Abstract
Background
Advantages of radial access over brachial/axillary or femoral access have been well described for several decades and include decreased cost, patient preference, and decreased major access site complications. Despite these advantages, radial access is rarely employed or even considered for neurointerventional procedures. This attitude should be reconsidered given several recent large, randomized, controlled trials from the cardiovascular literature proving that radial access is associated with statistically lower costs, decreased incidence of myocardial infarctions, strokes, and even decreased mortality. Radial access is now considered the standard of care for percutaneous coronary interventions in most US centers. Although radial access has been described for neurovascular procedures in the past, overall experience is limited. The two major challenges are the unique anatomy required to access the cerebral vasculature given very acute angles between the arm and craniocervical vessels and limitations in available technology.
Methods
We present a simplified approach to radial access for cerebrovascular procedures and provide a concise step-by-step approach for patient selection, ultrasound-guided single-wall access, recommended catheters/wires, and review of patent hemostasis. Additionally, we present a complex cerebrovascular intervention in which standard femoral access was unsuccessful, while radial access was quickly achieved to highlight the importance of familiarity with the radial approach for all neurointerventionalists.
Results
We have found that the learning curve is not too steep and that the radial access approach can be adopted smoothly for a large percentage of diagnostic and interventional neuroradiologic procedures.
Conclusions
Radial access should be considered in all patients undergoing a cerebrovascular procedure.
Keywords: Radial, technique, angiography, intervention
Introduction
Radial access for interventional procedures was first described in 1948 by Radner et al. This first access was surgical, and transradial catheterization was obtained with a radial artery cut-down approach.1 Percutaneous radial access was explored when Mandel and Dauchot as well as Slogoff et al. described the safety and potential complications of radial artery cannulation for the purpose of monitoring in a surgical and critical care setting in 1977.2,3 In 1989, Campeau published the first large series of selective coronary angiography in 100 patients via a transradial approach.4 Compared to conventional transfemoral and transbrachial access, radial access has been shown to have fewer complications at the access site, offer more post-procedural comfort, allow for earlier ambulation and return to daily activities, and has been proven to be more cost effective.5,6 Most recently, the radial approach has been associated with reduction in mortality, myocardial infarction, and stroke for patients undergoing coronary interventions at high-volume radial access centers.7,8 Experience with radial access for coronary interventions is now well established in many centers and is becoming the standard of care.
Radial access was first described for neurointerventional procedures in 2000, by Matsumoto et al.9 Although there are a few larger series showing high technical success and low complications, radial access is rarely used for neurovascular procedures.10–15 Some barriers to the radial approach for neurointerventional procedures include lack of comfort with the technique, concern about technical difficulty with selection of cervical and intracranial vessels, and concern about vessel injury and post-procedural hemostasis management. The radial approach has proven to be especially advantageous in patients requiring vertebrobasilar interventions, on anticoagulation, with bulky hardware (orthopedic external fixators or stereotactic frames) or patients in whom post-procedural groin care would be difficult.11
The purposes of this paper are to describe patient selection, illustrate simplified patient positioning, present an updated technique for radial access using ultrasound guidance, and discuss approaches to post-procedural hemostasis.
Case example
An 82-year-old woman presented with a four-week history of diplopia upon right lateral gaze, and was found to have a right cranial nerve VI palsy on exam. Noncontrast head computed tomography (CT) demonstrated a 2.5 cm mass in the right cerebellopontine angle compressing the pons, which was concerning for a giant aneurysm and was confirmed by computed tomography angiography (CTA). The patient was urgently admitted for further evaluation and treatment because of the symptomatic nature of this giant posterior circulation aneurysm.
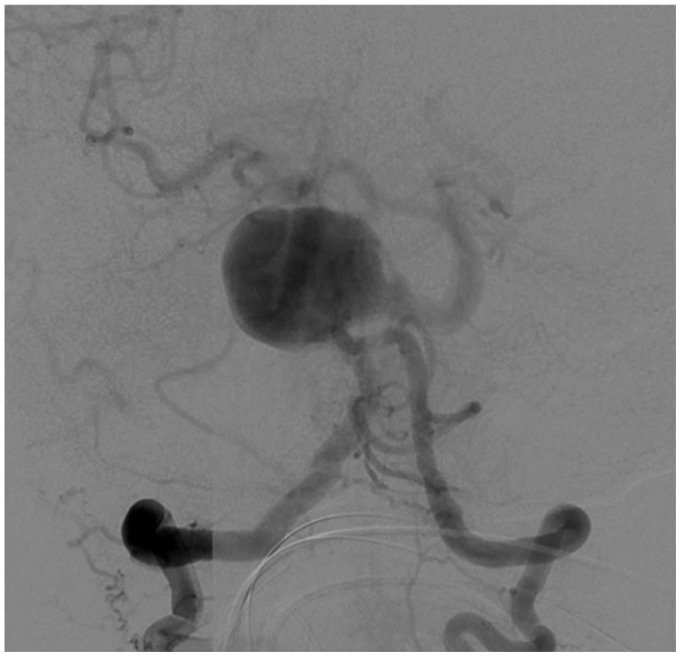
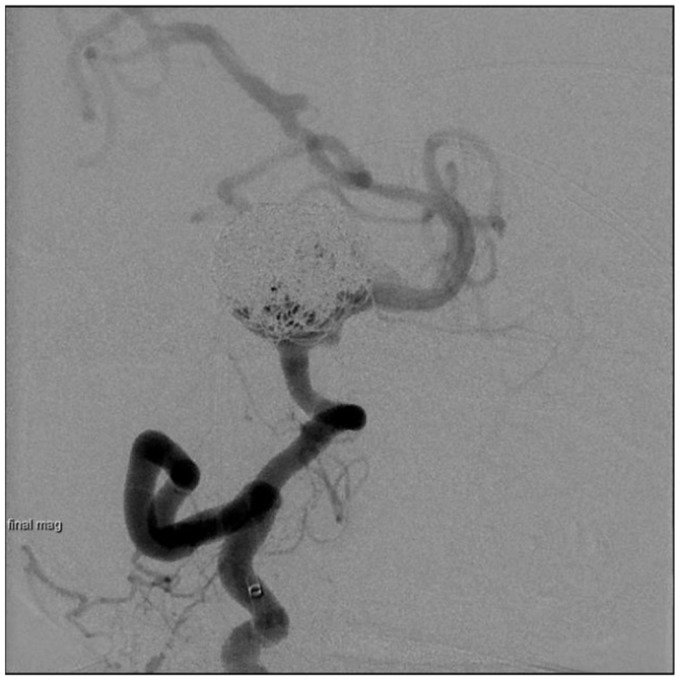
Diagnostic cerebral angiography from a right femoral artery approach confirmed a 2.5 cm vertebrobasilar junction aneurysm with separate inflow from both vertebral arteries, and outflow into the basilar artery, which arose from the left lateral aneurysm wall 8–10 mm away from the left vertebral artery inflow zone (Figure 1). The cervical vertebral arteries were relatively codominant, with a focal 70%–80% stenosis in the distal left vertebral artery at its junction with the aneurysm. Additional angiographic findings included a type 2 aortic arch with significant atheromatous change and marked tortuosity of the proximal great vessels (Figure 2). The patient also had a suspected chronic occlusion of the right internal carotid artery with retrograde flow through the right posterior communicating artery supplying the right middle cerebral artery territory.
Figure 1.
Right vertebral artery injection showing giant wide-neck vertebrobasilar junction aneurysm with severe tortuosity of the proximal basilar artery.
Figure 2.
Innominate artery injection (left anterior oblique (LAO) projection) showing extreme type II/III arch and extreme tortuosity of innominate and subclavian artery with moderate atherosclerotic disease (50% right vertebral artery stenosis not well visualized).
It was felt that the best therapeutic option would be placement of a stent from the right vertebral artery into the basilar trunk, with coil embolization of the aneurysm sac and sacrifice of the distal V4 segment of the left vertebral artery in order to reduce inflow. Severe atherosclerotic disease and tortuosity of the brachiocephalic and right subclavian arteries precluded navigation of a 5 Fr H1 diagnostic catheter into the right subclavian or vertebral arteries. After approximately one hour of attempting to select the right subclavian and vertebral arteries with a variety of diagnostic catheters over several different wires from the right femoral approach, it was felt that a right radial access would allow a more direct approach to the right vertebral artery resulting in a more stable guide catheter position.
The previously placed right radial arterial line and right wrist were then prepped and draped in sterile fashion, and the 4 Fr arterial line was exchanged over a 0.035-inch wire for a short 6 Fr sheath. A solution of nitroglycerin 200 mcg, verapamil 5 mg, and heparin 2000 units were injected through the sheath, which was then connected to a continuous heparinized saline flush after confirmation of vessel patency. The right femoral artery sheath was transduced and used as an arterial line throughout the case.
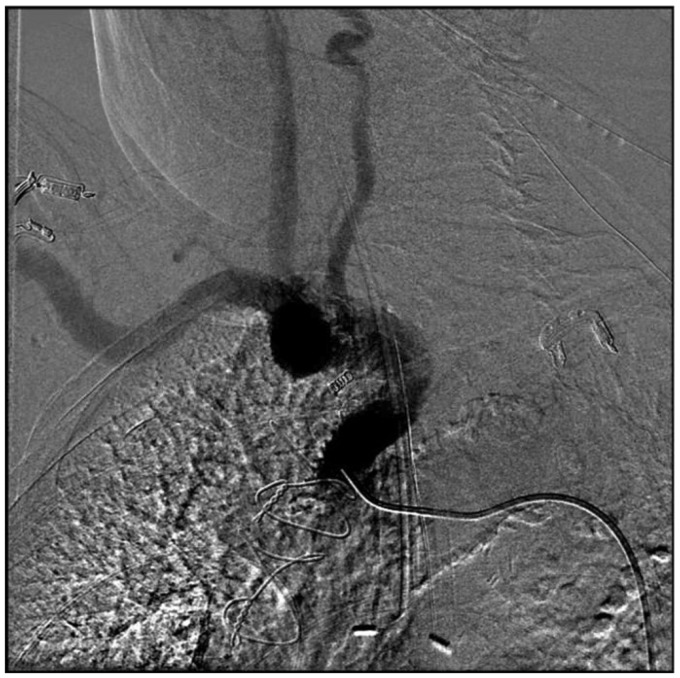
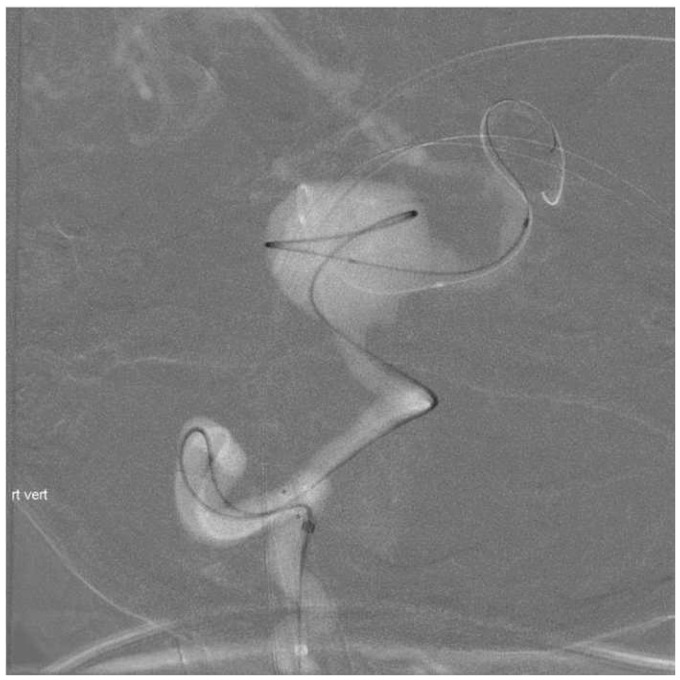
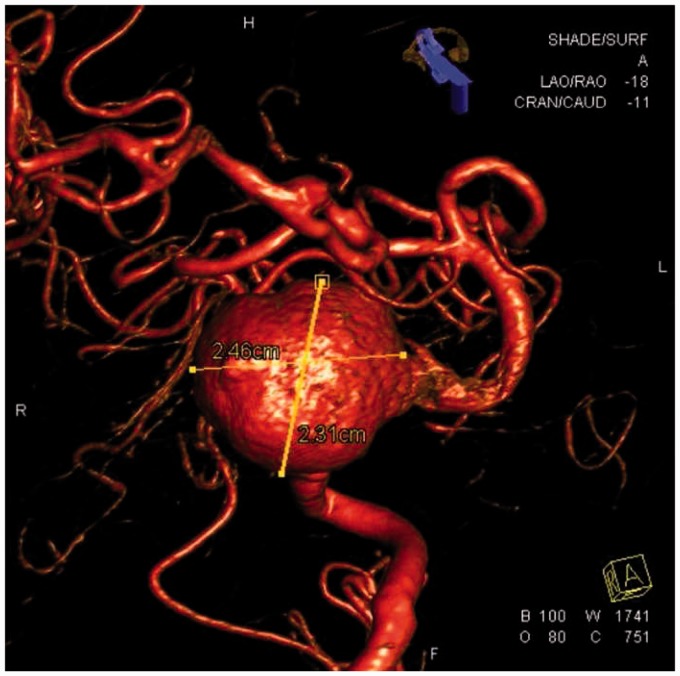
Using fluoroscopic and roadmap guidance, a 6 Fr Envoy DA-XB MPD guide catheter was advanced over an angled Glidewire into the right brachial artery, right subclavian artery, and subsequently into the cervical right vertebral artery without any difficulty (Figure 3). Time from right radial artery sheath placement to guide catheter positioning was approximately 10 minutes. Three-dimensional (3D) rotational angiography was performed and endovascular treatment was successful (Figure 4). An Enterprise stent was placed from the right vertebral artery into the mid-basilar trunk through a Prowler Select Plus microcatheter, and the aneurysm sac was embolized with a total of 40 bare platinum coils for a total coil length of 955 cm through an SL-10 microcatheter from the right vertebral artery approach (Figures 5 and 6). Owing to adequate occlusion of the aneurysm sac and lengthy nature of the procedure, it was decided to not pursue sacrifice of the distal left vertebral artery at the time of initial treatment. Leaving the left vertebral artery open would also allow access into the aneurysm sac in the event of significant recanalization. The patient received a total of 10 mg Decadron during the procedure, and did well clinically afterward, without significant headache and perhaps mild improvement in her diplopia.
Figure 3.
Right vertebral artery road map image showing position of Envoy DA 071 guiding catheter at C2/3 and extremely tortuous course of the Prowler Select Plus microcatheter taken through the cervical right vertebral artery and around the dome of the aneurysm into the left posterior cerebral artery over a Transcend Floppy 0.014 microwire.
Figure 4.
Three-dimensional rotational angiogram showing the wide-neck vertebrobasilar junction aneurysm and extreme tortuosity of the mid and distal basilar artery.
Figure 5.
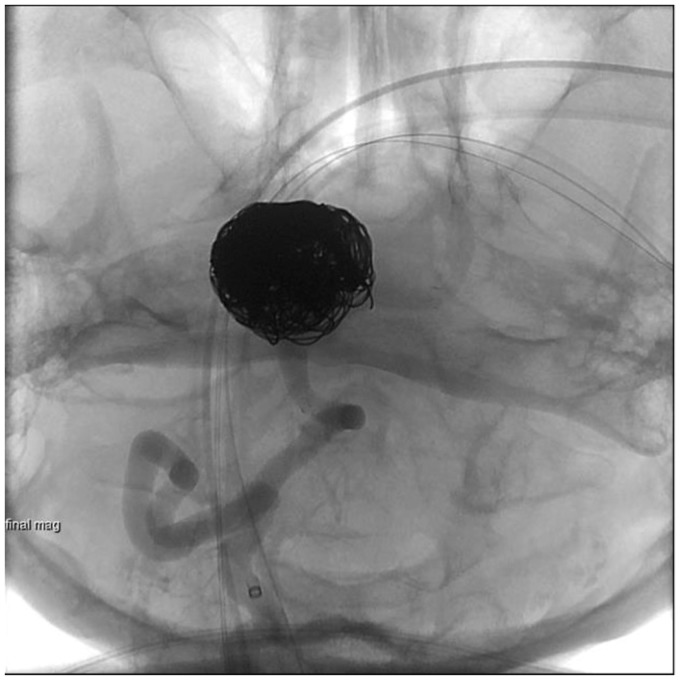
Unsubtracted right vertebral artery injection showing Enterprise reconstruction device from the right V4 segment into the mid-basilar artery across the neck of the aneurysm and coil mass in the aneurysm.
Figure 6.
Subtracted right vertebral artery injection post-stent coiling demonstrating residual filling in the coil mass and along the inferior right lateral base of aneurysm (Raymond Occlusion Classification 3a).
Patient selection
The key for low complication rates is careful patient selection, good technique, and appropriate pre-procedural assessment.11 Patients possibly requiring future arteriovenous fistula or arterial bypass graft should be excluded because of the potential need of a patent radial artery.
Prior to any invasive intervention involving the radial artery, collateral circulation to the hand must be assessed. A multitude of noninvasive methods have been described to assess collateral circulation to the hand including digital pressures, plethysmography, pulse oximetry, and duplex ultrasonography. Dr Edgar V. Allen first described his method in 1929 in three patients with thromboangiitis obliterans.16 The most commonly used modified Allen’s test, first described by Wright in 1952, is conducted by having the patient make a fist for 30 seconds.17 The radial and ulnar arteries are then occluded with manual pressure. The patient then opens the fist and manual pressure is released from the ulnar artery. Hyperextension of the hand or wide separation of the digits should be avoided as they may result in a false-positive or abnormal test. If sufficient collateral circulation is present, there should be normal return of color to the hand within about 3–12 seconds. If there is no return of color within the allotted time, this is called a positive modified Allen’s test and the radial artery should be avoided for interventional procedures.18 Cheng et al. first showed in 1989 that the plethysmographic component of the pulse oximetry might improve the assessment of collateral circulation of the hand over the sole utilization of the modified Allen’s test in the setting of critical care monitoring.19 We performed a technique using pulse oximetry in collaboration with the modified Allen’s test to ensure the most accurate evaluation.20 Adequate radial and ulnar artery compression is confirmed with loss of the normal pulse oximetry waveform. Subsequent return of an appropriate waveform and oxygen saturation following the release of the ulnar artery implies a negative test and adequate ulnar collateral circulation.
The transradial approach has a unique set of risks and complications aside from the traditional transfemoral approach that should be well understood prior to undertaking a procedure. There are a few well-documented complications of the transradial approach, primarily described in the interventional cardiology literature. Minor complications include ecchymosis at the puncture site in 2%–3% of patients.12 Arteriovenous fistulas and pseudoaneurysm formation with the transradial approach has been reported in 0.2%–0.4% of cases.6,7,16 More serious complications including significant local site hematoma secondary to arterial injury with resultant compartment syndrome requiring surgical decompression have rarely been described with an estimated incidence of less than 0.4%.21
The most common complication associated with the transradial approach is delayed radial artery occlusion, which has been reported to occur in 1.5%–33% of patients undergoing transradial access for coronary procedures, with the true incidence likely underestimated because of the asymptomatic nature in the vast majority of cases.5,6,22–24 Most modern studies suggest delayed radial artery occlusion rates between 3% in diagnostic and up to 6% in interventional cardiac procedures, which are related in part to the size of the access sheath. Radial artery occlusion can be prevented with careful vascular access, appropriate sizing of the sheath, adequate intra-procedural anticoagulation, and careful post-procedural patent hemostasis techniques.25,26 Critical ischemia with necrosis has never been reported with transradial access after appropriate evaluation of collateral palmar circulation; however, critical hand ischemia requiring surgery has been described related to distal embolization.3,27 In addition to direct site complications, transient (ischemic or neurologic) symptoms have been described in the literature with prolonged manipulation of guiding catheters, but are a relatively uncommon issue. This can be avoided with selection of the appropriate catheters according to the individual patient’s unique anatomic variations.28
Technique
Appropriate patient selection is crucial to achieve good outcomes and minimize complications. Whether in the office for an elective case or on the table in the angiography suite for an emergent case, the Barbeau test (or modified Allen’s test) should be performed to ensure adequate collateral circulation via the ulnar artery and patency of the deep/superficial palmar arch.23,29 Usually, the right radial artery is preferentially chosen because of the more comfortable proximity to the operator, but either side can be accessed with a similar technique. In the setting of a left vertebral intervention, the left radial artery is preferred. The patient should be positioned supine with the target upper extremity taped extended and slightly supinated over a gel pad, sheet and rolled towel under the wrist with an arm board to keep the patient’s arm near the body to allow the lateral tube to be properly positioned (Figure 7).
Figure 7.
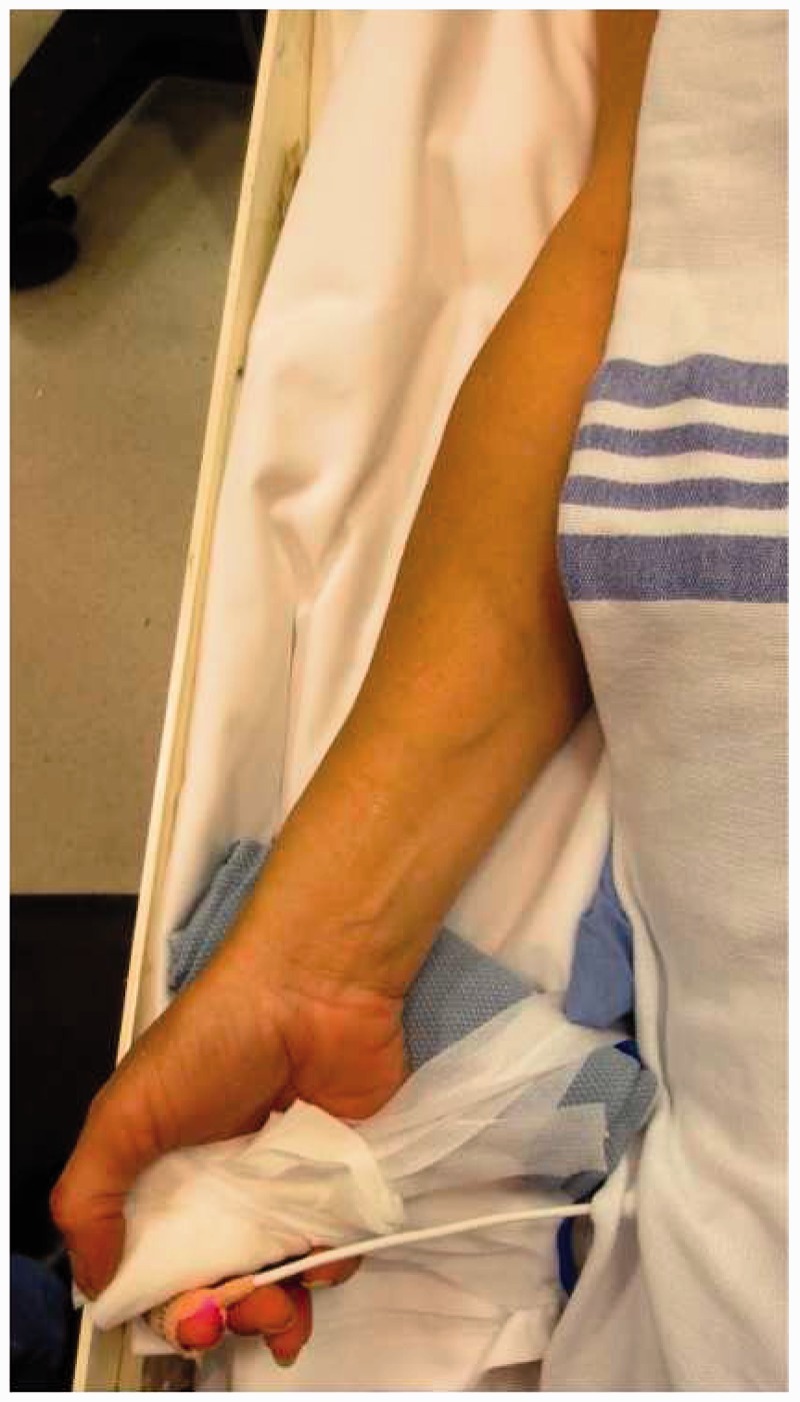
Supine position with extremity taped in an extended and slightly supinated position with pulse oximeter on index finger.
The pulse oximeter is placed on the ipsilateral index finger. The Barbeau test is again performed in the angiography suite by compressing the radial artery to occlusion while monitoring for a change in the plethysmographic waveform.23,29 While maintaining compression on the radial artery, the ulnar artery is then simultaneously compressed to confirm that the radial artery is truly compressed to avoid a false-negative exam. With both the radial and ulnar artery compressed, the plethysmographic waveform and pulse should be flat and zero, respectively. With the pulse oximeter on the ipsilateral index finger, adequate vascular flow can be continually monitored throughout the procedure for early detection of ischemia to the hand. Subsequently, the target extremity can then be prepped using aseptic technique and a standard femoral drape can be pulled over the target wrist. Risks of significant vascular injury including dissection, vessel perforation, or development of pseudoaneurysm with the transradial approach can be minimized with careful technique.
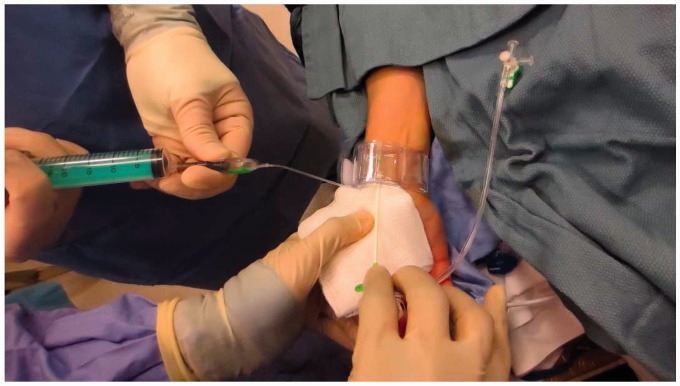
A sterilely prepped ultrasound probe is used to locate a target on the radial artery at the level of the radial styloid. Approximately 1 cm distal to the intended puncture site, a very superficial wheal is created using 1% lidocaine and a small incision made. Most of the previously published literature on radial artery access technique describes a traditional Seldinger double-wall puncture guided by palpation.11,13,30–33 The authors recommend an ultrasound-guided single-wall technique using a 21-gauge micropuncture needle and a 0.021-inch microwire (Figure 8). Careful puncture of the radial artery in the center of the vessel with confirmation of the tip of the needle in the middle of the vessel is mandatory prior to attempting wire access. Simply using blood return likely increases the risk of spasm and vessel injury/dissection because of the small caliber of the radial artery and risk of subintimal placement of the microwire. Attention to technique will minimize the need for repeated arterial sticks, decrease spasm, decrease vessel injury, and increase patient comfort. The 0.021-inch microwire is advanced to the level of the brachial artery (ideally above the elbow) under fluoroscopic guidance to confirm a straight course of the radial artery and provide adequate support in case of vessel tortuosity. The needle is then removed.
Figure 8.
A 21-gauge needle inserted under ultrasound guidance using modified Seldinger technique.
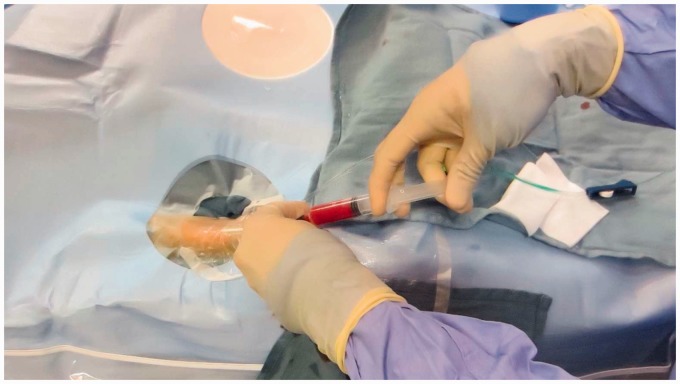
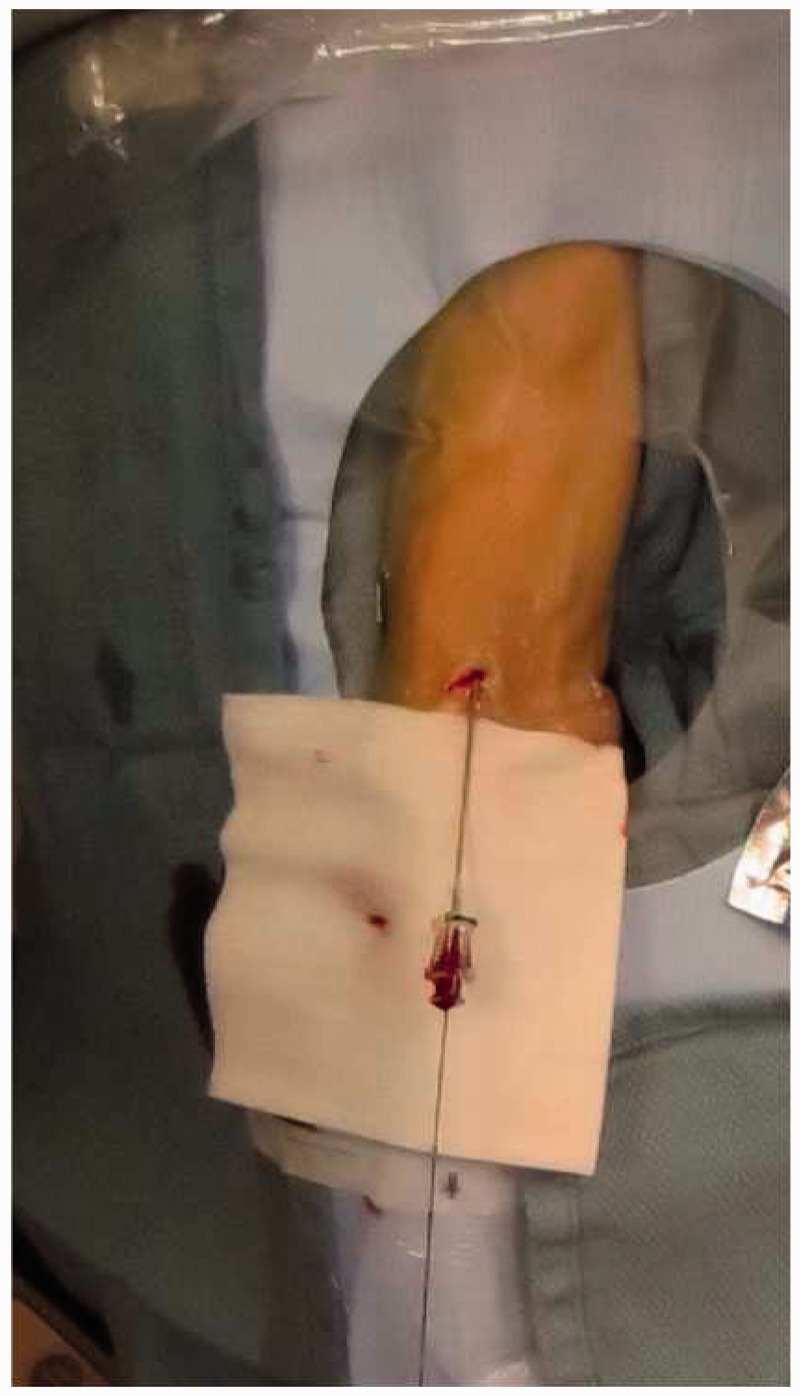
A sheath is then placed over the guidewire and the guidewire is removed.33,34 Generally the authors use a 4 French (F) Merit Prelude radial sheath for diagnostic procedures and a 5F Merit Prelude or 6F Terumo Slim sheath for interventional procedures. All of these sheaths are hydrophilic (should be re-wet immediately prior to insertion) and the provided obturator/dilator allows placement directly over the 0.021-inch wire without exchange for a 0.035-inch wire, which saves a step and may minimize vessel trauma and spasm. The sheath need be advanced only 4–5 cm and secured at the skin with several Tegaderm dressings. This will allow stable access without the need to hub the entire sheath as the sheath diameter increases proximally (Figure 9). Additionally, if the radial artery has a proximal loop, this approach may minimize risk of vessel injury. Once the sheath is in place and flushed, an intra-arterial radial cocktail is given consisting of 5 mg verapamil, 3000–5000 U of heparin sulfate, and 200 mcg of nitroglycerin (Figure 10). The drugs should be injected with intermittent aspiration of the patient’s blood to minimize discomfort. A retrograde radial road map is used to confirm the size of the radial artery, exclude any vascular injury, and identify flow-limiting spasm prior to insertion of any catheter.22 At this point, the procedure can be aborted or if there is a flow-limiting spasm, additional nitroglycerin can be administered as needed. Subsequently, the sheath is attached to a continuous flush with heparinized saline (at our institution 4000 U/liter).
Figure 9.
A 6F 10 cm sheath, not completely advanced to the hub.
Figure 10.
Radial cocktail with intermittent aspiration.
The main technical challenge associated with neurovascular procedures via the transradial approach is the orientation of the cerebral vasculature in relation to the axis of the innominate or left subclavian artery. Currently there are no dedicated catheter shapes and lengths designed for neurovascular procedures via a radial approach. Access of the left common carotid artery or left vertebral artery from a right radial approach is more challenging and generally requires use of a Simmons II catheter for angiography and a long exchange for intervention. This is becoming less of an issue because of improved catheters and wires and increased operator experience. Access of more distal cerebral vasculature may be difficult because of the need for longer guide catheters and microcatheters. One significant trend in neurointerventional technology is the development of devices that are deliverable through smaller microcatheters including stents, stent retrievers, and balloons. This allows for even complex neurovascular procedures to be performed through a 6F access. Additionally, radial access for diagnostic and interventional neurovascular procedures via the right vertebral and carotid systems may be simpler and more stable than a traditional transfemoral approach.11
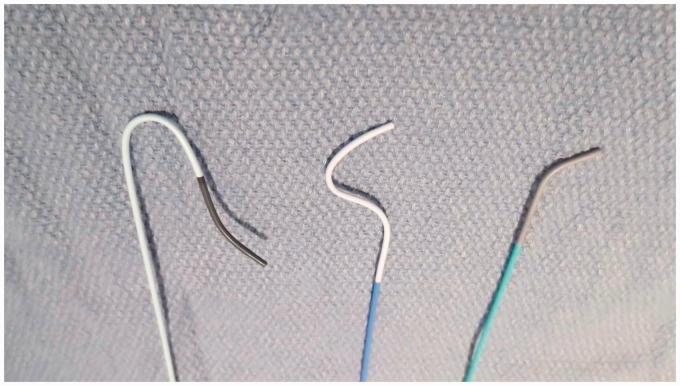
The authors have found diagnostic cerebral angiography of the right vertebral artery and right common carotid artery branches can easily be performed using a 100 cm Osborn braided catheter (Cook, Bloomington, IN) and a Terumo 150 cm shapeable Glidewire (Terumo, Somerset, NJ). If angiography is to include the left common carotid artery, a Simmons II glide catheter is generally used to perform angiography of the right vertebral artery and right common or internal carotid artery without forming the catheter (Figure 11). As the Simmons II catheter is withdrawn from the right common carotid artery, the catheter is formed in the innominate artery. In some cases, the Simmons II catheter must be formed in the aortic arch. Rarely, if the catheter cannot be formed or if the left common carotid artery or left vertebral artery cannot be successfully selected, a femoral approach may be required. The authors generally discuss this possibility with the patients prior to the procedure, but this is so rare that we do not routinely prepare the groin for these procedures.
Figure 11.
Catheters (from left to right: 4Fr Simmons 1 100 cm, 4Fr Braided Osborne 100 cm, 4Fr Berenstein 100 cm).
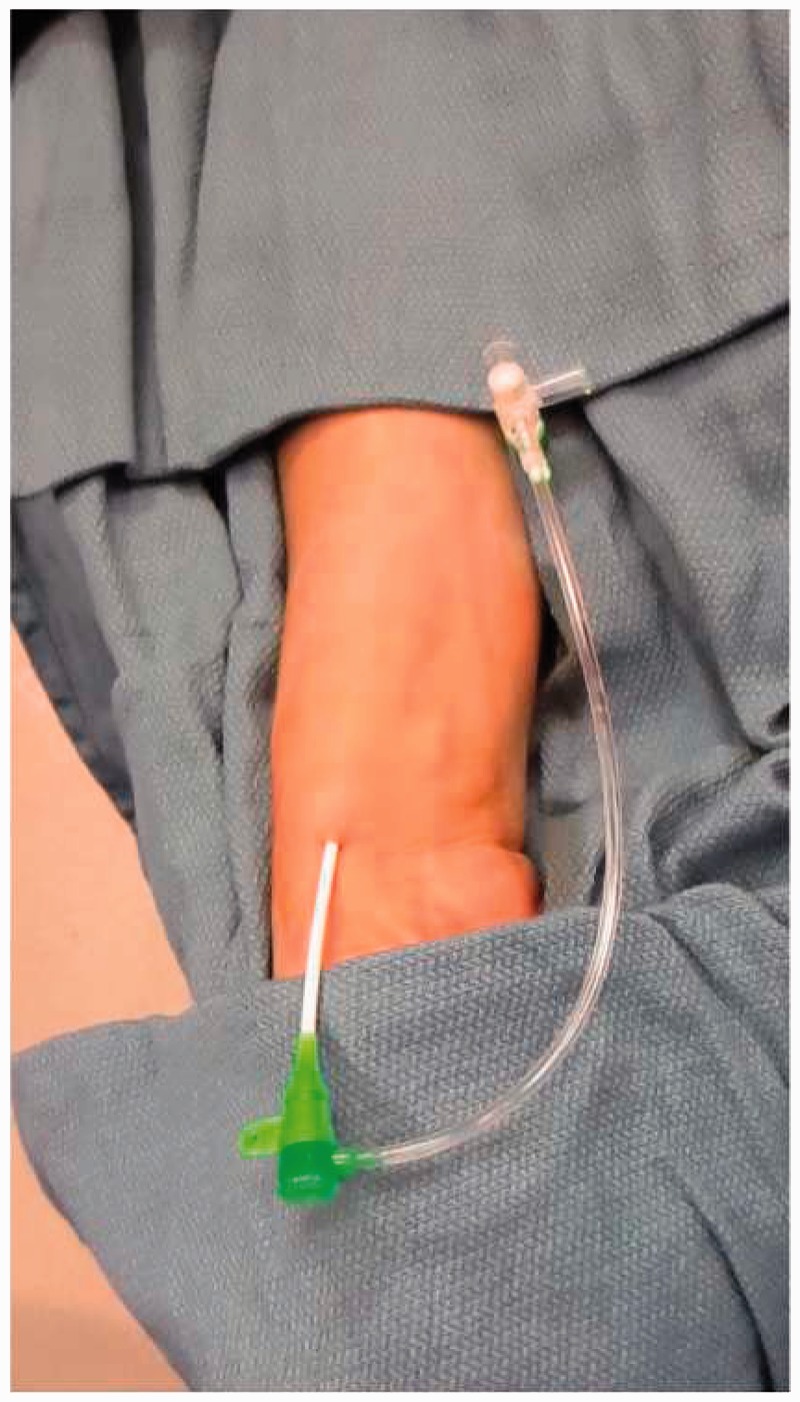
Once the procedure is complete, a patent hemostasis technique should be performed. There is a fine balance in the amount of pressure that should be applied as aggressive compression of the radial puncture site may increase the risk of radial artery occlusion.25,26 We remove the dressing from the wrist and leave the pulse oximeter in place. Then we apply the hemostatic compression device so that it is centered just proximal to the arteriotomy rather than the dermatotomy, to avoid subcutaneous hemorrhage (Figure 12). After inflating the balloon in the compression band, the arterial sheath is removed. The balloon in the compression device is slowly deflated until blood return is observed and then an additional 3 cc of air is injected (Figure 13). A good plethysmographic waveform and pulse oximeter reading is confirmed for several minutes and the patient and staff are reminded to keep the patient’s wrist straight. Although not routinely performed, a reverse Barbeau’s test may be performed using plethysmography by applying manual occlusive compression to the ipsilateral ulnar artery and subsequently observing the plethysmographic waveform. The presence of a normal pulsatile plethysmographic signal is compatible with a patent radial artery and appropriate radial compression band pressure. Loss of a normal pulsatile plethysmographic signal requires release of hemostatic pressure from the radial compression band until a pulsatile waveform is again achieved.26,35
Figure 13.
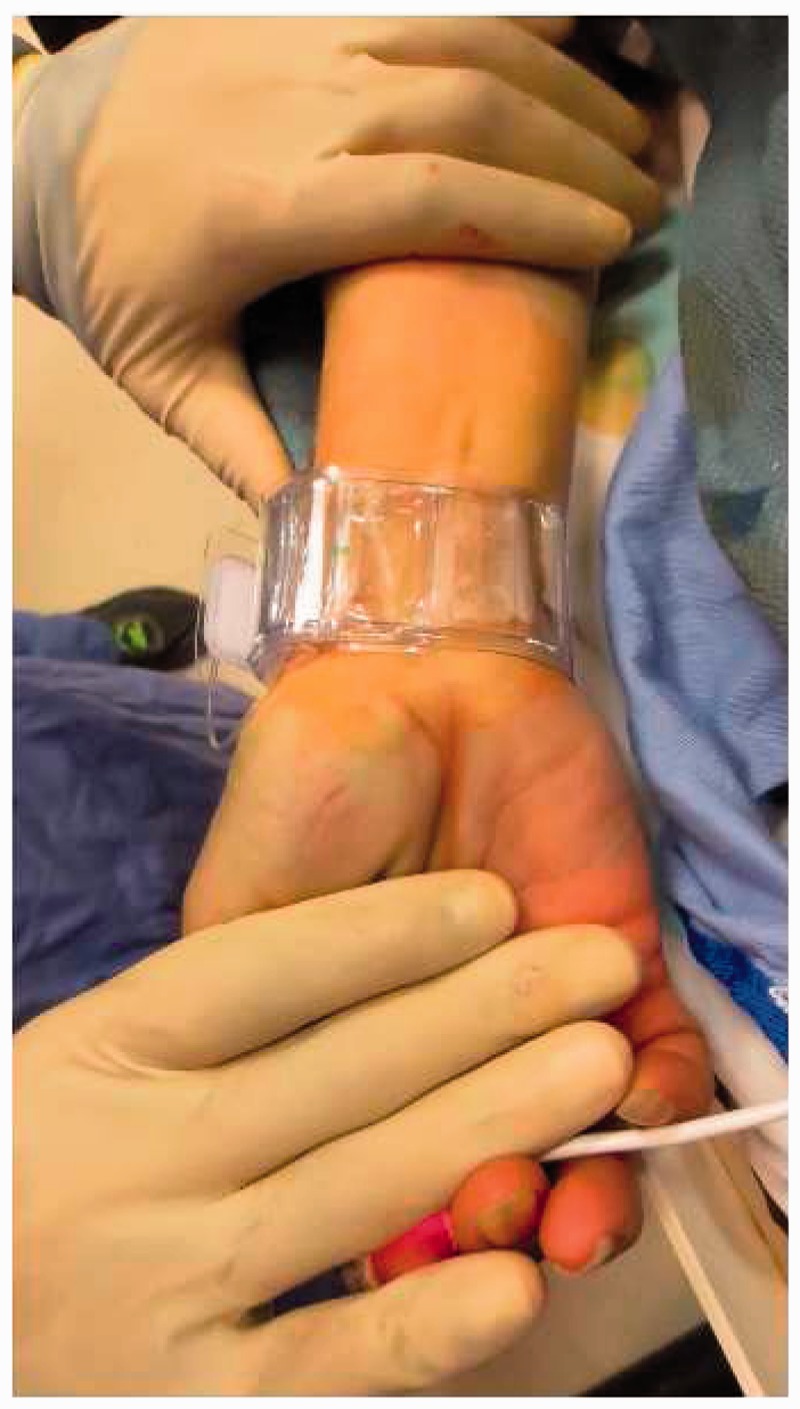
Patent hemostasis confirmed after sheath removal.
Figure 12.
Patent hemostasis. Withdrawal of sheath 1–2 cm and application of radial compression band.
This precise balance of hemostasis and radial artery patency should be checked every 15 minutes for at least 30 minutes. The first 15 minutes may be the most crucial as the patient’s blood pressure will likely fluctuate and the aforementioned balance may need to be monitored closely. The band should stay inflated for two to four hours, depending on the sizes of the devices used and the last dose of anticoagulant administered. Heparin will typically lose its effect within four hours of administration and thus extended radial artery compression beyond this time span may increase the risks of radial artery occlusion. If radial artery occlusion is noted upon removal of the compression band, transient compression of the ipsilateral ulnar artery has been found to aid in re-canalization of the occluded radial artery.36 Once the compression band is removed, a light dressing should be applied to ensure continued patency of the radial artery. Radial artery patency should again be assessed prior to discharge (Table 1).
Table 1.
Summary technique.
| 1) Patient selection: Pre-procedural assessment including risk of future dialysis and performing a Barbeau’s test or modified Allen’s test. |
| 2) Patient positioning: Slightly supinated, pulse oximeter on ipsilateral index finger. |
| 3) Draping and sterile technique: Femoral drape pulled over target extremity. Standard sterile preparation. |
| 4) Arterial access: Meticulous ultrasound-guided single wall radial arterial puncture using modified Seldinger technique. |
| 5) Sheaths: Merit 4F Prelude for diagnostic procedures and Merit 5F Prelude or Terumo 6F slim for interventions. |
| 6) Radial cocktail: Verapamil 5 mg, heparin 3000 U–5000 U and nitroglycerin 200 mcg with intermittent aspiration. |
| 7) Roadmap: Radial/Brachial roadmap to assess radial artery prior to catheter navigation. |
| 8) Suggested devices: Catheters: 4F 100–125 cm Berenstein diagnostic catheter, 4F Osborn braided 100 cm, 120 cm 4F Simmons 2 glide catheter. Wires: Terumo 150 cm standard, Terumo Benson wire or Terumo 150 cm Shapeable glide wire. Right vertebral access: Berenstein catheter and standard glide or Osborn and shapeable glide. Right common carotid access: Osborn and shapeable glide. Left common carotid access: 4F Simmons 120 glide (form in right common carotid artery (CCA) or right vertebral artery (RVA)). |
| 9) Closure: Patent hemostasis technique with hemostatic compression device. |
Not every patient or anatomy is suitable for radial access. Conversion to a femoral approach may be needed in some cases and should be anticipated.
Conclusion
Radial access has been well described in the cardiovascular literature with significant advantages including decreased incidence of myocardial infarctions, strokes, and even decreased mortality.7,8 Radial access has recently been described for neurovascular procedures as well and is rapidly becoming a safe and advantageous alternative to the traditional transfemoral approach. We have found that the learning curve is not too steep and that the radial access approach can be adopted smoothly using a structured technique resulting in reproducible successful results and increased patient satisfaction for nearly the entire spectrum of diagnostic and interventional neuroradiologic procedures.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Radner S. Thoracal aortography by catheterization from the radial artery; preliminary report of a new technique. Acta radiol 1948; 29: 178–180. [DOI] [PubMed] [Google Scholar]
- 2.Mandel MA, Dauchot PJ. Radial artery cannulation in 1,000 patients: Precautions and complications. J Hand Surg Am 1977; 2: 482–485. [DOI] [PubMed] [Google Scholar]
- 3.Slogoff S, Keats AS, Arlund C. On the safety of radial artery cannulation. Anesthesiology 1983; 59: 42–47. [DOI] [PubMed] [Google Scholar]
- 4.Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn 1989; 16: 3–7. [DOI] [PubMed] [Google Scholar]
- 5.Kiemeneij F, Laarman GJ, Odekerken D, et al. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: The access study. J Am Coll Cardiol 1997; 29: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 6.Hamon M, Pristipino C, Di Mario C, et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: Position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology. EuroIntervention 2013; 8: 1242–1251. [DOI] [PubMed] [Google Scholar]
- 7.Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet 2011; 377: 1409–1420. [DOI] [PubMed] [Google Scholar]
- 8.Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: The RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol 2012; 60: 2481–2489. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto Y, Hokama M, Nagashima H, et al. Transradial approach for selective cerebral angiography: Technical note. Neurol Res 2000; 22: 605–608. [DOI] [PubMed] [Google Scholar]
- 10.Levy EI, Boulos AS, Fessler RD, et al. Transradial cerebral angiography: An alternative route. Neurosurgery 2002; 51: 335–340; discussion 340–342. [PubMed] [Google Scholar]
- 11.Nohara AM, Kallmes DF. Transradial cerebral angiography: Technique and outcomes. AJNR Am J Neuroradiol 2003; 24: 1247–1250. [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto Y, Hongo K, Toriyama T, et al. Transradial approach for diagnostic selective cerebral angiography: Results of a consecutive series of 166 cases. AJNR Am J Neuroradiol 2001; 22: 704–708. [PMC free article] [PubMed] [Google Scholar]
- 13.Bendok BR, Przybylo JH, Parkinson R, et al. Neuroendovascular interventions for intracranial posterior circulation disease via the transradial approach: Technical case report. Neurosurgery 2005; 56: E626 discussion E626. [DOI] [PubMed] [Google Scholar]
- 14.Lawson MF, Velat GJ, Fargen KM, et al. Direct radial artery access with the 070 neuron guide catheter for aneurysm coiling: A novel application of the neuron catheter for cerebral interventions. Neurosurgery 2012; 71(2 Suppl Operative): onsE329–onsE334. discussion onsE334. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Kim DY, Kim JW, et al. Efficacy of transradial cerebral angiography in the elderly. J Korean Neurosurg Soc 2013; 53: 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen EV. Thromboangiitis obliterans: Methods of diagnosis of chronic occlusive arterial lesions distal to the wrist with illustrative cases. Am J Med Sci 1929; 178: 237–244. [Google Scholar]
- 17.Wright IS. Vascular diseases in clinical practice, 2nd ed Chicago, IL: Year Book Publishers Inc, 1952, pp. 552. [Google Scholar]
- 18.Habib J, Baetz L, Satiani B. Assessment of collateral circulation to the hand prior to radial artery harvest. Vasc Med 2012; 17: 352–361. [DOI] [PubMed] [Google Scholar]
- 19.Cheng EY, Lauer KK, Stommel KA, et al. Evaluation of the palmar circulation by pulse oximetry. J Clin Monit 1989; 5: 1–3. [DOI] [PubMed] [Google Scholar]
- 20.Paul BZ, Feeney CM. Combining the modified Allen’s test and pulse oximetry for evaluating ulnar collateral circulation to the hand for radial artery catheterization of the ED patient. Cal J Emerg Med 2003; 4: 89–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Eltahawy EA and Cooper CJ. Managing radial access vascular complications. Cardiac Inte Today 2010; 4: 46--49.
- 22.Goldberg SL, Renslo R, Sinow R, et al. Learning curve in the use of the radial artery as vascular access in the performance of percutaneous transluminal coronary angioplasty. Cathet Cardiovasc Diagn 1998; 44: 147–152. [DOI] [PubMed] [Google Scholar]
- 23.Barbeau GR, Arsenault F, Dugas L, et al. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: Comparison with the Allen’s test in 1010 patients. Am Heart J 2004; 147: 489–493. [DOI] [PubMed] [Google Scholar]
- 24.Mann JT, 3rd, Cubeddu MG, Schneider JE, et al. Right radial access for PTCA: A prospective study demonstrates reduced complications and hospital charges. J Invasive Cardiol 1996; 8(Suppl D): 40D–44D. [PubMed] [Google Scholar]
- 25.Sanmartin M, Gomez M, Rumoroso JR, et al. Interruption of blood flow during compression and radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv 2007; 70: 185–189. [DOI] [PubMed] [Google Scholar]
- 26.Pancholy SB. The fine points of radial hemostasis management: Incorporating a patent hemostasis technique can maximize radial artery patency. Cardiac Interventions Today 2013; July/August: 41–44. [Google Scholar]
- 27.Rademakers LM, Laarman GJ. Critical hand ischaemia after transradial cardiac catheterisation: An uncommon complication of a common procedure. Neth Heart J 2012; 20: 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu CJ, Hung WC, Chen SM, et al. Feasibility and safety of transradial artery approach for selective cerebral angiography. Catheter Cardiovasc Interv 2005; 66: 21–26. [DOI] [PubMed] [Google Scholar]
- 29.Kotowycz MA, Dzavik V. Radial artery patency after transradial catheterization. Circ Cardiovasc Interv 2012; 5: 127–133. [DOI] [PubMed] [Google Scholar]
- 30.Pancholy SB, Sanghvi KA, Patel TM. Radial artery access technique evaluation trial: Randomized comparison of Seldinger versus modified Seldinger technique for arterial access for transradial catheterization. Catheter Cardiovasc Interv 2012; 80: 288–291. [DOI] [PubMed] [Google Scholar]
- 31.Bertrand OF, Rao SV, Pancholy S, et al. Transradial approach for coronary angiography and interventions: Results of the first international transradial practice survey. JACC Cardiovasc Interv 2010; 3: 1022–1031. [DOI] [PubMed] [Google Scholar]
- 32.Yokoyama N, Takeshita S, Ochiai M, et al. Anatomic variations of the radial artery in patients undergoing transradial coronary intervention. Catheter Cardiovasc Interv 2000; 49: 357–362. [DOI] [PubMed] [Google Scholar]
- 33.Mangar D, Thrush DN, Connell GR, et al. Direct or modified Seldinger guide wire-directed technique for arterial catheter insertion. Anesth Analg 1993; 76: 714–717. [PubMed] [Google Scholar]
- 34.Caputo ND, Auld M. Placement of a central venous catheter in the antecubital vein using a modified Seldinger technique. Air Med J 2014; 33: 280–282. [DOI] [PubMed] [Google Scholar]
- 35.Rao SV, Tremmel JA, Gilchrist IC, et al. Best practices for transradial angiography and intervention: A consensus statement from the Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Catheter Cardiovasc Interv 2014; 83: 228–236. [DOI] [PubMed] [Google Scholar]
- 36.Bernat I, Bertrand OF, Rokyta R, et al. Efficacy and safety of transient ulnar artery compression to recanalize acute radial artery occlusion after transradial catheterization. Am J Cardiol 2011; 107: 1698–1701. [DOI] [PubMed] [Google Scholar]