Abstract
Introduction
Five randomized controlled trials (RCTs) on endovascular therapy (EVT) of stroke have proven a clinical benefit over conservative treatment or IV-thrombolysis alone. Lesional clot aspiration with a dedicated system can achieve revascularization without an additional retriever (a direct-aspiration first-pass technique, ADAPT), and the SOFIA has been shown to be both safe and efficacious in a multicentric retrospective study. We have evaluated a subset of these data acquired in two major stroke centers with regard to using the SOFIA for first-line lesional aspiration.
Methods
Thirty patients with large-vessel occlusions treated with first-line lesional aspiration were identified. Procedural data, clot length, reperfusion success (mTICI), procedural timings, complications, and clinical status at admission, discharge and at 90 days were analyzed.
Results
The median baseline NIHSS was 16. IV thrombolysis was administered in 15/30 patients. Ninety-three percent of occlusions were in the anterior circulation. TICI ≥ 2b was achieved in 90% of multimodality treatments; lesional aspiration was successful in 67% within a median time of 20 minutes. The highest first-attempt success rate was in MCA occlusions (median time to recanalization 10 minutes). There were no device-related events. Symptomatic intracerebral hemorrhage (sICH) occurred in 10%, but never with sole lesional aspiration. Embolization to new territories was recorded in 1/30 (3%). Median discharge NIHSS was 7; 30% were mRS ≤ 2 at discharge and 43% at 90-day follow-up.
Conclusions
Lesional aspiration with SOFIA is in line with published data. The SOFIA may be used as a first-line device, aiming at fast recanalization by sole aspiration with good safety and efficacy. If unsuccessful, it converts into part of a stent retriever-based multimodality treatment.
Keywords: Angiography, catheter, stroke, thrombectomy, lesional aspiration
Introduction
Intravenous thrombolysis (IVT) has been the standard of care for acute ischemic stroke within 4.5 hours after symptom onset for many years.1 Low effectiveness in large-artery occlusions is a major drawback of IVT since revascularization is paramount for a favorable clinical outcome.2–4 Stent retrievers have been shown to be both safe and effective for acute stroke treatment.3,4 Five randomized trials of thrombectomy vs. IVT or conservative treatment have proven the clinical benefit of mechanical thrombectomy. In these trials, all patients or at least the vast majority were treated with a stent retriever.5–9 As an addition to mechanical thrombectomy (MTE), first-line, direct-aspiration first-pass technique (ADAPT) performed with a large-bore aspiration catheter generated promising results.10–12 Recently, safety and efficacy data for a novel intermediate catheter (SOFIA 5F, Soft torqueable catheter Optimized for Intracranial Access, MicroVention Inc, Tustin, CA, USA) acquired at three large-volume stroke centers have been reported.13 Only two of these centers have applied the SOFIA with intentional first-line lesional aspiration. We report our analysis of this specific subset of data with regard to revascularization efficacy, duration of thrombectomy, procedural safety, and outcome at discharge.
Methods
At the university hospitals of Cologne and Heidelberg, 30 non-consecutive patients who were treated for acute stroke by means of aspiration thrombectomy with the SOFIA catheter were identified and analyzed from a database composed of 115 patients total. Fifty-two patients from Heidelberg included 10 aspiration-only cases. Thirty-eight cases from Cologne included 12 first-line aspiration cases. Eight additional treatments were performed in Cologne that did not enter the mutual database. In both centers, the use of the SOFIA was at the operator’s discretion; the total number of endovascular stroke treatments within the time span (January to December 2014) was 175 (92 and 83 per center). According to the guidelines of the local ethics committee, no approval was necessary.
Patient selection
Inclusion criteria were as follows: large cerebral artery occlusion; no age limit, and baseline National Institutes of Health Stroke Scale (NIHSS) score of ≥5 or aphasia if NIHSS was <5 at admission. Patients were eligible for endovascular therapy (EVT) within a time frame from symptom onset to treatment of ≤8 hours if salvageable brain parenchyma was identified by perfusion computed tomography (CT) mismatch.14,15 If time from symptom onset to admission was uncertain, patients were scanned with magnetic resonance imaging (MRI), applying diffusion-weighted imaging (DWI), perfusion scanning and fluid-attenuated inversion recovery (FLAIR) image to discern between salvageable and terminally infarcted tissue. All patients received IVT if eligible, according to the guidelines of the German Society of Neurology. Exclusion criteria were intracranial hemorrhage and cerebral infarction of a third or more of the middle cerebral artery (MCA) territory.
Imaging and clinical assessment
All angiographic images were re-evaluated according to the recommendations of the cerebral Angiographic Grading collaborators, including the target artery lesion, target downstream territory, and modified Thrombolysis in Cerebral Infarction (mTICI) scale, before and after the procedure, as described elsewhere.16 A control CT scan was performed 24 hours after intervention or after clinical deterioration to rule out any intracranial hemorrhage. At each hospital, two experienced interventional neuroradiologists who were blinded to all of the clinical data performed image evaluation of the patients treated at their respective site. A favorable revascularization result was defined as mTICI ≥2b. Procedural timings were taken from the angiographic records. NIHSS and modified Rankin scale (mRS) at admission and discharge were assessed by a consultant neurologist.
Endovascular procedure
EVT was performed as described previously.10,11 Briefly, in anterior circulation stroke a short 8F sheath was used to navigate an 8F guide catheter into the internal carotid artery (ICA). In vertebrobasilar occlusions the guide catheter was 6F. In all cases the SOFIA was advanced to the level of the occlusion. Accepting only the slightest resistance, the SOFIA was advanced over either a standard 0.021-inch microcatheter, a standard 0.014-inch microguidewire (Synchro14, Stryker, Fremont, CA, USA) or in select cases just by itself. After any coaxial guiding device had been removed, the aspiration was carried out using a 20-cc syringe locked to the SOFIA. The resulting vacuum was manually maintained for at least one minute, before slow retraction of the SOFIA was begun. If this resulted in backward flow inside the SOFIA, the catheter was left in place and checked for free aspiration to rule out remaining clot before injecting contrast to control for revascularization success. If the vacuum remained during catheter withdrawal, the SOFIA was completely removed from the patient—usually to find the clot nestled in its tip (Figure 2). If unsuccessful, this maneuver was repeated up to three times, before a stent retriever (Solitaire2, ev3, Irvine, CA, USA, or Trevo ProVue, Stryker, Fremont, CA, USA) was used together with lesional aspiration through the SOFIA intermediate catheter while retrieving the Solitaire or Trevo.
We defined the term “rescue treatment” as a switch of endovascular methods by adding a stent retriever after failure of the aspiration-only approach.
Statistical analysis
Continuous study parameters were compared between patients by either Welch’s t-test in case of normal distribution, or by the Mann–Whitney U test in case of non-normal or ordinal distribution. Categorical variables were compared using Fisher’s exact test. All statistical analyses were performed using GraphPad Prism software version 6.1 (GraphPad Software, La Jolla, CA, USA). The significance level for all tests was set to α = 0.05.
Results
We identified 30 patients with intracranial large-vessel occlusions who underwent EVT with the SOFIA aspiration catheter as a first-line approach. The median age of the patients was 75 (29–86) years; 50% were male. In 15 of 30 procedures (50%) IVT was administered in a bridging approach. The median baseline NIHSS score was 16 (range 13–23); the mean time from symptom onset to the beginning of the EVT was 189 minutes ± 70.
Sites of occlusion were: anterior circulation in 93% (28/30) of cases with MCA-M1 in 14/30 (47%), MCA M2 in 3/30 (10%), carotid T and carotid L in 11/30 (37%). Two of 30 lesions (7%) were located in the posterior circulation, both affecting the basilar artery. All lesions could be reached without effort using the 0.014-inch microguidewires or 0.021-inch microcatheters as guiding tools.
There was no significant difference regarding baseline characteristics between patients treated by direct aspiration only and those treated with an additional stent retriever (Table 1).
Table 1.
Patient baseline characteristics.
| Characteristic | All (n = 30) | Aspiration only (n = 21) | Aspiration + stent (n = 9) | p value | Test |
|---|---|---|---|---|---|
| Age years, median (range) | 75 (29–86) | 76 (39–86) | 71 (29–82) | 0.4 | Mann–Whitney |
| Male sex, n/N (%) | 15/30 (50%) | 10/21 (48%) | 5/9 (56%) | 1 | Fisher’s exact |
| Baseline NIHSS median, (range) | 16 (13–23) | 17 (14–23) | 16 (10–22) | 0.5 | Mann–Whitney |
| IVT, n/N (%) | 15/30 (50%) | 11/21 (52%) | 4/9 (44%) | 1 | Fisher’s exact |
| Atrial fibrillation, n/N (%) | 17/30 (57%) | 14/21 (67%) | 3/9 (33%) | 0.1 | Fisher’s exact |
| Thrombus length (mm), median (range) | 17 (5–144) | 17 (5–34) | 26 (8–144) | 0.3 | Mann–Whitney |
| Symptom onset to groin puncture, mean (±SD) | 189 (±70) | 194 (±72) | 176 (±72) | 0.6 | Unpaired t-test |
NIHSS: National Institutes of Health Stroke Scale; IVT: intravenous thrombolysis.
The pretreatment score was mTICI 0 in 28/30 (93%) and mTICI 1 in 2/30 (7%) cases. A final revascularization result of mTICI ≥ 2b was achieved in 90% of the cases (Table 2). SOFIA-aspiration alone was successful (mTICI ≥ 2b) in 20/30 cases (67%) with an average of 1.4 ± 0.7 attempts (Figures 1 and 2), resulting in 20/21 mTICI ≥ 2b revascularizations (95%). In 9 of all 30 cases (30%), a stent retriever was needed to achieve the final revascularization status. Here, the average number of aspiration attempts was 2.2 ± 1.3, finally resulting in a rate of 78% mTICI ≥ 2b revascularizations in SOFIA and stent retriever cases (Table 2).
Table 2.
Revascularization results, procedural timings, complications, and clinical results.
| Characteristic | All (n = 30) | Aspiration only (n = 21) | Aspiration + stent (n = 9) | p value | Test |
|---|---|---|---|---|---|
| mTICI ≥ 2b, n/N (%) | 27/30 (90%) | 20/21 (95%) | 7/9 (78%) | 0.2 | Fisher’s exact |
| mTICI 3, n/N (%) | 17/30 (57%) | 13/21 (62%) | 4/9 (44%) | 0.4 | Fisher’s exact |
| Time from groin puncture to recanalization, median (range) | 26 (6–240) | 20 (6–84) | 60 (35–240) (cave: rescue therapy after failed first-line aspiration) | <0.0001 | Mann–Whitney |
| sICH, n/N (%) | 3/30 (10%) | 0/21 (0%) | 3/9 (33%) | 0.02 | Fisher’s exact |
| ENT, n/N (%) | 1/30 (3%) | 1/21 (5%) | 0/9 (0%) | 1 | Fisher’s exact |
| NIHSS at discharge, median (range) | 7 (2–15) | 8 (2–15) | 5 (1–17) | 0.7 | Mann–Whitney |
| mRS ≤ 2 at discharge, n/N (%) | 9/30 (30%) | 6/21 (28.6%) | 3/9 (33%) | 1 | Fisher’s exact |
| mRS ≤ 2 90 days, n/N (%) | 13/30 (43.3%) | 8/21 (38.1%) | 5/9 (56%) | 0.7 | Fisher’s exact |
| Mortality, n/N (%) | 6/30 (20%) | 4/21 (19%) | 2/9 (22%) | 1 | Fisher’s exact |
mTICI: modified Thrombolysis in Cerebral Infarction; sICH: symptomatic intracerebral hemorrhage; ENT: ENT: embolization to new territory; NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin scale.
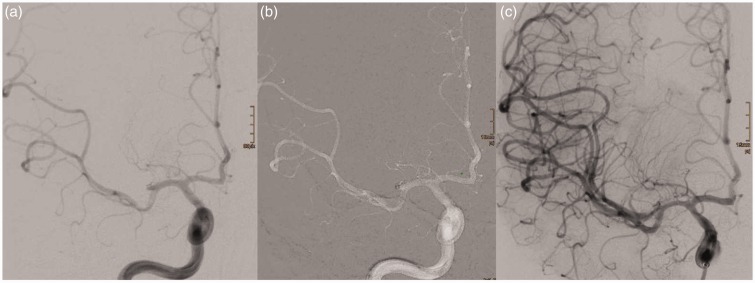
Figure 1.
Successful aspiration thrombectomy with a SOFIA: (a) post-bifurcation MCA occlusion (13:09 hours); (b) SOFIA located just proximal to the clot, no wire or microcatheter needed to advance the SOFIA (13:11 hours); (c) TICI3 reperfusion (13:14 hours). SOFIA: Soft torqueable catheter Optimized for Intracranial Access; MCA: middle cerebral artery; TICI: Thrombolysis in Cerebral Infarction.
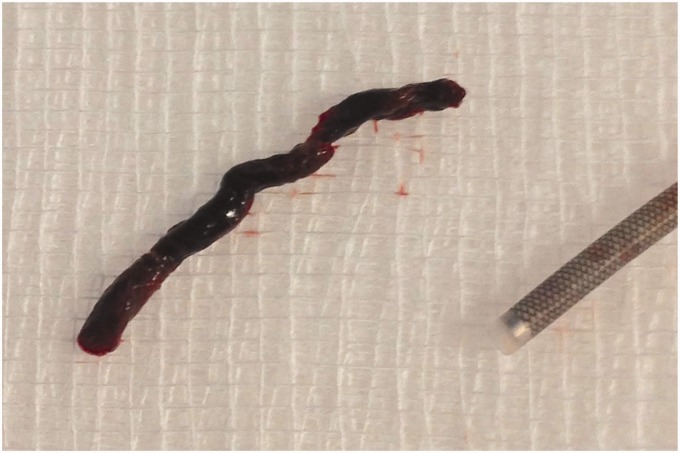
Figure 2.
Successful aspiration thrombectomy often yields the entire thrombus nestled in the tip of the Soft torqueable catheter Optimized for Intracranial Access (SOFIA).
In a total of 21 cases that were successfully recanalized with the SOFIA alone, a rate of 62% mTICI 3 was achieved compared to 44% when aspiration had failed and a stent retriever was introduced in addition. In 10 of these 21 cases (48%), mTICI 3 was achieved after just one aspiration attempt. In the majority of successful first-pass aspirations, the occlusion was located in the MCA (7/10, 70%).
Because of the nature of a stepwise escalating treatment approach, there was a significant difference between both groups regarding the time from groin puncture to final revascularization (20 minutes for aspiration alone vs. 60 minutes with rescue treatment, p < 0.0001). There were no significant differences between the groups (“aspiration only” (n = 21) vs. “rescue treatment” (n = 9)) regarding the rate of adverse events, clinical outcome at discharge and after 90 days (mRS ≤ 2). However, sICH was more frequent when direct lesional aspiration failed and an additional stent retriever was necessary (33% vs. 0%, p = 0.02). Good clinical outcome was correlated with patient age, initial NIHSS, and time from onset to recanalization (Tables 2 and 3). Thrombus length and the use of IV tissue plasminogen activator (tPA) had no significant effect on aspiration thrombectomy success, duration of treatment, or clinical outcome.
Table 3.
Comparison of patients with good and poor outcomes at 90 days (all, n = 30).
| Characteristic | mRS ≤ 2, n = 13 (43%) | mRS ≥ 3, n = 17 (57%) | p value | Test |
|---|---|---|---|---|
| Age, mean (±SD) | 64 (±16) | 75 (±7) | 0.03 | t-test with Welch’s correction |
| Male sex, n/N (%) | 8/13 (62%) | 7/17 (41%) | 0.5 | Fisher’s exact |
| Baseline NIHSS, median (range) | 11 (1–22) | 21 (13–35) | 0.0008 | Mann–Whitney |
| Time from symptom onset to revascularization, median (range) | 150 (60–365) | 180 (120–320) | 0.04 | Mann–Whitney |
| Time from groin puncture to recanalization (min) (median (range) | 33 (6–157) | 23 (8–240) | 0.3 | Mann–Whitney |
| Direct aspiration alone, n/N (%) | 8/13 (62%) | 13/17 (77%) | 0.4 | Fisher’s exact |
| mTICI ≥ 2b, n/N (%) | 13/13 (100%) | 14/17 (82%) | 0.2 | Fisher’s exact |
NIHSS: National Institutes of Health Stroke Scale; mTICI: modified Thrombolysis in Cerebral Infarction.
Discussion
Recently, major randomized trials5–9 have proven that thrombectomy results in good patient outcome more often than IVT in acute ischemic stroke due to large-artery occlusion. Stent retrievers used in these studies may well be viewed as the standard of care in EVT for stroke and can be applied in conjunction with flow-arresting or aspiration techniques, one of which is lesional aspiration via an intermediate catheter that is advanced to the site of occlusion. This facilitates deployment and retrieval of thrombectomy devices, potentially reduces the risk of clot migration to any previously unaffected proximal territory, and potentially enhances the mechanic effects of thrombectomy itself.
Though not altogether a new approach,17,18 lesional aspiration with flexible large-bore catheters has recently been suggested as a primary treatment.10–12 Particularly, advances in catheter manufacturing techniques have enabled the interventional neuroradiologist to apply this treatment seemingly without an increased effort or risk of adverse events. The potential benefits include reduced procedural duration and reduced treatment cost in cases for which no additional device is needed. These benefits have been addressed in previously published series under the term of the ADAPT technique with the 5MAX and 5MAX ACE system (Penumbra Inc, Alameda, CA, USA), respectively.10,11
There is of course a variety of intermediate catheters available that might serve the same purpose. Table 4 gives an overview of distal access catheters available for lesional aspiration-thrombectomy. One of the latest additions to the family of intermediate catheters is the SOFIA. The clinical safety and efficacy of the SOFIA have recently been reported by Stampfl et al. in a multicentric retrospective analysis of 115 treatments from three centers.13
Table 4.
Catheters feasible for lesional aspiration – thrombectomy.
| Sofia/Microvention | 5MAX/Penumbra | 5MAX ACEa/Penumbra | ACE64/Penumbra | |
|---|---|---|---|---|
| Inner diameter at tip (inch) | 0.055 | 0.054 | 0.060 | 0.064 |
| Proximal outer diameter (inch) | 0.068 | 0.080 | 0.080 | 0.080 |
| Distal outer diameter (inch) | 0.067 | 0.066 | 0.071 | 0.075 |
| Working length (cm) | 125 | 132 | 132 | 132 |
5MAX ACE replaced with the ACE64 system by the manufacturer.
Our intention was to evaluate whether the result of the so-called ADAPT technique that uses a dedicated catheter can be replicated with the SOFIA that, at least in the authors’ country, represents a less expensive alternative intermediate catheter.
The data reported herein to some extent represent a subset of those previously reported by Stampfl et al. However, in their paper lesional aspiration has not been a distinct criterion and was not further evaluated other than a mere mention of the potential technical opportunity to use the SOFIA analog to the ADAPT technique, resulting in 17 successful first-line aspiration cases. Our own cohort contains eight additional cases that have not been included by Stampfl et al., resulting in a series of 30 treatments from two centers. In these cases, we were able to achieve the final reperfusion result with the sole use of lesional aspiration in 70% of cases, taking an average of 20 minutes and resulting in 67% mTICI ≥ 2b. The additional use of a stent retriever increased this value to a total of 90%. These values compare favorably to large stent-retriever thrombectomy trials5,7,19,20 and are within the range of previously reported series on lesional aspiration10–12 that were in line with the percentage of mTICI 2b/3 reperfusion in the aspiration group with similar procedural timing (Table 5).
Table 5.
Revascularization results in comparison to other relevant studies.
| Characteristic | This series (n = 30) | Kowoll et al. (n = 54) | ADAPT (n = 37) | MR CLEAN (n = 196) | ESCAPE (n = 165) | NASA registry (n = 354) |
|---|---|---|---|---|---|---|
| Device mTICI ≥ 2b (lesional aspiration alone %) | 95 | 97 | 96 | NA | NA | 73 |
| Final mTICI ≥ 2b (aspiration + stent-retriever %) | 90 | 93 | 95 | 58.7 | 72.4 | 73 |
| Time to recanalization (minutes), all cases | 41.5 | 41 | 28.1 | 66 | 56 (time to first reperfusion) | 50 |
| Aspiration-only success (mTICI ≥ 2b) and time needed (mean) | 67% in 20 minutes | 54% in 30 minutes | 75% in 26 minutes | NA | NA | NA |
mTICI: modified Thrombolysis in Cerebral Infarction; ADAPT: a direct aspiration first-pass technique; MR CLEAN: Multicenter Randomized Clinical Trial of Endovascular treatment for Acute ischemic stroke in the Netherlands; ESCAPE: Endovascular treatment for Small Core and Anterior circulation Proximal occlusion with Emphasis on minimizing CT to recanalization times; NASA: North American Solitaire Stent Retriever Acute Stroke.
We found that aspiration only with the SOFIA had the highest rate of first-attempt success in MCA occlusions where 10/14 were successful (mTICI 2b/3)—7 of these 10 being mTICI3 results. The median time to recanalization in MCA occlusions with aspiration-only first-pass success was 10 minutes.
There were no device-related adverse events. However, we recorded one clot migration to a previously unaffected territory in the aspiration-only group, in which a thrombus at the tip of the SOFIA fractured during retrieval. The resulting proximal occlusion of A1 was left untreated, because the dependent anterior cerebral artery (ACA) territory was well collateralized from the contralateral A1. We recorded no subarachnoid hemorrhages (SAH); 3/30 patients developed sICH—all in the group in which an additional stent retriever had been used. This finding, though unique in our cohort, is not reflected in the published ADAPT literature, in which Turk et al. and Kowoll et al. reported no significant difference between groups.10–12 In addition, small sample size might create a bias.
In light of the major thrombectomy trials and the fact that we now have level-1 evidence for the use of stent retrievers in endovascular stroke treatment, we envision the role of lesional aspiration as the potential overture in the succession of thrombectomy devices. While the use of balloon-guide occlusion catheters (BGOC) has shown to achieve superior results, both angiographically and clinically20 when compared to standard guiding catheters, direct comparison of intermediate catheters and BGOC has not been made yet. Both aiming at a reduction of clot fragment distribution, lesional aspiration appears to be more efficient in preventing clot migration to proximal territories than BGOC. In the Multicenter Randomized Clinical Trial of Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN) trial, clot migration was seen in more than 8%.7 We saw only one such event, admitting that our sample size is too small to prove superiority of lesional aspiration. In any case, until proven otherwise intermediate catheters are a valuable tool in EVT of stroke, since they facilitate access and probably enhance the effect of mechanical thrombectomy in conjunction with stent retrievers. Regarding the aspiration maneuver itself, we felt that direct manual aspiration offers valuable tactile feedback, is very controllable, can be set up extremely fast, and eliminates additional potential drawbacks such as intermittent cleaning of the aspiration tubing once it has been contaminated with blood or scrutinizing an external pump setup. Using a 20-cc syringe appeared to be volumetrically sufficient, as the actual act of aspiration consisted of the mere maintenance of the vacuum, not the net movement of the aspirate itself within the lumen of the aspiration catheter.
In a multimodality approach, a high-performance, large-bore flexible catheter may well be used as a standalone first-line device, resulting in successful mTICI2b/3 reperfusion in at least two-thirds of cases, especially in MCA occlusions, in less than 15 minutes. This can be easily applied in all cases where the lesion itself can be reached with the tip of the SOFIA with adequate effort, which is largely facilitated by its suppleness and torqueability. Thereafter, if unsuccessful, it becomes helpful in the coaxial delivery of a stent retriever and yields high percentages of successful recanalization. This has been shown for the Penumbra 5Max-ACE system previously10–12 and could now be reproduced for the SOFIA in our series.
Limitations
We are aware of several limitations of this work. Some of the data had previously been included in a safety and efficacy study that did not, however, elucidate the possibility of first-line aspiration with the SOFIA. The number of patients is very limited, the treatments were non-consecutive, and no randomization process was involved. Also, there was no predefined endpoint in our study. We further included occlusions both in the anterior and in the posterior circulation. Thus, the validity of our clinical results may be impaired.
Conclusion
Based on our experience, lesional aspiration with the SOFIA is in line with previously published data for other aspiration catheters with regard to reperfusion success and procedural duration. It may be used as a first-line device, aiming at fast recanalization by means of aspiration only with safety and efficacy particularly in MCA occlusions. If unsuccessful, it converts into part of a stent retriever-based multimodality treatment.
Acknowledgments
All data can be made available in an anonymized manner on request. We declare that all human and animal studies have been approved by the local ethics committees of Heidelberg and Cologne University Hospital and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: Real-world experience and a call for action. Stroke 2010; 41: 2254–2258. [DOI] [PubMed] [Google Scholar]
- 3.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): A randomised trial. Lancet 2012; 380: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. Lancet 2012; 380: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 7.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, Bonafe A, et al. Solitaire™ with the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) trial: Protocol for a randomized, controlled, multicenter study comparing the Solitaire revascularization device with IV tPA with IV tPA alone in acute ischemic stroke. Int J Stroke 2015; 10: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 10.Turk AS, Spiotta A, Frei D, et al. Initial clinical experience with the ADAPT technique: A direct aspiration first pass technique for stroke thrombectomy. J Neurointerv Surg 2014; 6: 231–237. [DOI] [PubMed] [Google Scholar]
- 11.Turk AS, Frei D, Fiorella D, et al. ADAPT FAST study: A direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2014; 6: 260–264. [DOI] [PubMed] [Google Scholar]
- 12.Kowoll A, Weber A, Mpotsaris A, et al. Direct aspiration first pass technique for the treatment of acute ischemic stroke: Initial experience at a European stroke center. J Neurointerv Surg. Epub ahead of print 12 January 2015. DOI: 10.1136/neurintsurg-2014-011520. [DOI] [PubMed] [Google Scholar]
- 13.Stampfl S, Kabbasch C, Müller M, et al. Initial experience with a new distal intermediate and aspiration catheter in the treatment of acute ischemic stroke: Clinical safety and efficacy. J Neurointerv Surg. Epub ahead of print 29 May 2015. DOI: 10.1136/neurinstsurg-2015-011801. [DOI] [PubMed] [Google Scholar]
- 14.Kim JT, Yoon W, Park MS, et al. Early outcome of combined thrombolysis based on the mismatch on perfusion CT. Cerebrovasc Dis 2009; 28: 259–265. [DOI] [PubMed] [Google Scholar]
- 15.Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol 2013; 34: E117–E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankowitz BT, Aleu A, Lin R, et al. Endovascular treatment of basilar artery occlusion by manual aspiration thrombectomy. J Neurointerv Surg 2010; 2: 110–114. [DOI] [PubMed] [Google Scholar]
- 18.Jankowitz B, Aghaebrahim A, Zirra A, et al. Manual aspiration thrombectomy: Adjunctive endovascular recanalization technique in acute stroke interventions. Stroke 2012; 43: 1408–1411. [DOI] [PubMed] [Google Scholar]
- 19.Pereira VM, Gralla J, Davalos A, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire Flow Restoration in acute ischemic stroke. Stroke 2013; 44: 2802–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidat OO, Castonguay AC, Gupta R, et al. North American Solitaire Stent Retriever Acute Stroke registry: Post-marketing revascularization and clinical outcome results. J Neurointerv Surg 2014; 6: 584–588. [DOI] [PubMed] [Google Scholar]