Abstract
Background
Severe intracranial arterial stenosis results in more than 10% incidence of stroke and transient ischemic attack. Using undersized angioplasty with off-label closed-cell Enterprise stent may be a feasible alternative option for treating patients with intracranial atherosclerotic disease who fail dual-antiplatelet medical therapy. The results of the authors’ study are presented in this paper.
Materials and methods
Between January 2013 and July 2014, 24 symptomatic patients with a total of 30 intracranial arterial stenotic lesions refractory to medical therapy, who underwent undersized angioplasty and Enterprise stenting, were retrospectively reviewed in the authors’ institution. The results evaluated include technical success rate, clinical outcome measured as modified Rankin Scale at presentation and follow-up, peri-procedural morbidity within 30 days and 1 year, and follow-up vessel patency.
Results
Stent deployment was successfully achieved in all stenotic lesions (30/30). Mean pre-stent and post-stent diameter residual stenosis was 81% and 18%, respectively. The peri-procedural complication rate during 30 days after stenting was 10% per lesion (3/30), including intracranial hemorrhage, in-stent thrombosis and ischemic stroke. No further thromboembolic event or complication occurred in any patient more than 30 days after stenting. Modified Rankin scale ≤ 2 was observed in 64% and 83% of patients at initial presentation and follow-up (mean 15.8 months), respectively. Imaging follow-up was available in 17 of 24 patients (70.8%) and 20 of 30 treated lesions (66.6%) with a mean follow-up period of 15.4 months. Only one asymptomatic in-stent restenosis occurred in 20 available lesions (5.0%).
Conclusion
This preliminary study suggests that using undersized angioplasty and Enterprise stenting may effectively treat high-degree symptomatic intracranial arterial stenosis with favorable clinical and angiographic outcome.
Keywords: Intracranial stent, Enterprise, intracranial arterial stenosis, stroke, angioplasty
Introduction
Intracranial arterial stenosis (ICAS) is nowadays a major cause of ischemic stroke, and accounts for almost 10% of Caucasian and 30% of Chinese patients who suffer cerebral ischemia.1,2 The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial, in which patients with symptomatic intracranial stenosis (50–99%) were randomly treated with warfarin or aspirin, showed that ipsilateral vascular territory recurrent stroke occurred in 25% of patients with 70–99% stenosis and 11% of patients with 50–69% stenosis within 2 years.3 Due to the limitations of medical treatment of symptomatic ICAS, percutaneous transarterial balloon angioplasty (PTA) without stent deployment was initially investigated. However, the high risk of peri- and post-procedural vascular occlusion or arterial dissection was broadly documented in several studies.4,5 Therefore, a different strategy – self-expandable stent deployment – was attempted. The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial using the Wingspan (Stryker Neurovascular, Fremont, CA, USA) stent system showed a higher risk of procedure-related morbidity, stroke and death within 30 days than when treated with medication alone.6 Nevertheless, further studies reported percutaneous transarterial balloon angioplasty and stenting (PTAS) using the Wingspan stent with a good outcome in ICAS.7,8 However, the Wingspan system with its open-cell design has duplicated radial force, which stimulates neointimal hyperplasia.9,10 We hypothesized that intracranial stenting with the off-label Enterprise (Codman Neurovascular, Raynham, MA, USA) stent with closed-cell design and lower radial force would be a clinical alternative for ICAS patients refractory to medication. In this study, we retrospectively review a series of 24 patients with symptomatic ICAS, who had failed medication, treated with undersized angioplasty and self-expanding Enterprise stent and evaluated the technical success rate and the vascular and clinical outcome.
Materials and methods
Patient population and eligibility criteria
This study was approved by our institutional review board. A total of 24 consecutive ICAS patients (with more than 70% stenosis), after giving informed consent, were treated with undersized angioplasty and Enterprise stent in our institution from January 2013 to July 2014. The inclusion criteria and management guidelines were based on the WASID and SAMMPRIS trials. Patients were selected who presented with transient ischemic attack (neurologic symptoms completely resolved within 24 h) or non-disabling minor stroke (modified Rankin scale (mRS) ≤ 3) attributed to vessel stenosis ≥70%, including internal carotid artery, middle cerebral artery, vertebral artery and basilar artery, identified by conventional angiography and refractory to dual antiplatelet medication (aspirin and clopidogrel) for at least 3 months, or asymptomatic ICAS but progression of stenosis. The exclusion criteria included non-atherosclerotic stenosis (such as vasculitis or moya-moya disease), potential sources of cardiac embolism, tumor-related arterial stenosis, vascular malformation or PTAS performed previously.
Procedure
Brain magnetic resonance imaging (MRI) and diagnostic cerebral angiography were performed before PTAS. In order to reduce the risk of post-procedural thrombosis and restenosis, the loading dose of aspirin (325 mg) and clopidogrel (75 mg) was given daily for all patients at least 3 days prior to intervention. PTAS was performed by a neurointerventionist with 15 years’ experience by deploying the Enterprise stent with subsequent undersized balloon angioplasty (80% diameter of the parent artery). After general anesthesia and femoral artery cannulation, a 6-Fr shuttle sheath (Cook Medical, Bloomington, IN, USA) was placed at target distal cervical carotid artery. Under roadmap guidance, a 0.014 inch Traxcess microwire (Microvention, Tustin, CA, USA) associated with a Headaway 17 microcatheter (Microvention, USA) was navigated for guidance. Then the delivery microcatheter (Prowler Select Plus, Codman, USA) was advanced through the microwire to bypass the stenosis, and the Enterprise stent (22–37 mm) was deployed by the over-the-wire stent exchange technique while the Prowler was pulling back gently. After stent deployment, 2500 units of intravenous heparin was given for anticoagulation and to maintain a doubled activated clotting time. Then, subsequent PTA with a balloon catheter (Rafale, Hexacath, France or Tazuna, Terumo, Japan) with undersized diameter (approximately 80% of the parent artery) was chosen to cover the post-stenting stenotic lesion. Angioplasty was performed by gradually and gently inflating the balloon (at least 2 min) with the nominal pressure indicated on the product label. After PTAS, a post-stenting angiogram was obtained immediately and 30 min later to ensure no in-stent thrombosis. Also, an additional angioplasty with a normal-diameter balloon catheter larger than the initial one would be performed if ≥50% residual stenosis was observed. The technical success of stent placement was defined as a complete coverage of the stenotic lesion resulting in ≤50% residual stenosis. During the procedure, we kept the systolic pressure (SBP) of the patients under 120–140 mmHg to avoid hyperperfusion or reperfusion injury. However, in some patients with multiple stenotic lesions, we relaxed the criteria to keep the SBP under 150 mmHg to ensure enough blood perfusion. Patients were admitted to an intensive care unit for monitoring of their neurological status. Blood pressure was strictly controlled during and after the procedure. Once in a stable condition, the patient was transferred to the ordinary ward until we were confident that no new stroke event would occur. All patients were instructed to take 100 mg aspirin daily for life and 75 mg clopidogrel daily for 6 months.
Clinical follow-up
By reviewing the medical records from each patient, the occurrence of early post-procedural complications, defined as new neurological signs or an acute infarct in treated territory confirmed by neuroimaging such as MRI, was evaluated 30 days after PTAS as primary endpoint according to the guidelines of the SAMMPRIS trial. Late post-procedural complications (>30 days after PTAS) were considered to be stroke or hemorrhage within the treated territory or death attributed to the stent placement. Clinical prognosis was accessed by phone survey, review of medical record, or clinical examination before stenting and follow-up according to the mRS. The clinical outcome was classified into “good” (mRS ≤ 2), “moderate” (mRS = 3) and “poor” (mRS > 4).
Vascular follow-up
The MRI and magnetic resonance angiography (MRA) studies were performed in all patients on day 1 and day 3 after PTAS. A computed tomography angiography (CTA) study for evaluating in-stent restenosis (ISR) was performed at 6 months after PTAS, and an additional imaging check at 12, 18 or 24 months. ISR was defined as CTA-verified luminal diameter stenosis over 50%. The CTA image was obtained by 64-channel dual energy computed tomography (CT) (Discovery CT750 HD, GE Healthcare, Pittsburgh, PA, USA). Scan parameters for this spectral imaging included 0.6 s tube rotation, slice thickness of 5 mm, 70 and 140 kVp, 640 mA and pitch of 0.984. All the images were reconstructed with 0.625 mm section thickness and 0.625 mm slice interval. The reconstructed CT raw data were sent to a workstation for generating material decomposition density and monochromatic representation of data using viewer software (GE viewer 2.0 and GE VolumeShare4 AW 4.4, GE Healthcare, USA) determined at 70 keV and displayed as axial view and multiplanar reconstruction (Gemstone Spectral Imaging (GSI)-generated iodine maps with window width of 175 HU and a window level of 75 Hounsfield unit (HU)). The stenotic degree was determined as the narrowest diameter of the pre-stenting or post-stenting vessel. Two radiologists were requested to view these images and decide the restenotic rate. Disagreement was settled by consensus.
Results
The 24 patients undergoing PTAS were 20 males and four females with mean age of 61.8 ± 10.3 years (range 43–90). Six patients had double high-grade intracranial stenotic lesions, and a total of 30 stenotic intracranial arterial lesions were treated. The patients’ demographic data and clinical and angiographic outcome are shown in Table 1. The technical success rate was 100% (30 of 30 treated vessels) and the treated arteries consisted of distal internal carotid arteries (ICA) (n = 6, 20%), middle cerebral artery (MCA) (n = 14, 46.6%), vertebral artery (VA) (n = 7, 23.3%) and basilar arteries (BA) (n = 3, 10%) (Figure 1). Mean pre-stent stenosis was 81 ± 11.3 % (range, 70–99.0%) and mean post-PTAS residual arterial stenosis was 18 ± 6.8% (range 10–35%). The mean length of the deployed Enterprise stent was 23.5 ± 4.4 mm.
Table 1.
The patient’s demographic data, clinical and angiographic outcome.
| Characteristic | Results |
|---|---|
| Number of patients | 24 |
| Age | 62 ± 10.3 (43–90) |
| Gender | |
| Male | 20 (83.3%) |
| Female | 4 (16.7%) |
| Lesion location | 30 |
| ICA | 6 (20%) |
| MCA | 14 (46.6%) |
| BA | 3 (10%) |
| VA | 7 (23.3%) |
| Enterprise stent size | |
| Diameter (mm) | 4.5 |
| Length (mm) | 23.5 ± 4.4 (22–37) |
| Lesion characteristics | |
| Mean pre-treated stenosis degree | 81 ± 11.3% |
| Mean post-treated stenosis degree | 18 ± 6.8% |
| Clinical outcome (mean follow-up of 15.8 months) | |
| Morbidity (per lesion) | |
| Post-procedural complication within 30 days (Early) | 3 (10%) |
| Post-procedural complication 30 days later (Late) | 0 (0%) |
| Modified Rankin Score (At presentation / at follow-up of at least 6 months) | |
| Good (0–2) | 16 (66%)/20 (83%) |
| Moderate to poor (3–6) | 8 (34%)/4 (17 %) |
| Angiographic outcome (mean follow-up of 15.4 months) | |
| Follow-up patients | 17 |
| Available follow-up lesions | 20 |
| In-stent restenosis | 1/20 (5.0%) |
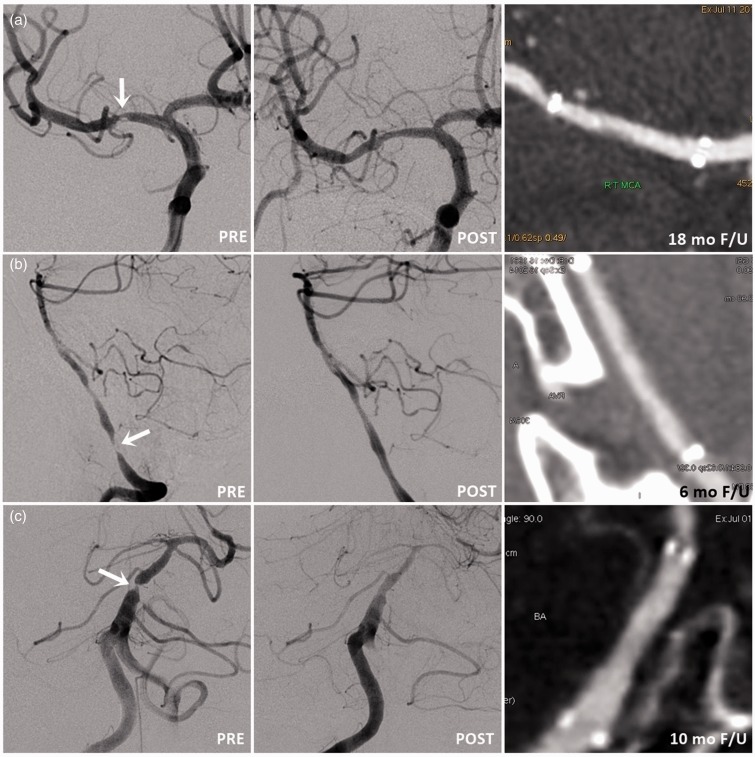
Figure 1.
Representative angiographic images of pre-stenting, post-stenting and follow-up CTA image in cases of high-grade intracranial arterial stenosis. (a) MCA, (b) intracranial VA, and (c) BA treated with PTA and Enterprise stent deployment. [Typesetter: please change A, B, C, D to (a), (b), (c), (d) in Figures].
Early post-procedural complications, within 30 days after the procedure, were reported in three of 30 treated lesions (10%). All the three adverse events were related to the treated angioplasty and stenting territory. The first post-stenting patient, with right VA tight stenosis, suffered from a stroke episode after 3 days and showed multiple newly diffusion-restricted lesions scattered in the bilateral cerebral hemisphere and occipital lobe on MRI. A perforator stroke due to migration of small plaques into distal branches was considered. The second patient, with right MCA tight stenosis, developed in-stent thrombosis 1 day after procedure, shown on follow-up MRA, and was re-treated with urokinase thrombolysis and an additional balloon-expandable stent (Helistent, Hexacath, Rueil-Malmaison, France) deployment (Figure 2), with a subsequent good outcome. The third patient, with right MCA tight stenosis, suffered from reperfusion hemorrhage after PTAS. During PTAS, the patient’s SBP was controlled under 120 mmHg, and post-stenting angiography showed patency of the treated segment without contrast extravasation or arterial dissection. However, this patient experienced dramatically elevated uncontrolled blood pressure (varying from 160/90 to 200/110 mmHg) and became restless during recovery from anesthesia. An emergent brain CT revealed extensive right putaminal hemorrhage extending into the ventricle. Surgical management was performed for hematoma evacuation. His consciousness deteriorated and he developed hyperventilation with severe hypocapnia due to brainstem dysfunction, and eventually died 10 days after the procedure. This was the only mortality shown in this study. No newly developed hemorrhagic and ischemic strokes or other procedure-related complications were noted in the rest of the treated patients from 30 days after PTAS until 1 year follow-up (late morbidity).
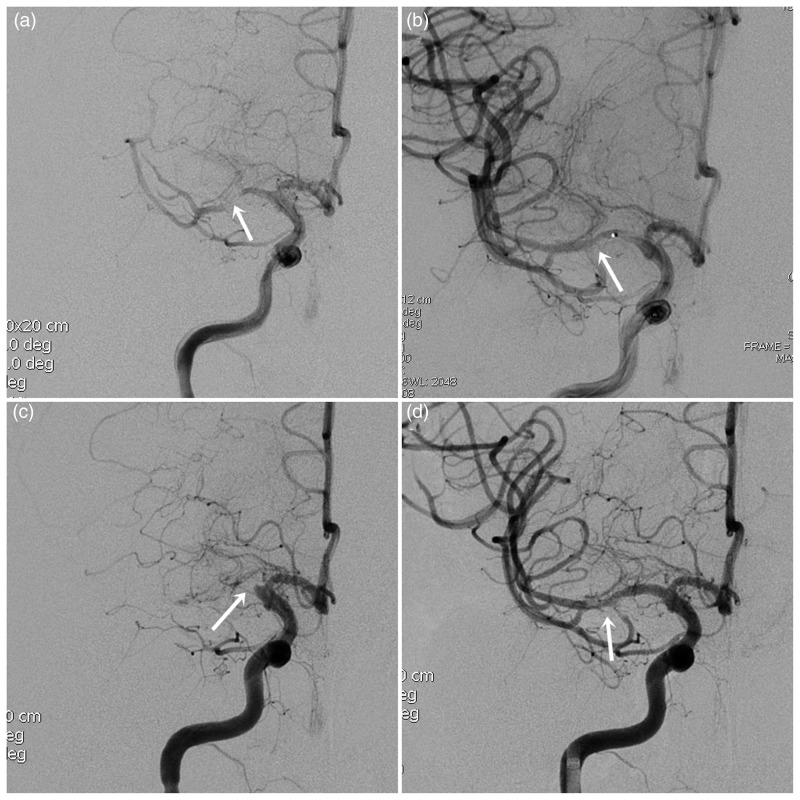
Figure 2.
A high-grade right MCA stenosis. (a) Pre-stenting right ICA angiogram reveals tight stenosis (99%) of M1 segment. (b) First-time post-stenting right ICA angiogram reveals resolution of the critical stenosis. (c) The follow-up (3 days) right ICA angiogram shows complete in-stent thrombosis. (d) After thrombolytic recanalization and balloon-expandable Helistent (2.5 × 7 mm) deployment, right ICA angiogram shows patent flow with some minimal filling defects representing thrombotic debris.
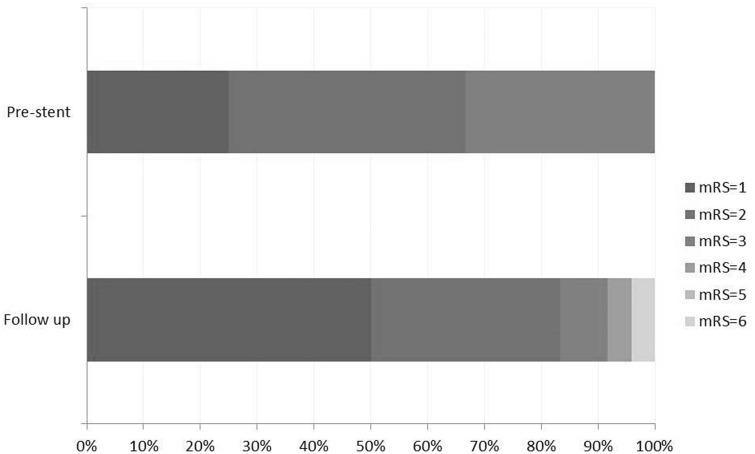
Clinical evaluation was performed by neurologists in all patients after least 12 months, with an average follow-up of 15.8 months. At presentation, 16 of 24 patients (66.6%) were scored mRS ≤ 2. At follow-up, 20 patients (83%) showed good neurological outcome with overall symptomatic improvement (Figure 3).
Figure 3.
Modified Rankin Scale (mRS) score for all patients evaluated at initial presentation (pre-stent) and after stenting follow-up of mean 15.8 months (24 patients).
Vascular outcomes were available in 17 of 24 patients (70.8%) with a total of 20 treated arteries evaluated by CTA. The mean follow-up was 15.4 ± 4.8 months (range 6–24 months) after PTAS. Nineteen of 20 treated arteries (95%) showed vessel patency, with only one treated vessel (5.0%) demonstrating moderate (55%) asymptomatic restenosis at 18 months’ follow-up (Figure 4). However, due to the stable condition of this patient, we continued medical treatment without further angioplasty or stent placement.
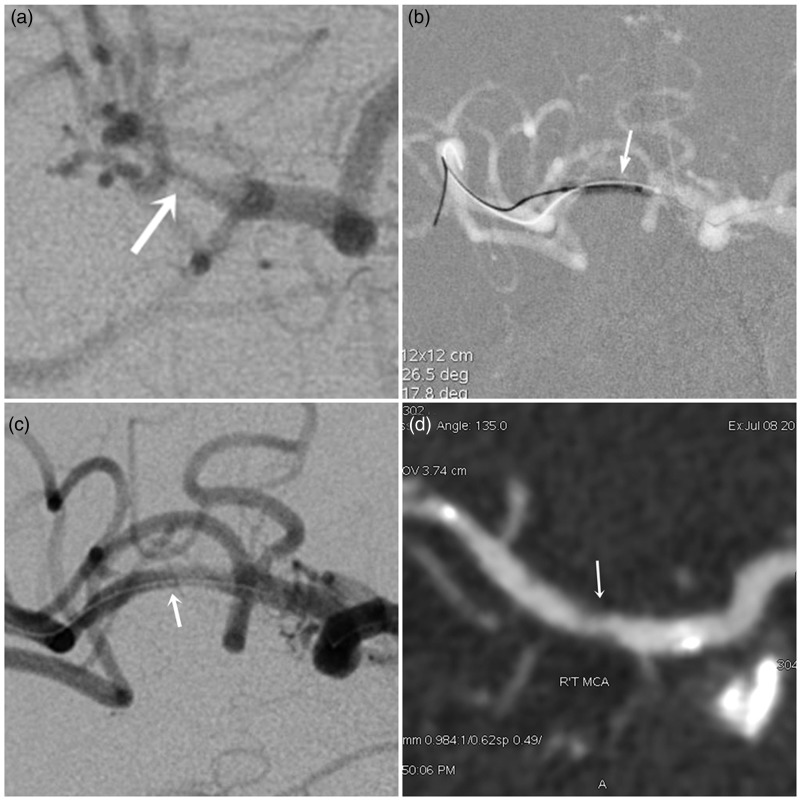
Figure 4.
A high-grade right MCA stenosis. (a) Pre-stenting right ICA angiogram shows right proximal M2 stenosis (90%). (b) Undersized PTA was performed by using a Rafale balloon (1.5 × 10 mm) for the M2 stenotic lesion. (c) Post-stenting angiogram after Enterprise stent (4.5 × 22 mm) deployment shows less than 10% diameter stenosis of right M2 segment. (d) Follow-up CTA 18 months after PTAS demonstrates in-stent restenosis with nearly 55% luminal narrowing.
Discussion
Our results demonstrated that patients with symptomatic ICAS undergoing undersized angioplasty and Enterprise stenting experience benefits in both functional recovery and procedural safety. Although this was an uncontrolled and subjective assessment, 20 of 24 patients (83%) showed good clinical outcome of mRS ≤ 2 and symptomatic improvement at a mean of 15.8 months after procedure. To some extent this may be attributed to the natural recovery course after a stroke episode, lifestyle change by the patients, and stent revascularization also preventing further ischemic injury.
Furthermore, we encountered no procedural failures, with a 100% (30/30) technical success rate. The high technical success rate may be attributed to the following reasons. First, all procedures was performed by a neurointerventionist with 15 years’ experience using a homogeneous technique. This may have ensured consistent procedural quality with no learning curve effect. Second, the delivery catheter of the Enterprise stent was originally developed for intracranial aneurysms. It has good accessibility, with a flexible tip that allows easier distal navigation, especially when the distal working length is short.10,11 Placement and unsheathing of the stent are possible in almost every tortuous segment of Will’s circle with conformable cell structure. What is more, recapturing and redeployment are also feasible if the stent deploys less than 70% of its length. Many studies have demonstrated a higher technical success rate with Enterprise (94–100%)10,12 than with Neuroform (Boston Scientific, Boston, MA USA) (80–82%)10,12,13 and a similar success rate to Wingspan (91.2–100%).9,14–19 Izar et al. also reported that in five of nine cases in which a Neuroform stent could not be navigated to the landing zone, an Enterprise stent was successfully deployed instead.13 The major drawback of the Enterprise stent was migration, which was documented in some previous studies.20,21 However, no stent migration happened in this study, probably because the shortest length (14 mm) of Enterprise stent was not used in our study.
Additionally, it is worth mentioning that in this study we deployed the stent first, followed by undersized angioplasty. We considered that, placed initially, the stent might play the role of a barrier to “stick” the disrupted atherosclerotic plaque between the stent and the vessel wall. According to our experience with carotid stenting, deploying the intracranial stent first, followed by delicate and undersized angioplasty, can reduce the risk of vessel injury (e.g. arterial dissection) and avoid squeezing the plaque more distally into the perforator branches. In addition, the design of a self-expandable stent system such as Enterprise may lower the risk of MCA perforator infarct compared with a balloon-expandable stent due to the snow-plough effect with disrupted thromboembolism.22 However, if the stenotic site is too tortuous, we may consider performing angioplasty first or choosing a longer stent to prevent Enterprise migration due to balloon dilatation in such a curved lumen.
The post-procedural complication rate at 30 days (early morbidity) was 10% (3/30 per lesion), which was lower than in the large prospective multicenter randomized SAMMPRIS trial (14.6%) and within the range of other Wingspan registries (6.1–20.6%).10,19,23–29 Our overall morbidity of 10% with an average follow-up of 15.8 months is also similar to the average reported overall morbidity of 8.8% (7–9.8%) in 282 patients.30,31 Although the early morbidity with Enterprise stenting is higher than in the medical arm of the SAMMPRIS trial (5.8%), there are some long-term benefits to this treatment. Patients in the SAMMPRIS medical arm showed a recurrent stroke rate of 12.2% at 1 year, which is higher than the overall morbidity in our study (10%) with an average follow-up of 15.8 months. In the recently published final results of the SAMMPRIS trial, the authors cite a 15% overall morbidity in the medical treatment arm over a median follow-up of 32.4 months.32 This may suggest that opening the stenosis by PTAS might lower the risk of long-term ischemic injury in the territory beyond severe stenosis, in conditions of either falling blood pressure or thromboembolism. Stenting of severe intracranial stenosis (70–90%) might not only improve the outcome, but also reduce the rate of recurrent ipsilateral stroke in patients. It has also been reported that perioperative stroke and death in patients treated with PTAS can be reduced to less than 4%.33
The major issue of PTAS for ICAS is restenosis. Intimal hyperplasia is the main cause of ISR, which may be due to balloon-dilatation vessel injury, thrombogenicity of the deployed stent, and inflammatory reactions of the vessel wall toward the stent.34 Several risk factors have been reported in previous studies: young age, lesion at anterior circulation, rapid balloon inflation, residual stenosis and longer lesion lengths were suggested to be associated with ISR after Wingspan stenting.35 Balloon angioplasty without simultaneous stenting may cause a greater risk of plaque rupture and may result in microdissections or macrodissections.36 It may, in turn, induce greater proliferative neointimal hyperplasia, resulting in a higher rate of ISR than slower balloon inflation.
Our result of a 5.0% ISR rate (1/20) with a mean follow-up of 15.4 months was similar to or even better than previously published Wingspan series (range 7.5–42.8%, mean follow-up of 6–13.2 months).18,19,24–26,37–39 This may be attributed to the closed-cell design of the Enterprise stent, with lower radial force than the first-generation open-cell designs such as Wingspan and Neuroform. The lower radial force may decrease development of intimal hyperplasia.40 In addition, undersized balloon angioplasty for the stenotic lesion may result in lower stimulation or pressure to the vessel.10 Second, the tip of the stent delivery system, Prowler Select Plus, was flexible and smooth with high accessibility, which may have avoided harming the vessel wall during operation. Besides, we inflated the balloon very gently to reduce the tension toward the target vessel during the procedure, which also lowers the risk of ISR.25 Third, we considered that deploying the stent first can prevent subtle vessel injury such as arterial microdissection and lower the stimulation of the vessel wall caused by balloon inflation.
In addition, recatheterization with a second-time angioplasty in the Enterprise system is also much easier than in open-cell design stents, which have a high risk of in-stent dissections as a result of hyperplastic in-stent tissue, especially in tortuous segments.10 Although the Enterprise stent system has several advantages in some cases, patients still need to be individually selected according to strict criteria, especially in some unstable cases. The adverse event rate and the restenosis rate of intracranial stent placement are definitely lower in the stable patient group compared with the unstable group.31
The angiographic follow-up studies were performed using a dual-energy CT machine with monochromatic image in this study. The optimal monochromatic level was determined at 70 keV, with Gemstone spectral imaging and metallic artifact reduction software to provide both image noise reduction and beam hardening artifact reduction arising from the intracranial stent compared with the conventional polychromatic images.41 We suggest that the monochromatic image might be useful to evaluate the post-stenting stenosis degree and vessel morphology. CTA follow-up studies may also increase acceptability to patients who are reluctant to accept invasive examinations such as conventional angiography.
The present study has some limitations. First, this is an uncontrolled, retrospective study, and the numbers of patients are small in comparison with other similar intracranial stent studies. Therefore, further results with a larger population need to be investigated. Second, CTA is not a routine follow-up modality for stenting vessel evaluation, unlike conventional digital subtraction angiography (DSA). Although CTA increases the inclination of patients to undergo follow-up investigation because of its non-invasiveness, and the dual-energy CT with gemstone spectral imaging and metallic artifact reduction software can markedly reduce artifacts as compared with the traditional polychromatic CT image, a comparative study between both modalities is still needed. Third, we did not check the consistency of the patient’s risk factor and lifestyle modification as in the SAMMPRIS trial. Additionally, the pre-procedural platelet reactivity test for clopidogrel was not done in this series. Response to clopidogrel might be a factor that would influence the decision on patient selection.
Conclusions
In this preliminary study, we suggest that undersized angioplasty and off-label use of the Enterprise stent system showed benefit for ICAS patients, with a high technical success rate, a satisfactory clinical outcome, a relatively low post-procedural morbidity rate, and a favorably low ISR rate. Besides, late morbidity, after 30 days up to 1 year, suggests that the Enterprise stent provides good long-term benefit and stroke prevention for severe ICAS. Further data on the Enterprise stent from prospective, randomized studies with a control group are still needed for investigation to determine safety and efficacy.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Ministry of Science and Technology, Taiwan (grant numbers NSC 98-2321-B-038-004-MY3 and NSC 101-2314-B-038-035-MY3) and Taipei Medical University, Taiwan (grant number 102TMU-SHH-20).
References
- 1.Feldmann E, Daneault N, Kwan E, et al. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology 1990; 40: 1541–1545. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Feldmann E, Gomez CR, et al. Intracranial atherosclerotic disease: An update. Ann Neurol 2009; 66: 730–738. [DOI] [PubMed] [Google Scholar]
- 3.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555–563. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Schumacher HC, Mangla S, et al. Urgent endovascular revascularization for symptomatic intracranial atherosclerotic stenosis. Neurology 2003; 61: 1729–1735. [DOI] [PubMed] [Google Scholar]
- 5.Marks MP, Wojak JC, Al-Ali F, et al. Angioplasty for symptomatic intracranial stenosis: Clinical outcome. Stroke 2006; 37: 1016–1020. [DOI] [PubMed] [Google Scholar]
- 6.Rahme RJ, Aoun SG, Batjer HH, et al. SAMMPRIS: End of intracranial stenting for atherosclerosis or back to the drawing board? Neurosurgery 2011; 69: N16–18. [DOI] [PubMed] [Google Scholar]
- 7.Henkes H, Miloslavski E, Lowens S, et al. Treatment of intracranial atherosclerotic stenoses with balloon dilatation and self-expanding stent deployment (Wingspan). Neuroradiology 2005; 47: 222–228. [DOI] [PubMed] [Google Scholar]
- 8.Fiorella D, Levy EI, Turk AS, et al. US multicenter experience with the Wingspan stent system for the treatment of intracranial atheromatous disease: Periprocedural results. Stroke 2007; 38: 881–887. [DOI] [PubMed] [Google Scholar]
- 9.Zhao LB, Park S, Lee D, et al. Mechanism of procedural failure related to Wingspan. Neurointervention 2012; 7: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vajda Z, Schmid E, Guthe T, et al. The modified Bose method for the endovascular treatment of intracranial atherosclerotic arterial stenoses using the Enterprise stent. Neurosurgery 2012; 70: 91–101; discussion. [DOI] [PubMed] [Google Scholar]
- 11.Higashida RT, Halbach VV, Dowd CF, et al. Initial clinical experience with a new self-expanding nitinol stent for the treatment of intracranial cerebral aneurysms: The Cordis Enterprise stent. AJNR Am J Neuroradiol 2005; 26: 1751–1756. [PMC free article] [PubMed] [Google Scholar]
- 12.Izar B, Rai A, Raghuram K, et al. Comparison of devices used for stent-assisted coiling of intracranial aneurysms. PLoS One 2011; 6: e24875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadkhodayan Y, Rhodes N, Blackburn S, et al. Comparison of Enterprise with Neuroform stent-assisted coiling of intracranial aneurysms. AJR Am J Roentgenol 2013; 200: 872–878. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Yun JK, Kim DW, et al. Clinical and angiographic outcomes of Wingspan stent placement for treatment of symptomatic intracranial stenosis: Single center experience with 19 cases. J Cerebrovasc Endovasc Neurosurg 2012; 14: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandini R, Chiaravalloti A, Pampana E, et al. Intracranial atheromatous disease treatment with the Wingspan stent system: Evaluation of clinical, procedural outcome and restenosis rate in a single-center series of 21 consecutive patients with acute and mid-term results. Clin Neurol Neurosurg 2013; 115: 741–747. [DOI] [PubMed] [Google Scholar]
- 16.Yu SC, Leung TW, Lee KT, et al. Learning curve of Wingspan stenting for intracranial atherosclerosis: Single-center experience of 95 consecutive patients. J Neurointerv Surg 2014; 6: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaidat OO, Kalia J, Nourollah-Zadeh E, et al. Stenting and angioplasty of small cerebral arteries in symptomatic intracranial atherosclerotic disease. Interv Neurol 2014; 2: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Huang Q, Zhang Y, et al. A single-center study of Wingspan stents for symptomatic atherosclerotic stenosis of the middle cerebral artery. J Clin Neurosci 2013; 20: 362–366. [DOI] [PubMed] [Google Scholar]
- 19.Costalat V, Maldonado IL, Vendrell JF, et al. Endovascular treatment of symptomatic intracranial stenosis with the Wingspan stent system and Gateway PTA balloon: A multicenter series of 60 patients with acute and midterm results. J Neurosurg 2011; 115: 686–693. [DOI] [PubMed] [Google Scholar]
- 20.Kelly ME, Turner RD 4th, Moskowitz SI, et al. Delayed migration of a self-expanding intracranial microstent. AJNR Am J Neuroradiol 2008; 29: 1959–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavine SD, Meyers PM, Connolly ES, et al. Spontaneous delayed proximal migration of Enterprise stent after staged treatment of wide-necked basilar aneurysm: Technical case report. Neurosurgery 2009; 64 E1012; discussion E. [DOI] [PubMed] [Google Scholar]
- 22.Jiang WJ, Srivastava T, Gao F, et al. Perforator stroke after elective stenting of symptomatic intracranial stenosis. Neurology 2006; 66: 1868–1872. [DOI] [PubMed] [Google Scholar]
- 23.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu SC, Leung TW, Lee KT, et al. Angioplasty and stenting of intracranial atherosclerosis with the Wingspan system: 1-Year clinical and radiological outcome in a single Asian center. J Neurointerv Surg 2014; 6: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin YS, Kim BM, Suh SH, et al. Wingspan stenting for intracranial atherosclerotic stenosis: Clinical outcomes and risk factors for in-stent restenosis. Neurosurgery 2013; 72: 596–604; discussion. [DOI] [PubMed] [Google Scholar]
- 26.Fiorella DJ, Turk AS, Levy EI, et al. U.S. Wingspan registry: 12-month follow-up results. Stroke 2011; 42: 1976–1981. [DOI] [PubMed] [Google Scholar]
- 27.Jiang WJ, Yu W, Du B, et al. Outcome of patients with >/ = 70% symptomatic intracranial stenosis after Wingspan stenting. Stroke 2011; 42: 1971–1975. [DOI] [PubMed] [Google Scholar]
- 28.Tarlov N, Jahan R, Saver JL, et al. Treatment of high risk symptomatic intracranial atherosclerosis with balloon mounted coronary stents and Wingspan stents: Single center experience over a 10 year period. J Neurointerv Surg 2012; 4: 34–39. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Huang QH, Zhang YW, et al. [Intracranial angioplasty with Wingspan stents for symptomatic atherosclerotic stenosis in small vessels: A study of long-term follow-up]. Zhonghua Yi Xue Za Zhi 2010; 90: 3323–3326. (in Chinese). [PubMed] [Google Scholar]
- 30.Alurkar A, Karanam LS, Oak S, et al. Role of balloon-expandable stents in intracranial atherosclerotic disease in a series of 182 patients. Stroke 2013; 44: 2000–2003. [DOI] [PubMed] [Google Scholar]
- 31.Suh DC, Kim JK, Choi JW, et al. Intracranial stenting of severe symptomatic intracranial stenosis: Results of 100 consecutive patients. AJNR Am J Neuroradiol 2008; 29: 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): The final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry SA, Watanabe M, Qureshi AI. The new standard for performance of intracranial angioplasty and stent placement after Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMMPRIS) trial. AJNR Am J Neuroradiol 2011; 32: E214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz RS, Henry TD. Pathophysiology of coronary artery restenosis. Rev Cardiovasc Med 2002; 3(Suppl 5): S4–9. [PubMed] [Google Scholar]
- 35.Albuquerque FC, Levy EI, Turk AS, et al. Angiographic patterns of Wingspan in-stent restenosis. Neurosurgery 2008; 63: 23–7; discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 36.Lafont A, Faxon D. Why do animal models of post-angioplasty restenosis sometimes poorly predict the outcome of clinical trials? Cardiovasc Res 1998; 39: 50–59. [DOI] [PubMed] [Google Scholar]
- 37.Yu SC, Leung TW, Lee KT, et al. Angioplasty and stenting of atherosclerotic middle cerebral arteries with Wingspan: Evaluation of clinical outcome, restenosis, and procedure outcome. AJNR Am J Neuroradiol 2011; 32: 753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Huang Q, Zhang Y, et al. Wingspan stents for the treatment of symptomatic atherosclerotic stenosis in small intracranial vessels: Safety and efficacy evaluation. AJNR Am J Neuroradiol 2012; 33: 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samaniego EA, Tari-Capone F, Linfante I, et al. Wingspan experience in the treatment of symptomatic intracranial atherosclerotic disease after antithrombotic failure. J Neurointerv Surg 2013; 5: 302–305. [DOI] [PubMed] [Google Scholar]
- 40.Krischek O, Miloslavski E, Fischer S, et al. A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg 2011; 54: 21–28. [DOI] [PubMed] [Google Scholar]
- 41.Lin XZ, Miao F, Li JY, et al. High-definition CT Gemstone spectral imaging of the brain: Initial results of selecting optimal monochromatic image for beam-hardening artifacts and image noise reduction. J Comput Assist Tomogr 2011; 35: 294–297. [DOI] [PubMed] [Google Scholar]