Abstract
Indole is an interspecies and interkingdom signaling molecule widespread in different environmental compartment. Although multifaceted roles of indole in different biological systems have been established, little information is available on the microbial utilization of indole in the context of combating odor emissions from different types of waste. The present study was aimed at identifying novel bacteria capable of utilizing indole as the sole carbon and energy source. From the selective enrichment of swine waste and cattle feces, we identified Gram-positive and Gram-negative bacteria belonging to the genera Arthrobacter and Alcaligenes. Bacteria belonging to the genus Alcaligenes showed higher rates of indole utilization than Arthrobacter. Indole at 1.0 mM for growth was completely utilized by Alcaligenes sp. in 16 h. Both strains produced two intermediates, anthranilic acid and isatin, during aerobic indole metabolism. These isolates were also able to grow on several indole derivatives. Interestingly, an adaptive response in terms of a decrease in cell size was observed in both strains in the presence of indole. The present study will help to explain the degradation of indole by different bacteria and also the pathways through which it is catabolized. Furthermore, these novel bacterial isolates could be potentially useful for the in situ attenuation of odorant indole and its derivatives emitted from different types of livestock waste.
Keywords: Alcaligenes sp., Arthrobacter sp., Biodegradation, Environmental biotechnology, Indole
Introduction
Indole is an N-heterocyclic compound produced by bacteria and some plants and was initially used as a diagnostic marker for the identification of E. coli. Indole is the metabolic product of tryptophan generated by tryptophanase enzymatic activity encoded by tnaA gene in E. coli [1]. It is also produced by bacteria in the rumen and many living systems as a product of tryptophan metabolism. Various industries that synthesize pharmaceuticals, cosmetics, pesticides, disinfectants, agrochemicals, and dyes are also considered sources of indole [2]. There are more than 85 Gram-positive and Gram-negative bacterial species that yield indole as the product of tryptophan metabolism [1]. Biochemical and molecular studies have shown that indole plays various roles in bacterial systems, for example, as an extracellular signal [1, 3], for multicopy plasmid maintenance [4], cell division, biofilm formation [5], and acid and drug resistance [5–8]. In addition, indole also controls the virulence of several pathogenic bacteria [7] and it has multifaceted properties and applications in prokaryotic systems [9–11] as well as eukaryotic systems [12].
Indole is a strong odorant and it is one of major odorous compounds from animal wastes and sewers. Also, indole and its derivatives form a class of toxic recalcitrant environmental pollutants [12]. Hence, it is required clean-up technologies to remove odorous and toxic indolic compounds from the environment.
Microbial metabolism or transformation of odorant could be used as an effective treatment to combat odorous compounds. Although several reports are available on indole degradation/transformation by bacteria (aerobic and anaerobic) and by bacterial consortia [13], little information is available on aerobic metabolism by isolates that utilize indole a sole carbon and energy source. Therefore, in the present study, we focused on the isolation of bacteria with the ability to utilize indole as the sole energy source.
Materials and Methods
Chemicals and Growth Medium
Analytical grade indole, catechol, isatin, 2-oxindole, and anthranilic acid (>99 % purity) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The indole derivatives used in the present study are listed in (Table 1), and were purchased from Sigma-Aldrich or Combi-blocks Inc. (San Diego, CA, USA). Minimal salt medium (MSM) was prepared as described previously [14]. Luria–Bertani-agar at one-quarter strength (1/4-LB) was used as a rich medium for bacterial growth and culture maintenance.
Table 1.
The growth properties of Arthrobacter sp. strain B1 and Alcaligenes sp. strain B5 on indole derivatives and intermediates
| Compounds | Arthrobacter sp. strain B1 (OD600) | Alcaligenes sp. strain B5 (OD600) |
|---|---|---|
| l-Tryptophan | 0.031 | 0.038 |
| Indole | 0.046 | 0.062 |
| Indole-3-acetic acid | – | 0.054 |
| 3-Indoleacetonitrile | – | – |
| Indole-3-carbinol | – | – |
| 3-Methyl indole | – | – |
| Indole-3-carboxyaldehyde | – | – |
| Indole-3-propionic acid | – | – |
| 3,3-Methylene bisindole | – | – |
| Indole-3-acetamide | – | – |
| 2-Oxindole | – | 0.037 |
| Isatin | 0.041 | 0.052 |
| Indole-3-butyric acid | – | – |
The working concentration of these compounds was 0.2 mM. Maximum optical densities (OD600) were measured. Initial OD600 was 0.005 and − indicates no growth
Selective Enrichment of Livestock Waste
Two different types of samples, swine waste and cattle feces, were collected from Daegu, Korea for the isolation of indole degrading bacteria using enrichment techniques. Enrichments were performed in carbon-free MSM. Samples (5.0 g) of swine or cattle feces were suspended in a 250 ml Erlenmeyer flask containing 50 ml of MSM supplemented with 0.1 mM of indole. Enrichment cultures were incubated under aerobic conditions on a rotary shaker at 250 rpm at 30 °C. Utilization of indole in the above enrichment cultures was monitored by HPLC. After 7 days of incubation, feces suspensions (1 %, v/v) were transferred into fresh MSM containing 0.1 mM indole. Three rounds of sub-culture enrichment were performed in the same way.
Isolation and Identification of Indole Degrading Bacteria
After three rounds of sub-culturing, serially diluted samples were spread plated on 1/4th-diluted LB-agar plates containing 0.5 mM indole and on an MSM-agar plate supplemented with 0.5 mM indole to isolate bacterial strains. After 2–4 days of incubation, bacterial colonies appeared on plates were selected and purified. These colonies were used for the initial screening. Overnight seed cultures grown in 1/4-LB were inoculated (1 %, v/v) into 10 ml McCartney vials containing 5 ml of carbon-free MSM supplemented with 0.2 mM indole as the sole carbon and energy source. Sequencing of the 16S rRNA gene was conducted by COSMO GENETECH (www.cosmogenetech.com/en) company. The full length 16S rRNA gene was amplified using universal bacterial 16S rRNA gene primers namely, forward primer 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and reverse primer 1492R (5′-GGTTACCTTGTTACGACTT-3′). In order to identify the phylogenetic neighbors of each isolate 16S rRNA gene sequences were subjected to a similarity search in the EzTaxon server (http://www.eztaxon.org/) [15]. The 16S rRNA gene sequence of each isolate and their respective closely related species were retrieved from the EzTaxon server and aligned using the CLUSTAL_W program in MEGA version 6.0 [16]. The phylogenetic tree of each strain was constructed using the neighbor-joining algorithm from MEGA 6.0 software. Bootstrap analysis was also performed to evaluate the branching confidence limits.
Growth Properties
The growth properties of all isolates were examined in 25 ml of carbon-free MSM supplemented with indole at different concentrations (ranging from 0.2 to 5.0 mM) by inoculating (1 %, v/v) overnight seed culture grown in 1/4-LB. Cultures were incubated on a rotary shaker at 250 rpm at 30 °C. At different times, aliquots were withdrawn and optical density was monitored at 600 nm using a UV–visible spectrophotometer (Optizen 2120 UV, Mecasys Co., Ltd. Korea). Each experiment was performed using at least six independent cultures.
Analytical Method for Indole
Indole degradation was carried out by HPLC as previously described [17]. The HPLC used was an YL9100HPLC unit (Young Lin Ltd., Anyang-Si, Korea) equipped with a PDA detector and a 4.6 × 100 mm Chromolith Performance RP-18e column (Merck KGaA, Darmstadt, Germany). The mobile phase used was water and acetonitrile (50:50, v/v) and the flow rate was 0.5 ml/min, and elutes were monitored at 271 nm for indole (retention time 5.1 min).
Metabolite Isolation and Identification
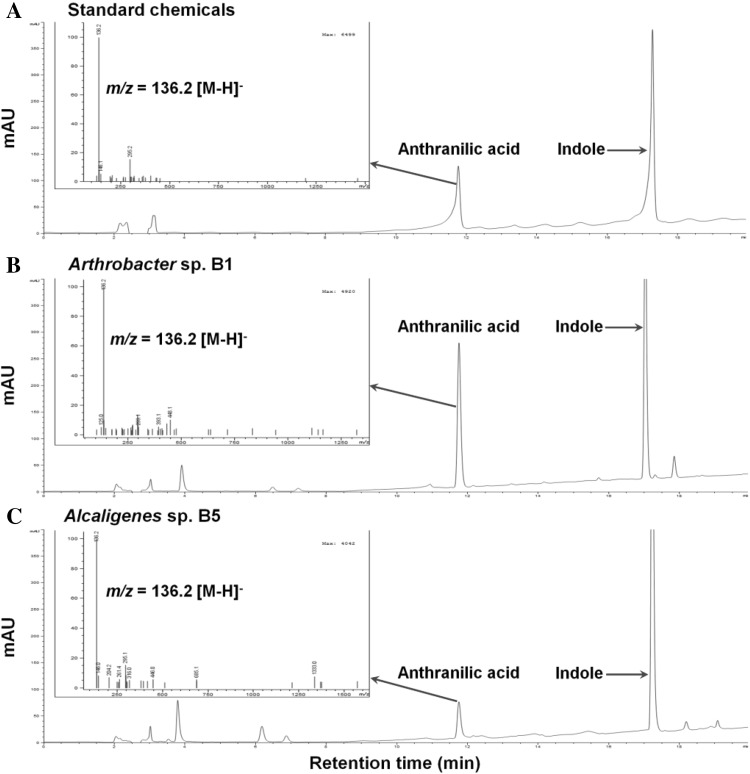
Two indole utilizing bacteria Arthrobacter sp. and Alcaligenes sp. were cultured for 7 h in 25 ml of carbon-free MSM supplemented with 1 mM indole on a rotary shaker at 250 rpm at 30 °C. Spent medium was passed through 0.45 μm filter to remove all bacteria and kept at −20 °C for further usage. To detect, catechol, isatin, and 2-oxindole, the above YL9100HPLC unit with 5 μm C18 column (Agilent Eclipse XD8-C18, 4.6 × 250 mm, Santa Clara, USA) was used. The mobile phase used was methanol and water (40:60, v/v) and the flow rate was 1.0 ml/min. Under these conditions, the retention times and the absorbance maxima were 4.5 min/274 nm for catechol, 5.2 min/310 nm for isatin, and 7.2 min/247 nm for 2-oxindole, respectively. To detect anthranilic acid, electrospray ionization mass spectrometry (ESIMS) studies were carried out with Agilent 6120 Quadruple LC/MS system (Santa Clara, USA). The mobile phases were comprised of 0.05 % formic acid with 5 % acetonitrile in deionized water (solvent A) and 0.05 % formic acid with acetonitrile (solvent B). Gradient solvent system was used as follows; 10 % solvent B (0–5 min) and 10–100 % solvent B (5–20 min) at 1.0 ml/min flow rate. Under these conditions, anthranilic acid was observed at 11.7 min (m/z [M–H]− 136.2). The presence of intermediates was confirmed with standard chemicals by comparison of retention time and maximal absorbance and also spiking experiment.
Scanning Electron Microscopy (SEM)
SEM was used to observe bacterial cell morphologies on a nylon filter, as previously described [18]. Briefly, a nylon filter (0.5 × 0.5 mm square) was exposed to bacterial suspension at an initial OD600 of 0.05. Cells and nylon filters were incubated together in the presence of indole (0.1 and 0.5 mM) or glucose (2 %) in MSM medium at 37 °C for 24 h without shaking to allow cells to attach on nylon filter membrane. As a control, cells were incubated in 1/4-LB medium without indole. After critical-point drying, specimens were examined using a scanning electron microscope (S-4100; Hitachi, Tokyo, Japan) at a voltage of 15 kV and magnifications ranging from 1000× to 10,000×.
Results
Enrichment and Identification of Indole Utilizing Bacteria
Selective enrichment was performed starting with swine and cattle feces samples. Seven isolates originated from swine waste and three isolates from cattle feces exhibited their ability to utilize indole as a sole carbon and energy source. Among ten isolates, the 16S rRNA gene sequences of two isolates showed the same sequence (named B1) which are similar to that of Arthrobacter, and eight isolates showed the same gene sequence (named B5) which are similar to that of Alcaligenes genus.
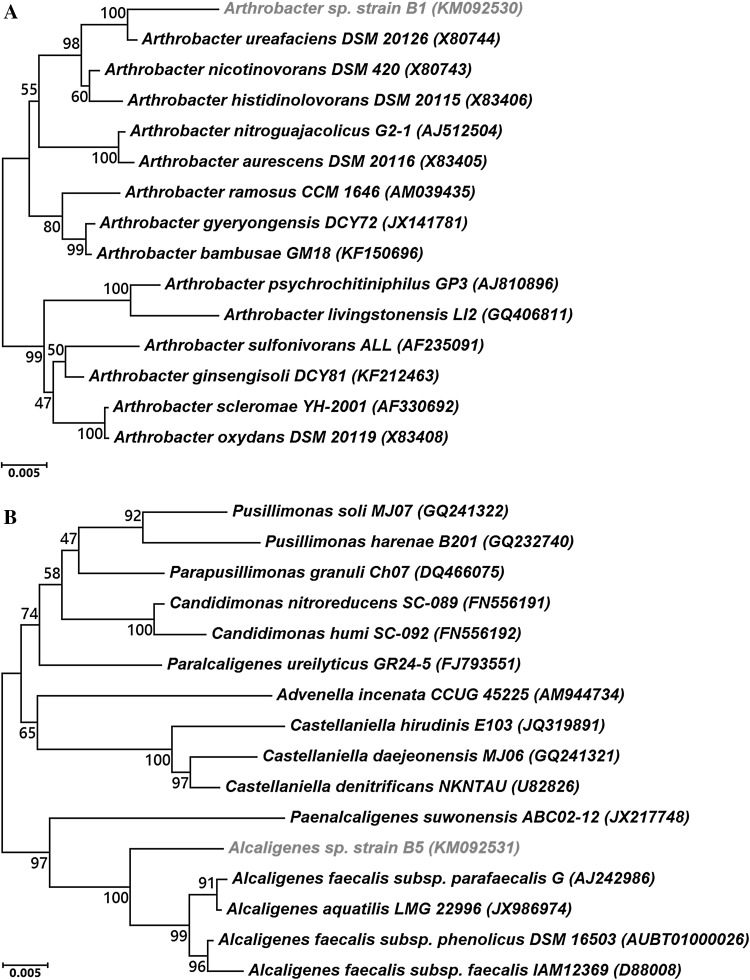
The 16S rRNA gene sequence of strain B1 (GenBank accession number: KM092530) showed highest identity with Arthrobacter ureafaciens (97.81 %), followed by A. nicotinovorans (97.08 %), A. histidinolovorans (96.73 %), A. ramosus (96.12 %) and A. psychrochitiniphilus (96.09 %) (Fig. 1a). The 16S rRNA gene sequence of strain B5 (GenBank accession number: KM092531) showed identity with Alcaligenes faecalis subsp. parafaecalis (97.50 %) followed by A. faecalis subsp. phenolicus (96.85 %), A. faecalis subsp. faecalis (96.37 %), and A. aquatilis (96.36 %) (Fig. 1b). Also, Gram-staining assay showed that the strain B1 is Gram-positive and the strain B5 is Gram-negative (data not shown). Thus, based on 16S rRNA gene sequence similarity, strains B1 and B5 were named Arthrobacter sp. strain B1 and Alcaligenes sp. strain B5, respectively.
Fig. 1.
Phylogenetic neighbor-joining tree of Arthrobacter sp. strain B1 (a) and Alcaligenes sp. strain B5 (b) based on 16S rRNA gene sequences. The phylogenetic tree shows the relationships between individual isolates and species within the respective genus. Bootstrap values (expressed as percentages of 1000 replications) greater than 50 % are given at nodes. Bar 0.005 % sequence variation. GenBank accession numbers are given in parentheses
Utilization of Indole by Strains Arthrobacter B1 and Alcaligenes B5
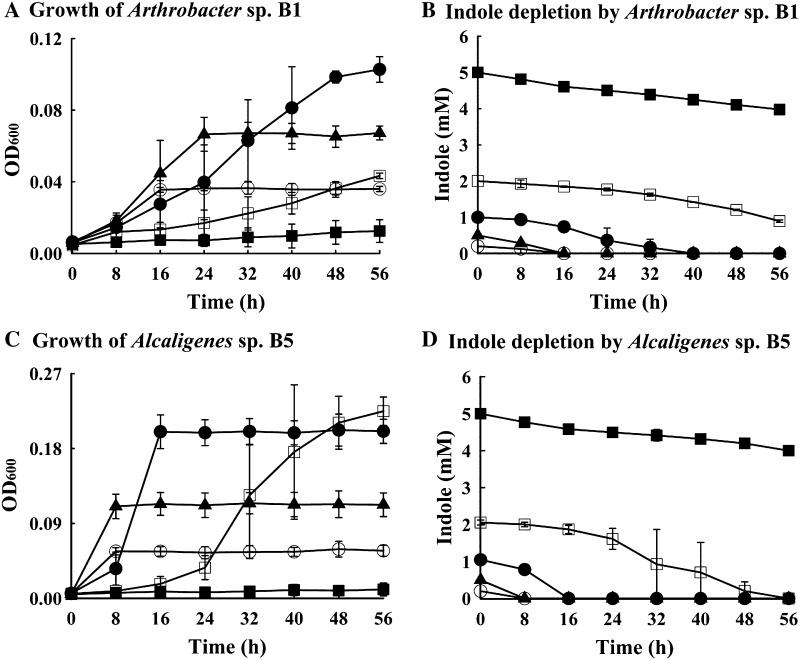
A range of indole concentrations (from 0.2 to 5.0 mM) was used to check the limits of indole utilization by strains B1 and B5 in carbon-free MSM medium (Fig. 2). Strain B1 at an indole concentration of 0.2 mM grew to achieve an optical density (OD600) of 0.04 in 24 h, and the final OD was increased when the indole concentration was increased to 1.0 mM (OD600 0.1 at 40 h) (Fig. 2a). However, when the indole concentration was increased to 2.0 mM strain B1 showed lower cell density (OD600 0.04), and at 5.0 mM growth inhibition occurred (Fig. 2a). The rate of indole depletion by strain B1 was also found to be concentration dependent (Fig. 2b). At concentrations of 0.2, 0.5 and 1.0 mM, indole was completely depleted after 40 h of incubation, whereas at concentrations of 2.0 and 5.0 mM indole depletions were 55 and 10 % after 56 h, respectively (Fig. 2b).
Fig. 2.
Growth characteristics and degradation kinetics of Arthrobacter sp. strain B1 and Alcaligenes sp. strain B5 in carbon-free MSM supplemented with different concentrations of indole. Circle, 0.2 mM; shaded triangle, 0.5 mM; shaded circle, 1.0 mM; square, 2.0 mM; shaded square, 5.0 mM. Growth of strain B1 (a), indole depletion by strain B1 (b), growth of strain B5 (c), and indole depletion by strain B5 (d)
The growth rate and the indole consumption rate of Alcaligenes B5 were greater than those of strain Arthobacter B1 (Fig. 2c). The optical density (OD600) of Alcaligenes B5 on 0.2 mM indole was found 0.06 after 8 h of incubation, and this increased to OD600 0.22 at 16 h on 1.0 mM indole (Fig. 2c). Also, Alcaligenes B5 completely utilized 1 mM indole for 16 h and 2 mM indole within 56 h (Fig. 2d).
Effects of Indole on Isolate Cell Morphologies
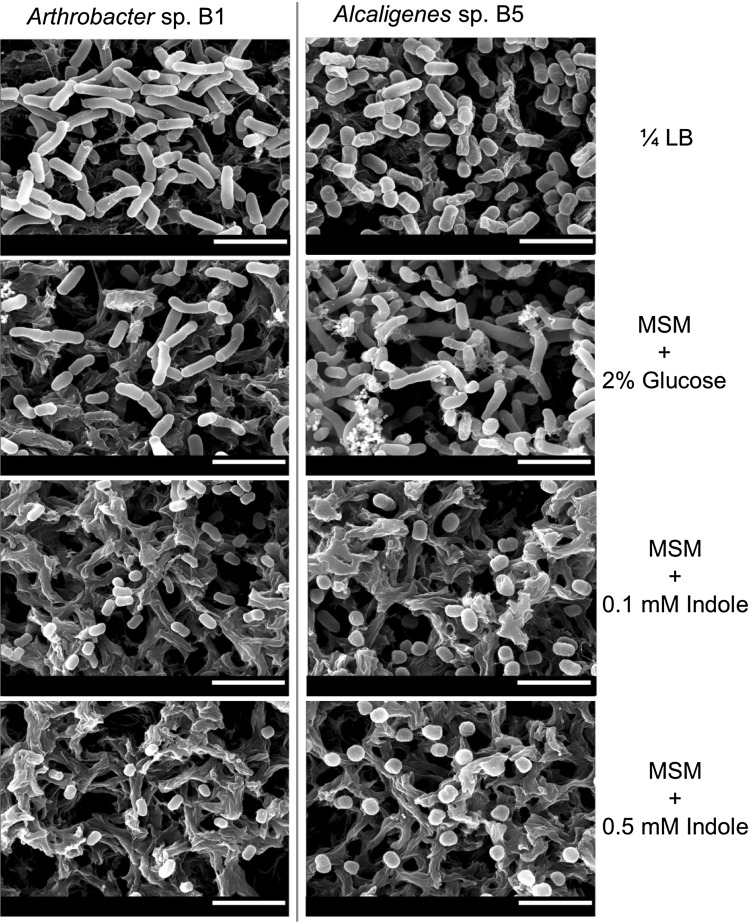
Effects of indole on the cell morphologies of Arthobacter sp. strain B1 and Alcaligenes sp. strain B5 were examined by culturing cells in the presence of indole. After culture, B1 and B5 cells in MSM medium without indole treatment were sized 1.6 ± 0.2 and 2.2 ± 0.3 μm in length, respectively and indole decreased cell size of two strains that became more spherical as compared with their normal rod-shaped structure. Specifically, the cell sizes of strains B1 and B5 reduced by 65 % (0.6 ± 0.1 μm) and 62 % (0.7 ± 0.1 μm) in the presence of 0.5 mM indole, respectively (Fig. 3). Based on the above results, it is clear that in addition to their indole utilizing properties, these strains exhibit adaptive response as indicated by reduction in cell size and changes in cell morphology when indole concentrations are high.
Fig. 3.
Scanning electron microscopic photographs of Arthrobacter sp. strain B1 and Alcaligenes sp. strain B5. SEM was used to examine the cell morphologies of cells grown on nylon filters in MSM medium containing indole (0.1 and 0.5 mM) or glucose (2 %) for 24 h at 37 °C. As a control, 1/4-LB medium was used without indole. The scale bar represents 3 μm. The experiment was performed in triplicate with two independent cultures and at least 20 random spots were observed
Growths of Arthrobacter sp. B1 and Alcaligenes sp. B5 on Indole Derivatives
The growth properties of Arthrobacter sp. B1 and Alcaligenes sp. B5 were also examined on different indole derivatives in carbon-free MSM. (Table 1) contains a list of the chemicals, which were applied at a concentration of 0.2 mM. Alcaligenes sp. B5 grew well on l-tryptophan, indole-3-acetic acid, 2-oxindole, and isatin as sole energy sources (Table 1). In contrast, Arthrobacter sp. strain B1 grew just on l-tryptophan and isatin (Table 1). These results show that Alcaligenes sp. B5 has broader substrate range than Arthrobacter sp. B1.
Identification of Intermediates in Aerobic Degradation of Indole
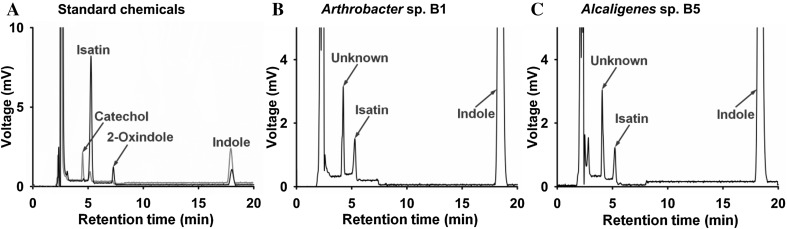
To study aerobic degradation pathways of indole by Arthrobacter sp. B1 and Alcaligenes sp. B5, additional HPLC and ESIMS analyses were performed, and two key intermediates, isatin and anthranilic acid, were found. During the indole metabolism, both strains produced double-oxidized indole isatin, while 2-oxindole was not detected (Fig. 4). In Fig. 4, there was an unidentified peak in both strains. While its retention time is similar to catechol, its UV spectrum was different from catechol. Hence, we performed LC/MS analysis to confirm that both strains produced anthranilic acid (Fig. 5), supporting the previous report in that Alcaligenes sp. strain In3 produced anthranilic acid [19].
Fig. 4.
HPLC chromatographs of culture filtrates during aerobic indole degradation by Arthrobacter sp. strain B1 and Alcaligenes sp. strain B5. Standard chemicals of catechol, isatin, 2-oxindole, and indole (a), Arthrobacter sp. strain B1 (b), and Alcaligenes sp. strain B5 (c). At the panel A, two wavelengths (243 nm and 271 nm) in the PDA detector were used to show chromatographs of four standard chemicals
Fig. 5.
LC chromatographs and ESIMS mass spectra of culture filtrates during aerobic indole degradation. Standard chemicals of anthranilic acid and indole (a), Arthrobacter sp. strain B1 (b), and Alcaligenes sp. strain B5 (c). Mass spectra of anthranilic acid are shown as insets
Discussions
Reports show that the major sources of indole produced by livestock are swine manure and cattle feces [20]. Thus, we have isolated two indole utilizing bacteria, Arthrobacter sp. strain B1 and Alcaligenes sp. strain B5, from cattle feces and swine waste. The 16S rRNA gene sequences of strains B1 and B5 showed relatively low identity levels with their best 16S neighbors with Arthrobacter ureafaciens (97.81 %) and Alcaligenes faecalis subsp. parafaecalis (97.50 %) indicating that they are probably novel species. In order to characterize the novel species of these isolates, a detailed polyphasic taxonomic study will be carried out in future.
Our growth data show that strains B1 and B5 utilize indole in a concentration dependent manner, and that indole above 2 mM is toxic to these isolates, which is in agreement with the values reported previously for other bacteria [19, 21, 22]. Indole is toxic to even indole producing bacteria like E. coli when the concentration is above 2.0 mM [23]. The minimal inhibitory concentration of indole in Pseudomonas sp. ST-200 was reported to be 2.56 mM [21]. Indole at 2.56 mM was also found to be toxic to Alcaligenes sp. strain In3 [19]. Similarly, cell growth and indole degradation by Pseudomonasaeruginosa Gs were inhibited when the concentration of indole was 4.0 mM [22], and Pseudomonas putida KT2440 cells did not grow well at above 3 mM indole [24].
Some previously characterized aerobic bacteria, such as, P. aeruginosa Gs [22], Pseudomonas sp. strain ST-200 [21], Alcaligenes sp. strain In3 [19], and Cupriavidus sp. strain [25] use indole as a growth substrate. While, Pseudomonas indoloxidans, P. aeruginosa [7], A. tumefaciens [10], and Burkholderia unamae strain CK43-B [26] can degrade indole without utilizing it as the sole carbon source. D. indolicum is the only anaerobic isolate identified to date that can utilize indole as a carbon and electron donor [27]. In this study, we have found two additional novel strains utilizing indole as the sole energy source.
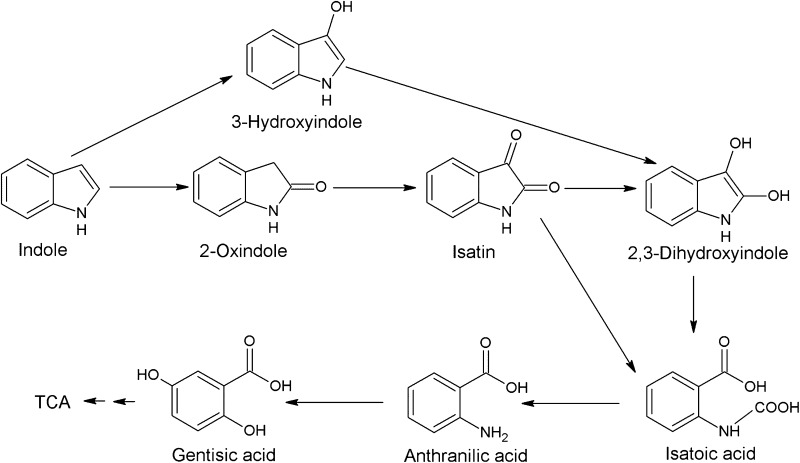
Microbial degradation of indole was investigated under aerobic and anaerobic conditions by several bacteria and fungi [13]. Aerobic indole degradation was generally initiated by oxidation of indole followed by heterocyclic ring cleavage [13]. Claus and Kutzner [19] reported that Alcaligenes sp. strain In3 degrades indole via the gentisate pathway. The pathway includes hydroxyindole, isatin, anthranilic acid and gentisic acid (Fig. 6), while the authors detected only anthranilic acid during the indole degradation by Alcaligenes sp. strain In3 [19]. This study detected another intermediate isatin (Fig. 4) as well as anthranilic acid (Fig. 5). Since anaerobic metabolite catechol in indole metabolism was not detected in both strains (Figs. 4, 5), the current indole utilization pathway is different from the catechol pathway [13] and probably occurs via the gentisate pathway.
Fig. 6.
Common metabolic pathways of aerobic degradation of indole by Arthrobacter sp. strain B1 and Alcaligenes sp. strain B5. Metabolic intermediates (isatin and anthranilic acid) of indole degradation were detected in this study
Various reports show that the hydroxylated and oxygenated products of indole degradation, such as, isatin, indoxyl, 7-hydroxyindole, 2-hydroxyindole, and 3-oxindole, which are used in the synthesis of indigo dyes by dimerization [28, 29]. Thus, those bacteria that are known to transform indole into hydroxylated indole intermediates might produce dimers. Recombinant technology has resulted in the cloning of some known or putative aromatic monooxygenases or dioxygenases in E. coli, and greatly enhanced indigo formation [28–31]. The accumulation of indigo as a dimer product during the indole degradation is the disadvantage for the application of those microorganisms in the in situ attenuation of indole odor emitted from different types of waste.
Therefore, complete mineralization of indole without the accumulation of any dimer product would provide a better means for the in situ decontamination of indole. Furthermore, isolates capable of this task could be potentially applicable for the in situ bioremediation of indole odor from different source of animal and industrial wastes.
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A6A1031189).
Footnotes
Minsu Kim and Jin-Hyung Lee have contributed equally to this article.
References
- 1.Lee J-H, Lee J. Indole as an intercellular signal in microbial community. FEMS Microbiol Rev. 2010;34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Xie XG, Ren CG, Dai CC. Degradation of N-heterocyclic indole by a novel endophytic fungus Phomopsis liquidambari. Bioresour Technol. 2013;129:568–574. doi: 10.1016/j.biortech.2012.11.100. [DOI] [PubMed] [Google Scholar]
- 3.Lee J, Zhang XS, Hegde M, Bentley WE, Jayaraman A, Wood TK. Indole cell signaling occurs primarily at low temperatures in Escherichia coli. ISME J. 2008;2:1007–1023. doi: 10.1038/ismej.2008.54. [DOI] [PubMed] [Google Scholar]
- 4.Chant EL, Summers DK. Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol Microbiol. 2007;63:35–43. doi: 10.1111/j.1365-2958.2006.05481.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Jayaraman A, Wood TK. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 2007;7:42. doi: 10.1186/1471-2180-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HH, Molla MN, Cantor CR, Collins JJ. Bacterial charity work leads to population-wide resistance. Nature. 2010;467:82–85. doi: 10.1038/nature09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Attila C, Cirillo SLG, Cirillo JD, Wood TK. Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microb Biotechnol. 2009;2:75–90. doi: 10.1111/j.1751-7915.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikaido E, Yamaguchi A, Nishino K. AcrAB multidrug efflux pump regulation in Salmonella enterica serovar Typhimurium by RamA in response to environmental signals. J Biol Chem. 2008;283:24245–24253. doi: 10.1074/jbc.M804544200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwidar M, Nam D, Mitchell RJ. Indole negatively impacts predation by Bdellovibrio bacteriovorus and its release from the bdelloplast. Environ Microbiol. 2014;17:1009–1022. doi: 10.1111/1462-2920.12463. [DOI] [PubMed] [Google Scholar]
- 10.Lee J-H, Kim Y-G, Baek KH, Cho MH, Lee J. The multifaceted roles of the interspecies signalling molecule indole in Agrobacterium tumefaciens. Environ Microbiol. 2014;17:1234–1244. doi: 10.1111/1462-2920.12560. [DOI] [PubMed] [Google Scholar]
- 11.Molina-Santiago C, Daddaoua A, Fillet S, Duque E, Ramos JL. Interspecies signalling: Pseudomonas putida efflux pump TtgGHI is activated by indole to increase antibiotic resistance. Environ Microbiol. 2014;16:1267–1281. doi: 10.1111/1462-2920.12368. [DOI] [PubMed] [Google Scholar]
- 12.Lee J-H, Wood TK, Lee J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015;23:707–718. doi: 10.1016/j.tim.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Arora PK, Sharma A, Bae H. Microbial degradation of indole and its derivatives. J Chem. 2015;2015:13. [Google Scholar]
- 14.Fazlurrahman Batra M, Pandey J, Suri CR, Jain RK. Isolation and characterization of an atrazine-degrading Rhodococcus sp. strain MB-P1 from contaminated soil. Lett Appl Microbiol. 2009;49:721–729. doi: 10.1111/j.1472-765X.2009.02724.x. [DOI] [PubMed] [Google Scholar]
- 15.Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol. 2007;57:2259–2261. doi: 10.1099/ijs.0.64915-0. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim Y-G, Lee J-H, Cho MH, Lee J. Indole and 3-indolylacetonitrile inhibit spore maturation in Paenibacillus alvei. BMC Microbiol. 2011;11:119. doi: 10.1186/1471-2180-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J-H, Cho MH, Lee J. 3-Indolylacetonitrile decreases Escherichia coli O157:H7 biofilm formation and Pseudomonas aeruginosa virulence. Environ Microbiol. 2011;13:62–73. doi: 10.1111/j.1462-2920.2010.02308.x. [DOI] [PubMed] [Google Scholar]
- 19.Claus G, Kutzner HJ. Degradation of indole by Alcaligenes spec. Syst Appl Microbiol. 1983;4:169–180. doi: 10.1016/S0723-2020(83)80046-0. [DOI] [PubMed] [Google Scholar]
- 20.Trabue S, Kerr B, Bearson B, Ziemer C. Swine odor analyzed by odor panels and chemical techniques. J Environ Qual. 2011;40:1510–1520. doi: 10.2134/jeq2010.0522. [DOI] [PubMed] [Google Scholar]
- 21.Doukyu N, Aono R. Biodegradation of indole at high concentration by persolvent fermentation with Pseudomonas sp. ST-200. Extremophiles. 1997;1:100–105. doi: 10.1007/s007920050021. [DOI] [PubMed] [Google Scholar]
- 22.Yin B, Gu JD, Wan N. Degradation of indole by enrichment culture and Pseudomonas aeruginosa Gs isolated from mangrove sediment. Int Biodeterior Biodegradation. 2005;56:243–248. doi: 10.1016/j.ibiod.2005.10.001. [DOI] [Google Scholar]
- 23.Garbe TR, Kobayashi M, Yukawa H. Indole-inducible proteins in bacteria suggest membrane and oxidant toxicity. Arch Microbiol. 2000;173:78–82. doi: 10.1007/s002030050012. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Hong H, Heo A, Park W. Indole toxicity involves the inhibition of adenosine triphosphate production and protein folding in Pseudomonas putida. FEMS Microbiol Lett. 2013;343:89–99. doi: 10.1111/1574-6968.12135. [DOI] [PubMed] [Google Scholar]
- 25.Qu Y, Shen E, Ma Q, Zhang Z, Liu Z, Shen W, Wang J, Li D, Li H, Zhou J. Biodegradation of indole by a newly isolated Cupriavidus sp. SHE. J Environ Sci (China) 2015;34:126–132. doi: 10.1016/j.jes.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Kim D, Rahman A, Sitepu IR, Hashidoko Y. Accelerated degradation of exogenous indole by Burkholderiaunamae strain CK43B exposed to pyrogallol-type polyphenols. Biosci Biotechnol Biochem. 2013;77:1722–1727. doi: 10.1271/bbb.130282. [DOI] [PubMed] [Google Scholar]
- 27.Bak F, Widdel F. Anaerobic degradation of indolic compounds by sulfate-reducing enrichment cultures, and description of Desulfobacterium indolicum gen. nov., sp. nov. Arch Microbiol. 1986;146:170–176. doi: 10.1007/BF00402346. [DOI] [Google Scholar]
- 28.McClay K, Boss C, Keresztes I, Steffan RJ. Mutations of toluene-4-monooxygenase that alter regiospecificity of indole oxidation and lead to production of novel indigoid pigments. Appl Environ Microbiol. 2005;71:5476–5483. doi: 10.1128/AEM.71.9.5476-5483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rui L, Reardon KF, Wood TK. Protein engineering of toluene ortho-monooxygenase of Burkholderia cepacia G4 for regiospecific hydroxylation of indole to form various indigoid compounds. Appl Microbiol Biotechnol. 2005;66:422–429. doi: 10.1007/s00253-004-1698-z. [DOI] [PubMed] [Google Scholar]
- 30.Doukyu N, Toyoda K, Aono R. Indigo production by Escherichia coli carrying the phenol hydroxylase gene from Acinetobacter sp. strain ST-550 in a water–organic solvent two-phase system. Appl Microbiol Biotechnol. 2003;60:720–725. doi: 10.1007/s00253-002-1187-1. [DOI] [PubMed] [Google Scholar]
- 31.Qu Y, Shi S, Zhou H, Ma Q, Li X, Zhang X, Zhou J. Characterization of a novel phenol hydroxylase in indoles biotranformation from a strain Arthrobacter sp. W1. PLoS One. 2012;7:e44313. doi: 10.1371/journal.pone.0044313. [DOI] [PMC free article] [PubMed] [Google Scholar]