evidence for two structural genes for alkaline phosphatase in Bacillus subtilis. J Bacteriol 172:735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffman CS, Winston F. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 51.Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A 74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ewing B, Green P. 1998. Base-calling of automated sequencer traces using Phred. II. Error probabilities. Genome Res 8:186–194. [PubMed] [Google Scholar]
- 53.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 54.Olsen GJ, Overbeek R, Larsen N, Marsh TL, McCaughey MJ, Maciukenas MA, Kuan WN, Macke TJ, Xing Y, Woese CR. 1992. The Ribosomal Database Project. Nucleic Acids Res 20:2199–2200. doi: 10.1093/nar/20.suppl.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollisterm EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letunic I, Bork P. 2011. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res 39:W475–W478. doi: 10.1093/nar/gkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunt D, David LA, Gevers D, Preheim SP, Alm EJ, Polz MF. 2008. Resource partitioning and sympatric differentiation among closely related bacterioplankton. Science 320:1081–1085. doi: 10.1126/science.1157890. [DOI] [PubMed] [Google Scholar]
- 58.StatSoft. 2000. Statistica version 6.0 for Windows. StatSoft, Tulsa, OK. [Google Scholar]
- 59.Aitkin M, Anderson D, Francis B, Hinde J. 1989. Statistical modelling in GLIM. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 60.Gómez-Lunar Z, Hernández-González I, Rodríguez-Torres MD, Souza V, Olmedo-Álvarez G. 2016. Microevolution analysis of Bacillus coahuilensis unveils differences in phosphorus acquisition strategies and their regulation. Front Microbiol 7:58. doi: 10.3389/fmicb.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.González-Pastor JE, Hobbs EC, Losick R. 2003. Cannibalism by sporulating bacteria. Science 301:510–513. doi: 10.1126/science.1086462. [DOI] [PubMed] [Google Scholar]
- 62.Tani K, Nasu M. 2010. Roles of extracellular DNA in bacterial ecosystem, p 25–37. In Kikuchi Y, Rykova E (ed), Extracellular nucleic acids, vol 25 Nucleic acids and molecular biology. Springer, New York, NY. [Google Scholar]
- 63.Jørgensen NOG, Kroer N, Coffin RB, Yang XH, Lee C. 1993. Dissolved free amino acids, combined amino acids, and DNA as sources of carbon and nitrogen to marine bacteria. Mar Ecol Prog Ser 98:135–148. doi: 10.3354/meps098135. [DOI] [Google Scholar]
- 64.Martinez A, Tyson GW, DeLong EF. 2010. Widespread known and novel phosphonates utilization pathways in marine bacteria revealed by functional screening and metagenomic analyses. Environ Microbiol 12:222–238. doi: 10.1111/j.1462-2920.2009.02062.x. [DOI] [PubMed] [Google Scholar]
- 65.Panas P, Ternan NG, Dooley JSG, McMullan G. 2006. Detection of phosphonoacetate degradation and phnA genes in soil bacteria from distinct geographical origins suggest its possible biogenic origin. Environ Microbiol 8:939–945. doi: 10.1111/j.1462-2920.2005.00974.x. [DOI] [PubMed] [Google Scholar]
- 66.McDonald AE, Grant BR, Plaxton WC. 2001. Phosphite (phosphorous acid): its relevance in the environment and agriculture and influence on plant phosphate starvation response. J Plant Nutr 24:1505–1519. doi: 10.1081/PLN-100106017. [DOI] [Google Scholar]
- 67.Plaxton WC. 1998. Metabolic aspects of phosphate starvation in plants, p 229–241. In Lynch JP, Deikman J (ed), Phosphorus in plant biology: regulatory roles in molecular, cellular, organismic, and ecosystem processes. American Society of Plant Physiologists, Rockville, MD. [Google Scholar]
- 68.Adams F, Conrad JP. 1953. Transition of phosphite to phosphate in soils. Soil Sci 75:361–371. doi: 10.1097/00010694-195305000-00004. [DOI] [Google Scholar]
- 69.Ohtake H, Wu H, Imazu K, Anbe Y, Kato J, Kuroda A. 1996. Bacterial phosphonate degradation, phosphite oxidation and polyphosphate accumulation. Resour Conserv Recycling 18:25–134. doi: 10.1016/S0921-3449(96)01173-1. [DOI] [Google Scholar]
- 70.Martiny AC, Treseder K, Pusch G. 2013. Phylogenetic conservatism of functional traits in microorganisms. ISME J 7:830–838. doi: 10.1038/ismej.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Imazu K, Tanaka S, Kuroda A, Anbe Y, Kato J, Ohtake H. 1998. Enhanced utilization of phosphonate and phosphite by Klebsiella aerogenes. Appl Environ Microbiol 64:3754–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moreno-Letelier A, Olmedo G, Eguiarte LE, Martinez-Castilla L, Souza V. 2011. Parallel evolution and horizontal gene transfer of the pst operon in Firmicutes from oligotrophic environments. Int J Evol Biol 2011:781642. doi: 10.4061/2011/781642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martiny AC, Coleman ML, Chisholm SW. 2006. Phosphate acquisition genes in Prochlorococcus ecotypes: evidence for genome-wide adaptation. Proc Natl Acad Sci U S A 103:12552–12557. doi: 10.1073/pnas.0601301103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villarreal-Chiu JF, Quinn JP, McGrath JW. 2012. The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front Microbiol 3:19. doi: 10.3389/fmicb.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.