Abstract
Background:
Dual red imaging (DRI), a novel image-enhanced endoscopic technique, is expected to improve visibility of thin vessels, but no reports of the clinical use of DRI in colorectal endoscopic submucosal dissection (ESD) have been published. We aimed to compare the visibility of vessels, demarcation line between the submucosal and muscle layers after injection of hyaluronate sodium with minute indigo carmine, and fibrosis on DRI with that on white light imaging (WLI). We applied the principle of DRI to the image of the submucosal layer during colorectal ESD as a pilot study.
Methods:
A total of seven physicians compared 17 DRI images to the corresponding WLI images in colorectal ESD. The physicians compared the number of arteries identified on DRI with the actual number of arteries. The physicians rated the visibility of vessels, the demarcation line between the submucosal and muscle layers after injection of hyaluronate sodium with minute indigo carmine, and fibrosis. Inter-observer agreement was also examined using the kappa statistic.
Results:
Visibility of vessels and the demarcation line between the submucosal and muscle layers after injection of hyaluronate sodium with minute indigo carmine improved with the use of DRI compared with that using WLI. DRI can discriminate between arteries and veins clearly through the color of the vessels.
Conclusions:
DRI improves the visibility of vessels, especially that of arteries, as they appear orange, and the demarcation line of the muscle layer. DRI may help to make colorectal ESD safer and faster.
Keywords: colorectal tumor, dual red imaging, endoscopic submucosal dissection, image-enhanced endoscopy
Introduction
Dual red imaging (DRI) is a novel image-enhanced endoscopy technique. DRI consists of three wavelengths different from narrow band images: the 540 nm light can visualize small blood vessels in shallow tissue, and the 600 nm and 630 nm lights can penetrate deep into the tissue. The principle of DRI is shown in Figure 1. The 600 nm and 630 nm lights can penetrate deep into the tissue because the scattering properties of these lights are not strong. If blood vessels are absent, each reflected light is detected by the charge-coupled device chip of an endoscope with little attenuation. On the other hand, if thick blood vessels exist in deep tissue, the reflected 600 nm light is attenuated because the light is absorbed strongly by hemoglobin in the blood vessels. Even with a certain level of absorption by hemoglobin, the 630 nm light is reflected with lower attenuation than the 600 nm light and the strong reflected light is detected. In short, DRI can display enhanced blood vessels in deep tissue when the following conditions are met: (1) the displayed image shows both the reflected 630 nm light and 600 nm light without attenuation; and (2) the displayed image shows that the 600 nm light is significantly more attenuated than 630 nm light. These enable visualization of thick vessels, which allows prompt identification of bleeding points in case of arterial hemorrhage.
Figure 1.
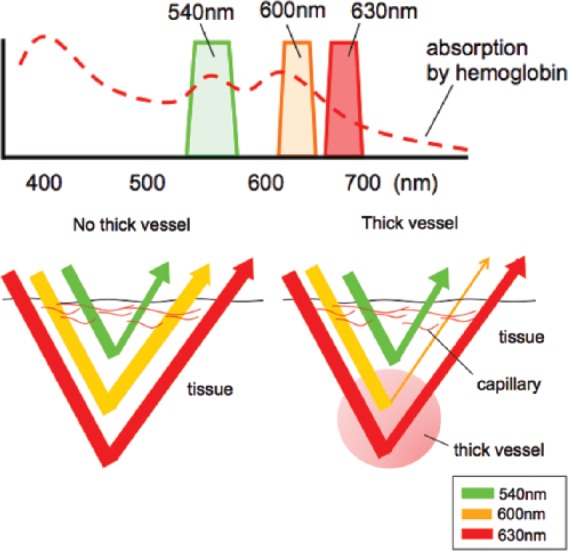
The principle of dual red imaging.
(a) DRI consists of three wavelengths: the 540 nm, 600 nm, and 630 nm lights. (b) If thick blood vessels exist in deep tissue, the reflected 600 nm light is attenuated because the light is absorbed strongly by hemoglobin in the blood vessels.
Endoscopic submucosal dissection (ESD) has been a reliable method for en-bloc resection of colorectal tumors regardless of lesion size [Tanaka et al. 2007, 2008, 2012, 2013; Tamegai et al. 2007; Saito et al. 2010; Oka et al. 2010, 2015a, 2015b; Terasaki et al. 2014; Ninomiya et al. 2015]. Although colorectal ESD has been established as a procedure with reproducible safety and efficacy, complications such as bleeding and perforation remain problematic. The clinical usefulness of DRI in colorectal ESD has not been reported. We conducted a retrospective study to compare the visibility of vessels, demarcation line between the submucosal and muscle layers after injection of hyaluronate sodium with minute indigo carmine, and fibrosis on DRI with that on white light imaging (WLI). We applied the principle of DRI to the image of submucosal layer during colorectal ESD as a pilot study.
Methods
A total of 17 endoscopic images of 10 patients who underwent colorectal ESD using DRI at Hiroshima University Hospital, Japan, during July 2014 were used for this study. We evaluated all of the images available to compare DRI with WLI in all patients who underwent ESD using DRI in our institution. We performed ESD using a DRI system scope (CF-H260AZI, PCF-Q260AZI, or CF-Y0044 [prototype], Olympus, Japan) and video processors (EVIS LUCERA ELITTE system, Olympus, Japan). For the injection solution, we used a mixture with a 1:1 ratio of 0.4% sodium hyaluronate (MucoUp®, Johnson & Johnson, USA) and 10% glycerin solution, plus minute indigo carmine. The DRI system can switch from WLI to DRI and narrow band imaging immediately using a button on the endoscope control head.
A total of seven physicians compared DRI images to the corresponding WLI images. The images of submucosal dissection during colorectal ESD were selected in all cases. The physicians counted the number of vessels with DRI and WLI. We also compared the number of arteries identified on DRI with the actual number of arteries identified by observing beating vessels or spurting bleeds on a recorded video. The physicians rated the visibility of vessels, demarcation line between the submucosal and muscle layers after injection of hyaluronate sodium with minute indigo carmine, and fibrosis (Figure 2). As previously described [Imagawa et al. 2011; Oka et al. 2015a, 2015b], the visibility on each image was rated as follows: +2 (improved), +1 (somewhat improved), 0 (equivalent to white light), −1 (somewhat decreased), and −2 (decreased). The seven scores for each image were totaled and evaluated. If an image earned a total score of +8 or more, the image was considered improved, a score between +7 and −7 indicated no change in visibility, and a score of −8 or less indicated decreased visibility. Inter-observer agreement was also examined using the kappa statistic.
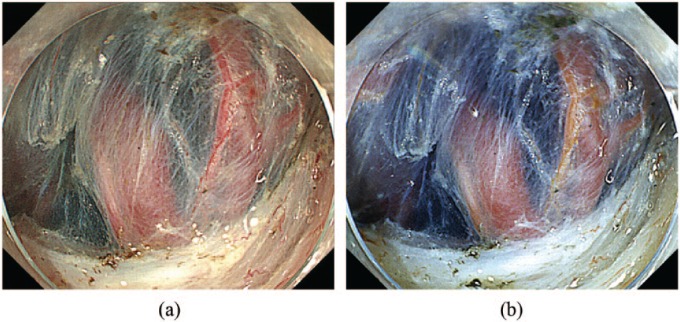
Figure 2.
Endoscopic images using dual red imaging.
(a) White light imaging. (b) Dual red imaging. The seven physicians evaluated the DRI image as improved visibility of vessels and the demarcation line of the muscle layer.
Use of patient data for the present study was approved by Hiroshima University’s Institutional Review Board, Japan.
Results
Visibility results are shown in Table 1. For vessels, improved visibility was seen in 59% (10/17) of images and equivalent visibility was seen in 41% (7/17) of images, and there was no decreased visibility. Vessels were classified into arteries and veins using DRI: arteries appeared orange and veins appeared red. The average number of arteries per image counted using DRI (2.2 ± 1.4) was statistically equivalent to that counted on observation of the video image (2.1 ± 1.5). For the demarcation line of the muscle layer, improved visibility was seen in 66% (11/17) of images and equivalent visibility was seen in 35% (6/17) of images, and there was no decreased visibility. For submucosal fibrosis, equivalent visibility was seen in all images and there was no improved or decreased visibility. Inter-observer agreement was 0.63 for vessels, 0.62 for the demarcation line of the muscle layer, and 0.47 for fibrosis.
Table 1.
Evaluation of visibility for DRI during colorectal ESD.
| Evaluation | Total (%) | Visibility, number of cases (%) |
||
|---|---|---|---|---|
| Improved | Equivalent | Decreased | ||
| Vessel | 17 (100) | 10 (58.8) | 7 (41.2) | 0 (0) |
| Demarcation line of the muscle layer | 17 (100) | 11 (64.7) | 6 (35.3) | 0 (0) |
| Submucosal fibrosis | 17 (100) | 0 (0) | 17 (100) | 0 (0) |
Kappa statistic: vessel, 0.63; demarcation line of the muscle layer, 0.62; submucosal fibrosis, 0.47.
DRI, dual red imaging; ESD, endoscopic submucosal dissection.
Discussion
This is the first report in the literature on the clinical use of DRI. Our study revealed that DRI images show a change in the color of the artery to orange and the vein to red; it is possible to discriminate between arteries and veins with clockwork precision, enhancing the safety and speed of colorectal ESD. The arteries and veins are recognizable as a result of the different degrees of oxygen saturation between the artery and vein reflected in the color tones of those blood vessels (Figure 3). There is no significant difference in the absorption of the 630 nm light displayed as a red channel on DRI between arterial blood and venous blood. On the other hand, the artery is displayed in bright orange, because hemoglobin absorption of the 600 nm light, which is displayed as a green channel on DRI, is weak. Because hemoglobin absorption of the 600 nm light in venous blood is strong, the vein is displayed in dark red. The 540 nm light, displayed as a blue channel on DRI, is strongly absorbed in both the artery and vein at the same level. In case of minor bleeding from a small vessel or vein, contact coagulation with the tip of a knife or coagulation with hemostatic forceps is usually used for hemostasis. In cases of severe bleeding from a large vessel or artery, hemostatic forceps are indispensable. To avoid delayed perforation, the bleeding point should be grasped precisely with hemostatic forceps [Tanaka et al. 2015]. Yahagi and colleagues reported that DRI visualizes the bleeding point clearly during gastric and colorectal ESD; it shortens the average time required for hemostasis, makes hemostasis less difficult, and decreases the operator’s psychological stress for hemostasis [Yahagi et al. 2014].
Figure 3.
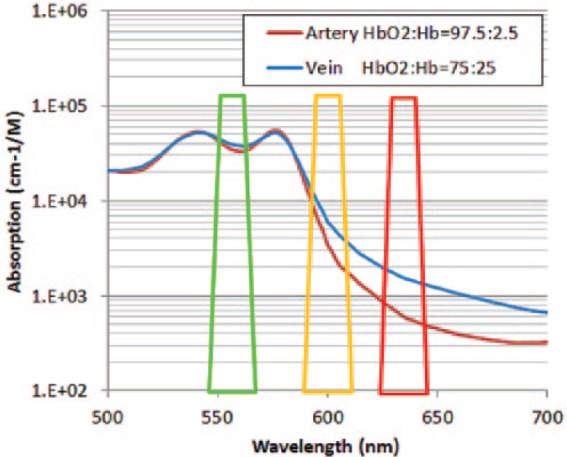
The principle of discrimination between arteries and veins using dual red imaging.
The arteries are displayed in bright orange, because hemoglobin absorption of the 600 nm light, which is displayed as a green channel on DRI, is weak. Because hemoglobin absorption of the 600 nm light in venous blood is strong, the vein is displayed in dark red.
Our study revealed that the visibility of vessels and the demarcation line of the muscle layer improved with the use of DRI. It is considered that different color tones between the submucosal and muscular layers are more enhanced on DRI than on WLI. On DRI, under the influence of absorption of the light by indigo carmine dye, the submucosal layer is displayed in dark blue as on WLI. On the other hand, DRI enhances the white element of the muscular layer more intensely than WLI. In our study for submucosal fibrosis, equivalent visibility was seen in all images and there was no improved visibility. Analysis was based on a small number of lesions with fibrosis. We will reanalyze the visibility of fibrosis using DRI in a larger number of patients.
The limitations of our study were its retrospective nature and the small number of cases. A prospective study including a large number of cases is needed in the near future to validate our results.
Conclusion
DRI improves the visibility of vessels, especially that of arteries as they appear orange, and the demarcation line of the muscle layer. DRI may help make colorectal ESD safer and faster than WLI.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Yuki Ninomiya, Department of Endoscopy, Hiroshima University Hospital, Hiroshima, Japan.
Shiro Oka, Department of Endoscopy, Hiroshima University Hospital, 1-2-3 Kasumi Minamiku, Hiroshima, Japan.
Shinji Tanaka, Department of Endoscopy, Hiroshima University Hospital, Hiroshima, Japan.
Daiki Hirano, Department of Gastroenterology and Metabolism, Hiroshima University Hospital, Hiroshima, Japan.
Kyoku Sumimoto, Department of Gastroenterology and Metabolism, Hiroshima University Hospital, Hiroshima, Japan.
Yuzuru Tamaru, Department of Gastroenterology and Metabolism, Hiroshima University Hospital, Hiroshima, Japan.
Naoki Asayama, Department of Gastroenterology and Metabolism, Hiroshima University Hospital, Hiroshima, Japan.
Kenjiro Shigita, Department of Gastroenterology and Metabolism, Hiroshima University Hospital, Hiroshima, Japan.
Soki Nishiyama, Department of Gastroenterology and Metabolism, Hiroshima University Hospital, Hiroshima, Japan.
Nana Hayashi, Department of Endoscopy, Hiroshima University Hospital, Hiroshima, Japan.
Kazuaki Chayama, Department of Gastroenterology and Metabolism, Hiroshima University Hospital, Hiroshima, Japan.
References
- Imagawa H., Oka S., Tanaka S., Noda I., Higashiyama M., Sanomura Y., et al. (2011) Improved visibility of lesions of the small intestine via capsule endoscopy with computed virtual chromoendoscopy. Gastrointest Endosc 73: 299–306. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Oka S., Tanaka S., Nishiyama S., Tamaru Y., Asayama N., et al. (2015) Risk of bleeding after endoscopic submucosal dissection for colorectal tumors in patients with continued use of low-dose aspirin. J Gastroenterol 50:1041–1046. [DOI] [PubMed] [Google Scholar]
- Oka S., Tamai N., Ikematsu H., Kawamura T., Sawaya M., Takeuchi T., et al. (2015a) Improved visibility of colorectal flat tumors using image-enhanced endoscopy. Dig Endosc 27: S35–S39. [DOI] [PubMed] [Google Scholar]
- Oka S., Tanaka S., Kanao H., Ishikawa H., Watanabe T., Igarashi M., et al. (2010) Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc 22: 376–380. [DOI] [PubMed] [Google Scholar]
- Oka S., Tanaka S., Saito Y., Iishi H., Kudo S., Ikematsu H., et al. (2015b) Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 110: 697–707. [DOI] [PubMed] [Google Scholar]
- Saito Y., Uraoka T., Yamaguchi Y., Hotta K., Sakamoto N., Ikematsu H., et al. (2010) A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 72: 1217–1225. [DOI] [PubMed] [Google Scholar]
- Tamegai Y., Saito Y., Masaki N., Hinohara C., Oshima T., Kogure E., et al. (2007) Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy 39: 418–422. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Kashida H., Saito Y., Yahagi N., Yamano H., Saito S., et al. (2015) JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 27: 417–434. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Oka S., Chayama K. (2008) Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection.J Gastroenterol 43: 641–651. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Oka S., Kaneko I., Hirata M., Mouri R., Kanao H., et al. (2007) Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 66: 100–107. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Terasaki M., Hayashi N., Oka S., Chayama K. (2013) Warning for unprincipled colorectal endoscopic submucosal dissection: accurate diagnosis and reasonable treatment strategy. Dig Endosc 25: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Terasaki M., Kanao H., Oka S., Chayama K. (2012) Current status and future perspectives of endoscopic submucosal dissection for colorectal tumors. Dig Endosc 24: S73–S79. [DOI] [PubMed] [Google Scholar]
- Terasaki M., Tanaka S., Shigita K., Asayama N., Nishiyama S., Hayashi N., et al. (2014) Risk factors for delayed bleeding after endoscopic submucosal dissection for colorectal neoplasms. Int J Colorectal Dis 29: 877–882. [DOI] [PubMed] [Google Scholar]
- Yahagi N., Horii J., Goto O. (2014) Dual red imaging; a new endoscopic imaging technology for clear visualization of bleeding points in endoscopic submucosal dissection. Gastrointest Endosc 79: AB464. [Google Scholar]