Abstract
Background:
Minimal hepatic encephalopathy (MHE) has a far-reaching impact on quality and function ability in daily life and may progress to overt hepatic encephalopathy. There is a synergistic effect between systemic oxidative stress and ammonia that is implicated in the pathogenesis of hepatic encephalopathy. The aim of this study is to investigate the effectiveness of oral supplementation of antioxidants and zinc gluconate on MHE versus lactulose.
Methods:
Our study included 58 patients with cirrhosis diagnosed as having MHE by neuropsychometric tests, including number connection test part A (NCT-A), digit symbol test (DST) and block design tests (BDTs). Patients were randomized to receive 175 mg zinc gluconate, 50,000 IU vitamin A, 500 mg vitamin C and 100 mg vitamin E once daily plus lactulose, dose 30–60 ml/day for 3 months [group A (n = 31)] or initiated and maintained on lactulose dose 30–60 ml/day for 3 months [group B (n = 27)]. Neuropsychometric tests and laboratory investigations were repeated after 3 months of therapy.
Results:
Compared with the baseline neuropsychometric tests, a significant improvement was reported in patients with MHE after 3 months of antioxidant and zinc therapy (group A) versus patients with lactulose therapy (group B) (NCT-A, p <0.001; DST, p = 0.006; BDT, p < 0.001). Antioxidant and zinc supplementation significantly decreased arterial ammonia level, alanine aminotransferase (ALT), aspartate aminotransferase (AST) (p < 0.001) and improved Child–Pugh score in MHE after 3 months of therapy (p= 0.024).
Conclusion:
Antioxidant and zinc supplementation can improve MHE in patients with liver cirrhosis.
Keywords: antioxidants, zinc, minimal hepatic encephalopathy
Introduction
Minimal hepatic encephalopathy (MHE) is a neurocognitive dysfunction, which occurs in patients with cirrhosis [Ortiz et al. 2005]. Prevalence of MHE has been reported to vary between 60% and 80% in patients with liver cirrhosis [Goeneweg et al. 2000; Amodio et al. 2001]. While overt hepatic encephalopathy (OHE) is often diagnosed clinically, MHE is more difficult to diagnose and more often requires the use of specialized testing to do so [Poordad, 2007]. The diagnostic methodologies were a combination of neuropsychometric (NP) and neurophysiologic testing strategies [Weissenborn et al. 2001; Ortiz et al. 2006]. MHE is manifested by impairment in specialized testing and is considered by most of the clinicians to predict the development of OHE [Poordad, 2007; Romero-Gómez et al. 2002]. Patients with evidence of MHE pose a potential danger to themselves or to the community in the operation of heavy equipment and motor vehicles [Bajaj et al. 2008]. Moreover, MHE can have a far-reaching impact on the quality of life and the ability to function in daily life [Romero-Gómez et al. 2007].
The pathogenesis of MHE is believed to be similar to that of OHE. Ammonia plays a key role in the pathogenesis of hepatic encephalopathy [Cordoba et al. 2001]. Oxidative stress is also a factor believed to play an important role in the pathogenesis of this syndrome. In different liver diseases, oxidative stress may represent a systemic phenomenon induced by several mechanisms including; increased systemic release of oxidant enzymes, decreased antioxidant synthesis, and generation of reactive oxygen species. Ammonia induces cerebral oxidative stress, thus contributing to hepatic encephalopathy [Bosoi and Rose, 2013].
Zinc, considered as a cofactor of urea cycle enzymes, is deficient in patients with cirrhosis, especially if associated with malnutrition or encephalopathy [Blei and Córdoba, 2001]. Zinc is essential for the synthesis of coenzymes that mediate biogenic amine synthesis and metabolism [Sharma et al. 2007]. Zinc deficiency also leads to alteration of neurotransmitters like γ aminobutyric acid and norepinephrine [Chetri and Choudhuri, 2003]. The possible causes of low serum zinc levels in patients with advanced cirrhosis are thought to be poor dietary intake via protein-restricted diet, excessive urinary losses, and impaired intestinal absorption [Dejong et al. 1994]. Data from previous studies show that there is a synergistic combined effect between systemic oxidative stress and ammonia that is implicated in the pathogenesis of hepatic encephalopathy [Bosoi and Rose, 2013]. So, we designed this study to assess the effects of oral supplementation of antioxidant and zinc gluconate in patients with cirrhosis and MHE.
Patients and methods
Consecutive patients with liver cirrhosis were screened for MHE. These patients were attending the Tropical and Neurology Outpatient clinics in Mansoura University Hospital, Mansoura, Egypt during the period from January 2013 to October 2013. The diagnosis of liver cirrhosis was based on history, clinical examination, laboratory findings, endoscopic evidence, Child–Pugh score and liver biopsy in some patients and radiological findings (abdominal ultrasound, triphasic computed tomography and magnetic resonance imaging). Chronic hepatitis B and chronic hepatitis C virus (HCV) were diagnosed by positive viral markers (Hepatitis B surface antigen (HBsAg) and anti-HCV respectively) using an enzyme-linked immunosorbent assay technique (ELISA Kit, Abbott Diagnostics, Santa Clara, CA, USA) and reverse transcriptase polymerase chain reaction (Rotor-Gene Q, Qiagen Co., Germany). Autoimmune hepatitis (AIH) was diagnosed if one of the autoimmune markers [antinuclear antibody, smooth muscle antibody or liver–kidney microsomal antibody] was positive and if liver histology suggested AIH [Czaja and Freese, 2002]. Cryptogenic cirrhosis was diagnosed when an extensive etiologic workup did not reveal any possible etiology for liver cirrhosis [Caldwell et al. 1999].
Ethics
The study was approved by the institutional review board of Mansoura University Hospital and informed consent was obtained from all patients in accordance with the guidelines of the Helsinki Declaration (1975).
Exclusion criteria
Patients were excluded if they had OHE, a history of OHE or any treatment for OHE before the start of the study; sensory or motor deficits; neurological causes of impaired cognition, electrolyte imbalances (serum sodium level < 125 mmol/liter; serum calcium level > 10 mg/dl and potassium level < 2.5 mmol/liter), ongoing systemic illnesses, intercurrent infection or active spontaneous bacterial peritonitis. Also, patients with recent history (<6 weeks) of alcohol intake, lactulose/lactitol, probiotics, metronidazole, rifaximin, patients on drugs affecting psychometric performances like benzodiazepines, antiepileptics or psychotropic drugs, antiviral treatment before or during the study, recent (<6 weeks) intake of antibiotics for gastrointestinal bleeding, history of shunt operation or transjugular intrahepatic portosystemic shunt for portal hypertension, or chronic renal impairment (creatinine level >2.0 mg/dl) were excluded. Another excluded group were patients with color blindness and mature cataract, diabetic retinopathy, hepatocellular carcinoma, or other comorbidities such as congestive heart failure and pulmonary disease. None of the study patients were specifically treated with other therapy for MHE within the study period (e.g. rifaximin).
Sample size and randomization
A prospective double-blind randomized controlled study was performed comparing the effect on MHE of antioxidant supplementation and zinc plus lactulose versus lactulose alone. Patients with MHE were randomly assigned either to receive zinc and antioxidant plus lactulose (group A; n = 31) or initiated and maintained on lactulose alone (group B, the control group; n = 27). Patients in group A received 175 mg zinc gluconate, 50,000 IU vitamin A, 500 mg vitamin C and 100 mg vitamin E once daily plus lactulose (30–60 ml in two or three divided doses). Patients in group B received 30–60 ml lactulose in two or three divided doses so that the patient passed two to three semi-soft stools per day. The therapy was taken daily for 3 months or until the patients discontinued the study drugs for any reason (e.g. noncompliance). All patients were followed up every month for treatment compliance and for development of any complications. Compliance with the therapy was assured primarily by ensuring increased stool frequency and its change to a softer consistency and by counting the number of bottles of lactulose consumed.
Dietary habits and concurrent therapy
As we have excluded patients with OHE, protein intake was not restricted in the enrolled patients. Salt was restricted in every patient to less than 2 g/day. Patients on diuretics or on β blockers for the prophylaxis of variceal bleeding continued their medication.
Clinical and laboratory assessment
The assessment included a thorough general physical, complete neurological and mental state examination using the Mini Mental State Examination to exclude the presence of any illness that could have caused or affected the neurological status or the quality of life. Abdominal ultrasonography and upper gastrointestinal endoscopy were carried out to look for stigmata of chronic liver disease. The Child–Pugh score was used to assess the severity of liver disease. After overnight fasting, venous blood was taken by conventional methods for hematologic tests, routine liver function tests and serum creatinine. The venous ammonia concentration was determined immediately after the psychometric testing by the ammonia test Kit II for the Pocket Chem BA device (Arkay Inc., Kyoto, Japan). The blood samples were collected and placed on ice and then were tested within 2 min of collection. The continuous measurement range is 8~285 µmol/liter and the normal blood ammonia level for healthy adults for this device is less than 54 μmol/liter.
NP tests
NP tests including the number connection test A (NCT-A), the block design test (BDT) and the digit symbol substitution test (DST) were done for all patients. We selected these tests because they were reported to be sensitive and easily administrated in outpatient clinics, and were not affected by sex, facility or education. All tests were conducted with a ‘lead-in’ of mock examples for patients before proceeding with the proper test with scoring. This was to ensure that patients had a full understanding of the test instructions and to reduce the possibility of false positive results. The tests were conducted in a quiet room with sufficient light. All tests were conducted in the subjects’ spoken language (Arabic language). Normal values of these tests were derived from 50 healthy volunteers. MHE was diagnosed if two of the three tests were impaired with two standard deviations beyond normative performance [Ferenci et al. 2002].
NCT-A: a test of visual-spatial orientation and psychomotor speed. The subject is shown a sheet of paper with 25 numbered circles which are randomly spread over the paper. The task is to connect the circles from 1 to 25 as quickly as possible. The test result is the time needed by the subject, including error correction time. Worse performance is indicated by a longer time for completion.
DST: the subject is given a series of double boxes with a number given in the upper part. The task is to draw a symbol pertinent to this number into the lower part of the boxes. Nine fixed pairs of numbers and symbols are given at the top of the test sheet. The test result is the number of boxes correctly filled in 90 s. Pathological test results indicate a deficit in visuoconstructive abilities. The number of correctly transcribed symbols indicates performance, that is, a low score means poor performance.
BDT: a test of visuospatial and motor skills. The task is to take six to nine blocks that have all white sides, all red sides, and red and white sides and arrange them according to a pattern formed by the examiner or shown on a card at a certain time (75–150 s).The scores are generated based on accurate construction of designs and a low score again indicates poor performance.
Statistical analysis
All the values were expressed as the mean ± SD. Student’s t test was used to check the differences among them. When p was less than 0.05, the difference was considered statistically significant. Software SPSS17.0 was used in all statistical analyses.
Results
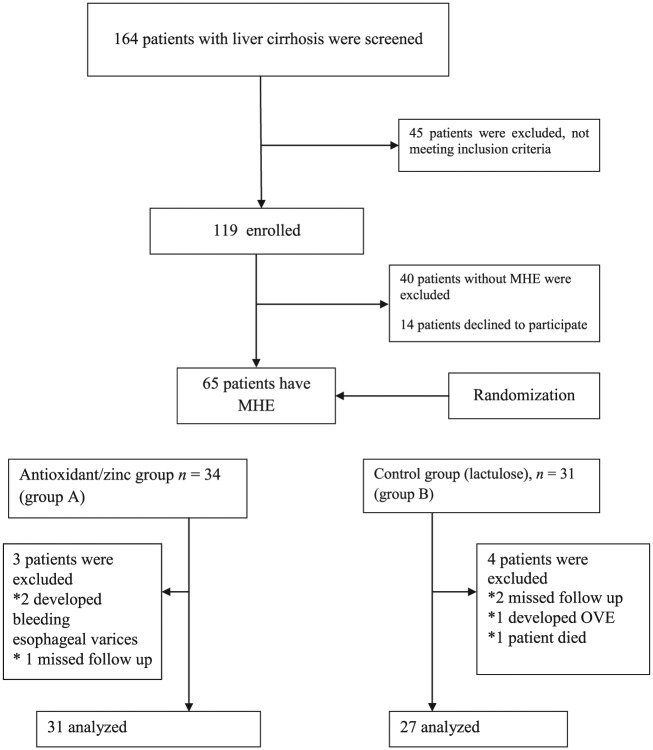
A total of 164 consecutive patients with liver cirrhosis of different etiology without overt encephalopathy were screened for MHE. Out of the screened patients, 119 (72.56%) patients who met the eligibility criteria were included in the study. Forty-five (27%) patients were excluded from the study (met an exclusion criterion) (Figure 1). The reasons why the 45 patients were excluded from the study were recent alcohol intake (one patient), drug addiction (nine patients), patient’s refusal (nine patients), age above 60 years (six patients), history of OHE (four patients), recent gastrointestinal bleeding (three patients), renal impairment (three patients), hepatocellular carcinoma (four patients), unfit to perform neuropsychological tests due to severe involuntary movements such as tremor (three patients) and recent antibiotic use (three patients). Many patients were excluded for one reason, 11 patients were excluded for two reasons and 24 were excluded for three reasons. From 119 patients who met the eligibility criteria and were included in the study, 54 patients again were excluded (40 patients without MHE according to NP tests and 14 patients declined to participate in the study).
Figure 1.
Flowchart demonstrating participants throughout the study procedures. MHE, minimal hepatic encephalopathy; OHE, overt hepatic encephalopathy.
The clinical course
According to the results of NP tests, 65 patients (54.62%) out of the 119 cases had at least two abnormal psychometric tests and were considered to have MHE, whereas the remaining 54 patients were not. Thirty-four patients who had MHE were assigned to zinc and antioxidant supplementation plus lactulose treatment (group A), whereas the remaining 31 (group B) were assigned to lactulose treatment (Figure 1). Seven patients with MHE were excluded during the 3-month course of therapy, three patients from group A (two developed bleeding esophageal varices and one missed follow up) and four patients from group B (one developed OHE, two missed follow up and one patient died). Ultimately, 31 patients received the full amount of supplement prescribed during the study period (175 mg zinc gluconate, 50,000 IU vitamin A and 100 mg vitamin E plus lactulose) versus 27 patients who were assigned to lactulose treatment only (Figure 1). There was no significant difference in the intake of medicines between groups.
Baseline characteristics
The clinical and demographic characteristics of the participants are shown in Table 1. MHE was found in 65 of 119 (54.62%) patients who fulfilled the inclusion criteria. The etiology of cirrhosis was due to chronic hepatitis C (n = 55), chronic hepatitis B (n = 7) and cryptogenic cirrhosis (n = 3). In group A, six patients had Child–Pugh grade A; 22 and 3 patients had Child–Pugh B and C grades respectively. In group B, four patients had Child–Pugh grade A; 20 and 3 patients had Child–Pugh B and C grades respectively. There were no statistically significant differences between both groups with respect to baseline characteristics.
Table 1.
Baseline characteristics of the patients.
| Antioxidants/zinc group (A) (N = 31) | Lactulose group (B) (N= 27) | p value | |
|---|---|---|---|
| Sex (M⁄F) | 16/14 | 15/12 | 0.92 |
| Age (years) | 54.5 ± 9.6 | 55.8 ± 9.2 | 0.6 |
| Etiology of liver disease | |||
| HCV related | 28 | 24 | 0.63 |
| HBV related | 2 | 3 | 0.94 |
| Cryptogenic | 1 | 2 | 0.95 |
| Child–Pugh score (N): A/B/C | 6⁄22⁄3 | 4⁄20⁄3 | 0.88 |
| Arterial ammonia (µmol/liter) | 65.1 ± 26.1 | 67.8 ± 25.5 | 0.69 |
| ALT (U/liter) | 69.6 ± 20.5 | 68.4 ± 18.2 | 0.82 |
| AST (U/liter) | 76.5 ± 22.7 | 67.56 ± 19.5 | 0.11 |
| Albumin (g/dl) | 3.45 ± 0.42 | 3.5 ± 0.44 | 0.59 |
| Bilirubin (mg⁄dl) | 1.38 ± 0.42 | 1.4 ± 0.42 | 0.89 |
| Zinc (µg/dl) | 49.6 ± 11.2 | 46.9 ± 10.5 | 0.34 |
ALT, alanine transaminase; AST, aspartate transaminase; HBV, hepatitis B virus; HCV, hepatitis C virus.
Neuropsychological evaluation
Table 2 summarizes the number of abnormal NP test results in each group. No statistically significant difference was found between patients in both groups at baseline.
Table 2.
Comparison between group A and group B as regard mean values of the neuropsychometric tests at baseline.
| Antioxidants/zinc group (A) | Lactulose group (B) | p value | |
|---|---|---|---|
| NCT-A (s) | 67.68 ± 4.86 | 67.93 ± 4.53 | 0.84 |
| Digit symbol test (points) | 6.13 ± 1.34 | 6.07 ± 1.52 | 0.89 |
| BDT (s) | 77.32 ± 3.65 | 77.78 ± 2.68 | 0.6 |
BDT, block design test; NCT-A, number connection test part A.
Impact of therapy on NP test results
After 3 months of therapy, all patients showed significant improvement in the mean value of all NP tests in both groups (group A: NCT-A, p < 0.001; DST, p < 0.001; BDT, p < 0.001; group B: NCT-A, p < 0.001; DST, p < 0.001; BDT,p = 0.002). Moreover, when comparing the NP test in both groups after 3 months of therapy, a significant improvement was reported in patients on zinc and antioxidant therapy (group A) versus patients on lactulose therapy (group B) (NCT-A, p < 0.001; DST, p = 0.006; BDT, p < 0.001) (Table 3).
Table 3.
Comparison of the mean values in both groups as regard clinical and laboratory parameters at baseline and after 3 months of follow up.
| Antioxidants/zinc group (A) |
Lactulose group (B) |
p3 value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 3 months | p1 value | Baseline | 3 months | p2 value | ||
| NCT-A (s) | 67.68 ± 4.86 | 56.80 ± 3.07 | <0.001 | 67.93 ± 4.53 | 64. 3 ± 5.31 | <0.001 | <0.001 |
| DST (points) | 10.11 ± 1.34 | 12.36 ± 0.87 | <0.001 | 10.07 ± 1.51 | 11.58 ± 1.19 | <0.001 | <0.006 |
| BDT (s) | 77.32 ± 3.65 | 68.35 ± 3.9 | <0.001 | 77.78 ± 2.68 | 74.52 ± 4.55 | 0.002 | <0.001 |
| Ammonia (µg⁄dl) | 65.1 ± 26.1 | 50.4 ± 18.3 | <0.001 | 67.8 ± 25.5 | 55.9 ± 16.7 | 0.048 | 0.24 |
| Total bilirubin (mg⁄dl) | 1.39 ± 0.32 | 1.21 ± 0.35 | 0.04 | 1.4 ± 0.42 | 1.29 ± 0.37 | 0.32 | 0.4 |
| Albumin (g⁄dl) | 3.45 ± 0.42 | 3.5 ± 0.42 | 0.81 | 3.5 ± 0.34 | 3.5 ± 0.44 | 0.11 | 0.47 |
| ALT (IU/ml) | 69.6 ± 20.5 | 52.19 ± 12.47 | <0.001 | 68.4 ± 18.2 | 62.2 ± 20.2 | 0.78 | 0.001 |
| AST (IU/ml) | 76.5 ± 22.7 | 53.3 ± 12.5 | <0.001 | 67.56 ± 19.5 | 68.4 ± 20.2 | 0.23 | 0.002 |
| Prothrombin concentration (%) | 70.4 ± 12.1 | 78.3 ± 11.4 | 0.01 | 70.6 ± 12.7 | 71.9 ± 12.4 | 0.71 | 0.045 |
| Zinc (µg⁄dl) | 49.6 ± 11.2 | 75.6 ± 16.8 | <0.001 | 46.9 ± 10.5 | 48.7 ± 9.2 | 0.7416 | < 0.001 |
| Child–Pugh score | 6⁄22⁄3 | 16⁄12⁄3 | 0.024 | 4⁄20⁄3 | 11⁄13⁄3 | 0.09 | 0.07 |
ALT, alanine transaminase; AST, aspartate transaminase; BDT, block design test; DST, digit symbol test; NCT-A, number connection test part A; p1, group A before and after therapy; p2, group B before and after therapy; p3, group A and B after therapy.
Impact of therapy on clinical and laboratory parameters
In group A, 3 months after therapy, we noticed a significant decrease in serum levels of arterial ammonia, ALT, AST (p < 0.001) and a significant increase in prothrombin concentration (p = 0.01) and serum zinc level (p < 0.001). Also, a significant improvement was noticed in Child–Pugh score after 3 months of treatment (p = 0.024). However, the improvement in serum bilirubin and albumin in comparison to baseline was nonsignificant (p= 0.4 and p= 0.81, respectively) (Table 3).
In group B, nonsignificant changes in the laboratory values were observed 3 months after therapy in comparison to baseline; apart from significant reduction of the arterial ammonia levels (p = 0.048). Also, a nonsignificant improvement in Child–Pugh score was observed (p = 0.09).
Furthermore, post-treatment, ALT and AST levels were decreased significantly and prothrombin concentration and serum zinc level were increased in group A versus group B (p = 0.001, p = 0.002, p = 0.045 and p < 0.001, respectively), However, nonsignificant changes were observed for serum ammonia, bilirubin, albumin levels and Child–Pugh score (p = 0.24, p = 0.4, p = 0.47 and p = 0.07, respectively).
Discussion
The present study confirmed the high prevalence of MHE among Egyptian patients with liver cirrhosis. The prevalence of MHE in our outpatient population with cirrhosis (54.62%) was documented by using a combination of psychometric tests. In this study, we have demonstrated that oral antioxidant and zinc supplementations clearly improved NP tests in patients with liver cirrhosis and MHE.
Studies reported that zinc supplementation improved psychometric performance with a reduction in blood ammonia level in patients with hepatic encephalopathy [Marchesini et al. 1996]. Although a recent study demonstrated that both rifaximin and lactulose improve health-related quality of life equally well in patients with cirrhosis and MHE [Sidhu et al. 2016], our study may be the first one confirming that a combination of antioxidant and zinc therapy is an effective treatment for patients with MHE in terms of improving patient psychometric tests (NCT-A, DST and BDT) and reducing blood ammonia level after 3 months of continuous therapy.
The possible explanations for our results are, first, extensive evidence suggests that the pathogenesis of MHE is similar to that of OHE and that ammonia plays a key role [Balata et al. 2003]. Therefore, patients with cirrhosis and hepatic encephalopathy showed a high incidence of zinc deficiency which was associated with increased ammonia level [Blei and Córdoba, 2001], hence, correction of zinc deficiency improved ammonia level and thus improved MHE. This is in agreement with Riggio and colleagues, who stated that zinc supplementation improves ammonia detoxification through the urea genesis by increasing liver ornithine transcarbamylase activity [Riggio et al. 1992]. This explanation is in accordance with Marchesini and colleagues who demonstrated that in patients with cirrhosis, zinc supplementation was shown to increase the hepatic activity of ornithine transcarbamylase, a key enzyme of the urea cycle. This was accompanied by increased urea formation and decreased ammonia levels, which might be the biochemical basis of the beneficial effects of zinc on the mental state in humans [Marchesini et al. 1996].
A second explanation is based on the fact that ammonia is the likely toxin and astrocytes are the main target of its neurotoxicity, although precisely how ammonia brings about cellular injury is poorly understood. Several studies over the past decade have invoked the concept of oxidative stress that leads to lipid peroxidation and free-radical generation as a pathogenic mechanism for ammonia neurotoxicity [Norenberg et al. 2004; Essa and Subramanian, 2006]. The present study demonstrated that antioxidants in patients with MHE were associated with improvement in psychometric tests, raising a new prospective in the therapeutic approach of this clinical condition. Antioxidants play a major role in protecting biological systems from reactive oxygen derived species [Irshad and Chaudhuri, 2002]. The beneficial effect of antioxidants in this study could be explained by counteracting the synergistic effect between systemic oxidative stress and ammonia that plays a pivotal role in the pathogenesis of hepatic encephalopathy. Also, this could be due to the prevention or inhibition of the lipid peroxidative system, maintenance of cellular integrity and the hepatoprotective effect [Thenmozhi and Subramanian, 2011].
Third, the presence of local and systemic inflammation and the release of reactive oxygen species further exacerbate the cerebral effects of ammonia. The anti-inflammatory and the antioxidative strategies may abrogate these effects and offer real treatment options to patients with hepatic encephalopathy in the future [Seyan et al. 2010]. However, the exact mechanism is still unclear and further research on the effect of the constituents of this antioxidant is needed. This study clearly demonstrated that 3 months of oral zinc and antioxidant supplementations were associated with a significant reduction of ammonia level. This was associated with an objective clinical and biochemical (liver function) improvement and performance of psychometric tests. The same results were obtained by Marchesini and colleagues [Marchesini et al. 1996].
In conclusion, patients with cirrhosis can exhibit motor impairments. Our study showed a robust protective effect of antioxidants and zinc against MHE. Thus, patients with cirrhosis who have MHE and who are at occupational risk, for example truck drivers and patients handling heavy machines, could benefit from this treatment.
Acknowledgments
We would like to express our sincere gratitude to the patients and staff of the Department of Tropical Medicine. Also to Dr Salma Mohkles and Dr Ahmed Taha Yonis for their great effort. Author contributions: Nasser Mousa and Magdy Hamed: study concept and design; Ahmed Abdelrazik and Ashraf Zaher: acquisition of data, analysis and interpretation of data, drafting the manuscript; Gamal Shiha and Narmin Effat: revision of the manuscript for important intellectual content; Waleed Eldars, Sherif Elbaz, Niveen El-Wakeel, Mona Arfa and Mohamed Hafez: statistical analysis, material support and study supervision. ClinicalTrials.gov identifier: NCT02520817.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Nasser Mousa, Tropical Medicine Department, Faculty of Medicine, Mansoura University, 35516 Mansoura, Egypt.
Ahmed Abdel-Razik, Department of Tropical Medicine, Faculty of Medicine, Mansoura University, Mansoura City, Egypt.
Ashraf Zaher, Department of Neurology, Faculty of Medicine, Mansoura University, Mansoura City, Egypt.
Magdy Hamed, Department of Internal Medicine, Faculty of Medicine, Mansoura University, Mansoura City, Egypt.
Gamal Shiha, Department of Internal Medicine, Faculty of Medicine, Mansoura University, Mansoura City, Egypt.
Narmin Effat, Department of Clinical Pathology, Faculty of Medicine, Mansoura University, Mansoura City, Egypt.
Sherif Elbaz, Endemic Diseases and Gastroenterology Department, Aswan University, Aswan, Egypt.
Rania Elhelaly, Department of Clinical Pathology, Faculty of Medicine, Mansoura University, Mansoura City, Egypt.
Mohamed Hafez, Department of Internal Medicine, Faculty of Medicine, Aswan University, Aswan, Egypt.
Niveen El-Wakeel, Department of Medical Microbiology and Immunology, Faculty of Medicine, Mansoura University, Mansoura City, Egypt.
Waleed Eldars, Department of Medical Microbiology and Immunology, Faculty of Medicine, Mansoura University, Mansoura City, Egypt.
References
- Amodio P., Del Piccolo F., Pettenò E., Mapelli D., Angeli P., Iemmolo R., et al. (2001) Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol 35: 37–45. [DOI] [PubMed] [Google Scholar]
- Bajaj J., Hafeezullah M., Franco J., Varma R., Hoffmann R., Knox J., et al. (2008) Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology 135: 1591–1600. [DOI] [PubMed] [Google Scholar]
- Balata S., Damink S., Ferguson K., Marshall I., Hayes P., Deutz N., et al. (2003) Induced hyperammonemia alters neuropsychology, brain MR spectroscopy and magnetization transfer in cirrhosis. Hepatology 37: 931–939. [DOI] [PubMed] [Google Scholar]
- Blei A., Córdoba J. (2001) Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol 96: 1968–1976. [DOI] [PubMed] [Google Scholar]
- Bosoi C., Rose C. (2013) Oxidative stress: a systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab Brain Dis 28: 175–178. [DOI] [PubMed] [Google Scholar]
- Caldwell S., Oelsner D., Iezzoni J. (1999) Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 3: 664–669. [DOI] [PubMed] [Google Scholar]
- Chetri K., Choudhuri G. (2003) Role of trace elements in hepatic encephalopathy: zinc and manganese. Indian J Gastroenterol 22(Suppl. 2): S28–S30. [PubMed] [Google Scholar]
- Cordoba J., Alonso J., Rovira A., Jacas C., Sanpedro F., Castells L., et al. (2001) The development of low-grade cerebral edema in cirrhosis is supported by the evolution of (1) H-magnetic resonance abnormalities after liver transplantation. J Hepatol 35: 598–604. [DOI] [PubMed] [Google Scholar]
- Czaja A., Freese D. (2002) American Association for the Study of Liver Disease. Diagnosis and treatment of autoimmune hepatitis. Hepatology 36: 479–497. [DOI] [PubMed] [Google Scholar]
- Dejong C., Deutz N., Soeters P. (1994) Muscle ammonia and glutamine exchange during chronic liver insufficiency in the rat. J Hepatol 21: 299–307. [DOI] [PubMed] [Google Scholar]
- Essa M., Subramanian P. (2006) Pongamiapinnata modulates oxidant–antioxidant imbalance during hyperammonemic rats. Fund Clin Pharmacol 3: 299–303. [DOI] [PubMed] [Google Scholar]
- Ferenci P., Lockwood A., Mullen K., Tarter R., Weissenborn K., Blei A. (2002) Hepatic encephalopathy, definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 35: 716–721. [DOI] [PubMed] [Google Scholar]
- Goeneweg M., Moerland W., Quero J. (2000) Screening of subclinical hepatic encephalopathy. J Hepatol 32: 748–753. [DOI] [PubMed] [Google Scholar]
- Irshad M., Chaudhuri S. (2002) Oxidant–antioxidant system: role and significance in human body. Ind J Exp Biol 40: 1233–1239. [PubMed] [Google Scholar]
- Marchesini G., Fabbri A., Bianchi G., Brizi M., Zoli M., et al. (1996) Zinc supplementation and amino acid–nitrogen metabolism in patients with advanced cirrhosis. Hepatology 23: 1084–1092. [DOI] [PubMed] [Google Scholar]
- Norenberg M., Jayakumar A., Rama Rao K. (2004) Oxidative stress in the pathogenesis of hepatic encephalopathy. Metab Brain Dis 19: 313–329. [DOI] [PubMed] [Google Scholar]
- OrtIz M., Jacas C., Cordoba J. (2005) Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol 42(Suppl.): S45–S53. [DOI] [PubMed] [Google Scholar]
- Ortiz M., Cordoba J., Jacas C., Flavia M., Esteban R., Guardia J. (2006) Neuropsychological abnormalities in cirrhosis include learning impairment. J Hepatol 44: 104–110. [DOI] [PubMed] [Google Scholar]
- Poordad F. (2007) Review article: The burden of hepatic encephalopathy. Aliment Pharmacol Ther 25(Suppl. 1): 3–9. [DOI] [PubMed] [Google Scholar]
- Riggio O., Merli M., Capocaccia L., Caschera M., Zullo A., Pinto G., et al. (1992) Zinc supplementation reduces blood ammonia and increases liver ornithine transcarbamylase activity in experimental cirrhosis. Hepatology 16: 785–789. [DOI] [PubMed] [Google Scholar]
- Romero Gómez M., Córdoba J., Jover R., del Olmo J., Ramírez M., Rey R., et al. (2007) Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology 45: 879–885. [DOI] [PubMed] [Google Scholar]
- Romero-Gómez M., Grande L., Camacho I., Benitez S., Irles J., Castro M. (2002) Altered response to oral glutamine challenge as prognostic factor for overt episodes in patients with minimal hepatic encephalopathy. J Hepatol 37: 781–787. [DOI] [PubMed] [Google Scholar]
- Sidhu S., Goyal O., Parker R., Kishore H., Sood A. (2016) Rifaximin vs. lactulose in treatment of minimal hepatic encephalopathy. Liver Int 36: 378–385. [DOI] [PubMed] [Google Scholar]
- Seyan A., Hughes R., Shawcross D. (2010) Changing face of hepatic encephalopathy: role of inflammation and oxidative stress. World J Gastroenterol 16: 3347–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Sharma B., Puri V., Sarin S., et al. (2007) Critical flicker frequency: diagnostic tool for minimal hepatic encephalopathy. J Hepatol 47: 67–73. [DOI] [PubMed] [Google Scholar]
- Thenmozhi A., Subramanian P. (2011) Antioxidant potential of Momordica charantia in ammonium chloride-induced hyperammonemic rats. Evid Based Complement Alternat Med 2011: 612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn K., Ennen J., Schomerus H., Ruckert N., Hecker H. (2001) Neuropsychological characterization of hepatic encephalopathy. J Hepatol 34: 768–773. [DOI] [PubMed] [Google Scholar]