Abstract
Introduction:
Patients with rectal cancer who exhibit a pathologic complete response to preoperative concurrent chemoradiotherapy have excellent oncologic outcomes. In this study, we evaluated the potential advantages of adding oxaliplatin to preoperative fluoropyrimidine-based chemoradiotherapy administered in rectal cancer patients.
Methods:
A total of 78 patients with rectal cancer were enrolled. Patients were administered chemoradiotherapy, which comprised radiotherapy and chemotherapy involving a 5-fluorouracil, leucovorin, and oxaliplatin regimen every 2 weeks. Surgery was performed 10–12 weeks after radiotherapy completion. Tumor regression, adverse events, surgical complications, and short-term clinical outcomes were recorded.
Results:
Two patients were excluded because of incomplete radiotherapy treatment or refusal of surgery. Eventually, 76 patients underwent total mesorectal excision and no perioperative mortality was observed. Of these, 20 patients (25.6%) developed grade 3 or 4 toxicity during concurrent chemoradiotherapy. Among the 76 patients who underwent surgery, 24 (31.6%) patients achieved a pathologic complete response. The sphincter preservation rate was 96.1% (73/76) in all patients and 92.2% (39/42) in patients with tumors located less than 5 cm from the anal verge. The 2-year overall and disease-free survivals were 94% and 87.4%, respectively.
Conclusion:
The intensified multimodality therapy was well tolerated in our cohort and resulted in a considerably high pathologic complete response rate. Regardless of favorable short-term clinical outcomes, long-term oncologic outcomes will be closely monitored among the patients with a pathologic complete response.
Keywords: oxaliplatin, pathologic complete response, rectal cancer, sphincter preservation rate
Introduction
Combined-modality treatment comprising concurrent chemoradiotherapy (CCRT) followed by total mesorectal excision (TME) is the standard of care for patients with locally advanced rectal cancer (LARC). Preoperative CCRT substantially improves local control and sphincter preservation rates, and decreases treatment-related toxicity compared with that in a postoperative setting [Sauer et al. 2004; Bosset et al. 2006]. However, the response rate to preoperative CCRT has varied in previous investigations [Goldberg et al. 2004; Sauer et al. 2004; Bosset et al. 2006; Kalady et al. 2009; Chen et al. 2012]. Approximately 8–15% of patients with rectal cancer receiving standard CCRT with 5-fluorouracil (5-FU) achieve a pathologic complete response (pCR), which has been associated with improved local control and disease-free survival (DFS) [Garcia-Aguilar et al. 2003; Capirci et al. 2008; Maas et al. 2010].
Combinations of 5-FU and oxaliplatin (FOLFOX) have been confirmed to improve survival in the adjuvant treatment of colon cancer and prolong time to progression in metastatic colorectal cancer [Goldberg et al. 2004; Andre et al. 2009]. Although promising results have been reported in phase I/II studies in which oxaliplatin has been added in a neoadjuvant setting, in five randomized phase III trials comparing the efficacy and safety of fluoropyrimidine-based CCRT with or without oxaliplatin, the results have been inconsistent [Dolinsky et al. 2007; Aschele et al. 2011; Gerard et al. 2012; O’Connell et al. 2014; Schmoll et al. 2014; Rödel et al. 2015]. Only the German study demonstrated that oxaliplatin-containing neoadjuvant CCRT could improve the pCR rate and DFS [Rödel et al. 2015]. Recently, we have observed that the FOLFOX regimen was used in preoperative CCRT with a longer time interval between radiotherapy completion and robotic surgery in 26 LARC patients. A pCR was obtained in 14 (28%) patients [Huang et al. 2016].
Numerous studies have suggested that the rectal cancer response to radiation was independently related to the interval between CCRT and surgery, and complete tumor regression might require months of waiting time [Francois et al. 1999; Kerr et al. 2008; Garcia-Aguilar et al. 2011]. Therefore, increasing the time interval between CCRT and surgery may increase the chance of a pCR. In the present study, to achieve a higher pCR rate, patients with rectal cancer were treated with preoperative FOLFOX-based CCRT to enhance tumor regression and prevent perioperative metastasis. This current study presents the pCR rate, neoadjuvant treatment-related toxicity, surgical complications, and short-term clinical outcomes among the patients.
Methods
This retrospective study evaluated consecutive rectal cancer patients receiving preoperative CCRT followed by radical resection at a single institution. Patients with histopathologically verified rectal cancer (clinical T3–4 or nodal involvement or cT2 lesion within 5 cm from the anal verge) located within 15 cm of the anal verge, an Eastern Cooperative Oncology Group score of 0–2, and no evidence of distant metastases at diagnosis were reviewed in the study. The exclusion criteria included a history of previous or synchronous malignancies and prior pelvic radiotherapy or systemic chemotherapy. The study was approved by the institutional ethics committee of our hospital (KMUHIRB-20150195). Pretreatment staging workup entailed a complete history review and physical examination, colonoscopy, tumor biopsy, chest radiography, abdominal and pelvic computed tomography (CT) scan or magnetic resonance imaging, and routine laboratory studies.
Preoperative therapy
Radiotherapy was delivered according to a previously published study [Huang et al. 2014a]. In brief, radiotherapy was administered to the whole pelvis at a dose of 45 Gy in 25 fractions, followed by a 5.4 Gy boost to the primary tumor in three fractions. The concurrent chemotherapy regimen was a biweekly schedule of FOLFOX administered concurrently with radiotherapy. Patients were administered 12 cycles of FOLFOX regimen, including the neoadjuvant- and adjuvant-chemotherapy setting. Patients undergo surgical intervention at 10–12 weeks after completion of the radiotherapy; hence, it would be six or seven cycles of FOLFOX at the neoadjuvant setting and six or five cycles at the adjuvant setting. Each cycle of FOLFOX consisted of oxaliplatin (85 mg/m2) on day 1, folinic acid (400 mg/m2), and a 46-hour infusion of 5-FU (2800 mg/m2) repeated every 2 weeks.
Surgery and pathology
Patients underwent standard TME 10–12 weeks after completing radiotherapy. First, we started to perform peritoneal incision at the level of the sacral promontory. Then, the dissection was extended upward and downward. Afterward, the inferior mesenteric artery was identified and ligated near the origin. The inferior mesenteric vein (IMV) was also identified, but was not ligated immediately. If there was tension during the colonic anastomosis, the IMV would be ligated by using endo clips and divided. The rectum was mobilized with TME down to the pelvic floor. Hand-sewn end-to-end anastomosis was performed extracorporeally. For a tumor located in the upper and mid rectum, the surgical procedure used was low-anterior resection (LAR) with the double-stapled technique. In cases of low rectal cancers, the surgical procedure used was intersphincteric resection. Then, the specimen was extracted and resected transanally (natural orifice specimen extraction). Coloanal anastomosis was performed using the hand-sewn method and a loop colostomy or ileostomy would be performed. The tumor regression grade (TRG) was evaluated according to the AJCC system [Mace et al. 2015]. A circumferential resection margin (CRM) of less than 1 mm was defined as a positive CRM [Nagtegaal and Quirke, 2008]. A pCR was defined as the absence of viable cancer cells in the pathological specimen, including primary tumor and lymph nodes (ypT0N0) after neoadjuvant treatment.
Adjuvant chemotherapy
Patients received adjuvant chemotherapy up to total 12 cycles of FOLFOX regimen after radical resection if they exhibited one of the following risk factors: (1) pathologic lymph node metastasis, (2) positive resection or circumferential margin, or (3) pathologic T3–4 lesion. Adjuvant chemotherapy involved additional five to six cycles of FOLFOX (12 perioperative cycles in total) repeated every 14 days. For patients with ypT1–2N0 lesions, fluoropyrimidine-based chemotherapy was administrated to up to 6 months of perioperative chemotherapy.
Toxicity and efficacy
During CCRT and postoperative follow up, adverse events were scored and recorded at each visit according to the Common Terminology Criteria for Adverse Events, Version 3.0 (available at: http://ctep.cancer.gov). Treatment efficacy was evaluated according to the pCR, TRG, and downstaging of the T or N stage. Downstaging was determined by comparing the pre-CCRT clinical T or N stage and the postoperative pathological T or N stage.
Statistical Analysis
The chi-square test and Fisher’s exact test were used to compare categorical data, and continuous variables were analyzed using the nonparametric test. DFS was measured from the treatment start date to the date of any type of recurrence or the final follow up. Overall survival (OS) was defined as the period from the start of treatment to death from any cause or the final follow up. The time to recurrence was determined from the day of the commencement of treatment. Survivals and local–regional control were estimated using the Kaplan–Meier method. The log rank test was used to compare survival between patients with pCR versus non-pCR. Data analyses were performed using JMP software (Version 9.0, SAS Institute Inc., Cary, NC).
Results
A total of 78 patients with rectal cancer undergoing FOLFOX-based CCRT were included in the study from January 2012 to October 2015. The clinical tumor stage was cT2 in 9 patients, cT3 in 53 patients, and cT4 in 16 patients; 52 patients had a clinical nodal-positive disease (cN+). Table 1 presents the patient demographics and tumor characteristics.
Table 1.
Patient characteristics (n = 78).
| Age, median (years, range) | 61 (32–93) |
|---|---|
| Gender | |
| Male | 47 (60.3) |
| Female | 31 (39.7) |
| Clinical tumor depth | |
| T2 | 9 (11.5) |
| T3 | 53 (67.9) |
| T4a | 8 (10.3) |
| T4b | 8 (10.3) |
| Clinical lymph node metastasis | |
| N0 | 26 (33.3) |
| N1 | 28 (35.5) |
| N2 | 24 (31.2) |
| AJCC staging | |
| I | 4 (5.1) |
| II | 22 (28.2) |
| III | 52 (66.7) |
| Pretreatment CEA (ng/ml) | |
| ≦5 | 48 (61.5) |
| >5 | 30 (38.5) |
| Tumor distance from anal verge (cm) | |
| ≦5 | 42 (53.8) |
| 6–10 | 28 (35.9) |
| 11–15 | 8 (10.3) |
Data are presented as n (%), unless otherwise indicated.
AJCC, American Joint Committee on Cancer; CEA, carcinoembryonic antigen.
Acute toxicity
Table 2 shows the acute toxicity profiles obtained from CCRT. Leukopenia was the most common grade 3 toxicity (11.5%) followed by diarrhea (5.6%), but they were manageable in all patients. The only grade 4 adverse event (1.3%) was anemia; however, the patient recovered uneventfully after a blood transfusion. No treatment-related death occurred in the current study.
Table 2.
Preoperative chemoradiotherapy toxicity profile (n = 78).
| n (%) | |
|---|---|
| Number of patients with grade 3 or 4 toxicities | 20 (25.6) |
| Nausea | |
| 1 | 23 (29.5) |
| 2 | 3 (3.8) |
| Vomiting | |
| 1 | 16 (20.5) |
| 2 | 2 (2.6) |
| Diarrhea | |
| 1 | 36 (46.2) |
| 2 | 13 (16.7) |
| 3 | 5 (6.4) |
| Leukopenia | |
| 1 | 15 (19.2) |
| 2 | 26 (33.3) |
| 3 | 9 (11.5) |
| Anemia | |
| 1 | 18 (23.1) |
| 2 | 16 (20.5) |
| 3 | 2 (2.6) |
| 4 | 1 (1.3) |
| Dermatitis | |
| 1 | 20 (25.6) |
| 2 | 7 (8.9) |
| 3 | 3 (3.8) |
| Paresthesia | |
| 1 | 21 (26.9) |
| 2 | 6 (7.7) |
| Fatigue | |
| 1 | 29 (37.2) |
Only one patient did not complete the scheduled radiation regimen; treatment was interrupted because of grade 3 diarrhea and he refused further treatment. Therefore, the patient was excluded from the pathologic response and surgical morbidity evaluation.
Surgery and pathology
The median interval between radiotherapy and surgery was 11 (range 10–19) weeks. One patient declined surgery after CCRT because anal pain had improved and one patient did not complete the scheduled radiation regimen. Finally, 76 patients could be evaluated with the surgical results. The number of patients who underwent open, laparoscopic, and robotic-assisted surgery were 6, 42, and 28, respectively. Protective-loop transverse colostomy was performed in 45 patients (59.2%), including 42 patients who received restorative proctectomy with coloanal anastomosis and three patients who received LAR. The sphincter preservation rate was 96.1% (73/76) in all patients and 92.9% (39/42) in patients with tumors located less than 5 cm from the anal verge. The CRM and distal resection margins were free of cancer cells in 74 (97.4%) and 75 (98.7%), respectively, of the 76 patients. Table 3 lists the surgical results.
Table 3.
Surgical results of 76 rectal cancer patients.
| n (%) | |
|---|---|
| Surgery | |
| Low-anterior resection | 31 (40.8) |
| Restorative proctectomy with coloanal anastomosis | 42 (55.3) |
| Abdominoperineal resection | 3 (3.9) |
| ypT | |
| 0 | 26 (34.2) |
| 1 | 5 (6.6) |
| 2 | 15 (19.7) |
| 3 | 30 (39.5) |
| ypN | |
| 0 | 60 (78.9) |
| 1 | 12 (15.3) |
| 2 | 4 (5.5) |
| Median number of resected nodes* | 8 (2–25) |
| Median number of involved nodes* | 0 (0–5) |
| Lymphovascular invasion | |
| Yes | 8 (10.5) |
| No | 68 (89.5) |
| Perineural invasion | |
| Yes | 9 (11.8) |
| No | 67 (88.2) |
| Tumour differentiation | |
| Well | 13 (17.2) |
| Moderately | 60 (78.9) |
| Poorly | 3 (3.9) |
| Distal-resection margin | |
| Negative | 75 (98.7) |
| Positive | 1 (1.3) |
| Circumferential-resection margin | |
| Negative | 74 (97.4) |
| Positive | 2 (2.6) |
Median (range).
ypT, the tumor (T) stage has been determined after neoadjuvant therapy; ypN, the regional lymph nodes (N) stage has been determined after neoadjuvant therapy.
Table 4 presents the pathologic evaluation of the primary tumor compared with that of the initial clinical stage. Three out of 16 patients (18.8%) with cT4 disease exhibited ypT0, and all T4 tumors exhibited tumor downstaging after intensified CCRT. Moreover, no patient progressed to ypT4 disease in the study. And 37.3% (19/51) cT3 patients achieved ypT0 after intensified CCRT.
Table 4.
Correlation between clinical T stage and pathological T stage (%) in 76 rectal cancer patients.
| ypT0 | ypT1 | ypT2 | ypT3 | ypT4a | ypT4b | Total | |
|---|---|---|---|---|---|---|---|
| cT1 | – | – | – | – | – | – | 0 |
| cT2 | 4 (5.3) | – | 2 (2.6) | 3 (3.9) | – | – | 9 (11.8) |
| cT3 | 19 (25.0) | 4 (4.3) | 11 (14.5) | 17 (22.4) | – | – | 51 (67.2) |
| cT4a | 2 (2.6) | 1 (1.3) | – | 5 (6.6) | – | – | 8 (10.5) |
| cT4b | 1 (1.3) | – | 2 (2.6) | 5 (6.6) | – | – | 8 (10.5) |
| Total | 26 (34.2) | 5 (6.6) | 15 (19.7) | 30 (39.5) | 0 | 0 | 76 (100) |
ypT, the tumor (T) stage has been determined after neoadjuvant therapy.
Table 5 shows a summary of the treatment efficacy. Of the patients, two (2.6%) exhibited ypT0N1a and ypT0N1c and neither of them experienced recurrence after 2 years of follow up. Among the 76 patients who underwent surgery, 24 (31.6%) exhibited a pCR. Among the patients who exhibited a pCR, longer interval (⩾11 weeks versus <11 weeks) between CCRT and surgery tended to result in favorable pCR, though statistical significance was not met (42.9% versus 20.6%, p = 0.064). The proportion of patients who exhibited major tumor regression (TRG 1), moderate tumor regression (TRG 2), and minimal tumor regression (TRG 3) was 40.8%, 19.7%, and 5.3%, respectively. The overall tumor response (TRG 0 + 1 + 2) rate was 94.7%, and 57 patients (75%) had TRG 0 or 1 in surgical specimens after neoadjuvant treatment.
Table 5.
Tumor response after preoperative chemoradiotherapy (n = 76).
| n (%) | |
|---|---|
| Pathologic complete response | |
| Yes | 24 (31.6) |
| No | 52 (68.4) |
| Tumour regression grade | |
| 0 | 26 (34.2) |
| 1 | 31 (40.8) |
| 2 | 15 (19.7) |
| 3 | 4 (5.3) |
| ypT 0–2 versus ypT 3–4 | |
| ypT 0–2 | 46 (60.5) |
| ypT 3–4 | 30 (39.5) |
| Pathologic T stage | |
| Downstaging | 54 (71.1) |
| Stable | 19 (25.0) |
| Progressive | 3 (3.9) |
| Pathologic N stage | |
| Downstaging | 43 (56.8) |
| Stable | 31 (40.8) |
| Progressive | 2 (2.4) |
| Pathologic TNM stage | |
| Downstaging | 55 (72.4) |
| Stable | 21 (27.1) |
| Progressive | 0 (0) |
ypT, the tumor (T) stage has been determined after neoadjuvant therapy; TNM, a cancer staging system that describes the characteristics of the tumor (T) and the presence of any lymph nodes metastases (N) or distant organ metastases (M).
Adjuvant chemotherapy
There were 44 patients who received adjuvant chemotherapy after radical resection. Of those patients, 84.1% completed the prescribed treatment of total 12 cycles; however, chemotherapy was discontinued in five patients because of grade 3 gastrointestinal toxicity and in two patients because of grade 3 leukopenia.
Postoperative complications
Surgical complications comprised three (3.9%) anastomotic leakages, two instances of delayed wound healing in coloanal anastomosis, one rectovesical fistula and one intestinal obstruction. Anastomotic leakage and fistula required diversion colostomy and others recovered uneventfully after conservative treatment. No hospital mortality within 30 days after surgery occurred.
Failure patterns and survival data
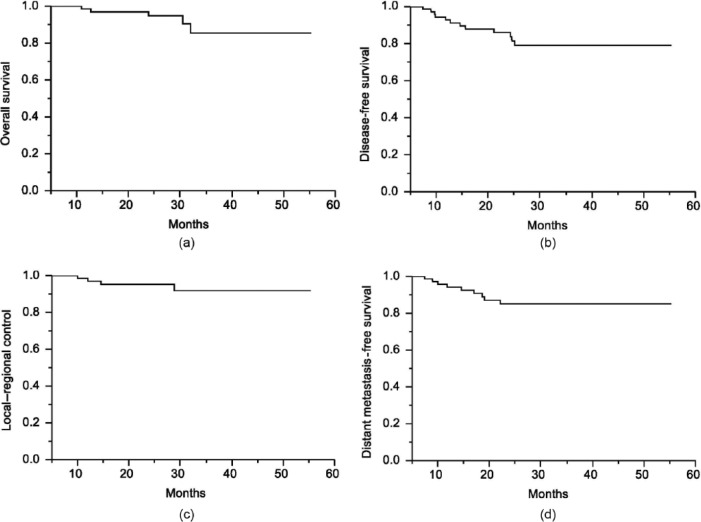
The median follow-up time was 28 (range, 5–52) months. The 2-year OS and DFS were 94% and 87.4%, respectively (Figure 1A and 1B). The 2-year local–regional control rate and distant metastasis-free survival rate were 95.3% and 85.1%, respectively (Figure 1C and 1D). Four patients (5.3%) had local recurrence and nine patients (11.8%) developed distant metastases. The lung (five patients) was the most common site for distant metastases, followed by the liver (three patients) and spine (one patient). Patients achieving pCR had an increased DFS when compared with non-pCR patients (94% versus 84%,p = 0.025; Figure 2).
Figure 1.
(A) Overall survival, (B) disease-free survival, (C) local–regional control rate, and (D) distant metastasis-free survival in 76 patients with rectal cancer patients after neoadjuvant chemoradiation and radical resection.
Figure 2.
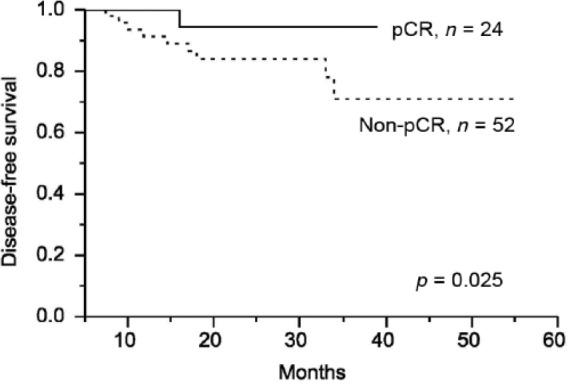
Cumulative two-year disease-free survival (DFS) between the pathologic complete response (pCR) group and the non-pCR group. The pCR group had a significantly improved DFS in comparison with the non-pCR group.
Discussion
In the current study, we added oxaliplatin to the 5-FU-based CCRT and maintained FOLFOX chemotherapy after CCRT until surgery for patients with rectal cancer. This intensified multimodality treatment resulted in a pCR rate of 31.6%, which is considerably higher than that obtained in similar published phase III clinical trials (14–20.9%) [Aschele et al. 2011; Gerard et al. 2012; O’Connell et al. 2014; Schmoll et al. 2014; Rödel et al. 2015]. Of equal importance, our study demonstrated that this regimen is safe and does not prominently increase the incidence of severe adverse events or surgical complications compared with approaches in other studies involving oxaliplatin added to neoadjuvant CCRT [Aschele et al. 2011; Gerard et al. 2012; Rödel et al. 2015]. Patients with LARC would benefit from the intensified treatment because a high pCR rate can not only improve the survival rate but also increase the proportion of those patients to receive less invasive surgery, which could improve quality of life.
To minimize the influence of various treatment approaches among different hospitals, we compared clinical results in the current study with those in our previous study regarding the efficacy and safety of capecitabine versus bolus infusional 5-FU-based preoperative CCRT in rectal cancer patients [Chen et al. 2012]. The pCR rate (31.6%) in the current study was considerably higher than that in the infusional 5-FU (22.2%) or oral capecitabine group (14.0%). The overall grade 3 or 4 toxicities in the oxaliplatin group (25.6%) are comparable with those in the infusional 5-FU (40.7%) and capecitabine (19.1%) groups in our previous study. Furthermore, the sphincter preservation rates for low-lying rectal cancer were as high as 92.9% in the oxaliplatin group, whereas they were 86.7% in the infusional 5-FU group and 80.0% in the capecitabine group [Chen et al. 2012].
In the current study, four cT2N0 patients underwent neoadjuvant treatment because of low-lying rectal tumors, and two of them exhibited a pCR. After those four patients were excluded from the analysis, 30.5% (22/72) of the stage II or III rectal cancer patients exhibited a pCR. In five phase III trials involving adding oxaliplatin to fluoropyrimidine-based CCRT, the pCR rates ranged from 15% to 20.9% [Aschele et al. 2011; Gerard et al. 2012; O’Connell et al. 2014; Schmoll et al. 2014; Rödel et al. 2015]. Unlike in the phase III trials, we not only added oxaliplatin to the CCRT regimen but also administered an additional three to four cycles of chemotherapy between the end of CCRT and surgery. In a multicenter trial, researchers investigated the effect of adding mFOLFOX6 after preoperative CCRT in LARC patients and determined that patients undergoing six cycles of mFOLFOX6 exhibited the highest pCR rate, 38% [Garcia-Aguilar et al. 2015], demonstrating that administering systemic chemotherapy between CCRT and surgery, and thereby expanding preoperative therapy and prolonging the interval between radiation and surgery, was effective. We applied the same rationale, extending FOLFOX after CCRT, thus enhancing pCR rates.
The optimal timing for surgery after CCRT for LARC remains unclear. Numerous retrospective studies have demonstrated that prolonging the interval between radiotherapy and surgery resulted in high pCR rates [Kalady et al. 2009; Sloothaak et al. 2013]. However, certain studies have shown no correlation between the pCR rates and the CCRT-to-surgery interval [Dolinsky et al. 2007; Lim et al. 2008]. The Lyon R90-01 trial is currently the only available randomized trial that investigated the interval between CCRT and surgery [Francois et al. 1999]. However, in the Lyon R90-01 trial, no radiosensitizing chemotherapy was used, the radiation schedule differed, and radiation and surgery were separated by only 8 weeks, rendering comparison with our study difficult. Therefore, delayed surgery after CCRT must be verified through a prospective, randomized clinical trial.
Because of an excellent tumor control and survival rate for patients who had a pCR after neoadjuvant treatment, nonoperative treatment has been suggested as management for such patients [Sauer et al. 2004; Bosset et al. 2006; Garcia-Aguilar et al. 2015]. Sparing TME could prevent surgery-related mortality, morbidity, and long-term sequelae,and thus may improve quality of life. Studies have revealed the feasibility of less-invasive surgery for patients with LARC who experienced a complete regression after CCRT [Habr-Gama et al. 2013, 2014; Garcia-Aguilar et al. 2015]. Although the debate on less-invasive surgery continues, deferring surgery for highly selective patients is acceptable. The high pCR rate achieved in the current study indicates that more patients may be eligible for less-invasive surgery. However, two out of 26 patients (7.7%) achieving pCR in primary tumor (pT0) had pathologically positive mesorectal nodes in our cohort. Our finding is consistent with the report in Memorial Sloan-Kettering Cancer Center. They investigated pathological results of 187 consecutive rectal cancer patients receiving preoperative CCRT followed by radical surgery, and demonstrated a 7% incidence of positive mesorectal among patients with a pCR of the primary tumor [Stipa et al. 2004].
Our results revealed that delivering systemic chemotherapy concurrently with, and following radiotherapy, as well as delaying surgery, did not create much difficulty in surgical resection and postoperative complications. Anastomotic leakage occurred in three patients (3.9%), a percentage that is comparable with that in previously reported data (2.6–10.3%) [Sauer et al. 2004; Bosset et al. 2006; Huang et al. 2014b; Rödel et al. 2015]. The wound complication rate was 2.6% in the present study, which is also consistent with the 1.1–37.9% reported in previous studies [Garcia-Aguilar et al. 2011; Chen et al. 2012; Huang et al. 2014b]. Radiation-induced pelvic fibrosis is a concern that has deterred surgeons from delaying surgery after radiotherapy. In the present study, we encountered no prominent surgical difficulty caused by pelvic fibrosis, and surgical complications did not increase substantially compared with those reported in previous studies [Sauer et al. 2004; Dolinsky et al. 2007; Garcia-Aguilar et al. 2011; Huang et al. 2014b; O’Connell et al. 2014].
Numerous studies have revealed that adding oxaliplatin to the CCRT regimen has engendered significant increases of 24% to 40% in grade 3 or 4 toxicities compared with those related to standard 5-FU-based CCRT [Aschele et al. 2011; Gerard et al. 2012; O’Connell et al. 2014; Schmoll et al. 2014]. In our results, the incidence of grade 3 or 4 toxicities was 25.6%, which was not higher than that reported in other studies [Aschele et al. 2011; Gerard et al. 2012; O’Connell et al. 2014; Schmoll et al. 2014; Rödel et al. 2015]. One possible explanation is that most patients (85.5%) in the current study received volumetric-modulated arc therapy (VMAT), an advanced radiation technique that produces excellent target-volume coverage of organs at risk of sparing in dosimetric studies [Richetti et al. 2010; Droge et al. 2015]. Clinical investigations using rectal-cancer irradiation demonstrated that VMAT reduced acute toxicity [Huang et al. 2014b; Droge et al. 2015]. Therefore, this might contribute to a lower toxicity profile and subsequent high compliance in our study.
In this study, 39 out of 42 patients (92.8%) with low-lying rectal cancer underwent sphincter-preserving surgery. The sphincter-preservation rate in the current study was higher than those reported in other studies (60.4–82%) [Aschele et al. 2011; Gerard et al. 2012; O’Connell et al. 2014; Rödel et al. 2015]. Possible reasons for the high sphincter-preservation rate in this study are: first, intensified preoperative treatment resulted in remarkable tumor regression, and therefore, a safe margin could be achieved without sacrificing the sphincter; second, further refinement of the surgical technique facilitated sphincter preservation [O’Connell et al. 2014]; and third, increasing the CCRT-to-surgery interval and extending chemotherapy until surgery might have facilitated further tumor shrinkage.
Numerous studies have demonstrated that an involved CRM is associated with high local and distant failure rates, as well as with miserable OS and DFS [Kelly et al. 2011; Russell et al. 2013; Amri et al. 2015]. In addition, evidence demonstrates that the clinical T4-stage is related to an increased risk of CRM involvement [Van Leersum et al. 2014; Homan et al. 2015]. In the current study, 16 patients (20.6%) had cT4 tumors; however, we still achieved a negative CRM in all but two patients (97.4%).
It is well known that patients with a pCR following neoadjuvant chemoradiotherapy have an excellent prognosis [Garcia-Aguilar et al. 2003; Kuo et al. 2007; Capirci et al. 2008]. Because of this observation, several researchers have asserted that adjuvant chemotherapy should be administered selectively, depending on the postoperative stage. Capirci and colleagues reported the long-term outcome of 566 pCR patients and showed that adjuvant chemotherapy did not improved survival [Capirci et al. 2008]. Based on a pooled analysis of 3313 rectal cancer patients, this report demonstrates that patients with a pCR after chemoradiotherapy may not benefit from adjuvant chemotherapy [Maas et al. 2015]. In these published studies, most of the pCR patients received chemotherapy during radiotherapy and it resulted in a favorable prognosis. In the current study, patients with a pCR received six or seven cycles of FOLFOX at the neoadjuvant setting. Although the cumulative dose of chemotherapy was lower in the pCR patients than in the non-pCR patients, patients with a pCR had a better DFS than those with a non-pCR (94% versus 84%, p = 0.025). Because of excellent oncologic outcome in pCR patients, we suggested a wait-and-see approach and omitted adjuvant chemotherapy for them. However, we have no relevant information of sustaining long-term remission and preventing distant metastasis with regard to the additional three to four cycles of FOLFOX in the neoadjuvant setting. Further studies are mandatory to elucidate this critical issue.
This study had certain limitations. First, our results may have been influenced by an external bias caused by the retrospective design. Second, the follow-up time was not long enough; therefore, long-term oncologic outcomes and late adverse events could not be fully addressed. Third, we used pCR as a surrogate for efficacy. Although evidence demonstrates that a pCR is correlated with an improved oncologic outcome, the current guidelines have not suggested that pCR is a predictor of long-term prognosis [Sauer et al. 2004; Kalady et al. 2009; Sloothaak et al. 2013]. Therefore, long-term follow up is mandatory to confirm the sustainability of the intensified regimen. Fourth, 60 patients underwent a CT scan for staging in this study. Because of some limitations in CT images for local staging of rectal cancers, the reported T-downstaging effect might be unreliable [Raman et al. 2015]. However, it should not affect the reported pCR rate. We could compare this downstaging effect with our previous report, for which CT scanning was primarily used for rectal cancer staging [Chen et al. 2012]. The T-downstaging rate in the FOLFOX group, 5-FU group, and capecitabine group were 71.1%, 51.9%, and 69.8%, respectively.
Conclusions
An intensified regimen of oxaliplatin and 5-FU administered concurrently with radiotherapy, as well as an extended interval between radiotherapy and surgery, was well tolerated in rectal cancer patients. This treatment may enhance the pCR rates without increasing adverse events. These promising results warrant further investigation in clinical trials.
Acknowledgments
The authors acknowledge the contribution made in data collection by the Colorectal Cancer Group from the Cancer Center of Kaohsiung Medical University Hospital.
Footnotes
Funding: This work was supported by grants from: the Excellence for Cancer Research Center (MOST104-2325-B-037-001); the Ministry of Health and Welfare (MOHW105-TDU-B-212-134007), Taiwan, Republic of China; Kaohsiung Medical University Hospital (KMUH102-2M47, KMUH104-4M44, KMUHS10422, KMUHS10418, KMUHS10405); the Center for Biomarkers and Biotech Drugs, Kaohsiung Medical University (KMU-TP104C00, KMU-TP104C03, KMU-TP104C07, D08-00005-10401, KMU-TP104A11, KMU-DK105001); and the Grant of Biosignature in Colorectal Cancers from Academia Sinica, Taiwan.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Chun-Ming Huang, Department of Radiation Oncology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Ming-Yii Huang, Department of Radiation Oncology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Department of Radiation Oncology, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Hsiang-Lin Tsai, Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan Division of General Surgery Medicine, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Division of Gastroenterology and General Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Department of Surgery, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Ching-Wen Huang, Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan Division of Gastroenterology and General Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Division of Colorectal Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Cheng-Jen Ma, Division of Gastroenterology and General Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Division of Colorectal Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Yung-Sung Yeh, Division of Gastroenterology and General Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan Division of Trauma, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Suh-Hang Juo, Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan Center for Biomarkers and Biotech Drugs, Kaohsiung Medical University, Kaohsiung, Taiwan.
Chih-Jen Huang, Department of Radiation Oncology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Department of Radiation Oncology, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Jaw-Yuan Wang, Division of Gastroenterology and General Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Department of Surgery, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan Division of Colorectal Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Cancer Center, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan Center for Biomarkers and Biotech Drugs, Kaohsiung Medical University, Kaohsiung, Taiwan Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
References
- Amri R., Bordeianou L., Sylla P., Berger D. (2015) Association of radial margin positivity with colon cancer. JAMA Surg 150: 890–898. [DOI] [PubMed] [Google Scholar]
- Andre T., Boni C., Navarro M., Tabernero J., Hickish T., Topham C., et al. (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the Mosaic trial. J Clin Oncol 27: 3109–3116. [DOI] [PubMed] [Google Scholar]
- Aschele C., Cionini L., Lonardi S., Pinto C., Cordio S., Rosati G., et al. (2011) Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the Star-01 randomized phase III trial. J Clin Oncol 29: 2773–2780. [DOI] [PubMed] [Google Scholar]
- Bosset J., Collette L., Calais G., Mineur L., Maingon P., Radosevic-Jelic L., et al. (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355: 1114–1123. [DOI] [PubMed] [Google Scholar]
- Capirci C., Valentini V., Cionini L., De Paoli A., Rodel C., Glynne Jones R., et al. (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 72: 99–107. [DOI] [PubMed] [Google Scholar]
- Chen C., Huang M., Huang C., Wu C., Yeh Y., Tsai H., et al. (2012) A observational study of the efficacy and safety of capecitabine versus bolus infusional 5-fluorouracil in pre-operative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 27: 727–736. [DOI] [PubMed] [Google Scholar]
- Dolinsky C., Mahmoud N., Mick R., Sun W., Whittington R., Solin L., et al. (2007) Effect of time interval between surgery and preoperative chemoradiotherapy with 5-fluorouracil or 5-fluorouracil and oxaliplatin on outcomes in rectal cancer. J Surg Oncol 96: 207–212. [DOI] [PubMed] [Google Scholar]
- Droge L., Weber H., Guhlich M., Leu M., Conradi L., Gaedcke J., et al. (2015) Reduced toxicity in the treatment of locally advanced rectal cancer: a comparison of volumetric modulated arc therapy and 3D conformal radiotherapy. BMC Cancer 15: 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois Y., Nemoz C., Baulieux J., Vignal J., Grandjean J., Partensky C., et al. (1999) Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90–01 Randomized Trial. J Clin Oncol 17: 2396. [DOI] [PubMed] [Google Scholar]
- Garcia-Aguilar J., Chow O., Smith D., Marcet J., Cataldo P., Varma M., et al. (2015) Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase II trial. Lancet Oncol 16: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Aguilar J., Hernandez de, Anda E., Sirivongs P., Lee S., Madoff R., Rothenberger D. (2003) A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum 46: 298–304. [DOI] [PubMed] [Google Scholar]
- Garcia-Aguilar J., Smith D., Avila K., Bergsland E., Chu P., Krieg R. (2011) Optimal timing of surgery after chemoradiation for advanced rectal cancer: preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann Surg 254: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard J., Azria D., Gourgou-Bourgade S., Martel-Lafay I., Hennequin C., Etienne P., et al. (2012) Clinical outcome of the ACCORD 12/0405 Prodige 2 randomized trial in rectal cancer. J Clin Oncol 30: 4558–4565. [DOI] [PubMed] [Google Scholar]
- Goldberg R., Sargent D., Morton R., Fuchs C., Ramanathan R., Williamson S., et al. (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22: 23–30. [DOI] [PubMed] [Google Scholar]
- Habr-Gama A., Gama-Rodrigues J., Sao Juliao G., Proscurshim I., Sabbagh C., Lynn P., et al. (2014) Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 88: 822–828. [DOI] [PubMed] [Google Scholar]
- Habr-Gama A., Sabbaga J., Gama-Rodrigues J., Sao Juliao G., Proscurshim I., Bailao Aguilar P., et al. (2013) Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum 56: 1109–1117. [DOI] [PubMed] [Google Scholar]
- Homan J., Bokkerink G., Aarts M., Lemmens V., Van Lijnschoten G., Rutten H., et al. (2015) Variation in circumferential resection margin: reporting and involvement in the South-Netherlands. Eur J Surg Oncol 41: 1485–1492. [DOI] [PubMed] [Google Scholar]
- Huang C., Huang C., Huang M., Lin C., Chen C., Yeh Y., et al. (2014a) Coexistence of perineural invasion and lymph node metastases is a poor prognostic factor in patients with locally advanced rectal cancer after preoperative chemoradiotherapy followed by radical resection and adjuvant chemotherapy. Med Princ Pract 23: 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Yeh Y., Su W., Tsai H., Choy T., Huang M., et al. (2016) Robotic surgery with high dissection and low ligation technique for consecutive patients with rectal cancer following preoperative concurrent chemoradiotherapy. Int J Colorectal Dis 31: 1169–1177. [DOI] [PubMed] [Google Scholar]
- Huang M., Chen C., Huang C., Tsai H., Yeh Y., Ma C., et al. (2014b) Helical tomotherapy combined with capecitabine in the preoperative treatment of locally advanced rectal cancer. Biomed Res Int 2014: 352083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalady M., De Campos-Lobato L., Stocchi L., Geisler D., Dietz D., Lavery I., et al. (2009) Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg 250: 582–589. [DOI] [PubMed] [Google Scholar]
- Kelly S., Mills S., Bradburn D., Ratcliffe A., Borowski D. (2011) Effect of the circumferential resection margin on survival following rectal cancer surgery. Br J Surg 98: 573–581. [DOI] [PubMed] [Google Scholar]
- Kerr S., Norton S., Glynne-Jones R. (2008) Delaying surgery after neoadjuvant chemoradiotherapy for rectal cancermay reduce postoperative morbidity without compromising prognosis. Br J Surg 95: 1534–1540. [DOI] [PubMed] [Google Scholar]
- Kuo L., Liu M., Jian J., Horng C., Cheng T., Chen C., et al. (2007) Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol 14: 2766–2772. [DOI] [PubMed] [Google Scholar]
- Lim S., Choi H., Jeong S., Kim D., Jung K., Hong Y., et al. (2008) Optimal surgery time after preoperative chemoradiotherapy for locally advanced rectal cancers. Ann Surg 248: 243–251. [DOI] [PubMed] [Google Scholar]
- Maas M., Nelemans P., Valentini V., Crane C., Capirci C., Rodel C., et al. (2015) Adjuvant chemotherapy in rectal cancer: defining subgroups who may benefit after neoadjuvant chemoradiation and resection: a pooled analysis of 3,313 patients. Int J Cancer 137: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas M., Nelemans P., Valentini V., Das P., Rödel C., Kuo L., et al. (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–844. [DOI] [PubMed] [Google Scholar]
- Mace A., Pai R., Stocchi L., Kalady M. (2015) American Joint Committee on Cancer and College of American Pathologists regression grade: a new prognostic factor in rectal cancer. Dis Colon Rectum 58: 32–44. [DOI] [PubMed] [Google Scholar]
- Nagtegaal I., Quirke P. (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 26: 303–312. [DOI] [PubMed] [Google Scholar]
- O’Connell M., Colangelo L., Beart R., Petrelli N., Allegra C., Sharif S., et al. (2014) Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol 32: 1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman S., Chen Y., Fishman E. (2015) Evolution of imaging in rectal cancer: multimodality imaging with MDCT, MRI, and PET. J Gastrointest Oncol 6: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetti A., Fogliata A., Clivio A., Nicolini G., Pesce G., Salati E., et al. (2010) Neo-adjuvant chemo-radiation of rectal cancer with volumetric modulated arc therapy: summary of technical and dosimetric features and early clinical experience. Radiat Oncol 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel C., Graeven U., Fietkau R., Hohenberger W., Hothorn T., Arnold D., et al. (2015) Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 Study): final results of the multicentre, open-label, randomised, phase III trial. Lancet Oncol 16: 979–989. [DOI] [PubMed] [Google Scholar]
- Russell M., You Y., Hu C., Cormier J., Feig B., Skibber J., et al. (2013) A novel risk-adjusted nomogram for rectal cancer surgery outcomes. JAMA Surg 148: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R., Becker H., Hohenberger W., Rodel C., Wittekind C., Fietkau R., et al. (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351: 1731–1740. [DOI] [PubMed] [Google Scholar]
- Schmoll H., Haustermans K., Price T., Nordlinger B., Hofheinz R., Daisne J., et al. (2014) Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: disease-free survival results at interim analysis. J Clin Oncol 32: 5s, 2014. (abstract 3501). [Google Scholar]
- Sloothaak D., Geijsen D., Van Leersum N., Punt C., Buskens C., Bemelman W., et al. (2013) Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 100: 933–939. [DOI] [PubMed] [Google Scholar]
- Stipa F., Zernecke A., Moore H., Minsky B., Wong W., Weiser M., et al. (2004) Residual mesorectal lymph node involvement following neoadjuvant combined-modality therapy: rationale for radical resection? Ann Surg Oncol 11:187–191. [DOI] [PubMed] [Google Scholar]
- Van Leersum N., Martijnse I., Den Dulk M., Kolfschoten N., Le Cessie S., Van De Velde C., et al. (2014) Differences in circumferential resection margin involvement after abdominoperineal excision and low anterior resection no longer significant. Ann Surg 259: 1150–1155. [DOI] [PubMed] [Google Scholar]