Abstract
Introduction
Sickle cell disease (SCD) is a clinical phenotype that presents a unique challenge to the interventionalist, particularly when stent therapy is contemplated. Homozygous individuals are likely at increased risk for thromboembolic complications. There are no formal guidelines regarding antiplatelet therapy in the short or long term for intracranial stent use in SCD. The authors describe the novel use of a pipeline embolization device (PED) to treat a ruptured dissecting bilobed/fusiform vertebral artery V4 aneurysm in an SCD patient complicated by tortuous proximal anatomy and the anterior spinal artery arising from the diseased segment. Considerations regarding antiplatelet therapy in this scenario are discussed.
Case report
A 50-year-old woman with homozygous recessive SCD was transported to the emergency department and presented with diffuse subarachnoid hemorrhage. CT angiography demonstrated a left-sided 3 × 5 mm fusiform bi-lobulated presumed dissecting vertebral artery aneurysm, immediately distal to the origin of the posterior inferior cerebellar artery (PICA). A PED was deployed within the V4 segment across the aneurysm. Post-treatment angiography showed patency of the parent artery, and patency of the “jailed” anterior spinal artery and of the PICA.
Discussion
Selecting a treatment method in SCD patients with a ruptured intracranial aneurysm is challenging and there are no clinical trials comparing treatment methods in this population. The authors demonstrate that flow diversion is feasible in SCD, which has not been described in the literature. Additionally, the case stresses the peri- and post-procedural management of SCD, as well as long-term considerations with a flow-diverting stent in place.
Keywords: Anemia, sickle cell, dissecting vertebral artery aneurysm, endovascular techniques, intracranial aneurysm/surgery, pipeline embolization device
Introduction
Sickle cell disease (SCD) is an autosomal recessive hemoglobinopathy that leads to hemolytic anemia, thrombosis, and vascular remodeling. An amino acid substitution of valine for glutamate in the position six of β-globin chain results in an abnormal hemoglobin: sickle hemoglobin (HbS) (α2 βs2). HbS is unstable and polymerizes under a number of stressors such as hypoxemia, acidosis, and operative interventions, causing sickling of erythrocytes as well as vaso-occlusive crises.1,2 End-organ damage may ensue including stroke, which is attributable to both thrombosis and vasculopathy.1 Moreover, these abnormal erythrocytes adhere to the endothelium and cause fragmentation of the internal elastic lamina thus weakening the vessel wall and predisposing to aneurysm formation. Consequently, SCD patients have a higher prevalence of intracranial aneurysms3 and their lesions tend to have complex morphologies.1,2 We present the novel use of a pipeline embolization device (PED) for the management of a ruptured fusiform/bilobed-dissecting intracranial aneurysm in a patient with SCD. Vessel sacrifice was felt to be less favorable because of the origin of the cervical anterior spinal artery from the diseased segment of V4. The literature concerning the use of intracranial stents in patients with SCD is reviewed along with discussion of antiplatelet management in this population.
Case report
A 50-year-old woman was transported to the emergency department with a sudden severe headache while at rest, nausea and vomiting. She had a longstanding diagnosis of homozygous sickle cell disease (HbSS) with known vaso-occlusive crises requiring hospital admissions in the past. On physical examination, she was agitated and photophobic with a Glasgow Coma Scale (GCS) score of 13 (E3 V4 M6) (World Federation of Neurosurgical Societies (WFNS) Grade 2) with no focal neurologic deficit. Her hemoglobin measured 61 g/l.
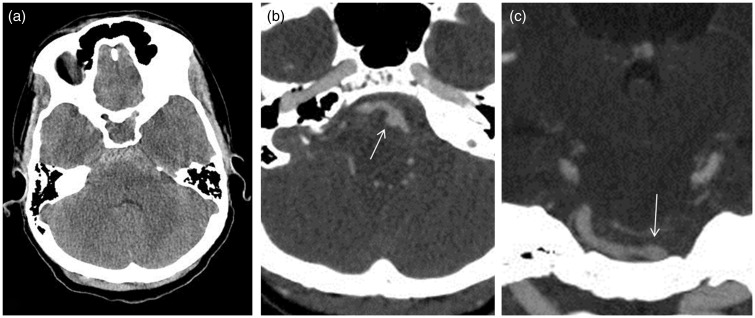
Non-contrast computed tomography (CT) of the head revealed subarachnoid hemorrhage (SAH) (Fisher Grade 3) in the basal cisterns (Figure 1(a)), with extension into the third and fourth ventricles. CT angiography (CTA) (Figure 1(b) and (c)) demonstrated a left-sided 3 × 5 mm fusiform bi-lobulated presumed dissecting vertebral artery aneurysm, immediately distal to the origin of the posterior inferior cerebellar artery (PICA). Codominant vertebral arteries were noted.
Figure 1.
(a) Noncontrast CT of the head showing SAH in basal cisterns. (b) Axial and (c) coronal CT angiogram images showing fusiform-bilobed wide-neck left V4 aneurysm. CT: computed tomography; SAH: subarachnoid hemorrhage.
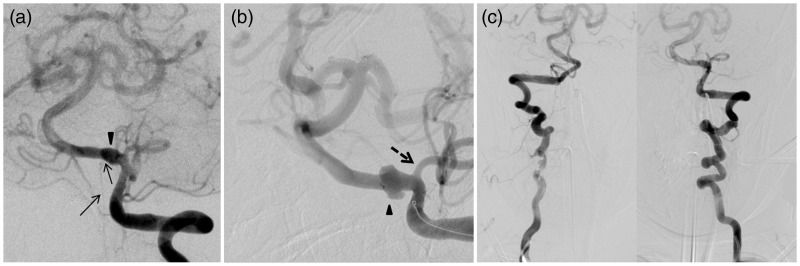
Prior to endovascular repair, the patient underwent external ventricular drain (EVD) placement that yielded cerebrospinal fluid under high pressure. The patient was brought to the angiography suite for planned parent vessel sacrifice, via coiling of the aneurysm together with parent artery. Cerebral catheter angiography confirmed the fusiform-bilobed aneurysm of the left V4 arising distal to the PICA (Figure 2(a) and (b)); however, there was also severe tortuosity of the vertebral access (Figure 2(c)) and the anterior spinal artery arose from the V4 segment of the vertebral artery bearing the aneurysm (Figure 2(a)). Hence, it was judged that parent artery sacrifice with occlusion of the anterior spinal artery may place the patient at risk of cord ischemia. Proximal (i.e. V3) sacrifice was also entertained but felt to be less favorable as there would be persistent albeit reversed flow through the aneurysm to keep the PICA open. Therefore, we selected a reconstructive approach with a flow-diverting technique.
Figure 2.
Images (a) and (b) of the AP oblique left vertebral artery injections show the relationship of the aneurysm (arrowhead) to the anterior spinal artery (arrows) and the PICA (dashed arrow). Note the ASA origin from the aneurysmal segment. (c) Shows the tortuosity of the vertebral artery access system. AP: anteroposterior; PICA: posterior inferior cerebellar artery; ASA: anterior spinal artery.
The patient was heparinized to an activated clotting time (ACT)>300. A nasogastric (NG) tube was placed and the patient was loaded with acetylsalicylic acid (ASA) 650 mg and Plavix 600 mg. A 6F Cook Shuttle 80 cm (Cook Medical, Bloomington, IN, USA) was placed in the left subclavian artery and a 105 cm 070 Neuron (Penumbra Inc, Alameda, CA, USA) was navigated into the cervical vertebral artery. Navigation was difficult because of tortuosity but ultimately a Marksman 150 cm (Covidien/Medtronic, Dublin, Ireland) was navigated over a microwire into the basilar artery past the aneurysm. A 4.75 × 18 mm PED (Covidien/Medtronic, Dublin, Ireland) was then deployed within the V4 segment across the aneurysm (Figure 3(a)). Post-treatment angiography showed patency of the parent artery and patency of the “jailed” anterior spinal artery and PICA (origin covered by PED) (Figure 3(b)) as well as mild stasis in the aneurysm sac (Figure 3(c)). Given the patient’s SCD and concern about thromboembolic complication with further stent coverage, we elected not to place additional stents telescopically.
Figure 3.
(a) Deployed PED prior to recapture of delivery wire; (b) post-deployment left vertebral arterial phase angiogram showing patency of V4 parent artery, anterior spinal artery and PICA; (c) delayed-phase image showing mild contrast stasis in the aneurysm. PED: pipeline embolization device; PICA: posterior inferior cerebellar artery.
The patient was managed in the Neurotrauma intensive care unit (ICU) according to standard post-SAH protocols. Prophylactic nimodipine was administered. The patient was kept hydrated with intravenous (IV) fluid management to mitigate risk of vasospasm as well as vaso-occlusive disease. Daily ASA and Plavix were maintained. On postoperative day 2, the patient was treated empirically for fever with intravenous vancomycin and ceftazidime as well as intravascular cooling; however, pancultures were negative.
Between postoperative day 1 and 5, the patient developed small asymptomatic hemorrhages surrounding the EVD tracts. The SAH and intraventricular hemorrhage (IVH) gradually resolved. The EVDs were removed by day 12 with further mild increase in asymptomatic hemorrhage along the tracks. By postoperative day 18, she was neurologically intact and close to her functional baseline with a Modified Rankin scale (mRS) score of 1 for mild headache. Hydroxyurea for SCD was started at 500 mg twice daily (bid) at the suggestion of the Hematology Department and the patient was discharged the following day.
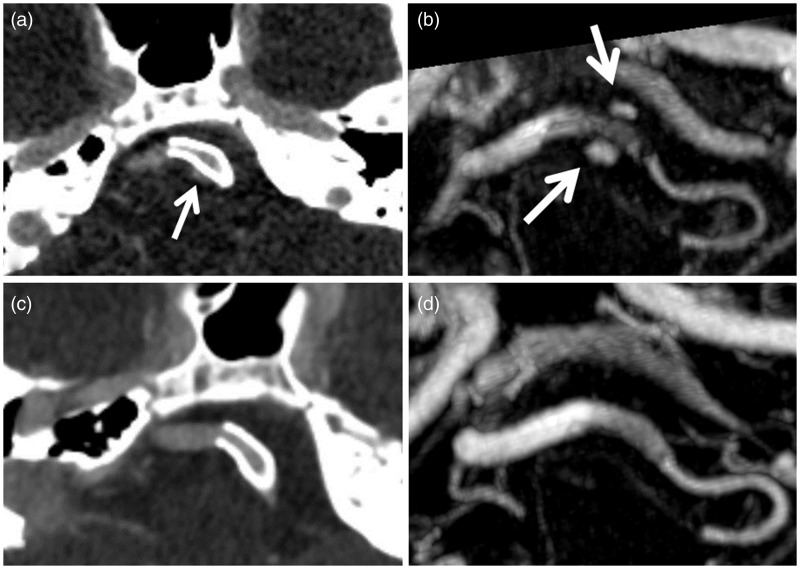
Initial post-treatment magnetic resonance angiography (MRA) and CTA performed in the first week postoperatively showed patency of the stented segment of the left vertebral artery with persistent filling of the aneurysm (Figure 4(a) and (b)). There were no post-procedural infarcts as detected by diffusion-weighted magnetic resonance imaging (MRI). Imaging follow-up (Figure 2(c) and (d)) at three months and subsequent follow-up imaging at one to two years’ postoperatively showed exclusion of the aneurysm and no evidence of in-stent stenosis or parent artery occlusion. She is clinically well at two years with no neurological deficits and her headaches have diminished in frequency and intensity. We have kept her on prolonged dual antiplatelet therapy with ASA and clopidogrel, in consultation with her hematologist.
Figure 4.
(a) CTA axial image and (b) Gd-enhanced MRA (axial MIP) immediately post-PED placement show persistent filling of the aneurysm (white arrows). Images (c) and (d) show interval thrombosis of the aneurysm at three-month follow-up with patency of the stented vertebral artery and PICA. CTA: computed tomography angiography; Gd: gadolinium; MRA: magnetic resonance angiography; MIP: maximum intensity projection; PICA: posterior inferior cerebellar artery; PED: pipeline embolization device.
Discussion
In SCD, stroke risk is directly related to anemia, elevated reticulocyte counts and lower hemoglobin F levels.4 This is felt to be due to microvascular dysfunction secondary to the binding of nitric oxide by released hemoglobin and also to hypoxemia, where ischemia results from the inability of intracranial vessels to dilate sufficiently in response to hypoxic stress.1 Additionally, released hemoglobin binds to nitric oxide resulting in microvascular dysfunction. The former mechanism is currently felt to be the primary player, in that released hemoglobin has been shown to also induce a prethrombotic state by altering phosphatidylserine on erythrocyte membranes.4 Moreover, the release becomes more prevalent with each subsequent sickling cycle. It has also been shown that platelets are chronically activated in individuals with SCD via elevated CD40L levels.5
Aneurysm formation via damage to the internal elastic lamina and smooth muscle layer of the arterial wall is caused by turbulent flow and is common in SCD, correlating with hemorrhage at an earlier age than the average individual with intracranial aneurysm.6 Moreover, these hemorrhages are not closely linked to conventional risk factors and indeed our patient had no hypertension, polycystic kidney disease or connective tissue disease.7 Though not extensively studied, it appears that individuals with SCD are more likely than the general population to have small aneurysms (3–7 mm), multiple aneurysms, and aneurysms of the posterior circulation.7,8 Lastly, individuals with SCD appear to be more likely to play host to complex aneurysm morphology including complex wall anatomy, giant aneurysms, and branches associated with the aneurysm.9 Moreover, when these aneurysms present with SAH, their outcomes are inferior.10 It is not clear whether dissection is more common in individuals with SCD.
Selecting a treatment method in SCD patients with a ruptured intracranial aneurysm is challenging and there are no clinical trials comparing treatment methods in this population. The treating physician must be wary of potential acidosis, dehydration, hypothermia and hypoxia, all of which can precipitate vaso-occlusion. With respect to endovascular repair, the contrast medium itself can precipitate vaso-occlusion.11 To this end, hydration is imperative and exchange transfusion to reduce HbS concentration can also be considered.6,12 Because of uncertainty about the thromboembolic risks of flow diversion in this patient population, we initially favored a deconstructive approach with endovascular sacrifice. However, given the anterior spinal artery supply from the aneurysmal segment, we elected to attempt flow diversion.
In addition to the usual systemic heparinization, we elected to load the patient with ASA and Plavix on the table. Another option would have been to use an IV glycoprotein (gp)IIb/IIIa inhibitor with bridging to ASA and Plavix.13 Plavix resistance has not been extensively studied in the SCD population. Currently, the gold standard is light transmission aggregometry, which assesses the effect of clopidogrel by quantifying response to adenosine diphosphate (ADP) stimulus at the P2Y12 platelet receptor. However, this is not currently available at our institution.
Our review of the literature did not reveal any other published cases of flow diversion in this population. In Ediriwickrema et al. a “blister”-like posterior cerebral artery (PCA) aneurysm was treated with a non-flow-diverting open cell stent (Neuroform 3) as monotherapy without coiling.14 In their case, it was felt that given the acute SAH, clopidogrel should be given only during the intervention and not subsequently; however, the stent used was not a flow diverter, which has a greater thromboembolic risk. In the setting of a ruptured aneurysm, the use of dual antiplatelet therapy is complicated given that the aneurysm may not have immediately occluded and thrombosis is usually gradual. This hemorrhagic risk must be weighed against the risk of thromboembolic complication. After consultation with our colleagues in Hematology, it was felt that in the setting of a PED our patient should be maintained on lifelong clopidogrel at the standard dose of 75 mg daily as well as ASA. While it is our practice usually to move to monotherapy at six to 12 months, so far this patient has been maintained on dual therapy.
The treatment of intracranial aneurysms in SCD patients presents a unique challenge, both because of the propensity toward complex morphology and risk of vaso-occlusion. We present the novel use of flow diversion with PED for a ruptured vertebral aneurysm in a patient with SCD with discussion of the related clinical issues. Our experience with this patient suggests that the use of flow-diverting stents is technically feasible and in this case provided a durable repair. Hematologic management in both the short and long term is paramount to minimize risk of vaso-occlusion.
Acknowledgment
Each author contributed equally and meaningfully to the preparation of this manuscript. This reflects both time and effort invested. Some examples are:
A.A. Dmytriw: Patient data acquisition, interpretation and analysis. Primary manuscript preparation and editing, critical review, and intellectual contribution. Approval of this version.
W. Montanera: Expert neurointerventional care and analysis. Expert radiological interpretation and analysis. Manuscript preparation and editing, critical review, and intellectual contribution. Approval of this version.
J. L. Martinez: Patient data acquisition, interpretation and analysis. Manuscript preparation and editing, critical review, and intellectual contribution. Approval of this version.
M. Cusimano: Expert clinical, neurosurgical and neurointerventional care and analysis. Manuscript preparation and editing, critical review, and intellectual contribution. Approval of this version.
T.R. Marotta: Expert neurointerventional care and analysis. Expert radiological interpretation and analysis. Manuscript preparation and editing, critical review, and intellectual contribution. Approval of this version.
A. Bharatha: Expert neurointerventional care and analysis. Expert radiological interpretation and analysis. Manuscript preparation and editing, critical review, and intellectual contribution. Approval of this version.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Switzer JA, Hess DC, Nichols FT, et al. Pathophysiology and treatment of stroke in sickle-cell disease: Present and future. Lancet Neurol 2006; 5: 501–512. [DOI] [PubMed] [Google Scholar]
- 2.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet 2004; 364: 1343–1360. [DOI] [PubMed] [Google Scholar]
- 3.Nabavizadeh SA, Vossough A, Ichord RN, et al. Intracranial aneurysms in sickle cell anemia: Clinical and imaging findings. J Neurointerv Surg. Epub ahead of print 19 March 2015. DOI: 10.1136/neurintsurg-2014-011572. [DOI] [PubMed]
- 4.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 2002; 8: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 5.Lee SP, Ataga KI, Orringer EP, et al. Biologically active CD40 ligand is elevated in sickle cell anemia: Potential role for platelet-mediated inflammation. Arterioscler Thromb Vasc Biol 2006; 26: 1626–1631. [DOI] [PubMed] [Google Scholar]
- 6.Firth PG, Peterfreund RA. Management of multiple intracranial aneurysms: Neuroanesthetic considerations of sickle cell disease. J Neurosurg Anesthesiol 2000; 12: 366–371. [DOI] [PubMed] [Google Scholar]
- 7.Liaquat I, Murphy M, Bassi S, et al. Paediatric and adult vascular intracranial complications of sickle-cell disease. Acta Neurochir (Wien) 2010; 152: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 8.Brandão RA, de Carvalho GT, Reis BL, et al. Intracranial aneurysms in sickle cell patients: Report of 2 cases and review of the literature. Surg Neurol 2009; 72: 296–299. discussion 299. [DOI] [PubMed] [Google Scholar]
- 9.Hanel RA, Spetzler RF. Surgical treatment of complex intracranial aneurysms. Neurosurgery 2008; 62(6 Suppl 3): 1289–1297. discussion 1297–1299. [DOI] [PubMed] [Google Scholar]
- 10.Andaluz N, Zuccarello M. Treatment strategies for complex intracranial aneurysms: Review of a 12-year experience at the University of Cincinnati. Skull Base 2011; 21: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zantomio D, Dowling RJ, Grigg A. Cerebral vaso-occlusive event with low-osmolar intravenous contrast in a patient with sickle cell disease. Am J Hematol 2006; 81: 383. [DOI] [PubMed] [Google Scholar]
- 12.Vicari P, Choairy AC, Siufi GC, et al. Embolization of intracranial aneurysms and sickle cell disease. Am J Hematol 2004; 76: 83–84. [DOI] [PubMed] [Google Scholar]
- 13.Lee SP, Ataga KI, Zayed M, et al. Phase I study of eptifibatide in patients with sickle cell anaemia. Br J Haematol 2007; 139: 612–620. [DOI] [PubMed] [Google Scholar]
- 14.Ediriwickrema A, Williamson T, Hebert R, et al. Intracranial stenting as monotherapy in subarachnoid hemorrhage and sickle cell disease. J Neurointerv Surg 2013; 5: e4. [DOI] [PubMed] [Google Scholar]