ABSTRACT
CMY-2 is the most common plasmid-mediated AmpC β-lactamase in Escherichia coli isolates of human and animal origin. The aim of this study was to elucidate the epidemiology of CMY-2-producing E. coli in Denmark. Strain and plasmid relatedness was studied in 93 CMY-2-producing clinical and commensal E. coli isolates collected from 2006 to 2012 from humans, retail poultry meat, broilers, and dogs. Multilocus sequence typing (MLST), antimicrobial susceptibility testing, and conjugation were performed in conjunction with plasmid replicon typing, plasmid multilocus sequence typing (pMLST), restriction fragment length polymorphism (RFLP), and sequencing of selected blaCMY-2-harboring plasmids. MLST revealed high strain diversity, with few E. coli lineages occurring in multiple host species and sample types. blaCMY-2 was detected on plasmids in 83 (89%) isolates. Most (75%) of the plasmids were conjugative and did not (96%) cotransfer resistance to antimicrobials other than cephalosporins. The main replicon types identified were IncI1-Iγ (55%) and IncK (39%). Isolates from different host species mainly carried distinct plasmid subtypes. Seven of the 18 human isolates harbored IncI1-Iγ/sequence type 2 (ST2), IncI1-Iγ/ST12, or IncK plasmids highly similar to those found among animal isolates, even though highly related human and animal plasmids differed by nonsynonymous single nucleotide polymorphisms (SNPs) or insertion sequence elements. This study clearly demonstrates that the epidemiology of CMY-2 can be understood only by thorough plasmid characterization. To date, the spread of this β-lactam resistance determinant in Denmark is mainly associated with IncK and IncI1-Iγ plasmids that are generally distributed according to host-specific patterns. These baseline data will be useful to assess the consequences of the increasing human exposure to CMY-2-producing E. coli via animal sources.
IMPORTANCE CMY-2 is the most common plasmid-mediated AmpC β-lactamase in Escherichia coli. This β-lactamase is poorly inhibited by clavulanic acid and confers resistance to cephamycins, third-generation cephalosporins, and aztreonam. Furthermore, resistance to carbapenems has been reported in E. coli as a result of production of plasmid-encoded CMY-2 β-lactamase in combination with decreased outer membrane permeability. The gene encoding CMY-2 generally resides on transferable plasmids belonging to different incompatibility groups. The prevalence of CMY-2-mediated cephalosporin resistance in E. coli varies significantly depending on the geographical region and host. This study demonstrates that the epidemiology of CMY-2 can be understood only by thorough plasmid characterization. To date, the spread of this β-lactam resistance determinant in Denmark is mainly associated with IncK and IncI1-Iγ plasmids, which are generally distributed according to host-specific patterns. These data will be useful to assess the consequences of the increasing human exposure to CMY-2-producing E. coli via animal sources.
INTRODUCTION
CMY-2 is the most common plasmid-mediated AmpC β-lactamase in Escherichia coli (1). This class C β-lactamase is poorly inhibited by clavulanic acid and confers resistance to cephamycins, third-generation cephalosporins, and aztreonam (2). Furthermore, resistance to carbapenems has been reported in E. coli as a result of production of plasmid-encoded CMY-2 β-lactamase in combination with decreased outer membrane permeability (3). The gene encoding CMY-2 (blaCMY-2) generally resides on transferable plasmids belonging to different incompatibility groups, including IncI1-Iγ, IncA/C, IncF, and IncK (4, 5).
The prevalence of CMY-2-mediated cephalosporin resistance in E. coli varies significantly depending on the geographical region and host (6, 7). In Denmark, the burden of resistance to third-generation cephalosporins in human infections is only marginally influenced by CMY-2, with an estimated prevalence lower than 1% (8). Among E. coli isolates of animal origin, CMY-2 was the most commonly detected cephalosporin resistance determinant in Danish (83%) and imported (42%) broiler meat until 2013 (9). However, in 2014, a reduction in the occurrence of CMY-2-producing E. coli was observed in both Danish (23%) and imported (33%) broiler meat, most likely as a result of discontinued use of third-generation cephalosporins in hatcheries at the top of the broiler production pyramid (10). On the other hand, blaCMY-2 is rarely detected in cephalosporin-resistant E. coli from Danish pigs and cattle (11). Poultry has been allegedly indicated as a possible source of blaCMY-2 in humans. In Sweden, similar blaCMY-2-carrying IncK plasmids were detected in genetically unrelated E. coli isolates from broilers, broiler meat, and human clinical isolates, suggesting possible plasmid-mediated zoonotic transmission of blaCMY-2 (12, 13). In the Netherlands, highly related IncI1-Iγ and IncK plasmids harboring blaCMY-2 were found in human, broiler, and broiler meat isolates (14, 15). Additionally, this resistance determinant occurs at high frequencies among E. coli isolates from dogs with an AmpC phenotype (16).
The objective of this study was to investigate possible epidemiological pathways of transmission of CMY-2 β-lactamase across humans, dogs, and poultry in Denmark.
MATERIALS AND METHODS
Strain collection.
The bacterial isolates characterized in this study were 93 blaCMY-2-positive E. coli samples collected in Denmark in the period from 2006 to 2012. The collection included one isolate from each of 18 humans (16 patients and 2 healthy individuals), 33 poultry meat products (25 broiler meat and 8 turkey meat), 25 chicken flocks (11 healthy broiler flocks and 14 healthy parent flocks), and 17 dogs (7 patients and 10 healthy individuals) (Table 1). The human clinical isolates were collected at the Department of Clinical Microbiology at Hvidovre Hospital (HH) as part of point prevalence studies carried out in October 2006, 2009, and 2011 (17). Isolates from healthy humans were collected in 2008 and kindly provided by the Statens Serum Institut (18). The isolates of poultry origin were kindly provided by the National Food Institute, Technical University of Denmark (DTU). The poultry meat isolates included (i) all available isolates (n = 13) from Danish broiler meat and a subset (n = 12) of isolates from imported broiler meat (representing different countries and, when applicable, different slaughterhouses within a country) obtained in the national surveillance program (DANMAP) in 2009 and 2010 (19–21) and (ii) isolates (n = 8) from turkey meat imported from Germany (the main source of turkey meat consumed in Denmark) representing all five batches positive for CMY-2-producing E. coli out of 69 batches randomly collected at Danish retail outlets in 2011. Isolates from Danish broiler flocks and parent flocks were collected in 2010 and 2011, respectively, and encompassed the majority (29 out of 35) of E. coli pulsed-field gel electrophoresis (PFGE) types previously identified (22). The clinical canine isolates were collected at the veterinary diagnostic laboratory of the University of Copenhagen between 2008 and 2012, whereas the isolates from healthy dogs originated from fecal samples collected in 2007 and 2008 (23). All the isolates originated from different individuals and were confirmed to carry blaCMY-2 by PCR and sequencing (Macrogen Inc.) using primers and under conditions previously described (24).
TABLE 1.
Characteristics of blaCMY-2-harboring plasmids among E. coli isolates from humans, animals, and meat in Denmark
| Isolatea | Host species | Isolation sample | Yr of isolation | Country of originp | Plasmid characterization |
Multilocus sequence type | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PBRTq | Plasmid size (kb)b | pMLSTc | pRFLPd | Transferability of blaCMY-2 | Cotransferred resistancer | ||||||
| C-6k | Human | Urine | 2009 | DK | I1-Iγ | 86 | 2 | A1 | Positive | None | 657 |
| R1AC | Dog | Feces | 2008 | DK | I1-Iγ | 86 | 2 | A1 | Positive | None | 2196 |
| R2AC | Dog | Feces | 2008 | DK | I1-Iγ | 86 | 2 | A1 | Positive | None | 2168 |
| R3AC | Dog | Feces | 2008 | DK | I1-Iγ | 86 | 2 | A1 | Positive | None | 746 |
| R7AC | Dog | Feces | 2008 | DK | I1-Iγ | 86 | 2 | A1 | Positive | None | 297 |
| R13AC | Dog | Feces | 2008 | DK | I1-Iγ | 86 | 2 | A1 | Positive | None | 457 |
| C-28397k | Dog | Urine | 2011 | DK | I1-Iγ | 86 | 2 | A1 | Negative | None | 68 |
| C-29199k | Dog | Urine | 2012 | DK | I1-Iγ | 86 | 2 | A1 | Positive | None | 2558 |
| C-30029k | Dog | Abdominal fluid | 2012 | DK | I1-Iγ | 86 | 2 | A1 | Positive | None | 3574 |
| C-30104k | Dog | Ear | 2012 | DK | I1-Iγ | 86 | 2 | A1 | Positive | None | 372 |
| R6AC | Dog | Feces | 2008 | DK | I1-Iγ | 86 | 23e | A1 | Positive | None | 963 |
| A1 | Dog | Feces | 2008 | DK | I1-Iγ | 86 | 2 | A2 | Positive | None | 68 |
| R5AC | Dog | Feces | 2008 | DK | I1-Iγ | 86 | 2 | NT | Positive | None | 546 |
| J20 | Dog | Feces | 2007 | DK | I1-Iγ | 86 | 2 | NT | Positive | None | 2144 |
| C-9k | Human | Urine | 2009 | DK | I1-Iγ | 86 | 116f | D1 | Negative | None | 963 |
| C-4k | Human | Urine | 2009 | DK | I1-Iγ | 82 | 55 | C1 | Positive | None | 1193 |
| C-21k | Human | Urine | 2011 | DK | I1-Iγ | 82 | 55 | C2 | Positive | None | 1193 |
| C-22k | Human | Urine | 2011 | DK | I1-Iγ | 100 | 12 | B1 | Negative | None | 484 |
| 1061-1 | Broiler | Meat | 2010 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 10m |
| 5499-28 | BFn | Feces | 2010 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 1056m |
| 5498-25 | BF | Feces | 2010 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 219m |
| 7077-63 | BF | Feces | 2010 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 115m |
| 5498-4 | BF | Feces | 2010 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 212m |
| 2028-8 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 10m |
| 2067-2 | PFo | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 131m |
| 2054-7 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 48m |
| 2028-4 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 4048 |
| 2028-7 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 745m |
| C-24716k | Dog | Skin | 2008 | DK | I1-Iγ | 100 | 12 | B1 | Positive | None | 405 |
| C-20k | Human | Urine | 2011 | DK | I1-Iγ | 100 | 12 | B2 | Positive | None | 155 |
| 5499-25 | BF | Feces | 2010 | DK | I1-Iγ | 100 | 12 | B3 | Positive | None | 1775m |
| 7077-60 | BF | Feces | 2010 | DK | I1-Iγ | 100 | 12 | B3 | Positive | None | 410m |
| 2123-1 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B3 | Positive | None | 350m |
| 2115-5 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B4 | Positive | None | 616m |
| 5499-19 | BF | Feces | 2010 | DK | I1-Iγ | 100 | 12 | B5 | Positive | None | 746m |
| 2028-14 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B6 | Positive | None | 3272m |
| 2067-1 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B7 | Negative | None | 88m |
| 2054-6 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B8 | Positive | None | 206m |
| 2028-10 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 12 | B9 | Positive | None | 1303m |
| 7075-31 | Broiler | Meat | 2010 | DK | I1-Iγ | 86 | 12 | B10 | Positive | None | 10m |
| 2054-3 | PF | Feces | 2011 | DK | I1-Iγ | 100 | 66g | B11 | Positive | None | 1585m |
| C-24k | Human | Urine | 2011 | DK | I1-Iγ | 65 | NTh | E1 | Negative | None | 155 |
| C-28464k | Dog | Biopsy | 2011 | DK | I1-Iγ | 65 | NTi | F1 | Negative | None | 361 |
| C-29870k | Dog | Skin | 2012 | DK | I1-Iγ | 55 | NTj | G1 | Negative | None | 448 |
| C-8k | Human | Skin | 2006 | DK | K | 78 | A1 | Positive | None | 46 | |
| 3786Z08 | Human | Feces | 2008 | DK | K | 78 | A1 | Positive | None | 1822 | |
| 3823Z08 | Human | Feces | 2008 | DK | K | 78 | A1 | Negative | None | 1800 | |
| C-12k | Human | Urine | 2009 | DK | K | 78 | A1 | Negative | None | 117 | |
| 637 | Turkey | Meat | 2011 | DE | K | 78 | A1 | Negative | None | 117 | |
| 671 | Turkey | Meat | 2011 | DE | K | 78 | A1 | Negative | None | 117 | |
| 885-01 | Broiler | Meat | 2009 | DK | K | 78 | A1 | Positive | None | 69m | |
| 1472-03 | Broiler | Meat | 2009 | DK | K | 78 | A1 | Positive | None | 4243l | |
| 2285-1 | Broiler | Meat | 2009 | DE | K | 78 | A1 | Positive | None | 115 | |
| 99-1 | Broiler | Meat | 2010 | DE | K | 78 | A1 | Positive | None | 115 | |
| 131-1 | Broiler | Meat | 2010 | DE | K | 78 | A1 | Positive | None | 1640 | |
| 2510-6 | Broiler | Meat | 2010 | DE | K | 78 | A1 | Positive | None | 1594 | |
| 1967-1 | Broiler | Meat | 2009 | FR | K | 86 | A1 | Positive | None | 1594 | |
| 821-1 | Broiler | Meat | 2009 | DE | K | 78 | A2 | Positive | None | 4124l | |
| 648-1 | Broiler | Meat | 2009 | BR | K | 78 | A3 | Positive | None | 38 | |
| 1239-2 | Broiler | Meat | 2010 | DK | K | 74 | B1 | Negative | None | 38 | |
| 6870-55 | Broiler | Meat | 2010 | DK | K | 74 | B1 | Positive | None | 38 | |
| 7075-4 | Broiler | Meat | 2010 | DK | K | 74 | B1 | Positive | None | 38 | |
| 7622-28 | Broiler | Meat | 2010 | DK | K | 74 | B1 | Negative | None | 38 | |
| 7625-26 | Broiler | Meat | 2010 | DK | K | 74 | B1 | Positive | None | 38 | |
| 9832-4 | Broiler | Meat | 2010 | DK | K | 74 | B1 | Positive | None | 38 | |
| 131-4 | Broiler | Meat | 2010 | DK | K | 74 | B1 | Positive | None | 38 | |
| 7077-57 | BF | Feces | 2010 | DK | K | 74 | B1 | Positive | None | 38m | |
| 2120-1 | PF | Feces | 2011 | DK | K | 74 | B1 | Positive | None | 38m | |
| 7075-19 | Broiler | Meat | 2010 | DK | K | 74 | B1 | Positive | None | 115m | |
| 7077-11 | BF | Feces | 2010 | DK | K | 74 | B1 | Positive | None | 23m | |
| 5499-9 | BF | Feces | 2010 | DK | K | 74 | B1 | Positive | None | 69m | |
| 7077-7 | BF | Feces | 2010 | DK | K | 74 | B1 | Negative | None | 1594m | |
| 2054-5 | PF | Feces | 2011 | DK | K | 74 | B1 | Positive | None | 1518m | |
| 1184-1 | Broiler | Meat | 2009 | FR | K | 78 | D1 | Negative | None | 93 | |
| 2075-10 | Broiler | Meat | 2010 | PL | K | 78 | C1 | Positive | None | 4125l | |
| C-1k | Human | Urine | 2006 | DK | A/C | 204 | A | Positive | CHL, GEN, TET | 48 | |
| 1428-1 | Broiler | Meat | 2009 | DE | A/C | 86 | B | Negative | TET | 117 | |
| 458 | Turkey | Meat | 2011 | DE | FII | 64 | 29 | Positive | None | 4240l | |
| 456 | Turkey | Meat | 2011 | DE | FII, R | 104 | Positive | CHL, SXT, TET | 1196 | ||
| C-23k | Human | Urine | 2011 | DK | NT | 82 | Negative | None | 131 | ||
| 7075-13 | Broiler | Meat | 2010 | DK | ND1 | 68, 91, 104 | Positive | 23 | |||
| 243-1 | Broiler | Meat | 2010 | DE | ND1 | 78, 138 | Positive | 115 | |||
| R4AC | Dog | Feces | 2008 | DK | ND1 | 24, 110, 128 | Positive | 38 | |||
| C-2k | Human | Urine | 2006 | DK | ND2 | ND2 | Positive | 652 | |||
| C-3k | Human | Urine | 2006 | DK | ND2 | ND2 | Negative | 448 | |||
| C-10k | Human | Urine | 2009 | DK | ND2 | ND2 | Negative | 448 | |||
| C-11k | Human | Urine | 2009 | DK | ND2 | ND2 | Negative | 117 | |||
| C-13k | Human | Urine | 2009 | DK | ND2 | ND2 | Negative | 410 | |||
| 6870-58 | Broiler | Meat | 2010 | DK | ND2 | ND2 | Positive | 2040l | |||
| 457 | Turkey | Meat | 2011 | DE | ND2 | ND2 | Negative | 1196 | |||
| 459 | Turkey | Meat | 2011 | DE | ND2 | ND2 | Negative | 1196 | |||
| 444 | Turkey | Meat | 2011 | DE | ND2 | ND2 | Positive | 428 | |||
| 683 | Turkey | Meat | 2011 | DE | ND2 | ND2 | Positive | 919 | |||
Isolates whose blaCMY-2-positive plasmids were sequenced are in boldface.
Plasmid size was deduced by comparing the migration of the transformant's bands with that of the closest corresponding marker's bands.
pMLST was performed on IncI1-Iγ and IncFII plasmids.
pRFLP was performed on plasmid types shared by different host species.
SLV of ST2.
Novel sequence type, SLV of ST2.
SLV of ST12.
No detection of the sogS allele; SLV of ST55.
Novel ardA allele (ardA, 20) and no detection of the sogS allele. The remaining allele variants were repI1, 4; trbA, 15; pilL, 3.
No detection of both the sogS and the pilL alleles. The remaining allele variants were repI1, 4; ardA, 5; trbA, 15.
Clinical isolate.
New ST identified in this study.
MLST data previously reported (22).
BF, broiler flock.
PF, parent flock.
DK, Denmark; DE, Germany; FR, France; BR, Brazil; PL, Poland.
NT, nontypeable; ND1, not determined, as more than one plasmid was present in the transformant; ND2, not determined, as the plasmid could not be transformed.
CHL, chloramphenicol; GEN, gentamicin; SXT, trimethoprim-sulfamethoxazole; TET, tetracycline.
Strain typing.
Multilocus sequence typing (MLST) analysis of four Danish broiler meat isolates and all but one isolate from broiler chickens was performed as part of a previous study (22). The remaining isolates were genotyped by MLST in this study according to published methods (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli/).
blaCMY-2 transferability and plasmid typing.
Transferability of blaCMY-2-harboring plasmids was analyzed by filter-mating experiments using a rifampin-resistant, lactose-negative E. coli K-12 J62-2 strain as the recipient (25). Transconjugants were selected on MacConkey agar (Merck, Denmark) supplemented with 2 mg/liter cefotaxime (CTX) and 25 mg/liter rifampin and were tested by colony PCR to confirm the presence of blaCMY-2. Plasmids carrying blaCMY-2 were typed following transfer of plasmid DNA purified either by the PureLink HiPure Plasmid Midiprep kit (Invitrogen, Denmark) or by alkaline lysis into electrocompetent Genehog E. coli (Invitrogen, Denmark) by electroporation. Transformants were selected on brain heart infusion agar (Oxoid, Denmark) supplemented with 2 mg/liter CTX and tested by colony PCR to confirm the presence of blaCMY-2. The number and sizes of plasmids were determined by PFGE after S1 nuclease (Thermo Scientific, Sweden) digestion of whole genomic DNA of transformants (26). Transformants positive for blaCMY-2 and carrying a single plasmid were further characterized by PCR-based replicon typing (PBRT) (27) using a commercially available kit (Diatheva, Italy). Plasmid multilocus sequence typing (pMLST) was performed for plasmids belonging to typeable groups (28). The sequences obtained were assembled with CLC Main Workbench 6.8.4 (CLC bio, Denmark) and compared to sequences deposited in the pMLST database (http://pubmlst.org/plasmid/). Plasmid types shared by different host species were further characterized by restriction fragment length polymorphism (RFLP) using the following FastDigest enzymes (Thermo Scientific, Sweden): (i) BglII and PstI for IncI1-Iγ plasmids; (ii) EcoRV and SalI for IncK plasmids; and (iii) BamHI, HindIII, and SalI for IncA/C plasmids. Restriction profiles were visualized on a 0.8% agarose gel, and band patterns were visually compared to define indistinguishable and closely related subtypes differing by two or three bands. Each RFLP type was assigned a capital letter code, followed by a number indicating closely related subtypes (e.g., A1, A2, and A3).
In order to detect cotransfer of resistance to antimicrobial agents other than cephalosporins, transformants were phenotypically tested by disk diffusion according to Clinical and Laboratory Standards Institute (CLSI) standards (29). The following discs (Oxoid, Denmark) were used: amoxicillin-clavulanic acid (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), imipenem (10 μg), trimethoprim-sulfamethoxazole (25 μg), and tetracycline (30 μg). E. coli ATCC 25922 was used as a quality control strain.
Selected indistinguishable and closely related plasmids found in both human and animal isolates were sequenced using either Illumina MiSeq (Illumina, USA) or the 454-Genome Sequencer FLX procedure (Roche Diagnostic, Monza, Milan). Plasmid DNA was extracted from transformants with the PureLink HiPure Plasmid Midiprep kit (Invitrogen, Denmark). For Illumina MiSeq sequencing, libraries were prepared using the Nextera XT DNA sample preparation kit (Illumina, USA) and sequenced using 150-bp paired-end reads. Contigs were assembled using Velvet (v1.0.11) and Velvet Optimizer (v2.1.7). For 454-generated sequences, contigs were obtained using the GS De Novo Assembler software and assembled in silico by the 454 ReadStatus output file identifying reads overlapping adjacent contigs. Open reading frames (ORFs) were predicted and annotated using Artemis software version 8 (Sanger Institute; http://www.sanger.ac.uk/resources/software/artemis/ngs/), and each predicted protein was compared to the all-protein database at the National Center for Biotechnology Information (NCBI) using BLASTP (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins). IncI1 plasmid R64 (GenBank accession no. NC_005014.1) and IncK plasmid pCT (GenBank accession no. FN868832.1) were used as references for annotating the plasmid sequences. Plasmid genome comparisons were performed using CLC Genomics Workbench v.7. Nucleotide BLAST at NCBI was used to determine the level of identity with publicly available plasmid sequences. ISfinder was used for identification of insertion sequences (ISs) (30).
Nucleotide sequence accession numbers.
Sequence data for pR7AC and pC-6 are available at GenBank under accession numbers KF434766 and KT186369, respectively. Sequence data for the remaining plasmids were deposited in the European Nucleotide Archives under the study accession number PRJEB9625.
RESULTS
Strain diversity.
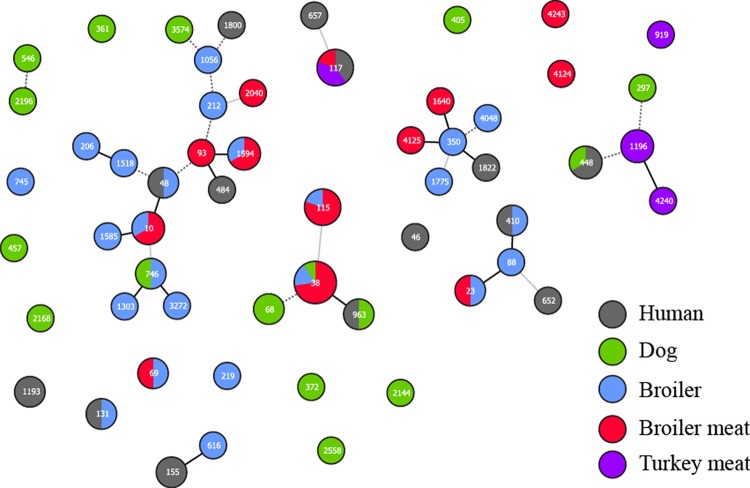
The 93 E. coli isolates belonged to 57 sequence types (STs), with five isolates (5%) displaying new STs (ST2040, ST4124, ST4125, ST4240, and ST4243) (Fig. 1). Overall, high strain diversity was observed across the different host species, with few exceptions. ST117 was detected among isolates from human patients (n = 2) and imported broiler (n = 1) and turkey (n = 2) meat. ST48, ST131, and ST410 occurred in single isolates from humans and broilers. ST448 and ST963 were detected in isolates of human and canine origin. ST38 strains were identified in broiler parents (n = 1), broilers (n = 1), Danish (n = 7) and imported (n = 1) broiler meat, and healthy dogs (n = 1) (Table 1). ST10 strains were identified in broiler parents (n = 1) and Danish broiler meat (n = 2). Finally, ST115 was identified in broilers (n = 1) and in Danish (n = 1) and imported (n = 2) broiler meat.
FIG 1.
Multilocus sequence typing patterns of blaCMY-2-carrying E. coli isolates from humans, dogs, broilers, and poultry meat in Denmark. Sequence types are shown as numbers. Black connecting lines indicate single-locus variants, gray connecting lines indicate double-locus variants, and dashed gray connecting lines indicate triple-locus variants. The figure was generated using PHYLOViZ software (46).
Plasmid transferability and diversity.
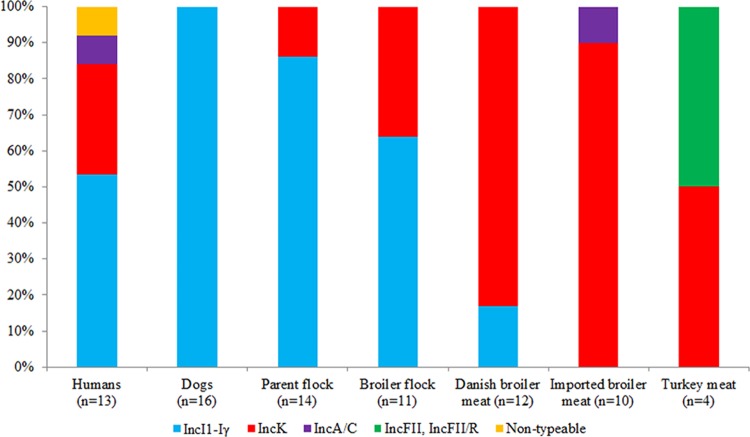
Conjugative transfer of blaCMY-2 was observed for 70 (75%) out of 93 isolates (Table 1). Transformation experiments indicated that blaCMY-2 was plasmid borne in at least 83 (89%) isolates. Three transformants harbored multiple plasmids and were not further analyzed. The remaining 80 plasmids ranged in size from approximately 55 kb to 204 kb and displayed the following incompatibility groups: IncI1-Iγ (n = 44; 55%); IncK (n = 31; 39%), IncA/C (n = 2; 3%), and IncFII (n = 1; 1%). Apparently one (1%) transformant harbored a plasmid positive for both IncFII and IncR, as suggested by the results of S1 PFGE (one band detected) and PBRT (two replicons detected), and one (1%) transformant was nontypeable by PBRT (Table 1). IncI1-Iγ and IncK plasmids were detected in seven (54%) and four (31%) of the 13 (72%) human isolates for which transfer of blaCMY-2 succeeded. IncI1-Iγ was the dominant replicon type among isolates from the parent flocks (86%), the broiler flocks (64%), and dogs (100%), while IncK was prevalent among isolates from Danish (83%) and imported (90%) broiler meat (Fig. 2).
FIG 2.
Replicons detected by PBRT in blaCMY-2-harboring plasmids among E. coli isolates from different host species for which transfer of blaCMY-2 succeeded.
Cotransfer of resistance to non-β-lactam antimicrobials was limited to three (4%) plasmids (Table 1). Both IncA/C plasmids cotransferred resistance to tetracycline, and one of them also cotransferred resistance to chloramphenicol and gentamicin. The IncFII/R multireplicon plasmid originating from imported turkey meat cotransferred resistance to chloramphenicol, trimethoprim-sulfamethoxazole, and tetracycline (Table 1).
IncI1-Iγ plasmids.
Subtyping of IncI1-Iγ plasmids by pMLST revealed two dominant sequence types: ST12 (n = 23; 52%) was identified in E. coli isolates from all sources except healthy humans and healthy dogs, and ST2 (n = 13; 30%) was found in E. coli isolates from a human patient and dogs (Table 1). The remaining STs were one single-locus variant (SLV) of ST12 (ST66) in a broiler parent isolate and different SLVs of ST2 (ST23, ST55, and the newly identified ST116) in human and canine isolates. Three IncI1-Iγ plasmids were not typeable by pMLST, as some target genes were not amplified (Table 1). Among the 23 IncI1-Iγ/ST12 plasmids examined by RFLP (see Fig. S1 and S2 in the supplemental material), 12 yielded indistinguishable RFLP profiles, designated subtype B1 (human patient, n = 1; Danish broiler meat, n = 1; broilers, n = 4; broiler parents, n = 5; and canine patient, n = 1) (Table 1). These plasmids measured approximately 100 kb by S1 PFGE. The remaining 11 IncI1-Iγ/ST12 plasmids mainly exhibited unique RFLP profiles (Table 1).
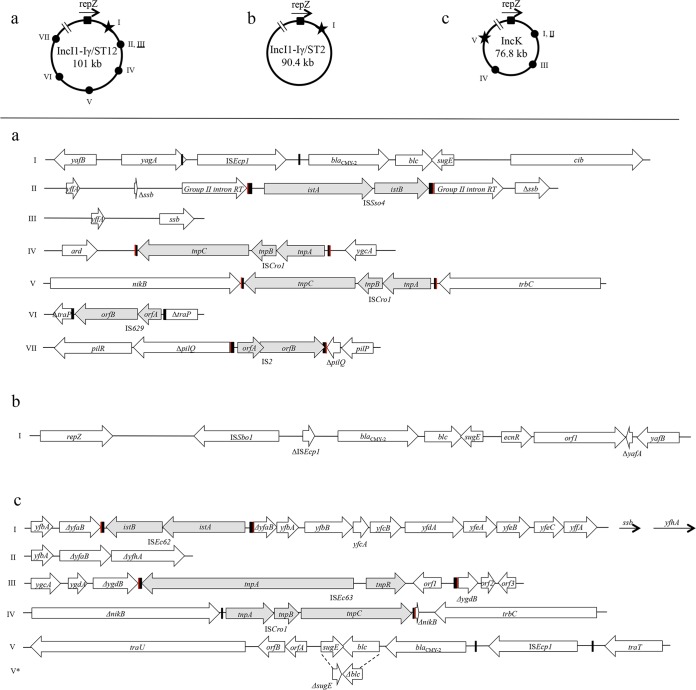
Sequences were obtained for 11 IncI1-Iγ/ST12 plasmids (Table 1). Sequence assembly generated contigs ranging from 99,835 bp (p2054-3) to 105,364 bp (p2028-14). These plasmids closely resembled the reference IncI1 plasmid, R64, in the organization of the backbone structure comprised of approximately 76 kbp, including the IncI1 plasmid replicon, conjugative-transfer regions (tra, trb, and pil operons), genes encoding plasmid partitioning and stability proteins (parAB, stbAB, and psiAB), and proteins involved in DNA modification and processing (nikAB and impAB). Comparative analysis of the plasmid sequences in this study showed high similarity, not only in the backbone structure, but also in the accessory region. Nine plasmids from human (n = 1), broiler parents (n = 6), broilers (n = 1), and broiler meat (n = 1) had a maximum of nine single nucleotide polymorphisms (SNPs) across 101 kbp and differed mainly in the integration of different ISs at different sites of the plasmid backbone (Fig. 3a). The remaining plasmids from broilers (p7077-60) and broiler parents (p2054-3) differed from the previous group by numerous SNPs accumulated in specific regions. The genetic context of blaCMY-2 consisted of an intact ISEcp1 upstream and blc followed by sugE downstream. This structure was inserted between a repZ-yafB region upstream and a colicin Ib-synthesizing gene downstream. These I1-Iγ/ST12 plasmids shared at least 94% of their nucleotide sequences (with 99% identity) with pCVM29188_101 (NC_011077.1) from a Salmonella enterica serovar Kentucky isolate from poultry.
FIG 3.
Representation of the genetic context of blaCMY-2 and insertions/deletions within otherwise identical plasmid sequences in E. coli across different host species. The diagrams at the top illustrate the positions of blaCMY-2 (indicated by a star) and of insertions/deletions (indicated by circles) in the different plasmids compared to repZ (indicated by a square, with arrowheads showing the direction of transcription). Distances from repZ are not drawn to scale. Each specific genetic context is identified by a roman numeral and is illustrated in the panels below. Underlined roman numerals indicate deletions. In the bottom panels, red and black rectangles represent direct repeats and inverted repeats, respectively, whereas gray shading indicates inserted genes. (a) IncI1-Iγ/ST12 plasmids. I, blaCMY-2 genetic context conserved across all sequenced plasmids; II, p2028-14 (parent flock origin); III, pC20 (human origin); IV, p2028-10 (parent flock origin); V, pC20 (human origin); VI, p2067-1 (parent flock origin); VII, p2054-6 (parent flock origin). (b) IncI1-Iγ/ST2 plasmids. I, blaCMY-2 genetic context conserved across all sequenced plasmids. (c) IncK plasmids. I, p2075-10 (Polish broiler meat origin) (ISEc62 is a new IS belonging to the IS21 family); II, p131-4 (Danish broiler meat origin), p2120-1 (parent flock origin), p5499-9 (broiler flock origin); III, p1967-1 (French broiler meat origin) (ISEc63 is a novel element belonging to the Tn3 transposon family); IV, p1184-1 (French broiler meat origin); V, blaCMY-2 genetic context conserved across all sequenced plasmids except V*. orfA and orfB code for hypothetical proteins and have not been associated with blaCMY-2 previously. V*, p1184-1 (broiler meat origin), in which deletions of 253 bp at the 3′ end of blc and 132 bp at the 3′ end of sugE were observed.
Among the 13 IncI1-Iγ/ST2 plasmids examined by RFLP (see Fig. S1 and S2 in the supplemental material), plasmids from one human patient and nine dogs (canine patients, n = 4; healthy individuals, n = 5) yielded indistinguishable RFLP profiles (subtype A1). Additionally, an IncI1-Iγ/ST23 plasmid from a healthy dog also displayed RFLP subtype A1. The RFLP profiles among the remaining 10 plasmids belonging to IncI1-Iγ were unique, or the plasmids were nontypeable by RFLP despite repeated analysis (Table 1). Sequencing of a human (pC-6) and a canine (pR7AC) IncI1-Iγ/ST2/A1 plasmid yielded partial sequences of 90,486 and 90,449 bp, respectively, closely resembling (86% coverage; 99% nucleotide sequence identity) the reference IncI1 plasmid, R64, in the organization of the backbone structure. Such sequences were identical except for (i) 36 additional nucleotides in pC-6 representing part of the shufflon region (not included in the pR7AC sequence) and (ii) a nonsynonymous SNP in both ychA and repA4. blaCMY-2 was inserted within the repZ-yafB region and had an ISSboI-ΔISEcp1 upstream and a blc-sugE-orf1 segment downstream (Fig. 3b). These plasmids exhibited 99% nucleotide sequence identity (96% coverage) to pCVM22462 (GenBank accession no. CP009566.1) from an S. enterica serovar Newport isolate from a dog in the United States.
IncK plasmids.
Two major clusters of IncK plasmids were identified by RFLP (see Fig. S3 and S4 in the supplemental material): subtypes A1 (42%) and B1 (45%). Subtype A1 was identified in four human (patients, n = 2; healthy individuals, n = 2) and nine poultry meat (Danish broiler meat, n = 2; imported broiler meat, n = 5; turkey meat, n = 2) plasmids. These plasmids measured approximately 78 kb, with the exception of an 86-kb plasmid from imported broiler meat (1967-1) (Table 1). RFLP subtype B1 was identified in plasmids from Danish broiler meat (n = 9), broilers (n = 4), and broiler parents (n = 2). The remaining four IncK plasmids yielded unique restriction profiles. Sequences were obtained for 12 IncK plasmids (Table 1) and ranged from 79,431 bp (p2120-1) to 90,439 bp (p1967-1). These plasmids closely resembled the reference pCT plasmid in the organization of the backbone structure comprised of approximately 68 kbp, including conjugative-transfer regions (tra, trb, and pil operons), genes encoding an endonuclease (parB), proteins involved in DNA modification and processing (nikAB and impABC), and proteins involved in inhibition of the SOS response (psiAB). The target of the PCR for IncK replicon typing differed from pCT by three SNPs (5G→A, 10T→C, and 135C→G) identified in all sequenced plasmids and one additional SNP identified in a subset of plasmids only (13G→A in p2120-1, p5499-9, p131-4, and p1967-1; 138G→T in p637). Comparative analysis showed high homology across the plasmid sequences in this study. Within 76,863 bp identified in all sequenced plasmids, between 1 and 46 SNPs were identified, and this difference was reduced to a maximum of 14 SNPs by excluding the most divergent plasmid (p648-1) from the comparison. In p648-1, SNPs were mainly accumulated in three specific regions (conceptually translated as hypothetical proteins). These plasmids mainly differed in various insertions/deletions represented in Fig. 3c. The genetic context of blaCMY-2 consisted of an intact ISEcp1 upstream and blc-sugE elements downstream. Interestingly, in p1184-1, deletion of the last 253 bp of blc and the last 132 bp of sugE was observed. Further downstream, two open reading frames coding for hypothetical proteins not previously associated with blaCMY-2 were detected. These elements were embedded between traT and traU. The plasmids exhibited 99% nucleotide sequence identity (96% to 99% coverage) to p53C_3 (BioProject accession no. PRJNA260957) from an E. coli isolate from chicken meat in the Netherlands in 2010 and 99% nucleotide sequence identity (88% to 100% coverage) to pNVI1292 (GenBank accession no. KU312044) from an E. coli isolate from chicken meat in Norway in 2012.
IncA/C plasmids.
Subtyping of IncA/C plasmids by RFLP showed distinct plasmid subtypes in E. coli isolates from a human patient and imported broiler meat (Table 1).
DISCUSSION
This study investigated the epidemiology of CMY-2-producing E. coli in Denmark in a set (n = 93) of isolates collected between 2006 and 2012 from humans and from the main animal reservoirs of CMY-2 known to date, namely, chickens, chicken meat, and dogs (7). The study provides molecular epidemiology data supporting the results of Carmo et al. (31), who concluded that the relative contribution of meat to infections by extended-spectrum β-lactamase (ESBL)/AmpC-producing E. coli in humans in Denmark was limited and indicated broiler meat as the main food source contributing to human exposure to this type of bacteria, especially CMY-2-producing E. coli. Indeed, only 4 of the 16 human clinical isolates analyzed in our study harbored the specific IncI1-Iγ/ST12 and IncK plasmid types linked to broiler meat (Table 1). Altogether, the results show that the occurrence of CMY-2-producing E. coli in different host species is mostly shaped by independent processes, as indicated by the presence of blaCMY-2 on distinct plasmid vectors shared to a minor extent across different host species.
CMY-2-encoding plasmids in human isolates showed a relatively high level of diversity, with at least four plasmid types and five plasmid subtypes detected in 13 isolates, which may indicate plasmid acquisition from multiple sources and various selective pressures favoring different E. coli strains and plasmids in individual hosts. Similar findings were reported in studies from the United States, Canada, and Norway (5, 16, 32). On the other hand, CMY-2-encoding plasmids identified in Danish dogs and Danish poultry showed limited diversity within the respective host species. The majority (n = 12; 71%) of canine isolates harbored blaCMY-2 on IncI1-Iγ/ST2 plasmids, also a predominant plasmid type in E. coli isolates from healthy dogs in France (33). This finding differs from reports of blaCMY-2 on a broad variety of plasmid types in canine isolates from the United States, Italy, and South Korea (16, 28, 34), highlighting geographical differences in regard to the plasmid vectors carrying blaCMY-2 in E. coli of canine origin. The reasons for these geographical differences remain unexplained, though they might be linked to different practices in antimicrobial use. In Danish broiler and broiler meat isolates, blaCMY-2 was nearly exclusively associated with IncI1-Iγ/ST12 and IncK plasmids. Notably, the majority (83%) of Danish broiler meat isolates harbored blaCMY-2 on IncK plasmids, while IncI1-Iγ seemed to be the prevalent plasmid among Danish broilers. However, this observation is biased by the criteria used for strain selection, as each isolate from Danish broilers represented a different E. coli PFGE type but the distribution of isolates within PFGE types was uneven. Indeed, a single PFGE type associated with IncK plasmids encompassed approximately 40% of blaCMY-2-producing E. coli isolates from Danish broilers in the previous study (22).
Plasmid sequencing allowed us to discover that human and animal plasmids indistinguishable by RFLP presented a limited number of nonsynonymous SNPs and the presence/absence of ISs, which may have biological significance. One example is a nonsynonymous SNP in repA4 found in human and canine IncI1-Iγ/ST2 plasmids. Such a difference may affect vertical plasmid transfer, as it has been previously shown that repA4 mutations cause unstable inheritance of IncF plasmids. However, no such studies have been performed on IncI1-Iγ plasmids to date (35). A further example is p2067-1, an IncI1-Iγ/ST12 plasmid isolated from the broiler parent flock that was unique for the presence of IS629 elements disrupting traP. Insertion mutations in traP have been shown to completely abolish transfer activity of R64, the IncI1 prototype plasmid (36), and indeed, p2067-1 did not transfer by conjugation (Table 1). Similarly, the IncK plasmid p1184-1 isolated from imported broiler meat had IS66 elements causing partial deletion of NikB, which is indispensable for transfer of R64 (37), and accordingly, it did not transfer by conjugation. Identification of different genetic environments of blaCMY-2 among IncI1-Iγ/ST2, IncI1-Iγ/ST12, and IncK plasmids suggested that at least IncI1-Iγ/ST2 and IncI1-Iγ/ST12 plasmids acquired the resistance gene independently, while the possibility that IncK plasmids acquired the ISEcp1-sugE structure from I1-Iγ/ST12 plasmids or vice versa cannot be excluded. The genetic context of blaCMY-2 in IncI1-Iγ/ST2 plasmids was nearly identical to previous descriptions (38–40), except for three gaps and four SNPs that were either synonymous or in intergenic regions. The genetic context of blaCMY-2 in IncI1-Iγ/ST12 was highly similar to that of pNF1358 (GenBank accession no. DQ017661) (38), while the genetic context of blaCMY-2 in IncK was unique for the presence of two genes coding for hypothetical proteins downstream of sugE. An IncK plasmid (p1184-1) had an additional unique feature consisting of partial deletion of blc and sugE.
The sequenced IncI1-Iγ/ST2, IncI1-Iγ/ST12, and IncK plasmids were highly similar (99% identity; 88% to 100% coverage) to plasmids detected in Enterobacteriaceae isolated from dogs (I1-Iγ/ST2) and poultry (IncI1-Iγ/ST12 and IncK) in the United States, the Netherlands, and Norway (14, 41–43). The recovery of highly similar plasmids from different E. coli lineages and from unrelated individuals of the same host species suggests that these plasmids may possess traits enhancing adaptation of E. coli to the intestinal tracts of specific animal hosts.
We detected a high diversity of STs within and between different sources and no linkage of blaCMY-2-positive plasmids to specific E. coli lineages. The criteria for the selection of poultry strains may have influenced this finding. Nevertheless, the finding was expected based on a previous study (44). Further characterization of STs shared by human and animal sources by plasmid typing demonstrated that such strains were diverse (Table 1), supporting the current notion that there is no evidence of whole-bacterium transmission of cephalosporin-resistant E. coli from poultry meat to humans (45). ST10, ST38, and ST115 strains were detected at different levels of Danish broiler meat production. Taking into consideration previous PFGE typing data (22) and our extensive plasmid characterization, it appears that clonal transmission of CMY-2-producing E. coli within the Danish poultry production system is mainly attributable to an E. coli ST38 lineage susceptible to non-β-lactam antimicrobials and carrying blaCMY-2 on IncK plasmids. CMY-2-producing E. coli ST38 harboring blaCMY-2 on IncK plasmids has also been reported in Swedish and Norwegian broiler meat production (13, 43), even though plasmid-subtyping data were not available in the Swedish study. Vertical transmission of this clone between Scandinavian countries is likely, since 1-day-old chickens for Danish parent flock production are acquired from Sweden. None of the human isolates belonged to this clone, possibly due to fact that isolates from broilers and broiler meat were collected in 2010 while most human isolates were collected in previous years.
The main limitations of the study were the fact that human isolates were collected before the sharp increase in the prevalence of CMY-2-producing E. coli in Danish poultry meat products in 2011 (11) and the relatively low numbers of isolates from humans and dogs compared to the number of isolates from poultry. CMY-2-producing E. coli isolates are not commonly isolated from human patients in Denmark and are not collected in national surveillance of ESBLs in E. coli bacteremia and urinary tract infections (11). Thus, these isolates are not easily available in Denmark. Continuous monitoring is warranted in consideration of the occurrence of these bacteria as contaminants in poultry products and as commensals in dogs (11, 33). In this respect, our study provides useful baseline data that will enable future studies to assess whether the exposure of humans to blaCMY-2 of poultry origin will result in an increase in the frequency of this β-lactam resistance determinant in human clinical isolates.
A further limitation of the study is the fact that plasmid sequences were not fully assembled. However, only very short parts of the sequences were missing, because the contigs obtained covered the length expected on the basis of the S1 PFGE results and all conserved elements expected in the respective plasmid types were identified.
In conclusion, the spread of blaCMY-2 in Denmark is mainly associated with IncK and IncI1-Iγ plasmids, with limited similarity of plasmid subtypes across different host species. These plasmids were not linked to a specific E. coli genetic background, and no apparent clonal transfer of CMY-2-producing E. coli across host species was observed. The absence of linkage between plasmids and the E. coli genetic background confirms that plasmid horizontal transfer is more important than clonal dissemination for transmission of CMY-2-mediated cephalosporin resistance between animals and humans. The most common plasmids detected in Danish isolates were highly similar to plasmids detected in distant geographical regions, indicating that plasmid diversity is remarkably limited compared to E. coli strain diversity in the epidemiology of this β-lactamase.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ljudmila Troianova and Manja Koudal Hanegård for dedicated technical assistance, Anette M. Hammerum at Statens Serum Institute for providing isolates from healthy humans, and Heidi Kira Gumpert Brodersen for her contribution to the illustration of MLST results.
Funding Statement
This work, including the efforts of Valeria Bortolaia and Katrine Hartung Hansen, was funded by European Union (EvoTAR) (HEALTH-F3-2011-282004) and by the University of Copenhagen Research Centre for Control of Antibiotic Resistance (http://www.uc-care.ku.dk).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00495-16.
REFERENCES
- 1.Denisuik AJ, Lagacé-Wiens PR, Pitout JD, Mulvey MR, Simner PJ, Tailor F, Karlowsky JA, Hoban DJ, Adam HJ, Zhanel GG. 2013. Molecular epidemiology of extended-spectrum β-lactamase-, AmpC β-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007-11. J Antimicrob Chemother 68(Suppl 1):i57–i65. doi: 10.1093/jac/dkt027. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby GA. 2009. AmpC β-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poirel L, Héritier C, Spicq C, Nordmann P. 2004. In vivo acquisition of high-level resistance to imipenem in Escherichia coli. J Clin Microbiol 42:3831–3833. doi: 10.1128/JCM.42.8.3831-3833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naseer U, Haldorsen B, Simonsen GS, Sundsfjord A. 2010. Sporadic occurrence of CMY-2-producing multidrug-resistant Escherichia coli of ST-complexes 38 and 448, and ST131 in Norway. Clin Microbiol Infect 16:171–178. doi: 10.1111/j.1469-0691.2009.02861.x. [DOI] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. 2011. Annual epidemiological report 2011. Reporting on 2009 surveillance data and 2010 epidemic intelligence data. European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 7.Ewers C, Bethe A, Semmler T, Guenther S, Wieler LH. 2012. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect 18:646–655. doi: 10.1111/j.1469-0691.2012.03850.x. [DOI] [PubMed] [Google Scholar]
- 8.Jørgensen RL, Nielsen JB, Friis-Møller A, Fjeldsøe-Nielsen H, Schønning K. 2010. Prevalence and molecular characterization of clinical isolates of Escherichia coli expressing an AmpC phenotype. J Antimicrob Chemother 65:460–464. doi: 10.1093/jac/dkp484. [DOI] [PubMed] [Google Scholar]
- 9.Agersø Y, Bager F, Boel J, Helwigh B, Borck Høg B, Jensen LB, de Knegt L, Korsgaard H, Larsen LS, Sørensen AIV, Dalby T, Hammerum AM, Hoffmann S, Gaardbo Kuhn K, Rhod Larsen A, Laursen M, Nielsen EM, Olsen SS, Petersen A, Bagger-Skjøt L, Skov RL, Slotved H-C, Torpdahl M. 2014. DANMAP 2013: use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Statens Serum Institut, Copenhagen, Denmark. [Google Scholar]
- 10.Baron S, Jouy E, Larvor E, Eono F, Bougeard S, Kempf I. 2014. Impact of third-generation-cephalosporin administration in hatcheries on fecal Escherichia coli antimicrobial resistance in broilers and layers. Antimicrob Agents Chemother 58:5428–5434. doi: 10.1128/AAC.03106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bager F, Birk T, Borck Høg B, Jensen LB, Jensen AN, de Knegt L, Korsgaard H, Dalby T, Hammerum A, Hoffmann S, Gaardbo Kuhn K, Rhod Larsen A, Laursen M, Nielsen EM, Schytte Olsen S, Petersen A, Wolff Sönksen U. 2015. DANMAP 2014: use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Statens Serum Institut, Copenhagen, Denmark. [Google Scholar]
- 12.Börjesson S, Jernberg C, Brolund A, Edquist P, Finn M, Landén A, Olsson-Liljequist B, Wisell KT, Bengtsson B, Englund S. 2013. Characterization of plasmid-mediated AmpC-producing E. coli from Swedish broilers and association with human clinical isolates. Clin Microbiol Infect 19:E309–E311. doi: 10.1111/1469-0691.12192. [DOI] [PubMed] [Google Scholar]
- 13.Börjesson S, Egervärn M, Lindblad M, Englund S. 2013. Frequent occurrence of extended-spectrum beta-lactamase- and transferable AmpC beta-lactamase-producing Escherichia coli on domestic chicken meat in Sweden. Appl Environ Microbiol 79:2463–2466. doi: 10.1128/AEM.03893-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Been M, Lanza VF, de Scharringa J, Dohmen W, Du Y, Hu J, Lei Y, Li N, Tooming-Klunderud A, Heederik DJ, Fluit AC, Bonten MJ, Willems RJ, de la Cruz F, van Schaik W. 2014. Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet 10:e1004776. doi: 10.1371/journal.pgen.1004776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dierikx C, van der Goot J, Fabri T, van Essen-Zandbergen A, Smith H, Mevius D. 2013. Extended-spectrum-β-lactamase- and AmpC-β-lactamase-producing Escherichia coli in Dutch broilers and broiler farmers. J Antimicrob Chemother 68:60–67. doi: 10.1093/jac/dks349. [DOI] [PubMed] [Google Scholar]
- 16.Bortolaia V, Hansen KH, Nielsen CA, Fritsche TR, Guardabassi L. 2014. High diversity of plasmids harbouring blaCMY-2 among clinical Escherichia coli isolates from humans and companion animals in the upper Midwestern USA. J Antimicrob Chemother 69:1492–1496. doi: 10.1093/jac/dku011. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen JB, Albayati A, Jørgensen RL, Hansen KH, Lundgren B, Schønning K. 2013. An abbreviated MLVA identifies Escherichia coli ST131 as the major extended-spectrum β-lactamase-producing lineage in the Copenhagen area. Eur J Clin Microbiol Infect Dis 32:431–436. doi: 10.1007/s10096-012-1764-x. [DOI] [PubMed] [Google Scholar]
- 18.Hammerum AM, Lester CH, Jakobsen L, Porsbo LJ. 2011. Faecal carriage of extended-spectrum β-lactamase-producing and AmpC β-lactamase-producing bacteria among Danish army recruits. Clin Microbiol Infect 17:566–568. doi: 10.1111/j.1469-0691.2010.03340.x. [DOI] [PubMed] [Google Scholar]
- 19.Agersø Y, Aarestrup FM, Pedersen K, Seyfarth AM, Struve T, Hasman H. 2012. Prevalence of extended-spectrum cephalosporinase (ESC)-producing Escherichia coli in Danish slaughter pigs and retail meat identified by selective enrichment and association with cephalosporin usage. J Antimicrob Chemother 67:582–588. doi: 10.1093/jac/dkr507. [DOI] [PubMed] [Google Scholar]
- 20.Jensen VF, Larsen LS, Seyfarth AM, Agersø Y, Jensen LB, Struve T, Christiansen P, Jensen US, Olsen SS, Lester CH, Hammerich AM, Skjøt-Rasmussen L, Petersen A, Lambertsen LM, Skov RL, Frimodt-Møller N. 2010. DANMAP 2009: use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark. Statens Serum Institut, Copenhagen, Denmark. [Google Scholar]
- 21.Agersø Y, Hald T, Borck Høg B, Jensen LB, Jensen VF, Korsgaard H, Larsen LS, Pires SM, Seyfarth AM, Struve T, Hammerum AM, Jensen US, Lambertsen LM, Rhod Larsen A, Møller Nielsen E, Olsen SS, Petersen A, Skjøt-Rasmussen L, Skov RL, Sørum M. 2011. DANMAP 2010: use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. Statens Serum Institut, Copenhagen, Denmark. [Google Scholar]
- 22.Agersø Y, Jensen JD, Hasman H, Pedersen K. 2014. Spread of extended spectrum cephalosporinase-producing Escherichia coli clones and plasmids from parent animals to broilers and to broiler meat in a production without use of cephalosporins. Foodborne Pathog Dis 11:740–746. doi: 10.1089/fpd.2014.1742. [DOI] [PubMed] [Google Scholar]
- 23.Damborg P, Gaustad IB, Olsen JE, Guardabassi L. 2011. Selection of CMY-2 producing Escherichia coli in the faecal flora of dogs treated with cephalexin. Vet Microbiol 151:404–408. doi: 10.1016/j.vetmic.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Dierikx C, van Essen-Zandbergen A, Veldman K, Smith H, Mevius D. 2010. Increased detection of extended spectrum beta-lactamase producing Salmonella enterica and Escherichia coli isolates from poultry. Vet Microbiol 145:273–278. doi: 10.1016/j.vetmic.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Call DR, Singer RS, Meng D, Broschat SL, Orfe LH, Anderson JM, Herndon DR, Kappmeyer LS, Daniels JB, Besser TE. 2010. blaCMY-2-positive IncA/C plasmids from Escherichia coli and Salmonella enterica are a distinct component of a larger lineage of plasmids. Antimicrob Agents Chemother 54:590–596. doi: 10.1128/AAC.00055-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 27.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 28.García-Fernández A, Chiaretto G, Bertini A, Villa L, Fortini D, Ricci A, Carattoli A. 2008. Multilocus sequence typing of IncI1 plasmids carrying extended-spectrum β-lactamases in Escherichia coli and Salmonella of human and animal origin. J Antimicrob Chemother 61:1229–1233. doi: 10.1093/jac/dkn131. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing, 26th ed, CLSI supplement M100S CLSI, Wayne, PA. [Google Scholar]
- 30.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmo LP, Nielsen LR, da Costa PM, Alban L. 2014. Exposure assessment of extended-spectrum beta-lactamases/AmpC beta-lactamases-producing Escherichia coli in meat in Denmark. Infect Ecol Epidemiol 44:22924. doi: 10.3402/iee.v4.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mataseje LF, Baudry PJ, Zhanel GG, Morck DW, Read RR, Louie M, Mulvey MR. 2010. Comparison of CMY-2 plasmids isolated from human, animal, and environmental Escherichia coli and Salmonella spp. from Canada. Diagn Microbiol Infect Dis 67:387–391. doi: 10.1016/j.diagmicrobio.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Haenni M, Saras E, Métayer V, Médaille C, Madec JY. 2014. High prevalence of blaCTX-M-1/IncI1/ST3 and blaCMY-2/IncI1/ST2 plasmids in healthy urban dogs in France. Antimicrob Agents Chemother 58:5358–5362. doi: 10.1128/AAC.02545-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamang MD, Nam HM, Jang GC, Kim SR, Chae MH, Jung SC, Byun JW, Park YH, Lim SK. 2012. Molecular characterization of extended-spectrum-β-lactamase-producing and plasmid-mediated AmpC β-lactamase-producing Escherichia coli isolated from stray dogs in South Korea. Antimicrob Agents Chemother 56:2705–2712. doi: 10.1128/AAC.05598-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang T, Min YN, Liu W, Womble DD, Rownd RH. 1993. Insertion and deletion mutations in the repA4 region of the IncFII plasmid NR1 cause unstable inheritance. J Bacteriol 175:5350–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komano T, Yoshida T, Narahara K, Furuya N. 2000. The transfer region of IncI1 plasmid R64: similarities between R64 tra and Legionella icm/dot genes. Mol Microbiol 35:1348–1359. [DOI] [PubMed] [Google Scholar]
- 37.Furuya N, Nisioka T, Komano T. 1991. Nucleotide sequence and functions of the oriT operon in IncI1 plasmid R64. J Bacteriol 173:2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verdet C, Gautier V, Chachaty E, Ronco E, Hidri N, Decré D, Arlet G. 2009. Genetic context of plasmid-carried blaCMY-2-like genes in Enterobacteriaceae. Antimicrob Agents Chemother 53:4002–4006. doi: 10.1128/AAC.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tagg KA, Iredell JR, Partridge SR. 2014. Complete sequencing of IncI1 sequence type 2 plasmid pJIE512b indicates mobilization of blaCMY-2 from an IncA/C plasmid. Antimicrob Agents Chemother 58:4949–4952. doi: 10.1128/AAC.02773-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yassine H, Bientz L, Cros J, Goret J, Bébéar C, Quentin C, Arpin C. 2015. Experimental evidence for IS1294b-mediated transposition of the blaCMY-2 cephalosporinase gene in Enterobacteriaceae. J Antimicrob Chemother 70:697–700. doi: 10.1093/jac/dku472. [DOI] [PubMed] [Google Scholar]
- 41.Fricke WF, McDermott PF, Mammel MK, Zhao S, Johnson TJ, Rasko DA, Fedorka-Cray PJ, Pedroso A, Whichard JM, Leclerc JE, White DG, Cebula TA, Ravel J. 2009. Antimicrobial resistance-conferring plasmids with similarity to virulence plasmids from avian pathogenic Escherichia coli strains in Salmonella enterica serovar Kentucky isolates from poultry. Appl Environ Microbiol 75:5963–5971. doi: 10.1128/AEM.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao G, Allard MW, Hoffmann M, Monday SR, Muruvanda T, Luo Y, Payne J, Rump L, Meng K, Zhao S, McDermott PF, Brown EW, Meng J. 2015. Complete sequences of six IncA/C plasmids of multidrug-resistant Salmonella enterica subsp. enterica serotype newport. Genome Announc 3:e00027-15. doi: 10.1128/genomeA.00027-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mo SS, Slettemeås JS, Berg ES, Norström M, Sunde M. 2016. Plasmid and host strain characreristics of Escherichia coli resistant to extended-spectrum cephalosporins in the Norwegian broiler production. PLoS One 11:e0154019. doi: 10.1371/journal.pone.0154019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidjabat HE, Paterson DL, Qureshi ZA, Adams-Haduch JM, O'Keefe A, Pascual A, Rodríguez-Baño J, Doi Y. 2009. Clinical features and molecular epidemiology of CMY-type β-lactamase-producing Escherichia coli. Clin Infect Dis 48:739–744. doi: 10.1086/597037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonten MJ, Mevius D. 2015. Less evidence for an important role of food-producing animals as source of antibiotic resistance in humans. Clin Infect Dis 60:1867. doi: 10.1093/cid/civ275. [DOI] [PubMed] [Google Scholar]
- 46.Francisco AP, Vaz C, Monteiro PT, Melo-Cristino J, Ramirez M, Carriço JA. 2012. PHYLOViZ: phylogenetic inference and data visualization for sequence based typing methods. BMC Bioinform 13:87. doi: 10.1186/1471-2105-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.