Abstract
Eight thermophilic bacterial strains were isolated from Tattapani Hot spring and screened for various hydrolytic enzymes including cellulases. The isolated bacterial strains were identified as Geobacillus thermodenitrificans IP_WH1(KP842609), Bacillus licheniformis IP_WH2(KP842610), B. aerius IP_WH3(KP842611), B. licheniformis IP_WH4(KP842612), B. licheniformis IP_60Y(KP842613), G. thermodenitrificans IP_60A1(KP842614), Geobacillus sp. IP_60A2(KP842615) and Geobacillus sp. IP_80TP(KP842616) after 16S ribotying. Out of the eight isolates Geobacillus sp. IP_80TP grew best at 80 °C whereas rest of the isolates showed optimal growth at 60 °C. G. thermodenitrificans IP_WH1 produced a thermotolerant cellulase with maximum activity at 60 °C.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-016-0578-4) contains supplementary material, which is available to authorized users.
Keywords: Tattapani hot spring, Thermotolerant cellulase, Geobacillus thermodenitrificans, North West Himalayas and thermophilic bacterial strains
Introduction
Thermophilic bacteria have been isolated from hot springs and hydrothermal vents where the temperature ranges between 40 and 122 °C [1]. Cellulases are important industrial enzymes and their applications include manufacture of paper, textile, pulp, food and biofuel [2]. As most of the industrial enzymatic reactions are carried out at high temperature, therefore cellulases isolated from thermophilic bacterial strains are important for industrial applications [3].
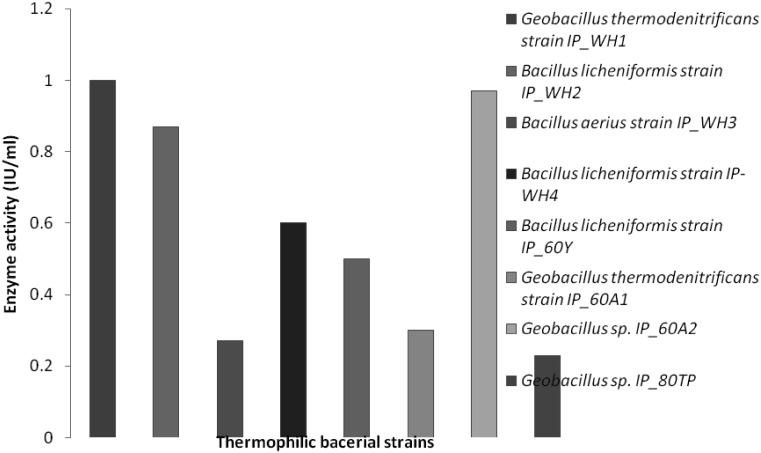
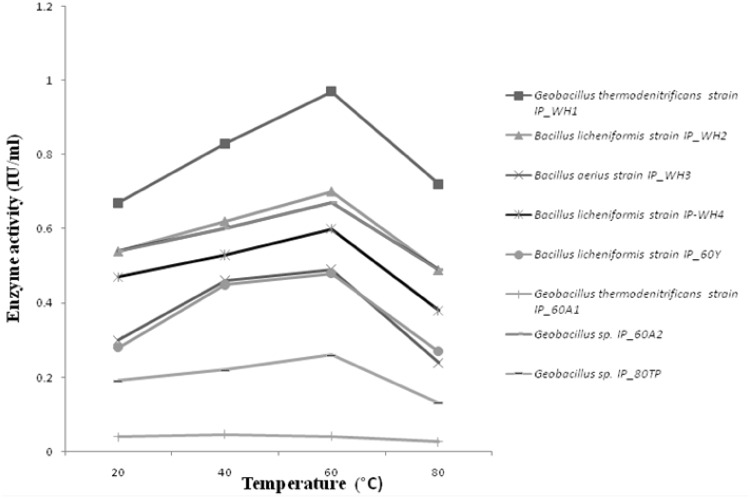
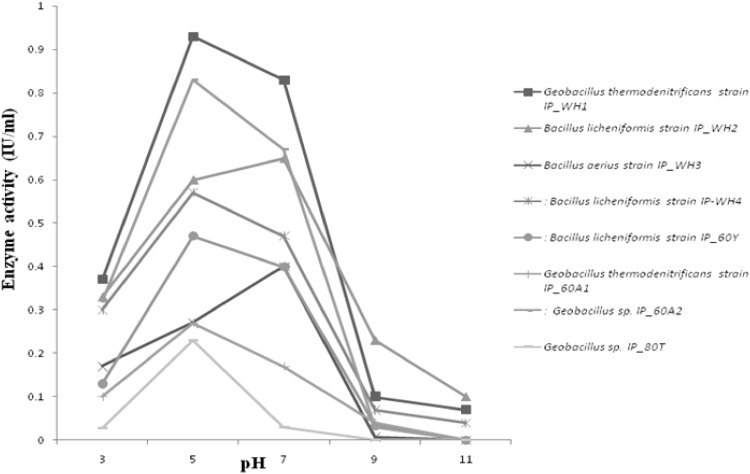
North West Himalayas have abundant unexplored environmental niches (both extreme hot and cold) that can be a source of novel organisms and biomolecules. With this background, thermophilic bacteria were isolated from previously unexplored Tattapani hot spring sediment. Tattapani hot spring (Lat. 33.2500°N, Long. 74.2500°E) is situated in Rajouri district of Jammu and Kashmir (India). The temperature of this hot spring ranges between 50 and 100 °C. The sediment was collected by using the protocol developed by Cummins [4]. At the time of the sample collection the temperature of the sediment was 60 °C and pH was 7. The samples were immediately shifted on dry ice and transported to the laboratory for further use. The bacterial strains (incubated for 48 h) were isolated by dilution plate method on the nutrient agar at 60 and 80 °C [5]. The bacterial load calculated at 60 °C and pH 7 was 62 × 105 cfu/g of sediment that is much higher than Moroccan hot springs 6 × 102 cfu/g [6] but relatively higher than Uttranchal hot springs 2 × 105 cfu/g [7]. All the bacterial isolates were identified by amplifying 16S rRNA gene [8] and their sequences were submitted to GenBank (Accession Numbers: KP842609, KP842610, KP842611, KP842612, (KP842614, KP842615 and KP842616). Among the isolates Geobacillus thermodenitrificans IP_WH1, Bacillus licheniformis IP_WH2, B. aerius IP_WH3, B. licheniformis IP_WH4, B. licheniformis IP60Y, G. thermodenitrificans IP_60A1 were obtained at 60 °C and two strains Geobacillus sp. IP_60A2 and Geobacillus sp. IP_80TP at 80 °C. The phylogenetic tree (Supplementary Fig. S1) of the isolated bacterial strains was constructed by using MEGA6 [9] to investigate their relationships with each other. The isolated strains were screened for various hydrolytic enzymes including cellulases at 60 °C by well diffusion method [10] and all were found to be cellulase producers. All the isolated strains were grown at different temperatures viz, 80, 60, 40 and 20 °C for 48 h to check the temperature optima for growth. Geobacillus sp. IP_80TP showed optimum growth at 80 °C, while others had optimum growth at 60 °C. The optimal cellulases activity of all the bacilli were assayed quantitatively at 60 °C by following the protocol developed by Miller [11] (Fig. 1). Geobacillus sp. IP_80TP though grew best at 80 °C but the cellulase was produced at 60 °C which was less in comparison to all others that grew and produced cellulase at 60 °C. Maximum cellulase was produced by G. thermodenitrificans IP_WH1 (0.94 IU/ml) which was higher than other thermophilic cellulases reported in literature such as Bacillus sp. with 0.14–0.37 IU/ml [12] and Bacillus sp. SMIA-2 with 0.29 IU/ml [13] at 50 °C and pH 7.0. Though G. thermodenitrificans has been reported in the literature for the production of thermostable lipase [14], alpha-amylase and alpha-glucosidase [15] but there are no reports of cellulase production. However, there are reports on cellulase production from Bacillus sp. and other Geobacillus sp. [16–18]. In addition all the thermophillic cellulases produced were analyzed for temperature optima (Fig. 2) and pH optima (Fig. 3). Interestingly, cellulases produced by all the bacteria isolated in present study, had temperature optima at 60 °C, even for Geobacillus sp. IP_80TP that has optimal growth at 80 °C. pH optima for cellulases from all the bacterial strains was 5.0 except B. licheniformis IP_WH2 and B. aerius IP_WH3 which showed pH optima near 7. G. thermodenitrificans IP_WH1 has pH optima at 5.0 as is true for many thermostable cellulase produced by various species of Geobacilli [16, 17].
Fig. 1.
Comparative cellulase production by eight thermophilic bacteria
Fig. 2.
Temperature profile of the cellulases produced from the isolated bacterial strains
Fig. 3.
pH profile of the cellulases produced from the bacterial strains
This is the first report of G. thermodenitrificans as acid active cellulase producer at 60 °C, approximately 10 °C higher than most reported cellulases. Though the present study indicates that G. thermodenitrificans IP_WH1 is the most suitable thermostable acid active cellulase producer but other isolates from Tattapani hot spring can further be screened for the presence of other thermophilic/stable enzymes of industrial importance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The work was conducted by the financial support provided under the UGC-SAP programme.
Footnotes
M. K. Dhar, B. K. Bajaj and Sanjana Koul have contributed equally to this work.
References
- 1.Takai K, Nakamura K, Toki T, Tsunogai U, Miyazaki M, Miyazaki J, Hirayama H, Nakagawa S, Nunoura T, Horikoshi K. Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci. 2008;105:10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Res, Article ID 280696. doi:10.4061/2011/280696 [DOI] [PMC free article] [PubMed]
- 3.Zamost BL, Nielsen HK, Starnes RL. Thermostable enzymes for industrial applications. J Ind Microbiol. 1991;8:71–81. doi: 10.1007/BF01578757. [DOI] [Google Scholar]
- 4.Cummins KW. An evaluation of some techniques for the collection and analysis of benthic samples with special emphasis on lotic waters. Am Midl Nat. 1962;67:477–504. doi: 10.2307/2422722. [DOI] [Google Scholar]
- 5.Adiguzel A, Inan K, Sahin F, Arasoglu T, Gulluce M. Molecular diversity of thermophilic bacteria isolated from Pasinler hot spring (Erzurum, Turkey) Turk J Biol. 2011;35:267–274. [Google Scholar]
- 6.Aanniz T, Ouadghiri M, Melloul M, Swings J, Elfahime E, Ibijbijen J, Ismaili M, Amar M. Thermophilic bacteria in Moroccan hot springs, salt marshes and desert soils. Braz J Microbiol. 2015;46:443–453. doi: 10.1590/S1517-838246220140219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar B, Trivedi P, Mishra AK, Pandey A, Palni LMS. Microbial diversity of soil from two hot springs in Uttaranchal Himalaya. Microbiol Res. 2004;159:141–146. doi: 10.1016/j.micres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Piterina AV, Bartlett J, Pembroke JT. Molecular analysis of bacterial community DNA in sludge undergoing autothermal thermophilic aerobic digestion (ATAD): pitfalls and improved methodology to enhance diversity recovery. Diversity. 2010;2:505–526. doi: 10.3390/d2040505. [DOI] [Google Scholar]
- 9.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan A, Chauhan G, Gupta PC, Goyal P, Kaushik P. In vitro antibacterial evaluation of Anabaena sp. against several clinically significant microflora and HPTLC analysis of its active crude extracts. Indian J pharmacol. 2010;42:105–107. doi: 10.4103/0253-7613.64490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 12.Padilha IQM, Carvalho LCT, Dias PVS, Grisi TCSL, Silva FLH, Santos SFM, Araujo DAM. Production and characterization of thermophilic carboxymethyl cellulase synthesized by Bacillus sp. Growing on sugarcane bagasse in submerged fermentation. Braz J Chem Eng. 2015;32:35–42. doi: 10.1590/0104-6632.20150321s00003298. [DOI] [Google Scholar]
- 13.Ladeira SA, Cruz E, Delatorre AB, Barbosa JB, Martins MLL. Cellulase production by thermophilic Bacillus sp. SMIA-2 and its detergent compatibility. Electron J Biotechnol. 2015;18:110–115. doi: 10.1016/j.ejbt.2014.12.008. [DOI] [Google Scholar]
- 14.Christopher LP, Zambare VP, Zambare A, Kumar H, Malek L. A thermo-alkaline lipase from a new thermophile Geobacillus thermodenitrificans AV-5 with potential application in biodiesel production. J Chem Technol Biotechnol. 2015;90:2007–2016. doi: 10.1002/jctb.4678. [DOI] [Google Scholar]
- 15.Ezeji TC, Wolf A, Bahl H. Isolation, characterization, and identification of Geobacillus thermodenitrificans HRO10, an alpha-amylase and alpha-glucosidase producing thermophile. Can J Microbiol. 2005;51:685–693. doi: 10.1139/w05-054. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi G, Bhalla A, Adhikari A, Bischoff KM, Hughes SR, Christopher LP, Sani RK. Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains. Bioresour Technol. 2010;101:8798–8806. doi: 10.1016/j.biortech.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Mawadza C, Hatti-Kaul R, Zvauya R, Mattiasson B. Purification and characterization of cellulases produced by two Bacillus strains. J Biotechnol. 2000;83:177–187. doi: 10.1016/S0168-1656(00)00305-9. [DOI] [PubMed] [Google Scholar]
- 18.Tai SK, Lin HPP, Kuo J, Liu JK. Isolation and characterization of a cellulolytic Geobacillus thermoleovorans T4 strain from sugar refinery wastewater. Extremophiles. 2004;8:345–349. doi: 10.1007/s00792-004-0395-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.