Abstract
Background and purpose
A patient with a previously Y-stented giant left middle cerebral artery (MCA) bifurcation aneurysm returned with a recurrence.
Materials and methods
A flow diverter (FD) was deployed through one limb of the high-porosity Y-stenting construction. The proximal FD failed to expand and an attempt at balloon angioplasty led to fatal rupture of the MCA.
Results
Autopsy demonstrated subarachnoid hemorrhage, vessel rupture and fracture of the proximal high-porosity stent. Microscopic photographs showed that the FD had failed to open because the guiding wire had inadvertently exited and re-entered the proximal segment of the high-porosity stent partially incorporated to the wall of the MCA. Balloon dilatation of the FD which had remained collapsed between the stent and the vessel wall caused fracture of the stent and rupture of the artery.
Conclusion
Angioplasty and flow-diversion of previously Y-stented aneurysms can lead to serious complications.
Keywords: Flow diverter, intracranial aneurysm, complication, stenting, coiling
Introduction
Stent-assisted coiling, including Y-stenting of bifurcation aneurysms, has been associated with a lesser risk of aneurysm recurrence at follow-up.1–4 Yet, little is known about the efficacy or safety of re-treating these aneurysms when they recur.
Flow diverters (FDs) have been approved for the treatment of large and giant proximal carotid aneurysms.5–10 Clinical applications have expanded to diverse clinical situations, including middle cerebral artery (MCA) bifurcation aneurysms and recurrences after coiling or stent-assisted coiling.11–21
We report a fatal complication related to angioplasty of an FD that failed to open properly during retreatment of a recurrent, previously coiled and Y-stented unruptured giant MCA bifurcation aneurysm.
Clinical case
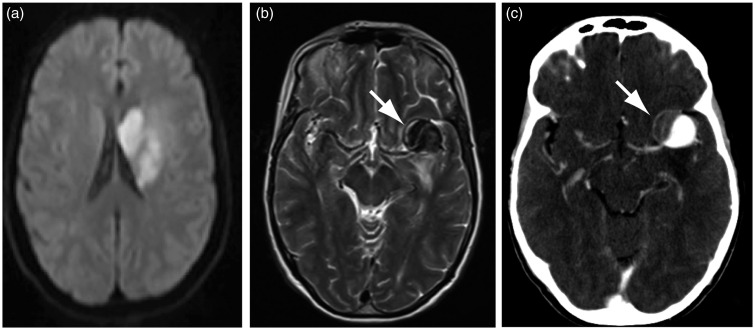
A 68-year-old woman was found in June 2012 to harbor a 25 mm left partially thrombosed MCA aneurysm at the time she presented with an ipsilateral subcortical ischemic stroke, which had led to a mild hemiparesis (Figure 1(a)–(c)). She made an excellent recovery. Endovascular treatment of the aneurysm was proposed within the Flow Diversion in Aneurysms trial (FIAT),22 and she was randomly allocated to treatment with flow diversion (with coiling).
Figure 1.
(a) Initial MRI (DWI) showing the ischemic lesion that revealed the aneurysm (white arrow in (b)). (c): CTA showing the partially thrombosed aneurysm of the left MCA bifurcation (white arrow). MRI: magnetic resonance imaging; DWI: diffusion weighted imaging; CTA: computed tomography angiography; MCA: middle cerebral artery.
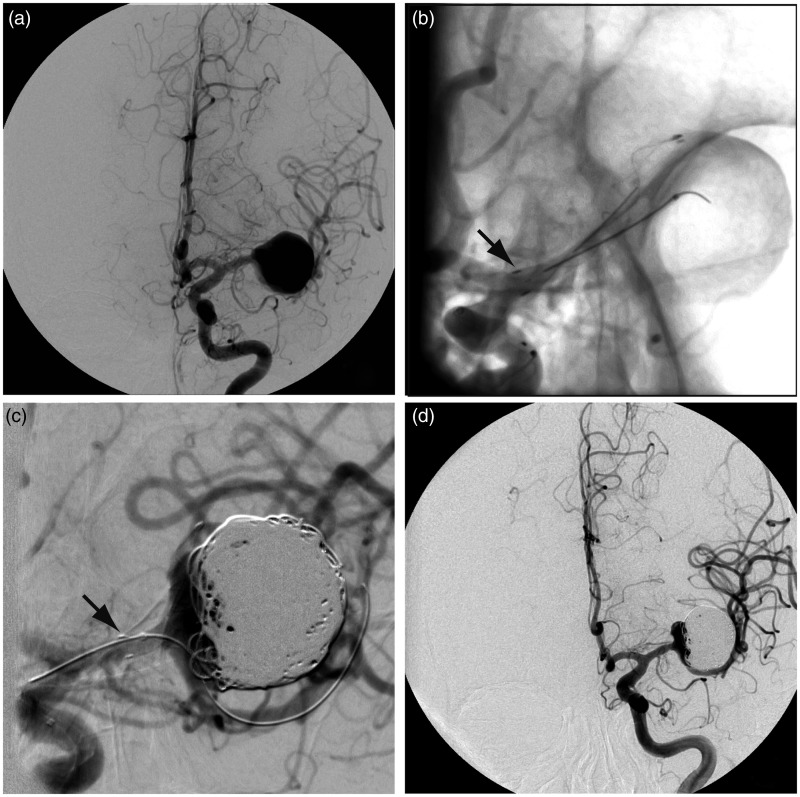
The initial treatment strategy, which eventually proved not possible, was to reconstruct the bifurcation using a combination of low-porosity stenting (LVIS® stent (MicroVention, Aliso Viejo, CA, USA)) in the superior, anterior M2 branch and an FD in the inferior, posterior and larger M2 branch, trapping a second microcatheter inside the aneurysm sac for coiling. Stenting of the upper M2 (LVIS 2.5 × 25 mm) proceeded without difficulty (Figure 2(b)), but tracking of the delivery catheter (Vasco® (Balt Extrusion, Montmorency, France)) over the exchange wire positioned in the lower M2 branch proved impossible (Figure 2(c)). Instead, the patient was eventually treated with a single low-porosity stent in the upper M2 and 616 cm of platinum coils inside the aneurysm sac (mostly helical caliber 18 coils (MicroVention, Aliso Viejo, CA, USA)). She tolerated this procedure without complication, although a residual aneurysm neck remained (Figure 2(d)).
Figure 2.
First treatment. (a) Angiography before and after (b) deployment of a 2.5 × 25 mm LVIS JR stent in the upper branch of the MCA bifurcation, with its proximal end located in the proximal M1 segment (black arrow in (b)). (c) The attempt at catheterization of the lower branch in order to deploy a flow diverter resulted in shortening and compaction of the stent (black arrow in (c)). The catheter could not be advanced in the lower branch. The aneurysm was filled with 616 cm of platinum coils (d). MCA: middle cerebral artery.
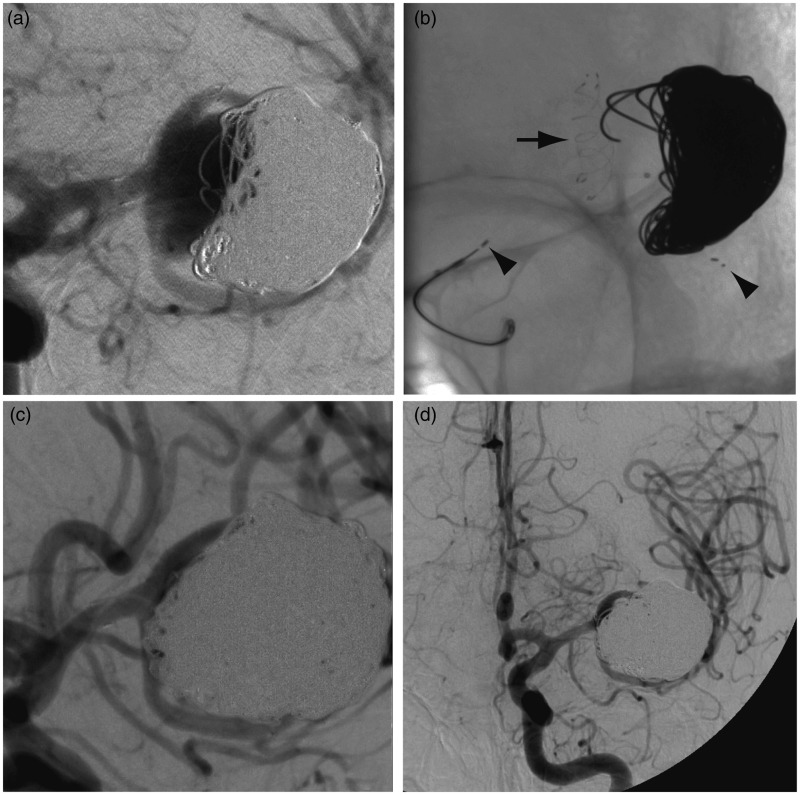
A follow-up angiography was performed five months later: the aneurysm was found to be enlarging in spite of stent-assisted coiling (Figure 3(a)), and a second endovascular treatment attempt was made in March 2013. This time, the inferior M2 branch was successfully catheterized with a Prowler Select Plus microcatheter (Codman Neurovascular, Raynham, MA, USA); a 3 × 20 mm Solitaire® AB stent (Covidien, Irvine, CA, USA) was deployed in the inferior M2 trunk (Figure 3(b)) and the recurrent aneurysm coiled with 365 additional cm of coils. Near-complete angiographic occlusion of the aneurysm was achieved, without complication (Figure 3(c), (d)).
Figure 3.
Second treatment. (a) Follow-up angiography revealed a recurrence. (b) A 3 × 20 mm Solitaire AB stent was deployed in the lower branch of the MCA bifurcation (proximal and distal end of the stent shown with black arrowheads) and the aneurysm was packed with 365 cm of platinum coils ((c), (d)). MCA: middle cerebral artery.
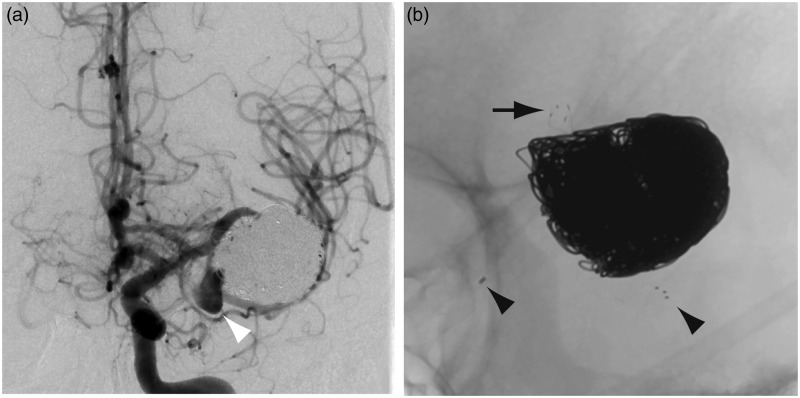
In October 2013 (seven months later) the aneurysm was found to have again recurred on follow-up magnetic resonance (MR) imaging, with the development of a daughter sac in the vicinity of the inferior branch. This finding was confirmed on a follow-up digital subtraction angiography (DSA) (Figure 4). Recoiling and flow diversion of the inferior M2 trunk was considered as a treatment option.
Figure 4.
(a) Follow-up angiography seven months later showed the development of a daughter sac in the vicinity of the inferior branch (white arrowhead). (b) A close-up view shows the distal end of the LVIS Junior stent (black arrow in (b)) and the proximal and distal end of the Solitaire AB stent (black arrowheads in (b)).
In November 2013, the recurrent portion of the aneurysm sac could not be catheterized for additional coiling. Catheterization of the inferior M2 branch proved impossible, and the decision was made to instead deploy the FD (SILK® 3.5 × 25 mm (Balt Extrusion, Montmorency, France)) in the superior M2 trunk. This would entail starting the deployment of the FD in and beyond the LVIS stent positioned in the upper branch, passing through one cell of the Solitaire stent, with potential stenosis of the FD within that cell, landing the proximal FD within the Solitaire inside the M1 segment of the MCA. The deployment of the FD went well in the upper M2 trunk, across the expected mild stenosis upon entering the Solitaire, and in the distal M1 segment, but the FD did not open into the proximal M1 segment (Figure 5(a)).
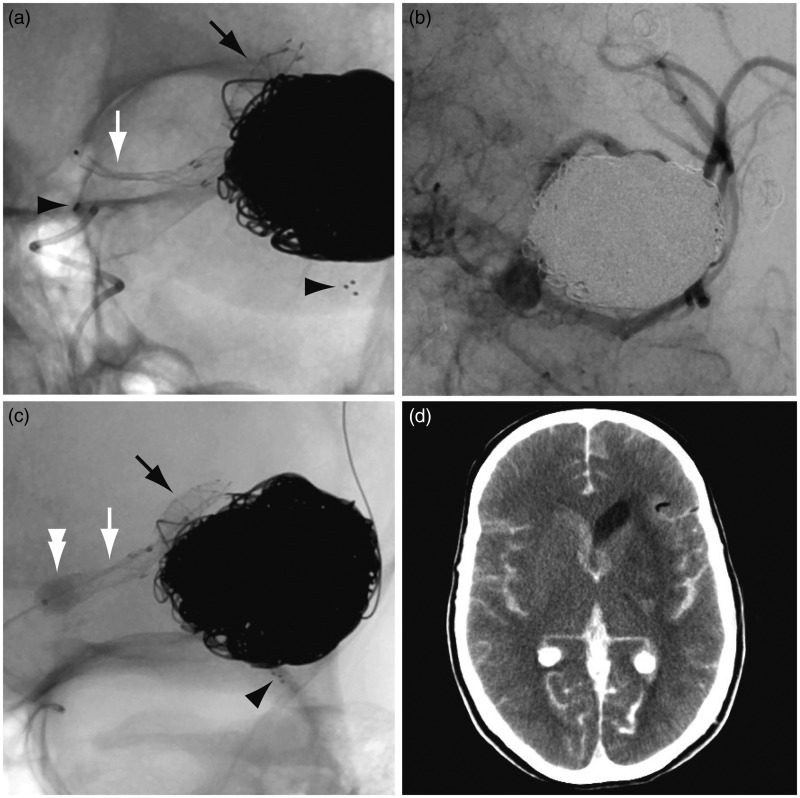
Figure 5.
Third treatment. (a) The FD opened distally (black arrow), across the expected narrowing upon entering the Solitaire, and in the distal M1 segment, but remained collapsed in the proximal M1 segment (white arrow). (b) Angiography showed slow blood flow in the MCA branches. (c) The Scepter balloon catheter is inflated (white double arrowhead). (d) Postoperative CT shows subarachnoid hemorrhage. FD: flow diverter; MCA: middle cerebral artery; CT: computed tomography.
Angiographic series at this point showed slow blood flow in all the MCA branches (Figure 5(b)). The FD could not be re-sheathed. It was finally decided to deliver the collapsed SILK. The delivery catheter was repositioned inside the distal FD over the pusher wire, the pusher wire replaced with an exchange wire, the delivery catheter retrieved and a Scepter® 4 × 10 mm balloon catheter (MicroVention, Aliso Viejo, CA, USA) was inflated inside the FD in an attempt to open the SILK, first within the distal and then the proximal M1 segments of the FD (Figure 5(c)). When the proximal FD was opened by balloon inflation, sudden hemodynamic changes occurred and immediate angiography demonstrated intracranial hypertension with flow arrest. The Scepter balloon was inflated, protamine administered and an urgent external ventricular drain placed, but it did not suffice to re-establish normal intracranial circulation. Immediate computed tomography (CT) scanning confirmed massive subarachnoid hemorrhage (Figure 5(d)). In retrospective, examination of the last angiographic series demonstrated that the proximal marker of the Solitaire stent had migrated proximally in the petrous carotid region, suggesting rupture of the stent during balloon inflation. The patient died the following day.
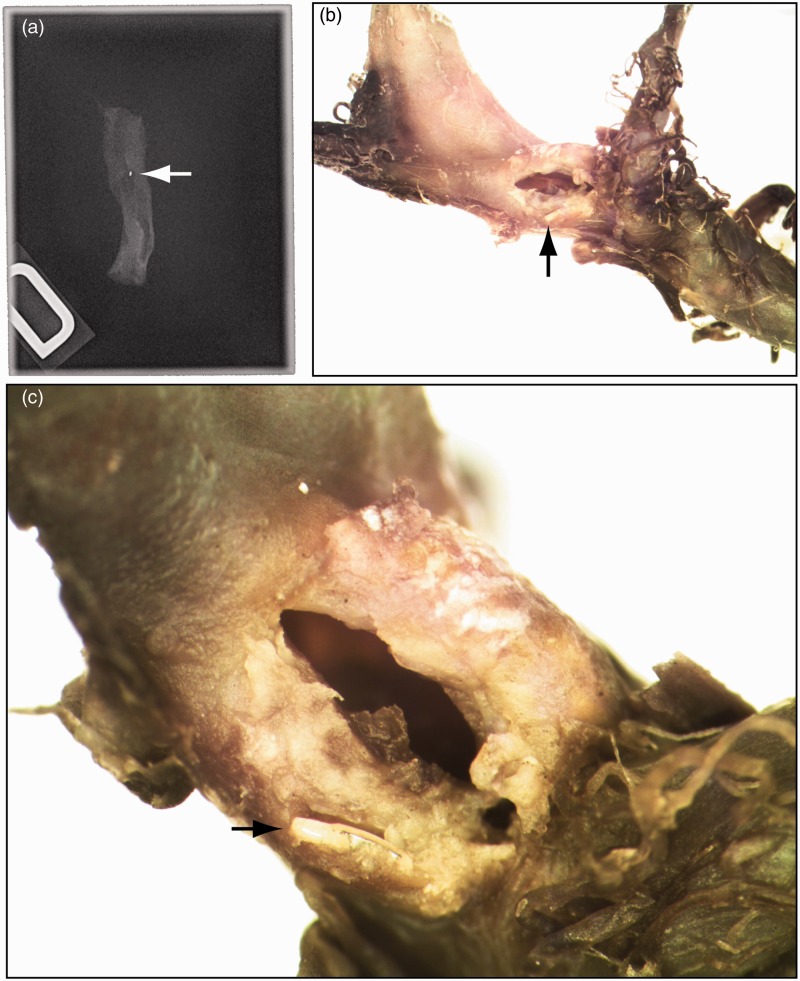
Autopsy showed massive subarachnoid hemorrhage. Radiographs of the resected brain and skull base confirmed the fracture of the Solitaire stent, with separation of the proximal marker recovered at the level of the petrous carotid artery (Figure 6(a)). After four months of fixation in formalin, microscopic dissection of the MCA aneurysm complex was performed under a stereomicroscope. A 2 mm point of rupture was found in the proximal M1 (Figure 6(b), (c)). The FD was found to have passed through a proximal cell of the Solitaire, resulting in the failure of the FD to open between the Solitaire and the vessel wall, within the proximal M1. When the balloon was inflated, the Solitaire stent, which had become partially integrated into the wall of the parent vessel, was fractured, with avulsion and tearing of the wall of the MCA. Figures 7 and 8 show an in vitro reconstruction of the stent set-up.
Figure 6.
Pathology: (a) The proximal marker of the Solitaire stent was found at the level of the petrous carotid artery (white arrow). The rupture was found in the proximal M1 artery (black arrow in (b)). Note the metallic fragment embedded in the vessel wall (black arrow in (c)).
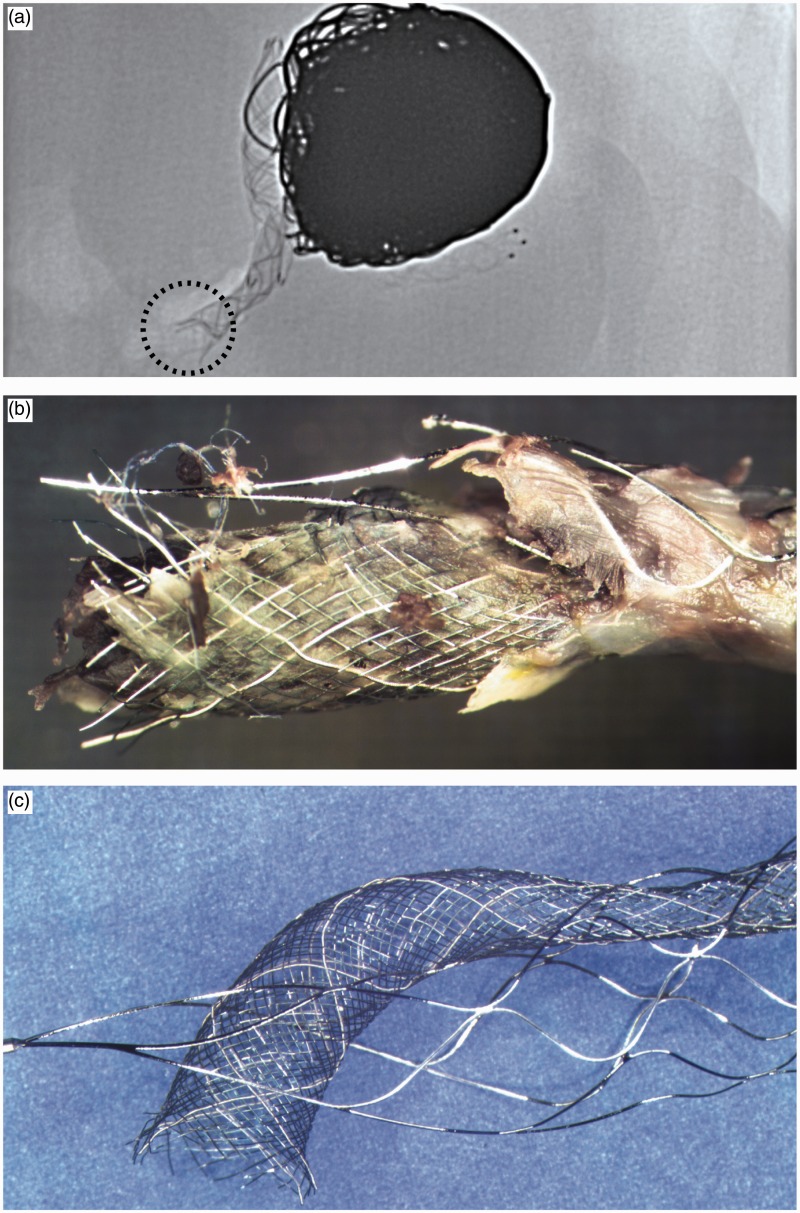
Figure 7.
Proximal stent and flow diverter: Radiography (a) and photography (b) of the specimen reveal a fracture of the proximal Solitaire stent and the proximal end of the flow diverter. The bench reconstruction shows how the flow diverter entered the Solitaire stent and exited through one of its proximal cells (c).
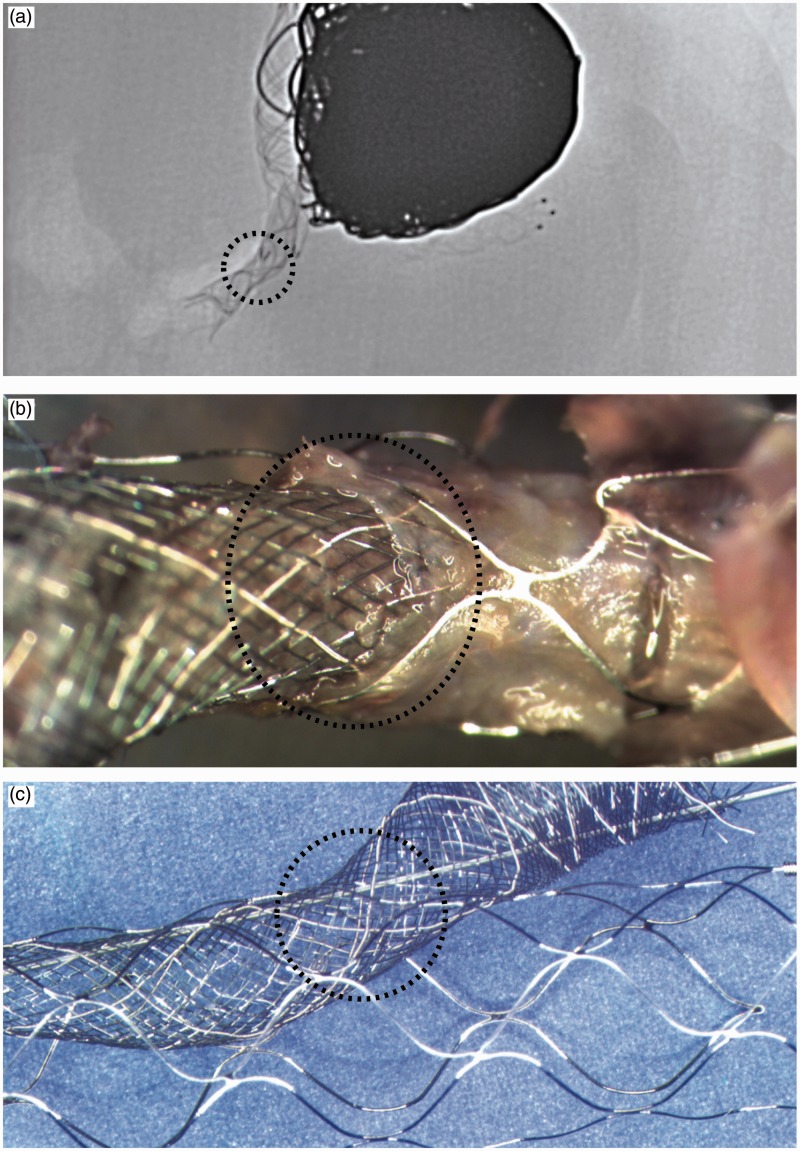
Figure 8.
Reconstruction of the stent and flow diverter: Radiography (a) and photography (b) of the specimen reveal how the flow diverter exited the Solitaire through a more distal cell, resulting in a mild stenosis. A bench reconstruction is shown in (c).
Discussion
Peri-procedural complications have been reported in 5%–35% of flow diversion procedures.23 Complex constructions, including flow diversion after Y-stenting, may be associated with unique complications.
Braided self-expanding stents such as the first stent (LVIS) used in this particular case are able, in bench-top studies, to accommodate other stents and FDs passing through to construct Y-stented bifurcations in situ, without resulting in significant device stenosis.24 However, some laser-cut stents, with closed cells, including the Solitaire (the second stent used in this case), can partially restrict expansion of FDs inserted through them.25 The resulting mild stenosis is not necessarily cause for concern; in this case, the SILK crossed through the cells of the distal Solitaire stent without significant stenosis (Figure 8), and may well have led to an acceptable result. However, when stents have been deployed for weeks or months in vivo, some stent segments may have become embedded into the neointima attempting to incorporate the foreign body to the vessel wall, while other wires (and cells) may still be suspended within the vessel lumen (Figure 7). In this situation, it is nearly impossible to ensure the guidewire used to re-catheterize the stented vessel has not been threaded through a suspended cell composed of wires that have been partially embedded in neointima. The winding path of the guidewire or the delivery catheter navigating through the poorly visible stent wires could perhaps have been better understood had advanced imaging methods been used.26 The deployment of intracranial stents can be examined with flat-panel detector CT.27 We speculate that the large coil mass would have produced many artifacts, but metal artifact reduction algorithms have been proposed.28,29
While the delivery catheter may follow the wire, the FD will fail to open when deployed. Complete or near-complete re-sheathability of FDs is important, but stent wires may obstruct the re-sheathing of a misplaced FD that fails to deploy properly, as in this case. Rescue strategies and removal of the device30,31 may become impossible once the distal FD has expanded beyond the stent.
Balloon dilatation of the collapsed FD, trapped between the embedded stent and the vessel wall, should not be attempted as it can cause vessel rupture. It is probably better to leave the collapsed FD in place when it cannot be re-sheathed. Slow blood flow (which now has to cross the FD twice to feed MCA branches) may cause ischemic complications. Perhaps a surgical bypass procedure should have been considered.
Conclusion
Physicians should be warned that catheterization of a previously deployed stent may be dangerous, because the micro-guidewire may exit and re-enter the stent. Angioplasty of a stent or an FD that would subsequently fail to open may lead to a fatal arterial rupture.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Hong Y, Wang YJ, Deng Z, et al. Stent-assisted coiling versus coiling in treatment of intracranial aneurysm: A systematic review and meta-analysis. PLoS One 2014; 9: e82311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piotin M, Blanc R, Spelle L, et al. Stent-assisted coiling of intracranial aneurysms: Clinical and angiographic results in 216 consecutive aneurysms. Stroke 2010; 41: 110–115. [DOI] [PubMed] [Google Scholar]
- 3.Piotin M, Blanc R. Balloons and stents in the endovascular treatment of cerebral aneurysms: Vascular anatomy remodeled. Front Neurol 2014; 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalouhi N, Jabbour P, Singhal S, et al. Stent-assisted coiling of intracranial aneurysms: Predictors of complications, recanalization, and outcome in 508 cases. Stroke 2013; 44: 1348–1353. [DOI] [PubMed] [Google Scholar]
- 5.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 6.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 7.Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: The Budapest experience with the Pipeline embolization device. AJNR Am J Neuroradiol 2010; 31: 1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the Pipeline embolization device: The Buenos Aires experience. Neurosurgery 2009; 64: 632–642. discussion 642–643; quiz N6. [DOI] [PubMed] [Google Scholar]
- 9.D’Urso PI, Lanzino G, Cloft HJ, et al. Flow diversion for intracranial aneurysms: A review. Stroke 2011; 42: 2363–2368. [DOI] [PubMed] [Google Scholar]
- 10.Arrese I, Sarabia R, Pintado R, et al. Flow-diverter devices for intracranial aneurysms: Systematic review and meta-analysis. Neurosurgery 2013; 73: 193–199. discussion 199–200. [DOI] [PubMed] [Google Scholar]
- 11.Pistocchi S, Blanc R, Bartolini B, et al. Flow diverters at and beyond the level of the circle of Willis for the treatment of intracranial aneurysms. Stroke 2012; 43: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 12.Saleme S, Iosif C, Ponomarjova S, et al. Flow-diverting stents for intracranial bifurcation aneurysm treatment. Neurosurgery 2014; 75: 623–631. quiz 631. [DOI] [PubMed] [Google Scholar]
- 13.Benaissa A, Januel AC, Herbreteau D, et al. Endovascular treatment with flow diverters of recanalized and multitreated aneurysms initially treated by endovascular approach. J Neurointerv Surg 2015; 7: 44–49. [DOI] [PubMed] [Google Scholar]
- 14.Daou B, Starke RM, Chalouhi N, et al. The use of the Pipeline embolization device in the management of recurrent previously coiled cerebral aneurysms. Neurosurgery 2015; 77: 692–697. [DOI] [PubMed] [Google Scholar]
- 15.Gawlitza M, Januel AC, Tall P, et al. Flow diversion treatment of complex bifurcation aneurysms beyond the circle of Willis: A single-center series with special emphasis on covered cortical branches and perforating arteries. J Neurointerv Surg. Epub ahead of print 15 April 2015. DOI: 10.1136/neurintsurg-2015-011682. [DOI] [PubMed] [Google Scholar]
- 16.Zanaty M, Chalouhi N, Tjoumakaris SI, et al. Flow-diversion panacea or poison? Front Neurol 2014; 5: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalouhi N, Chitale R, Starke RM, et al. Treatment of recurrent intracranial aneurysms with the Pipeline embolization device. J Neurointerv Surg 2014; 6: 19–23. [DOI] [PubMed] [Google Scholar]
- 18.Leung GK, Tsang AC, Lui WM. Pipeline embolization device for intracranial aneurysm: A systematic review. Clin Neuroradiol 2012; 22: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caroff J, Neki H, Mihalea C, et al. Flow-Diverter stents for the treatment of saccular middle cerebral artery bifurcation aneurysms. AJNR Am J Neuroradiol. Epub ahead of print 24 September 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briganti F, Delehaye L, Leone G, et al. Flow diverter device for the treatment of small middle cerebral artery aneurysms. J Neurointerv Surg. Epub ahead of print 20 January 2015. DOI: 10.1136/neurintsurg-2014-011460. [DOI] [PubMed] [Google Scholar]
- 21.Yavuz K, Geyik S, Saatci I, et al. Endovascular treatment of middle cerebral artery aneurysms with flow modification with the use of the Pipeline embolization device. AJNR Am J Neuroradiol 2014; 35: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raymond J, Darsaut TE, Guilbert F, et al. Flow diversion in aneurysms trial: The design of the FIAT study. Interv Neuroradiol 2011; 17: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burrows AM, Cloft H, Kallmes DF, et al. Periprocedural and mid-term technical and clinical events after flow diversion for intracranial aneurysms. J Neurointerv Surg 2015; 7: 646–651. [DOI] [PubMed] [Google Scholar]
- 24.Makoyeva A, Darsaut TE, Salazkin I, et al. Y-crossing of braided stents with stents and flow diverters does not cause significant stenosis in bench-top studies. Interv Neuroradiol 2013; 19: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazzaro MA, Zaidat OO. X-configuration intersecting Enterprise stents for vascular remodeling and assisted coil embolization of a wide neck anterior communicating artery aneurysm. J Neurointerv Surg 2011; 3: 348–351. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell BD, Chinnadurai P, Chintalapani G, et al. Endovascular recanalization in acute ischemic stroke using the Solitaire FR revascularization device with adjunctive C-arm CT imaging. AJNR Am J Neuroradiol 2015; 36: 1317–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarençon F, Piotin M, Pistocchi S, et al. Evaluation of stent visibility by flat panel detector CT in patients treated for intracranial aneurysms. Neuroradiology 2012; 54: 1121–1125. [DOI] [PubMed] [Google Scholar]
- 28.van der Bom IM, Hou SY, Puri AS, et al. Reduction of coil mass artifacts in high-resolution flat detector conebeam CT of cerebral stent-assisted coiling. AJNR Am J Neuroradiol 2013; 34: 2163–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Psychogios MN, Scholz B, Rohkohl C, et al. Impact of a new metal artefact reduction algorithm in the noninvasive follow-up of intracranial clips, coils, and stents with flat-panel angiographic CTA: Initial results. Neuroradiology 2013; 55: 813–818. [DOI] [PubMed] [Google Scholar]
- 30.Colby GP, Gomez JF, Lin LM, et al. In situ removal of the Pipeline embolization device: The ‘corking’ and ‘pseudo-corking’ techniques. J Neurointerv Surg 2013; 5: e6. [DOI] [PubMed] [Google Scholar]
- 31.Lin LM, Colby GP, Jiang B, et al. Intra-DIC (distal intracranial catheter) deployment of the Pipeline embolization device: A novel rescue strategy for failed device expansion. J Neurointerv Surg. Epub ahead of print 22 June 2015. DOI: 10.1136/neurintsurg-2015-011771. [DOI] [PubMed] [Google Scholar]