Abstract
Background:
It is recommended that ileocolonoscopy is performed within 1 year after resection for Crohn’s disease (CD). Nevertheless, optimal monitoring strategies for recurrence after the ileocolonoscopy remain to be elucidated. This prospective study was to evaluate the value of serial monitoring of faecal calprotectin (FC) after ileocolonoscopy for the assessment of endoscopic recurrence in asymptomatic patients.
Methods:
Patients in clinical remission who had no endoscopic recurrence at ileocolonoscopy 6–12 months after ileocolonic resection were studied. FC levels were measured every 2 months up to 24 months after the ileocolonoscopy. When the FC level was elevated (⩾140 µg/g), a second ileocolonoscopy was immediately undertaken. In contrast, patients who maintained low FC levels (<140 µg/g) during the 24-month follow up underwent a second ileocolonoscopy at the end of the study. Endoscopic recurrence was defined as a Rutgeerts score ⩾i2.
Results:
A total of 30 patients were studied. In eight patients, the FC level was raised during the 24-month follow up. Six of the eight patients (75%) had endoscopic recurrence. Of 22 patients who maintained low FC levels, 20 (91%) had no endoscopic recurrence, whereas two showed endoscopic recurrence at the end of the follow up. The incidence of endoscopic recurrence was significantly higher in patients with elevation of FC levels versus those with maintained low FC levels (75% versus 9%). A cut-off value of 140 µg/g for FC had a sensitivity of 75%, a specificity of 91%, a positive predictive value of 75%, a negative predictive value of 91% and a diagnostic accuracy of 87% to detect endoscopic recurrence.
Conclusions:
Consecutive monitoring of FC is useful for the assessment of endoscopic recurrence after the initial ileocolonoscopy. Increased FC levels indicate a need for repeat ileocolonoscopy, while sustained low FC levels predict a low risk of endoscopic recurrence. In patients maintaining low FC levels, unnecessary invasive endoscopic examinations can be avoided.
Keywords: Crohn’s disease, endoscopic recurrence, faecal calprotectin, ileocolonoscopy, postoperative recurrence
Introduction
The severity of endoscopic lesions in the neoterminal ileum during the first year after ileocolonic resection for Crohn’s disease (CD) is a reliable predictor for future clinical recurrence [Rutgeerts et al. 1990; Yamamoto et al. 2013a]. Patients with severe endoscopic lesions within 1 year after surgery develop early clinical recurrence. In contrast, patients with no or mild endoscopic lesions have a low frequency of subsequent clinical recurrence. In our clinical practice, ileocolonoscopy is therefore recommended to be performed within the first year after surgery where treatment decisions may be affected [Van Assche et al. 2010]. Nevertheless, optimal monitoring strategies for postoperative recurrence after the initial ileocolonoscopy have yet to be established. Although an ideal approach may be to repeat ileocolonoscopy, patients are reluctant to undergo repeated endoscopic examinations when their disease is inactive. Endoscopy is a time-consuming and invasive procedure. Simple and noninvasive methods for the detection of postoperative recurrence are desirable.
Calprotectin is a neutrophil-derived protein that is stable in faeces and can be detected in small stool samples [Lamb and Mansfield, 2011; Foell et al. 2009]. Faecal calprotectin (FC) showed a close correlation with endoscopic inflammation in patients with inflammatory bowel disease (IBD) [Lamb and Mansfield, 2011; Foell et al. 2009; D’Haens et al. 2012]. Accordingly, FC appears to be useful for the assessment of endoscopic disease activity of IBD. A number of studies found that levels of FC were associated with the severity of endoscopic inflammation in asymptomatic postoperative patients with CD [Yamamoto et al. 2013b; Wright et al. 2015; Boschetti et al. 2015]. In contrast, several studies failed to show a significant relationship between endoscopic severity and the values of FC [Lasson et al. 2014]. However, to our knowledge, few studies have consecutively measured the levels of FC after resection for CD. The aim of this prospective study was to evaluate the value of consecutive monitoring of FC after the initial ileocolonoscopy performed within the first year following surgery for the assessment of postoperative endoscopic recurrence in asymptomatic patients.
Patients and methods
Study design
This was a prospective, single-centre study undertaken at the Yokkaichi Hazu Medical Centre, a referral centre treating a large number of patients with IBD in the Mie Prefecture of Japan. Our study protocol was reviewed and approved by the Institutional Review Board at our hospital.
Patients
Inclusion criteria were: (1) patients who were between 20 and 70 years of age; (2) patients who had undergone ileocolonic resection and primary anastomosis for active CD; (3) patients in clinical remission [CD activity index (CDAI) score [Best et al. 1976] < 150], who had no endoscopic recurrence at ileocolonoscopy 6–12 months after surgery; and (4) patients who agreed to undergo endoscopic examination during the study period. Exclusion criteria were: (1) patients in whom a defunctioning stoma was created for the protection of ileocolonic anastomosis; (2) patients who had gastroduodenal, jejunal, or proximal ileal disease at the time of surgery; and (3) patients with active colonic or anorectal disease at ileocolonoscopy.
Endoscopic assessment
The endoscopic severity of inflammation in the neoterminal ileum was graded according to Rutgeerts and colleagues [Rutgeerts et al. 1990]. The Rutgeerts score is a well established endoscopic scoring system based on examination of the ileal segment proximal to ileocolonic anastomosis: i0, no lesions; i1, ⩽5 aphthous lesions; i2, >5 aphthous lesions with normal mucosa between lesions, or skip areas of larger lesions or lesions confined to the ileocolonic anastomosis; i3, diffuse aphthous ileitis with diffusely inflamed mucosa; i4, diffuse inflammation with larger ulcers, nodules, with or without narrowing. Endoscopic findings were recorded on colour pictures, and evaluated by two specialist physicians who were blinded to the patients’ clinical details. Endoscopic recurrence was defined as a Rutgeerts score ⩾ i2. In this study, circumferential ulcer confined to the anastomosis was considered to be ischemic change, but not to be inflammation.
Medical treatment
After surgery, all patients were treated with standard prophylactic medications (mesalazine, azathioprine, or biologics) for the prevention of CD recurrence. Because the updated meta-analysis remained in favour of treatment with mesalazine in patients with postoperative CD [Van Assche et al. 2010], mesalazine was given in the majority of patients at our centre. Prophylactic treatment with azathioprine, with or without a biologic agent (infliximab or adalimumab) was started within 4 weeks after surgery in patients at a high risk of postoperative recurrence, such as smokers, those with perforating disease, or those with multiple surgeries, and those who had received azathioprine or biologics immediately before surgery [De Cruz et al. 2012; Yamamoto and Watanabe, 2013]. In contrast, mesalazine treatment was started soon after surgery in patients at a low risk of recurrence (nonsmokers, those with nonperforating disease, or those without previous surgery) [De Cruz et al. 2012; Yamamoto and Watanabe, 2013]. These prophylactic medications were continued without any changes unless there was postoperative recurrence confirmed by endoscopy.
Follow up
All patients were reviewed every 2 months in our clinic for 24 months after the initial ileocolonoscopy at 6–12 months after surgery. Patients were required to record their symptoms in a diary. At clinic visits, body weight, general well-being, body temperature, stool frequency and consistency, presence or absence of abdominal discomfort, tenderness, tenesmus and haematochezia were recorded. Peripheral blood samples were taken for the measurement of white blood cell count (WBC), platelet count, C-reactive protein (CRP), and albumin.
Measurement of faecal calprotectin
FC measurement was performed at clinical visits, every 2 months during the study period. Patients were advised to collect a stool sample in the early morning within 5 days before their clinic visits and store it at room temperature. Patients were advised not to take nonsteroidal anti-inflammatory drugs before collecting stool samples. At the clinic, the sample was submitted to our laboratory, and FC was measured using a commercially available NS-Prime automatic analyser (Alfresa Pharma Corporation, Osaka, Japan) as previously described [Inoue et al. 2014]. A sample (0.01 ml) and reaction buffer (0.14 ml) were pipetted into a cuvette at 37°C. After about 1 minute, 0.05 ml of the solution of colloidal gold particles coated with the anticalprotectin antibody was added and mixed. Reactions between the particles and any calprotectin in the samples resulted in the formation of agglutinates and a concomitant change in the absorbance signal. The change ratio for the absorbance values at 660 nm and 546 nm (secondary and primary wavelengths, respectively) was measured for about 6 minutes after the start of the agglutination reaction. The calprotectin concentration in the sample was determined by comparison with a standard curve. This assay can provide results in about 10 minutes. Laboratory investigators were blinded to the clinical data. Stool culture and sensitivity tests were not performed with each FC analysis.
Timing of second ileocolonoscopy
When the FC level was elevated (⩾140 µg/g), a second ileocolonoscopy was immediately (within 1 week) undertaken. In contrast, patients maintaining low FC levels (<140 µg/g) during the 24-month follow up underwent a second ileocolonoscopy at the end of the study. The cut-off level of 140 µg/g for the detection of endoscopic recurrence was determined based on our own experiences and relevant studies by other researchers [Inoue et al. 2014].
Statistical analysis
Comparisons of frequencies were analysed using the Chi-square test with Yates’ correction. Differences between median values were compared using the Mann–Whitney U test or the Kruskal–Wallis test if more than two groups were compared. Correlations were calculated using the Spearman’s rank correlation coefficient test. A p value < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 36 eligible patients were monitored in this study. Six patients developed clinical recurrence (CDAI score ⩾ 150), and dropped out of the study because our study was intended for patients in clinical remission. Eventually, 30 patients were included in the analysis. Baseline characteristics of the 30 patients are presented in Table 1.
Table 1.
Baseline characteristics of the 30 patients included in this study.
| Median (range) age at entry | 32 (21–48) years |
| Male:female | 17:13 |
| Median (range) duration of Crohn’s disease before entry | 36 (12–97) months |
| Smoker (n) | 4 |
| Previous bowel resection (n) | 12 |
| Indication for surgery (n) | |
| Stricture | 22 |
| Abscess and/or fistula | 8 |
| Postoperative medications (n) | |
| Mesalazine | 28 |
| Azathioprine | 12 |
| Biologics (infliximab:adalimumab) | 9:5 |
Faecal calprotectin and endoscopic recurrence
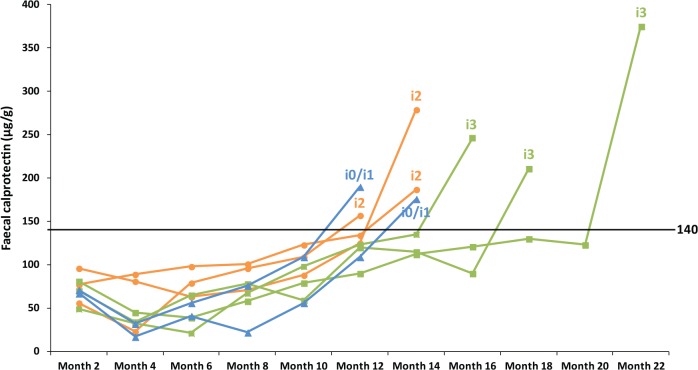
In eight patients (27%), FC level was elevated (⩾140 µg/g) during the 24-month follow up (Figure 1). The second ileocolonoscopy was undertaken when the FC level was raised. Six of the eight patients (75%) had endoscopic recurrence (Rutgeerts score ⩾ i2). The endoscopic score was i2 in three patients and i3 in three patients. In contrast, 22 patients (73%) maintained low FC levels (<140 µg/g) during the entire study period. At the end of the follow-up period, 20 of the 22 patients (91%) had no endoscopic recurrence (i0 or i1), whereas two patients (9%) showed endoscopic recurrence (both i2). A cut-off value of 140 µg/g for FC had a sensitivity of 75%, a specificity of 91%, a positive predictive value of 75%, a negative predictive value of 91% and a diagnostic accuracy of 87% to detect endoscopic recurrence. Overall, eight patients (27%) developed endoscopic recurrence during the 24-month follow up after the initial ileocolonoscopy. The incidence of endoscopic recurrence was significantly higher in patients with elevation of FC levels (6/8 patients; 75%) versus those with maintained low FC levels (2/22 patients; 9%) (p = 0.002). The FC level was significantly associated with the severity of endoscopic inflammation in the neoterminal ileum (Figure 2). The level of FC was significantly higher in patients with higher endoscopic scores [median (range): i0 or i1, 82.5 (10–190) µg/g; i2, 157 (87–279) µg/g; i3, 246 (211–375) µg/g; p = 0.004]. The FC level was significantly higher in patients with endoscopic recurrence when compared with those in endoscopic remission [median (range): 199 (87–375) µg/g versus 82.5 (10–190) µg/g; p = 0.002].
Figure 1.
Changes in faecal calprotectin levels in eight patients with elevation (⩾140 µg/g) during the 24-month follow up. The endoscopic score at the second ileocolonoscopy is presented for each patient.
Figure 2.
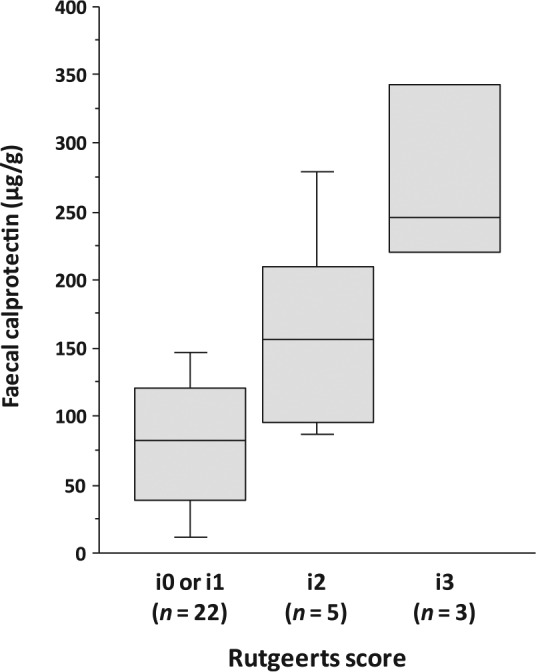
The relationship between the levels of faecal calprotectin and the severity of endoscopic inflammation in the neoterminal ileum at the second ileocolonoscopy. Boxes indicate interquartile ranges, with horizontal lines indicating medians and whiskers indicating the upper and lower limits.
Laboratory parameters
There was no significant correlation between the levels of FC and laboratory measurements including WBC, platelet count, CRP, and albumin throughout the whole study period (all comparisons: p > 0.05). Laboratory parameters (WBC, platelet count, CRP, and albumin) at the second ileocolonoscopy were not significantly different between patients with and without endoscopic recurrence (all comparisons: p > 0.05).
Discussion
This prospective study included asymptomatic patients without endoscopic recurrence at the initial ileocolonoscopy performed at 6–12 months after ileocolonic resection, and evaluated the value of FC measurement during a 24-month period. Our study has several advantages over what was done previously. First, we consecutively measured FC levels (every 2 months) for a relatively long time. In earlier studies, FC level was determined by only one test using a single stool sample [D’Haens et al. 2012; Yamamoto et al. 2013b; Boschetti et al. 2015]. In recent studies, we found that consecutive monitoring of FC was useful for the early detection of intestinal inflammation in patients with IBD [Yamamoto et al. 2015a, 2015b]. Another advantage is that all of our patients underwent a second ileocolonoscopy during the study period. It was performed immediately (within 1 week) when FC levels were increased, and even for patients who maintained low FC levels, it was conducted at the end of the study.
In previous research, FC measurement appeared to be useful for the assessment of endoscopic inflammation in the neoterminal ileum after resection for CD [Yamamoto et al. 2013b; Wright et al. 2015; Boschetti et al. 2015]. There was a close correlation between FC levels and endoscopic severity at 6–12 months after ileocolonic resection for CD. Wright and colleagues [Wright et al. 2015] and Boschetti and colleagues [Boschetti et al. 2015] found that the most valuable cut-off value for the detection of endoscopic recurrence was 100 µg/g. In contrast, the optimal cut-off value in our study was determined to be 140 µg/g, based on our own experiences and relevant studies by other researchers [Inoue et al. 2014]. For the diagnosis of endoscopic inflammation, the most suitable cut-off levels are different between studies because there is wide variation of FC values according to the assay used for the measurement [Whitehead et al. 2013; Labaere et al. 2014]. The standardization of assays used for measurement is urgently needed, and method-specific cut-off values should be determined.
Further, there are intraindividual variations of FC in stool samples collected during a single day. One study reported that since the levels of FC increase with longer time between the bowel movements, it seems most appropriate to analyse stool from the first bowel movement in the morning in one study [Lasson et al. 2015]. In another study, stool sample collection from the first bowel movement in the morning does not ensure the highest or lowest within-day FC value, and a single FC determination should not be used as the basis for therapeutic strategies [Calafat et al. 2015]. Thus, an intraindividual variation of FC should be thoroughly evaluated before FC testing is routinely used in our clinical practice.
In the present study, we found that 75% of patients with elevation of FC levels (⩾140 µg/g) had endoscopic recurrence. In contrast, 91% of patients with sustained low FC levels (<140 µg/g) had no endoscopic recurrence. The incidence of endoscopic recurrence was significantly higher in patients with elevated FC levels (75%) versus those with maintained low levels (9%). These results indicate that a cut-off value of 140 µg/g had a sensitivity of 75%, a specificity of 91%, a positive predictive value of 75%, a negative predictive value of 91% and a diagnostic accuracy of 87% to detect endoscopic recurrence. The diagnostic value of FC (⩾140 µg/g) in this study appears to be comparable with that in the previous research [Yamamoto et al. 2013b; Wright et al. 2015; Boschetti et al. 2015]. Therefore, our findings confirm that the FC test is also useful for the assessment of endoscopic recurrence after the initial ileocolonoscopy. Further, the level of FC was significantly associated with the severity of endoscopic inflammation. FC is derived from polymorphonuclear neutrophils in the inflamed intestinal mucosa, and it is a highly sensitive and specific biomarker for detecting mucosal inflammation. Consecutive monitoring of FC can detect an ongoing escalating inflammatory process in the gut that gives clinical symptoms when sufficiently severe.
Further, FC testing is a simple, low cost, and noninvasive method to avoid complicated invasive procedures. We do believe that FC is an ideal biomarker for the assessment of endoscopic disease activity particularly after the initial ileocolonoscopy performed within the first year of surgery because repeated endoscopic examinations are unpleasant for patient. The incidence of endoscopic recurrence is 27% in this study, which is much lower than approximately 70% observed within the first year after surgery in the previous study [Rutgeerts et al. 1990]. Thus, endoscopic recurrence, in our small cohort, is not common after the initial ileocolonoscopy performed within 1 year following surgery. With the introduction of FC testing, many patients can potentially avoid unnecessary invasive endoscopic examinations. Figure 3 shows our long-term strategy for the management of patients with CD after curative ileocolonic resection. After the conventional ileocolonoscopy at 6–12 months following surgery, FC testing could spare patients from going through complicated endoscopy procedures. As the previous study suggested [De Cruz et al. 2015], therapeutic decisions are made according to the severity of endoscopic inflammation. In patients without endoscopic lesions or with mild lesions (Rutgeerts score i0 or i1), the ongoing medication is continued. For patients who develop endoscopic recurrence (⩾i2), the medical treatment should be stepped up. Our strategy should be helpful in the management of postoperative CD for a long term.
Figure 3.
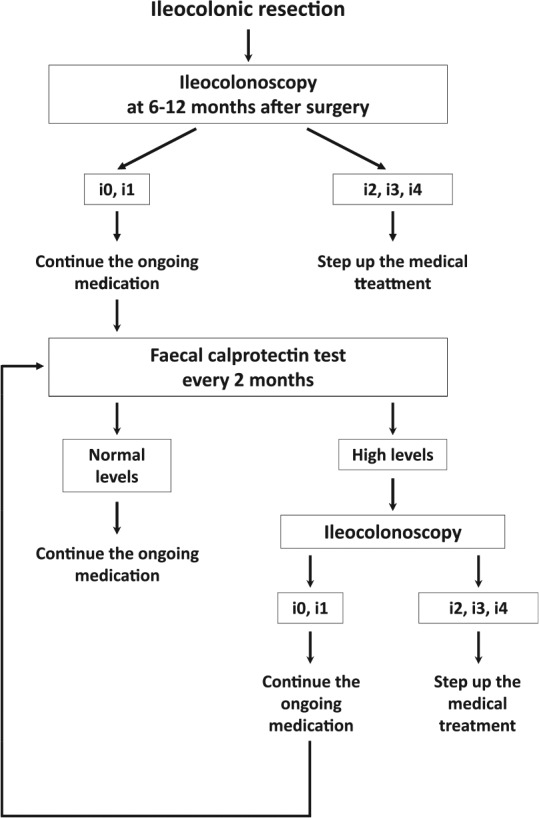
Our long-term strategy for the management of patients with Crohn’s disease after curative ileocolonic resection.
This study is limited by small case numbers and even smaller case numbers who actually had endoscopic recurrence that may limit the generalizability of the results. In this study, the sample size calculation was not done based on a primary statistical endpoint. We believe that the findings of this investigation should be strengthened by a future study involving a large cohort of patients. Further, an interval (2 months) for FC measurements in our study is imposed by the study design. The optimal interval remains unknown. Consecutive measurement of FC may be effective in a research setting; however, we should know how practical this is in the real world in terms of cost and the number of samples a patient would have to provide. We need to conduct a cost-effectiveness study to look at what the optimal measurement strategy would be based on incidence of postoperative recurrence, cost, and accuracy of the tests [Yamamoto et al. 2015a, 2015b].
In conclusion, consecutive monitoring of FC is useful for the assessment of endoscopic recurrence after the initial ileocolonoscopy performed within 1 year following surgery. Increased FC levels indicate a need for repeat ileocolonoscopy, while sustained low FC levels predict a low risk of endoscopic recurrence. In patients maintaining low FC levels, unnecessary invasive endoscopic examinations can be avoided. Further well designed large trials should strengthen the findings of the present investigation. The optimal FC test interval also needs to be determined. Further, future studies should investigate whether early medical intervention is useful for the prevention of subsequent recurrence in asymptomatic patients with elevated FC levels.
Acknowledgments
The authors would like to thank all colleagues who helped conducting this study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Takayuki Yamamoto, Inflammatory Bowel Disease Centre, Yokkaichi Hazu Medical Centre, 10-8 Hazuyamacho, Yokkaichi, Mie 510-0016, Japan.
Takahiro Shimoyama, Inflammatory Bowel Disease Centre, Yokkaichi Hazu Medical Centre, Yokkaichi, Mie, Japan.
Satoru Umegae, Inflammatory Bowel Disease Centre, Yokkaichi Hazu Medical Centre, Yokkaichi, Mie, Japan.
Koichi Matsumoto, Inflammatory Bowel Disease Centre, Yokkaichi Hazu Medical Centre, Yokkaichi, Mie, Japan.
References
- Best W., Becktel J., Singleton J., Kern F., Jr. (1976) Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 70: 439–444. [PubMed] [Google Scholar]
- Boschetti G., Laidet M., Moussata D., Stefanescu C., Roblin X., Phelip G., et al. (2015) Levels of fecal calprotectin are associated with the severity of postoperative endoscopic recurrence in asymptomatic patients with Crohn’s disease. Am J Gastroenterol 110: 865–872. [DOI] [PubMed] [Google Scholar]
- Calafat M., Cabré E., Mañosa M., Lobatón T., Marín L., Domènech E. (2015) High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis 21: 1072–1076. [DOI] [PubMed] [Google Scholar]
- De Cruz P., Kamm M., Hamilton A., Ritchie K., Krejany E., Gorelik A., et al. (2015) Crohn’s disease management after intestinal resection: a randomised trial. Lancet 385: 1406–1417. [DOI] [PubMed] [Google Scholar]
- De Cruz P., Kamm M., Prideaux L., Allen P., Desmond P. (2012) Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis 18: 758–777. [DOI] [PubMed] [Google Scholar]
- D’Haens G., Ferrante M., Vermeire S., Baert F., Noman M., Moortgat L., et al. (2012) Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 18: 2218–2224. [DOI] [PubMed] [Google Scholar]
- Foell D., Wittkowski H., Roth J. (2009) Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut 58: 859–868. [DOI] [PubMed] [Google Scholar]
- Inoue K., Aomatsu T., Yoden A., Okuhira T., Kaji E., Tamai H. (2014) Usefulness of a novel and rapid assay system for fecal calprotectin in pediatric patients with inflammatory bowel diseases. J Gastroenterol Hepatol 29: 1406–1412. [DOI] [PubMed] [Google Scholar]
- Labaere D., Smismans A., Van Olmen A., Christiaens P., D’Haens G., Moons V., et al. (2014) Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United European Gastroenterol J 2: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C., Mansfield J. (2011) Measurement of faecal calprotectin and lactoferrin in inflammatory bowel disease. Frontline Gastroenterol 2: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasson A., Stotzer P., Öhman L., Isaksson S., Sapnara M., Strid H. (2015) The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis 2015: 26–32. [DOI] [PubMed] [Google Scholar]
- Lasson A., Strid H., Ohman L., Isaksson S., Olsson M., Rydström B., et al. (2014) Fecal calprotectin one year after ileocaecal resection for Crohn’s disease - a comparison with findings at ileocolonoscopy. J Crohns Colitis 8: 789–795. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Geboes K., Vantrappen G., Beyls J., Kerremans R., Hiele M. (1990) Predictability of the postoperative course of Crohn’s disease. Gastroenterology 99: 956–963. [DOI] [PubMed] [Google Scholar]
- Van Assche G., Dignass A., Reinisch W., van der Woude C., Sturm A., De Vos M., et al. : European Crohn’s and Colitis Organisation (ECCO). (2010) The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: special situations. J Crohns Colitis 4: 63–101. [DOI] [PubMed] [Google Scholar]
- Whitehead S., French J., Brookes M., Ford C., Gama R. (2013) Between-assay variability of faecal calprotectin enzyme-linked immunosorbent assay kits. Ann Clin Biochem 50: 53–61. [DOI] [PubMed] [Google Scholar]
- Wright E., Kamm M., De Cruz P., Hamilton A., Ritchie K., Krejany E., et al. (2015) Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology 148: 938–947. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Bamba T., Umegae S., Matsumoto K. (2013a) The impact of early endoscopic lesions on the clinical course of patients following ileocolonic resection for Crohn’s disease: a 5-year prospective cohort study. United European Gastroenterol J 1: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Shimoyama T., Bamba T., Matsumoto K. (2015a) Consecutive monitoring of fecal calprotectin and lactoferrin for the early diagnosis and prediction of pouchitis after restorative proctocolectomy for ulcerative colitis. Am J Gastroenterol 110: 881–887. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Shimoyama T., Matsumoto K. (2015b) Consecutive monitoring of faecal calprotectin during mesalazine suppository therapy for active rectal inflammation in ulcerative colitis. Aliment Pharmacol Ther 42: 549–558. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Shiraki M., Bamba T., Umegae S., Matsumoto K. (2013b) Faecal calprotectin and lactoferrin as markers for monitoring disease activity and predicting clinical recurrence in patients with Crohn’s disease after ileocolonic resection: a prospective pilot study. United European Gastroenterol J 1: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Watanabe T. (2013) Strategies for the prevention of postoperative recurrence of Crohn’s disease. Colorectal Dis 15: 1471–1480. [DOI] [PubMed] [Google Scholar]