Abstract
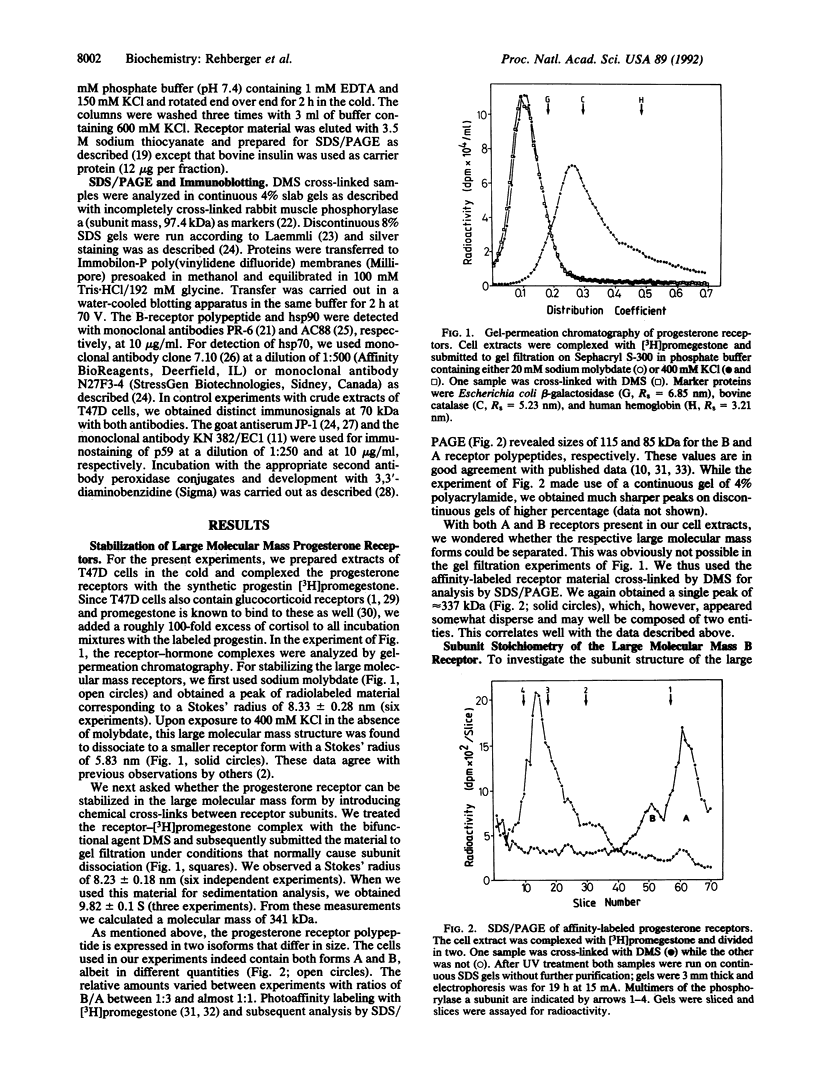
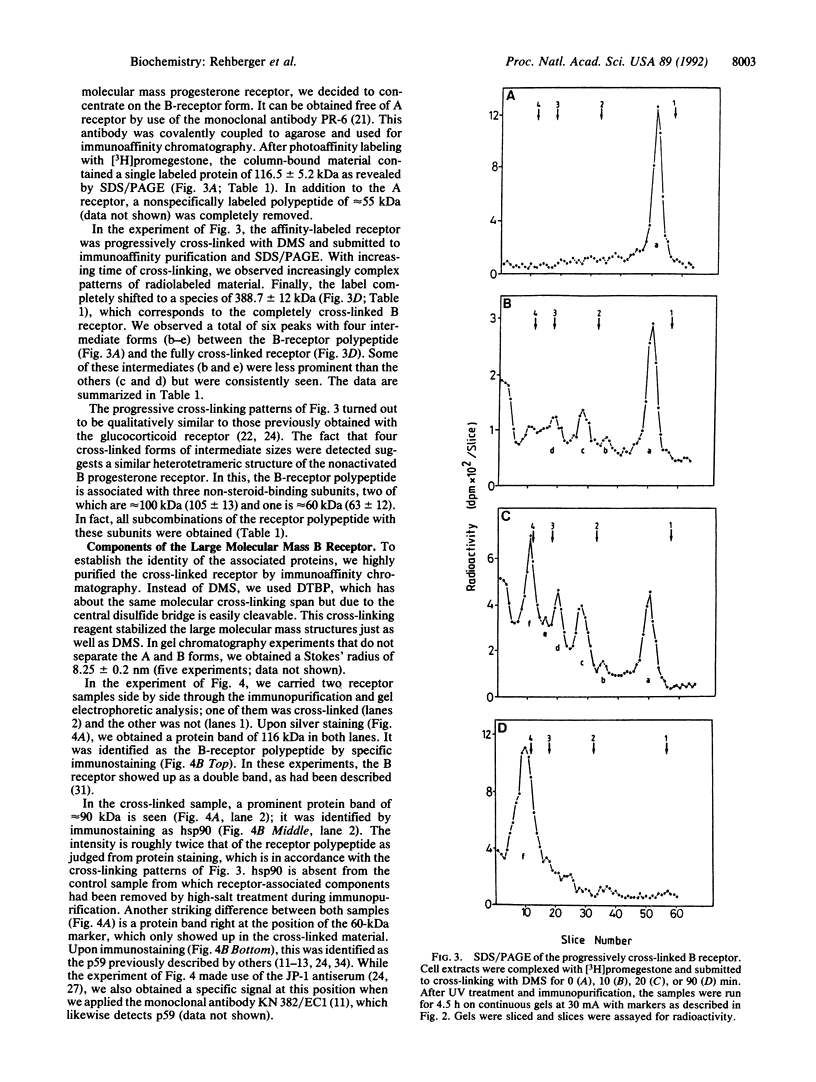
Nonactivated progesterone receptors in extracts of human T47D mammary carcinoma cells were investigated. Chemical cross-linking with dimethyl suberimidate resulted in complete stabilization of the A and B receptors with an average molecular mass of 340 kDa. For analyzing the subunit structure, we concentrated on the larger B receptor, which was separated from the A form by immunoaffinity chromatography. Progressive cross-linking of the photoaffinity-labeled receptor resulted in patterns of labeled bands in SDS gels, which are indicative of a heterotetrameric structure. It consists of one receptor polypeptide in association with two 90-kDa subunits and one polypeptide of approximately 60 kDa. The completely cross-linked B receptor has a molecular mass of approximately 390 kDa. To identify the subunits, the oligomeric B receptor was cross-linked with a cleavable bisimidate, highly purified by immunoaffinity chromatography, and analyzed by gel electrophoresis and immunoblotting. The receptor polypeptide has a mass of 116.5 kDa. The 90-kDa band was identified as the heat shock protein hsp90 and was roughly twice as intense as the receptor polypeptide. By use of specific antibodies, we identified the fourth receptor subunit as a 59-kDa protein (p59); we did not obtain any evidence for the heat shock protein hsp70 being a receptor component. We suggest an analogous heterotetrameric structure for the nonactivated A receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arànyi P., Radanyi C., Renoir M., Devin J., Baulieu E. E. Covalent stabilization of the nontransformed chick oviduct cytosol progesterone receptor by chemical cross-linking. Biochemistry. 1988 Feb 23;27(4):1330–1336. doi: 10.1021/bi00404a036. [DOI] [PubMed] [Google Scholar]
- Conneely O. M., Maxwell B. L., Toft D. O., Schrader W. T., O'Malley B. W. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987 Dec 16;149(2):493–501. doi: 10.1016/0006-291x(87)90395-0. [DOI] [PubMed] [Google Scholar]
- Dougherty J. J., Toft D. O. Characterization of two 8 S forms of chick oviduct progesterone receptor. J Biol Chem. 1982 Mar 25;257(6):3113–3119. [PubMed] [Google Scholar]
- Estes P. A., Suba E. J., Lawler-Heavner J., Elashry-Stowers D., Wei L. L., Toft D. O., Sullivan W. P., Horwitz K. B., Edwards D. P. Immunologic analysis of human breast cancer progesterone receptors. 1. Immunoaffinity purification of transformed receptors and production of monoclonal antibodies. Biochemistry. 1987 Sep 22;26(19):6250–6262. doi: 10.1021/bi00393a045. [DOI] [PubMed] [Google Scholar]
- Fischer G., Schmid F. X. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry. 1990 Mar 6;29(9):2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Gehring U., Mugele K., Arndt H., Busch W. Subunit dissociation and activation of wild-type and mutant glucocorticoid receptors. Mol Cell Endocrinol. 1987 Sep;53(1-2):33–44. doi: 10.1016/0303-7207(87)90189-4. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Alexander P. S. In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology. 1983 Dec;113(6):2195–2201. doi: 10.1210/endo-113-6-2195. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Zava D. T., Thilagar A. K., Jensen E. M., McGuire W. L. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978 Aug;38(8):2434–2437. [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990 May;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- Kurtz S., Rossi J., Petko L., Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986 Mar 7;231(4742):1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Herrick J., Faber L. E., Renoir J. M., Radanyi C., Baulieu E. E. P59, an hsp 90-binding protein. Cloning and sequencing of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992 Mar 5;267(7):4281–4284. [PubMed] [Google Scholar]
- Lessey B. A., Alexander P. S., Horwitz K. B. The subunit structure of human breast cancer progesterone receptors: characterization by chromatography and photoaffinity labeling. Endocrinology. 1983 Apr;112(4):1267–1274. doi: 10.1210/endo-112-4-1267. [DOI] [PubMed] [Google Scholar]
- Lorenzo F., Jolivet A., Loosfelt H., Thu vu Hai M., Brailly S., Perrot-Applanat M., Milgrom E. A rapid method of epitope mapping. Application to the study of immunogenic domains and to the characterization of various forms of rabbit progesterone receptor. Eur J Biochem. 1988 Sep 1;176(1):53–60. doi: 10.1111/j.1432-1033.1988.tb14250.x. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K., Lan N. C., Showers M. O., Baxter J. D. Photoaffinity labeling of glucocorticoid receptors. J Biol Chem. 1981 Oct 25;256(20):10503–10508. [PubMed] [Google Scholar]
- Perdew G. H., Whitelaw M. L. Evidence that the 90-kDa heat shock protein (HSP90) exists in cytosol in heteromeric complexes containing HSP70 and three other proteins with Mr of 63,000, 56,000, and 50,000. J Biol Chem. 1991 Apr 15;266(11):6708–6713. [PubMed] [Google Scholar]
- Pratt W. B. Transformation of glucocorticoid and progesterone receptors to the DNA-binding state. J Cell Biochem. 1987 Sep;35(1):51–68. doi: 10.1002/jcb.240350105. [DOI] [PubMed] [Google Scholar]
- Renoir J. M., Mester J. Chick oviduct progesterone receptor: structure, immunology, function. Mol Cell Endocrinol. 1984 Aug;37(1):1–13. doi: 10.1016/0303-7207(84)90123-0. [DOI] [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Faber L. E., Baulieu E. E. The non-DNA-binding heterooligomeric form of mammalian steroid hormone receptors contains a hsp90-bound 59-kilodalton protein. J Biol Chem. 1990 Jun 25;265(18):10740–10745. [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Jung-Testas I., Faber L. E., Baulieu E. E. The nonactivated progesterone receptor is a nuclear heterooligomer. J Biol Chem. 1990 Aug 25;265(24):14402–14406. [PubMed] [Google Scholar]
- Rexin M., Busch W., Gehring U. Chemical cross-linking of heteromeric glucocorticoid receptors. Biochemistry. 1988 Jul 26;27(15):5593–5601. doi: 10.1021/bi00415a030. [DOI] [PubMed] [Google Scholar]
- Rexin M., Busch W., Gehring U. Protein components of the nonactivated glucocorticoid receptor. J Biol Chem. 1991 Dec 25;266(36):24601–24605. [PubMed] [Google Scholar]
- Rexin M., Busch W., Segnitz B., Gehring U. Structure of the glucocorticoid receptor in intact cells in the absence of hormone. J Biol Chem. 1992 May 15;267(14):9619–9621. [PubMed] [Google Scholar]
- Rexin M., Busch W., Segnitz B., Gehring U. Tetrameric structure of the nonactivated glucocorticoid receptor in cell extracts and intact cells. FEBS Lett. 1988 Dec 5;241(1-2):234–238. doi: 10.1016/0014-5793(88)81068-8. [DOI] [PubMed] [Google Scholar]
- Riehl R. M., Sullivan W. P., Vroman B. T., Bauer V. J., Pearson G. R., Toft D. O. Immunological evidence that the nonhormone binding component of avian steroid receptors exists in a wide range of tissues and species. Biochemistry. 1985 Nov 5;24(23):6586–6591. doi: 10.1021/bi00344a042. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Faber L. E., Henzel W. J., Pratt W. B. The 56-59-kilodalton protein identified in untransformed steroid receptor complexes is a unique protein that exists in cytosol in a complex with both the 70- and 90-kilodalton heat shock proteins. Biochemistry. 1990 May 29;29(21):5145–5152. doi: 10.1021/bi00473a021. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Hirst M., Scherrer L. C., Tang H. Y., Welsh M. J., Harmon J. M., Simons S. S., Jr, Ringold G. M., Pratt W. B. Hormone-free mouse glucocorticoid receptors overexpressed in Chinese hamster ovary cells are localized to the nucleus and are associated with both hsp70 and hsp90. J Biol Chem. 1990 Nov 25;265(33):20123–20130. [PubMed] [Google Scholar]
- Sanchez E. R. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990 Dec 25;265(36):22067–22070. [PubMed] [Google Scholar]
- Schrader W. T., Birnbaumer M. E., Hughes M. R., Weigel N. L., Grody W. W., O'Malley B. W. Studies on the structure and function of the chicken progesterone receptor. Recent Prog Horm Res. 1981;37:583–633. doi: 10.1016/b978-0-12-571137-1.50017-7. [DOI] [PubMed] [Google Scholar]
- Sherman M. R. Physical-chemical analysis of steroid hormone receptors. Methods Enzymol. 1975;36:211–234. doi: 10.1016/s0076-6879(75)36021-7. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Faber L. E., Toft D. O. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990 Mar 5;265(7):3996–4003. [PubMed] [Google Scholar]
- Smith D. F., Stensgard B. A., Welch W. J., Toft D. O. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J Biol Chem. 1992 Jan 15;267(2):1350–1356. [PubMed] [Google Scholar]
- Sullivan W. P., Beito T. G., Proper J., Krco C. J., Toft D. O. Preparation of monoclonal antibodies to the avian progesterone receptor. Endocrinology. 1986 Oct;119(4):1549–1557. doi: 10.1210/endo-119-4-1549. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Maeda Y., Nakao K., Wakim N. G., Duhring J. L., Faber L. E. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986 Sep 9;25(18):5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- Tora L., Gronemeyer H., Turcotte B., Gaub M. P., Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988 May 12;333(6169):185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Vu Hai M. T., Jolivet A., Ravet V., Lorenzo F., Perrot-Applanat M., Citerne M., Milgrom E. Novel monoclonal antibodies against human uterine progesterone receptor. Mapping of receptor immunogenic domains. Biochem J. 1989 Jun 1;260(2):371–376. doi: 10.1042/bj2600371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. L., Sheridan P. L., Krett N. L., Francis M. D., Toft D. O., Edwards D. P., Horwitz K. B. Immunologic analysis of human breast cancer progesterone receptors. 2. Structure, phosphorylation, and processing. Biochemistry. 1987 Sep 22;26(19):6262–6272. doi: 10.1021/bi00393a046. [DOI] [PubMed] [Google Scholar]




