Abstract
Many toxic compounds are produced and released in the hemicellulosic hydrolyzates during the acid pretreatment step, which are required for the disruption of the lignocelluloses matrix and sugars release. The conventional methods of detoxification i.e. overliming, activated charcoal, ion exchange or even membrane-based separations have the limitations in removal of these toxic inhibitors in fermentation process. Hence, it is imperative to explore biological methods to overcome the inhibitors by minimizing the filtration steps, sugar loss and chemical additions. In the present study we screened sixty-four strains of yeasts to select potential strains for detoxification of furfural, acetic acid, ferulic acid, 5-hydroxymethyl furfural (5-HMF) as carbon and energy source. Among these strains Pichia occidentalis M1, Y1′a, Y1′b and Y3′ showed a significant decrease in the toxic compounds but we selected two best yeast strains i.e. P. occidentalis Y1′a and P.occidentalis M1 for the further experiments with an aim to remove the fermentation inhibitors. The yeasts P. occidentalis Y1′a and P.occidentalis M1 were grown aerobically in sugarcane bagasse hemicellulose hydrolysate under submerged cultivation. For each yeast, a 22 full factorial design was performed considering the variables—pH (4.0 or 5.0) and agitation rate (100 or 300 rpm), and the percentage removal of HMF, furfural, acetic acid and phenols from hemicellulosic hydrolysates were responsive variables. After 96 h of biological treatment, P. occidentalis M1 and P. occidentalis Y1′a showed 42.89 and 46.04 % cumulative removal of inhibitors, respectively.
Keywords: Microbial detoxification, Sugarcane bagasse, Dilute acid hydrolysis, Inhibitors
Introduction
Lignocellulosic materials are mainly composed of cellulose, hemicellulose and lignin [1, 2]. The carbohydrate fraction of lignocellulosic materials, if harnessed judiciously, can serve as an excellent building block for the production of renewable ethanol and other commodity chemicals via microbial fermentation. Production of fuel ethanol from lignocellulosic biomass so called second-generation (2G) ethanol is an important necessity due to the heavy usage of gasoline worldwide and environmental pollution. The 2G ethanol is completely renewable and is able to reduce atmospheric pollution due to the lower release of carbon dioxide, while offering several geo-political benefits [3]. Sugarcane bagasse (SB), a fibrous product after extraction of juice is an excellent carbohydrate source (~67 %) for 2G ethanol production [3].
Conventional process of ethanol production from lignocellulose biomass comprises of four major steps: pretreatment (responsible for the disruption of lignocellulosic matrix), enzymatic hydrolysis (leading to depolymerization of cellulose into glucose by the action of cellulolytic enzymes), the fermentation (which is the conversion of sugars to ethanol, generally carried out by yeast); distillation, rectification and dehydration steps (for separation and purification of the final product) [4]. The pretreatment of SB is an inevitable step to increase the accessibility of carbohydrate fraction towards enzymatic action [3]. Dilute acid hydrolysis of lignocellulosics is a fast and effective process but, it has bottlenecks, such as, by-product formation and non-selectivity [5]. Dilute sulfuric acid pretreatment precisely acts on hemicellulose releasing hemicellulosic monomers and releasing some undesired compounds like furans (5-hydroxymethylfurfural and hydroxymethylfurfural), phenolics, weak acids, and others. The dilute acid pretreatment is conducted at high temperature and pressure, and the reaction time takes for seconds or minutes [3]. Sulfuric acid (H2SO4) is mostly used catalyst, but other weak acids, such as: hydrochloric acid (HCl) and nitric acid (HNO3) have also been used for the hemicellulose removal from variety of lignocellulosic materials [6].
Inhibitory compounds generated in lignocellulosic bioethanol production are main hurdle faced during the hydrolysis process. One of the main challenges associated with 2G ethanol production is to overcome the cumulative negative effect of cell wall derived inhibitors on the fermenting microorganisms during fermentation reactions [7–11]. Inhibitory compounds like acids, furans and phenolic compounds interferes the fermenting microorganisms and complete process by reducing ethanol yield and productivity during fermentation. These inhibitors negatively affect the performance of microorganisms in the fermentation process [5]. Concentration of these inhibitors produced depends on process conditions and raw materials used for hydrolysis. Furans and phenol monomers produced during hydrolysis of lignin are also major cofactors which inhibit or slow down the hydrolysis process. Generally, furans and phenolic compounds constrain growth and rate of ethanol production. Therefore, it is essential, to remove these inhibitors prior to fermentation in order to obtain the desired ethanol or any other metabolite. Detoxification process can be categorized into physical, chemical and biological methods. Biological methods certainly have unique advantages. Further, to consolidate the detoxification and ethanol or xylitol fermentation so called simultaneous detoxification and fermentation (SDF) is also possible by the appropriate use of microorganisms in a single vessel [11].
This study was aimed to develop a biological detoxification method for sugarcane bagasse hemicellulosic hydrolysates. For this, the yeasts Pichia occidentalis Y1′a and P. occidentalis M1 [12] were grown in concentrated sugarcane bagasse hemicellulosic hydrolysate. For both the strains of yeast, a 22 full factorial design was carried out. The variables, pH (4.0 or 5.0) and agitation rate (100 rpm or 300 rpm), were evaluated and the percentage removal of HMF, furfural, acetic acid and phenols after 96-h of biological treatment were responsive variables.
Materials and Methods
Yeast Screening
Sixty-four strains of yeasts were screened to search for potential strains for detoxification of furfural, acetic acid, ferulic acid, HMF as carbon and energy source. Of these, only two strains of Picchia occidentalis were selected for detoxification (ISMD) of sugarcane bagasse hemicellulosic hydrolysate.
The growth medium for yeasts was composed of 6.7 g/l of yeast nitrogen base (YNB), 18.0 g/l agar and with different concentrations of individual (furfural, 0.2820 g/l; 5-HMF, 0.0149 g/l; total phenolics, 3.13 g/l) and combined toxic compounds (furfural, 0.2820 g/l + 5-HMF, 0.0149 g/l + total phenolics, 3.13 g/l). The medium containing the toxic compounds (hydroxymethyl furfural, furfural, acetic acid, ferulic acid and syringaldehyde) was prepared for the screening of the yeasts.
Preparation of Sugarcane Bagasse Hemicellulosic Hydrolysates
The sugarcane bagasse was hydrolyzed in vertical rotary drum reactor of 50 L capacity at 121 °C for 10 min with H2SO4 (98 %) at 1:10 solid/liquid ratio (100 mg of H2SO4 g−1 dry sugarcane bagasse). The hydrolyzed hemicellulose was recovered after filtration and subsequently concentrated at 70 °C under vacuum to obtain a fivefold increase in the xylose content.
The pH of the hemicellulosic hydrolysate was adjusted with 2 M NaOH according to the values of design experiment. It was filtered and subsequently autoclaved at 110 °C for 15 min. Then, hydrolysate was centrifuged under aseptic conditions at 2600×g for 20 min to remove the suspended solids, in order to use the hydrolysates for biological detoxification experiments.
Inoculum Preparation
Pichia occidentalis Y1′a (Genbank accession number: KP033405) and P. occidentalis M1 (Genbank accession number: KP033404) were obtained from stock strains of Social Insects Study Center, Rio Claro Biosciences Institute, São Paulo State University (UNESP—Universidade Estadual Paulista, Rio Claro, São Paulo State, Brazil). Both the strains were identified according to the standard methods described by Melo et al. [8]. All the strains were maintained at 5 °C in 2 % Sabouraud Agar Medium. The medium used for inoculum preparation contained 10 g/l of yeast extract, 20 g/l of peptone and 20 g/l of glucose. For inoculum preparation, loopful of cultures were transferred to 250 ml Erlenmeyer flasks containing 100 ml of YPX medium (10.0 g/l yeast extract, 20.0 g/l peptone, 30.0 g/l xylose, pH 6.0). The flasks were incubated at 30 °C, 200 rpm for 24 h. After 24 h of incubation, the cells were recovered by centrifugation (2000×g, 20 min) at room temperature, washed, centrifuged again and suspended in sterile distilled water to obtain an initial concentration of 0.5 g/l. Erlenmeyer flasks (500 ml) each containing 200 ml of medium closed with cotton plugs were incubated in rotary shaker (New Brunswick Scientific, Edison, NJ, USA) at 200 rpm for 24 h at 30 °C.
Medium and Detoxification Conditions
The concentrated sugarcane bagasse hemicellulosic hydrolysate was supplemented with 6.7 g/l of YNB (Sigma-Aldrich, USA). Fifty milliliter of medium was taken in Erlenmeyer flasks (125 ml capacity), and inoculated with 0.5 g/l of cells. The biological detoxification was conducted at different agitation at 30 °C for 96 h on rotary shaker (New Brunswick Scientific—Edison, NJ, USA). Assays were performed in triplicates. Aliquots of 50 ml samples were taken at times 0, 24, 48, 72 and 96 h for the quantification of d-glucose, d-xylose, d-arabinose, HMF, furfural, phenols and acetic acid, and pH and cell growth determination.
Analytical Methods
The concentrations of glucose, xylose, arabinose and acetic acid were determined by HPLC with a refraction index detector (Waters 410; Milford, MA, USA). The samples were diluted in a ratio of 1:10 and filtered through a Sep Pak C18 filter. Subsequently, samples were injected into the chromatograph, using the following conditions: column BIO-RAD Aminex HPX-87H (7.8 × 300 mm) (Bio-Rad, Hecules, CA, USA), a temperature of 45 °C, eluent: 0.005 M sulfuric acid, flow 0.6 ml/min in a sample volume of 20 μL. Furfural and 5-HMF was also determined by HPLC with UV detector (Waters 2487/USA). The samples were filtered through Schleicher and Schuell membrane, 0.45 μm and subsequently injected into the chromatograph using the following conditions: column Eclipse XDB-C18 5 μm (4.6 × 150 mm), a temperature of 25 °C, eluent: acetonitrile and water at a ratio of 1:8 with 1 % acetic acid and phosphoric acid, flow 0.9 ml/min, sample volume of 25 μl. Total concentration of phenolics was determined by method described by Gouveia et al. [13]. Cell growth was determined by measuring absorbance at 600 nm using a Bioespectro SP-220 spectrophotometer. Cell concentration was calculated based on the relationship of optical density and cell dry weight through a calibration curve. Statistical analysis of the experiments was performed using Statistica 8.
Results
Preparation of Sugarcane Bagasse Hemicellulosic Hydrolysates
After hydrolysis, hemicellulose hydrolysate was concentrated by vacuum concentration process to increase the concentration of sugar in hydrolysate. Table 1 shows the composition of hemicellulose hydrolysates before and after the vacuum concentration process. Xylose was the major constituent in the hydrolysate (14.19 g/l) showing the maximum depolymerisation of hemicellulosic fraction from sugarcane bagasse. Also, glucose has been released from the hemicellulose or/and cellulose fractions. However, most of the cellulose remained in substrate and is not hydrolyzed by dilute acid hydrolysis. Fermentation inhibitors i.e. acetic acid, furfural, 5-HMF were also produced in hydrolysate. The non-concentrated acid hydrolysate also contained furfural (0.2820 g/l), 5-HMF (0.0149 g/l) and total phenolics (3.13 g/l). The vacuum evaporated acid hydrolysate represents almost fivefold increase of each component present in native hydrolysate except acetic acid, furfural and total phenolics.
Table 1.
Composition of sugarcane bagasse hemicellulosic hydrolysates after acid hydrolysis and concentrated fivefold by vacuum evaporation
| Compounds | Hemicellulose hydrolysates before concentration (g/l) | Hemicellulose hydrolyzates After concentration (g/l) |
|---|---|---|
| Xylose | 14.19 | 69.65 |
| Glucose | 1.52 | 7.32 |
| Arabinose | 1.43 | 7.08 |
| Acetic acid | 0.85 | 3.15 |
| Furfural | 0.2820 | 0.1680 |
| 5-HMF | 0.0149 | 0.0378 |
| Phenolics | 3.13 | 8.87 |
| pH | 0.99 | 0.24 |
Screening of Yeast for Detoxification
We have screened sixty-four yeast strains for their potential to use as detoxification of compounds present in hemicellulose hydrolysates (Fig. 1; Table 2). Among these, P. occidentalis M1, Y1′a, Y1′b and Y3′ showed a significant decrease in the combination of toxic compounds acetic acid, syring aldehyde, ferulic acid, furfural and HMF in synthetic culture medium (Fig. 2). Out of these tested strains, we selected two yeast strains i.e. P. occidentalis M1 and Y1′a for further experiments with an aim to remove the fermentation inhibitors from sugarcane bagasse hemicellulosic hydrolysates. A 22 full factorial design with three repetitions in central point was carried out considering two process variables-pH and agitation. Cumulative inhibitors removal (%) refers to the difference between the initial and final concentrations of 5-HMF, furfural, phenols and acetic acid concentrations from hemicellulose hydrolysates after vacuum concentration process. Tables 3 and 4 show the initial and final concentrations in g/l of toxic compounds present in hemicellulosic hydrolyzate before and after the biological treatment step employing the strains of Picchia occidentalis M1 and Y1′a all experiments.
Fig. 1.
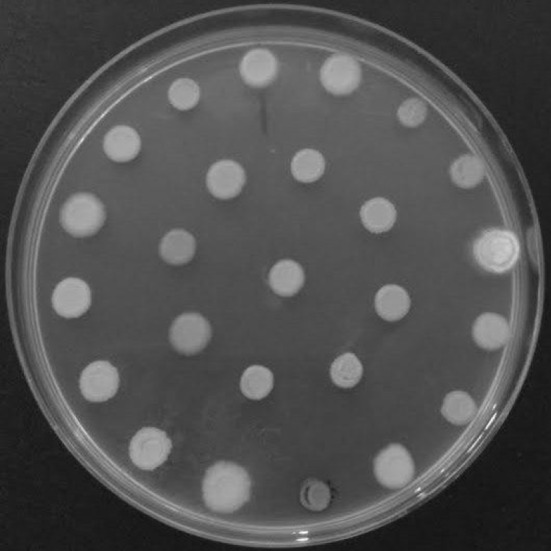
Yeasts growth tested in positive control (glucose as carbon source), after 21 days of incubation at 25 °C
Table 2.
Screening of different yeasts for their ability to utilize toxic compounds
| Strain | Species | Substrate where strain was obtained | Results | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Syringaldehyde | Ferulic acid | Furfural | 5-hydroxymethylfurfural | Combination of compounds | ||||||||||
| 1 g/L | 5 g/L | 0.2 g/L | 0.7 g/L | 8 × 10−2 g/L | 0.4 g/L | 1.8 × 10−3 g/L | 1.6 × 10−2 g/L | 4.0 × 10−3 g/L | 2.0 × 10−2 g/L | 1a | 2b | ||||
| 1 | PBM 63 | Candida metapsilosis ATCC 96144 | Atta laevigata | + | − | − | − | − | − | − | − | − | − | + | − |
| 2 | PBM 52 | Candida guilliermondii TJY14a | Atta laevigata | + | − | − | − | − | − | − | − | − | − | + | − |
| 3 | TO 100 | Aureobasidium pullulans | Acromyrmex balzani | − | − | − | − | − | − | − | − | − | − | − | − |
| 4 | TO 182 | Aureobasidium sp. | Acromyrmex balzani | − | − | − | − | − | − | − | − | − | − | − | − |
| 5 | TO 178 | Aureobasidium pullulans | Acromyrmex balzani | − | − | − | − | − | − | − | − | − | − | − | − |
| 6 | TO 047 | Cryptococcus laurentii | Acromyrmex balzani | + | − | − | − | − | − | − | − | − | − | − | − |
| 7 | PBM 44 | Pichia caribbica | Atta laevigata | + | − | − | − | − | − | − | − | − | − | + | − |
| 8 | PBM 64 | Candida parapsilopsis | Atta laevigata | + | − | − | − | − | − | − | − | − | − | + | − |
| 9 | PBM 30 | Pichia burtonii | Atta laevigata | + | − | − | − | − | − | − | − | − | − | + | − |
| 10 | BR3-3BY | Candida silvae | Vriesia sp. | + | − | − | − | − | − | − | − | − | − | + | − |
| 11 | TO 050 | Rhodosporidium toruloides | Acromyrmex balzani | + | − | − | − | − | + | − | − | − | − | + | − |
| 12 | TO 049 | Sporisorium elionuri | Acromyrmex balzani | − | − | − | − | − | + | − | − | − | − | + | − |
| 13 | TO 217 | Trichosporon mycotoxinivorans | Acromyrmex balzani | − | − | − | − | − | + | − | − | − | − | − | − |
| 14 | TO 023 | Cryptococcus laurentii | Acromyrmex balzani | + | − | − | − | − | − | − | − | − | − | − | − |
| 15 | TO 018 | Rhodotorula mucilaginosa | Acromyrmex balzani | + | − | − | − | − | + | − | − | − | − | − | − |
| 16 | SIA 24.1 | Sporobolomyces japonicus | Fallen vegetation litter | − | − | − | − | − | − | − | − | − | − | − | − |
| 17 | TO 057 | Sporisorium elionuri | Acromyrmex balzani | − | − | − | − | − | + | − | − | − | − | − | − |
| 18 | BR6-2AI | Candida shehatae | Bromeliad water | − | − | − | − | − | − | − | − | − | − | − | − |
| 19 | 54 | Trichosporon chiarellii | Myrmicocrypta sp. | − | − | − | − | − | − | − | − | − | − | − | − |
| 20 | CG8-8BY | Candida shehatae | Mushroom | − | − | − | − | − | − | − | − | − | − | − | − |
| 21 | PT1-1BASP | Candida shehatae | Euterpe sp. | − | − | − | − | − | − | − | − | − | − | − | − |
| 22 | BR6-2AY | Candida shehatae | Bromeliad water | − | − | − | − | − | − | − | − | − | − | − | − |
| 23 | N8a | Meyerozyma guilliermondii | – | + | − | − | − | − | − | − | − | − | − | + | − |
| 24 | N12a2 | Trichosporon asahii | – | − | − | − | − | − | − | − | − | − | − | − | − |
| 25 | N4ap2 | Trichosporon asahii | – | − | − | − | − | − | − | − | − | − | − | − | − |
| 26 | PI-II5 | Aureobasidium pullulans | Polybia ignobilis | − | − | − | − | − | − | − | − | − | − | − | − |
| 27 | PI-II63 | Aureobasidium pullulans | Polybia ignobilis | − | − | − | − | − | + | − | − | − | − | − | − |
| 28 | 32 | Aureobasidium pullulans | Polybia ignobilis | − | − | − | − | − | − | − | − | − | − | − | − |
| 29 | MP2-2CB | Pseudozyma hubeiensis | – | − | − | − | − | − | + | − | − | − | + | + | − |
| 30 | PBM 39 | Meira Arqovae CBS 110053 | Atta laevigata | + | − | − | − | − | − | − | − | − | − | + | − |
| 31 | A1 M-A12 | Pichia caribbica | Atta sp. | + | − | − | − | − | − | − | − | − | − | + | − |
| 32 | PBM 3 | Aureobasidium pullulans | Atta laevigata | − | − | − | − | − | − | − | − | − | − | − | − |
| 33 | FB1-1DASP | Candida sp. | Campanulaceae | − | − | − | − | − | − | − | − | − | − | − | − |
| 34 | H10-10AY | NI | Hedychium coronarium koening | − | − | − | − | − | − | − | − | − | − | − | − |
| 35 | HFLS-5BASP | Candida tenuis | Hedychium coronarium koenig | − | − | − | − | − | − | − | − | − | − | − | − |
| 36 | TO O11 | Cryptococcus mangaliensis | Acromyrmex balzani | − | − | − | − | − | − | − | − | − | − | − | − |
| 37 | TO 23 | Cryptococcus laurentii | Acromyrmex balzani | − | − | − | − | − | − | − | − | − | − | − | − |
| 38 | A1M-A7 | Exophiala sp. | – | − | − | − | − | − | − | − | − | − | − | − | − |
| 39 | FB8 | NI | – | + | − | − | − | − | − | − | − | − | − | + | − |
| 40 | Lj-3 | Issatchenkia occidentalis CCTCC M 206097 | Wastewater and sludge | + | + | − | − | + | − | − | − | − | − | + | − |
| 41 | S-7 | Issatchenkia orientalis CCTCC M 206098 | Wastewater and sludge | + | − | − | − | − | − | − | − | − | − | + | + |
| 42 | W7 | Lecythophora sp. | Polybia ignob ilis | − | − | − | − | − | − | − | − | − | − | − | − |
| 43 | B22a2 | Candida azyma | Polybia ignob ilis | − | − | − | − | − | − | − | − | − | − | − | − |
| 44 | S11 | Saccharomycopsis crataegensis | Polybia ignob ilis | + | − | − | − | + | − | − | − | − | − | + | − |
| 45 | Mel 28 | Pichia caribbica | Polybia ignob ilis | + | − | − | − | + | − | − | − | − | − | + | − |
| 46 | YS4 | Candida kofuensis | Polybia ignob ilis | − | − | − | − | − | − | − | − | − | − | − | − |
| 47 | W13a1b | Pichia guilliermondii | Polybia ignob ilis | − | − | − | − | − | − | − | − | − | − | + | − |
| 48 | Mel 10 | Aureobasidium pullulans | Polybia ignob ilis | − | − | − | − | − | − | − | − | − | − | − | − |
| 49 | Ml | Issatchenkia occidentalis | Syzygium jambolanum | + | + | − | − | − | − | − | − | − | − | + | + |
| 50 | Yl′a | Issatchenkia occidentalis | Syzygium j amb olanum | + | + | − | − | − | − | − | − | − | − | + | + |
| 51 | Yl′b | Issatchenkia occidentalis | Syzygium jambolanum | + | + | − | − | − | − | − | − | − | − | + | + |
| 52 | Y3′ | Issatchenkia occidentalis | Syzygium jambolanum | + | + | − | − | − | − | − | − | − | − | + | + |
| 53 | JP26/06a | Issatchenkia terricola | Syzygium jambolanum | + | − | − | − | − | − | − | − | − | − | + | − |
| 54 | W14 | Pichia guilliermondii | Polybia ignobilis | + | − | − | − | − | − | − | − | − | − | + | − |
| 55 | BD149 | Cryptococcus sp. (in description) | Electrical wiring of lamp post | − | − | − | − | − | − | − | − | − | − | + | − |
| 56 | A2-1 | Cryptococcus sp. (in description) | Electrical wiring of lamp post | + | − | − | − | − | − | − | − | − | − | + | − |
| 57 | A2-4 | Cryptococcus sp. (in description) | Electrical wiring of lamp post | − | − | − | − | − | − | − | − | − | − | − | − |
| 58 | TO 622 | NI | Acromyrmex balzani | + | − | − | − | + | + | − | − | − | − | − | − |
| 59 | TO 623 | NI | Acromyrmex balzani | − | − | − | − | − | − | − | − | − | − | − | − |
| 60 | TO 621 | NI | Acromyrmex balzani | + | − | − | − | + | − | − | − | − | − | + | − |
| 61 | TO 443 | NI | Acromyrmex balzani | + | − | − | − | − | − | − | − | − | − | − | − |
| 62 | TO 440 | NI | Acromyrmex balzani | − | − | − | − | − | − | − | − | − | − | − | − |
| 63 | TO 753 | NI | Acromyrmex balzani | − | − | − | − | − | − | − | − | − | − | − | − |
| 64 | CBS 5813 | NI | – | − | − | − | − | − | − | − | − | − | − | − | − |
aCombination 1: Acetic acid (1 g/L) + Syringaldehyde (0.2 g/L) + ferulic acid (8 × 10−2 g/L) + furfural (1.8 × 10−3 g/L) + HMF (4.0 × 10−3 g/L)
bCombination 2: Acetic acid (5 g/L) + Syringaldehyde (0.7 g/L) + ferulic acid (0.40 g/L) + furfural (1.6 × 10−2 g/L) + HMF (2.0 × 10−2 g/L)
+ Microorganism growth in presenting the selected compound. − Microorganism no growth under the selected compound
Strains 40—Lj-3 Issatchenkia occidentalis CCTCC M 206097 e 41—S-7 Issatchenkia orientalis CCTCC M 206098 were used as positive control [22]
Fig. 2.
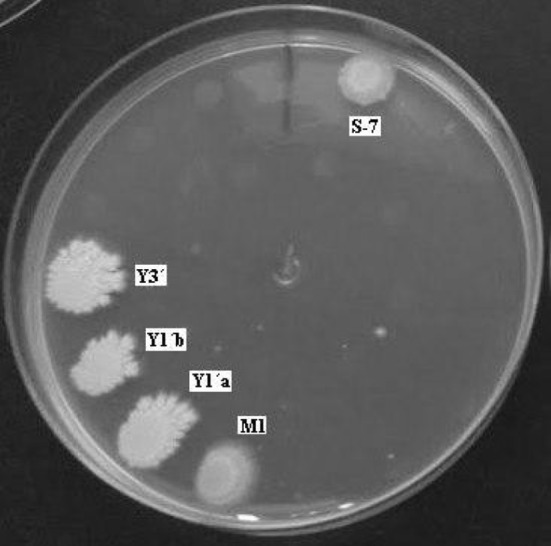
Yeast strains M1 Y1′a, Y1′b, and Y3′ and Pichia occidentalis S-7, CCTCC M2006098 used as standard growth observed in the combination with higher concentrations of the toxic compounds tested
Table 3.
Initial and final concentrations of toxic compounds in hemicellulose hydrolyzate treated biologically by Picchia occidentalis M1 (g/L)
| Exp. | Concentration | HMF (g/L) | Furfural (g/L) | Acetic acid (g/L) | Total phenols (g/L) | Total concentration (g/L)a | Removed amount of toxic compounds (%)b |
|---|---|---|---|---|---|---|---|
| 1 | Initial | 0.0364 | 0.1402 | 1.3 | 6.373 | 7.85 | 19.43 |
| Final | 0.0297 | 0.1256 | 0.91 | 5.26 | 6.325 | ||
| 2 | Initial | 0.0324 | 0.0153 | 1.47 | 6.04 | 7.557 | 22.87 |
| Final | 0.002 | 0.0043 | 1.2 | 4.623 | 5.829 | ||
| 3 | Initial | 0.0371 | 0.1135 | 1.12 | 6.51 | 7.781 | 36.29 |
| Final | 0.0005 | 0.0065 | 0 | 4.95 | 4.957 | ||
| 4 | Initial | 0.032 | 0.0925 | 1.42 | 6.41 | 7.954 | 42.89 |
| Final | 0.0017 | 0.0007 | 0 | 4.54 | 4.542 | ||
| 5 | Initial | 0.0341 | 0.1359 | 2.06 | 5.31 | 7.538 | 39.52 |
| Final | 0.002 | 0.007 | 0 | 4.55 | 4.559 | ||
| 6 | Initial | 0.0341 | 0.1284 | 2.04 | 5.356 | 7.562 | 39.98 |
| Final | 0.002 | 0.007 | 0 | 4.53 | 4.539 | ||
| 7 | Initial | 0.0335 | 0.1229 | 2.09 | 5.27 | 7.521 | 39.01 |
| Final | 0.002 | 0.005 | 0 | 4.58 | 4.587 |
a“Total concentration” (g/L) refers to the sum of the concentrations of HMF, furfural, acetic acid and total phenols
b“Removed amount of toxic compounds” (%) refers to the difference between initial concentration and final total concentration (g/L)
Table 4.
Initial and final concentrations of toxic compounds in hemicellulose hydrolyzate treated biologically by Picchia occidentalis Y1′a (g/L)
| Exp. | Concentration | HMF (g/L) | Furfural (g/L) | Acetic acid (g/L) | Total phenols (g/L) | Total concentration (g/L)a | Removed amount of toxic compounds (%)b |
|---|---|---|---|---|---|---|---|
| 1 | Initial | 0.037 | 0.1443 | 1.02 | 6.495 | 7.696 | 17.69 |
| Final | 0.035 | 0.0293 | 0.92 | 5.35 | 6.334 | ||
| 2 | Initial | 0.0322 | 0.0575 | 1.57 | 6.02 | 7.679 | 20.5 |
| Final | 0.0018 | 0.0035 | 1.33 | 4.77 | 6.105 | ||
| 3 | Initial | 0.037 | 0.1283 | 2.13 | 6.52 | 8.815 | 46.04 |
| Final | 0.0009 | 0.0051 | 0 | 4.75 | 4.755 | ||
| 4 | Initial | 0.0317 | 0.1002 | 1.43 | 5.9 | 7.462 | 38.97 |
| Final | 0.0015 | 0.002 | 0 | 4.55 | 4.553 | ||
| 5 | Initial | 0.034 | 0.1394 | 2.02 | 5.69 | 7.882 | 43.08 |
| Final | 0.0016 | 0.005 | 0 | 4.48 | 4.486 | ||
| 6 | Initial | 0.0347 | 0.1415 | 2.04 | 5.63 | 7.842 | 42.79 |
| Final | 0.0016 | 0.0049 | 0 | 4.48 | 4.486 | ||
| 7 | Initial | 0.034 | 0.1374 | 1.9 | 5.7 | 7.769 | 42.41 |
| Final | 0.0015 | 0.0031 | 0 | 4.47 | 4.474 |
a“Total concentration” (g/L) refers to the sum of the concentrations of HMF, furfural, acetic acid and total phenols
b“Removed amount of toxic compounds” (%) refers to the difference between initial concentration and final total concentration (g/L)
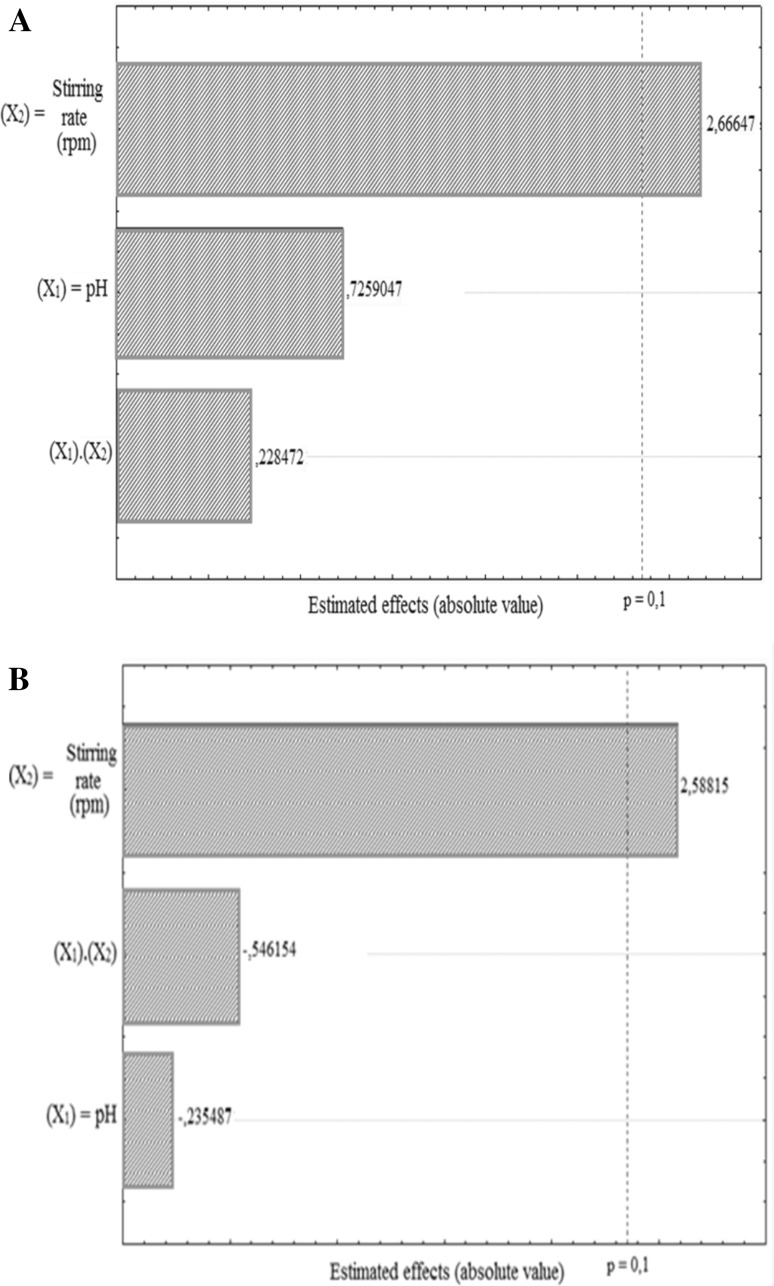
According to Pareto diagram, agitation (X2) showed significant reduction of inhibitors (90 %). However, pH (X1) and the interaction factor (X1 and X2) showed no significant removal of inhibitors for both strains of P. occidentalis. The agitation variable (X2) acted positively in the removal of toxic compounds (Fig. 3). Experiment 4 (pH 5.0, 300 rpm) demonstrated the most significant cumulative removal (42.89 %) of toxic compounds by P. occidentalis M1 (Table 5) allowing the removal of 94.77 % of 0.032 g/l 5-HMF, 99.19 % of 0.0925 g/l furfural, 29.17 % of 6.41 g/l phenolics and 100 % of 1.42 g/l acetic acid. On the other hand, experiment 3 (pH 4.0 and 300 rpm) showed the most significant cumulative removal (42.89 %) of toxic compounds by P. occidentalis Y1′a (46.04 %), i.e. 97.68 % of 0.037 g/l HMF, 96.06 % of 0.128 g/l furfural, 27.15 % of 6.52 g/l phenol and 100 % of 2.13 g/l acetic acid.
Fig. 3.
Pareto diagram representing the estimated effects of the variables and their interactions to a confidence level of 90 % (p = 0.1) for removal of toxic compounds by Pichia occidentalis strains M1 (a) and Y1′a (b)
Table 5.
Experimental matrix of full factorial 22 design of experiments with the variables’ values and the cumulative inhibitors removal (%) in each experiment by Pichia occidentalis M1 and P. occidentalis Y1′a
| Experiment | Real levels | Cumulative inhibitors removal (%) | ||
|---|---|---|---|---|
| X1 (pH) | X2 (rpm) | P. occidentalis M1 | P. occidentalis Y1′a | |
| 1 | 4.0 | 100 | 19.43 | 17.69 |
| 2 | 5.0 | 100 | 22.87 | 20.50 |
| 3 | 4.0 | 300 | 36.29 | 46.04 |
| 4 | 5.0 | 300 | 42.89 | 38.97 |
| 5 | 4.5 | 200 | 39.52 | 43.08 |
| 6 | 4.5 | 200 | 39.98 | 42.79 |
| 7 | 4.5 | 200 | 39.01 | 42.41 |
Discussions
Xylose and arabinose are the monomeric pentose sugars from hydrolysis of the hemicellulose fraction [9, 14]. Also, glucose produced in hydrolysate is from hemicellulose or/and cellulose fractions [15]. Acetic acid is derived from the hydrolysis of the acetyl groups linked to the monomers of this polymer [9]. Xylose sugar has the highest concentration in the hemicellulosic hydrolysates due to the considerable amount of xylan present in hemicellulose [15, 16]. After vacuum evaporation of hydrolysate, acetic acid, furfural and total phenolic compounds might have evaporated or eliminated during the filtration of concentrated hydrolysates, therefore did not show the fivefold increase in concentarion. The pH of the concentrated hydrolysate was slightly decreased. These results are in well accordance with the studies of Martiniano et al. [17] and Milessi et al. [18].
Vacuum concentration process is essential to increase the concentration of sugars in the lignocellulosic hydrolysates [19]. The concentration of xylose, glucose and arabinose increased in accordance with the concentration factor employed (fivefold). However, acetic acid, 5-HMF, furfural and phenolics concentrations did not show the same behavior. The concentration of acetic acid was increased, but not according to concentration factor used, which may be related, in part, to the volatility of it. Phenolics and 5-HMF concentrations showed the same behavior, suggesting a partial volatilization and/or degradation of 5-HMF [20]. Also, Furfural concentration might be reduced due to its volatile nature. Our results showed resemblance with the report of Parajó et al. [19] and Carvalho et al. [20]. Detoxification results of hdrolysates by four strains i.e. P. occidentalis M1, Y1′a, Y1′b and Y3′ corroborates with the work of Fonesca et al. [21].
Hou-Rui et al. [22] also, used P. occidentalis CCTCC M 206097 to remove fermentation inhibitors from sugarcane bagasse hemicellulosic hydrolysates and confirmed that this strain was able to remove 100 % of 2.0 g/l acetic acid and 0.02 g/l furfural. Likewise, Fonseca et al. [21] employed the same yeast strain P. occidentalis CCTCC M 206097 and reported 6.1 % removal of 3.3 g/l acetic acid, 100 % of 0.02 g/l of HMF and 100 % of 0.16 g/l furfural, after 72 h of biological detoxification. These results showed that P. occidentalis is a promising candidate for the in situ removal of toxic compounds from hemicellulosic hydrolysate. It is important to note that 5-HMF and furfural removal are not only related to the type of yeast used in the process, but also the kind of lignocelullosic material used to obtain the hemicellulosic hydrolysates. The different hemicellulosic hydrolysates provide different percentages removal by P. occidentalis [21].
This study demonstrates the potential of the yeasts for removal of inhibitors under the experimental conditions, pH (4.0 or 5.0) and agitation rate (100 rpm or 300 rpm), employing microbial detoxification approach. P. occidentalis M1 and P. occidentalis Y1′a exhibited the significant cumulative removal of inhibitors by 42.89 and 46.04 %, respectively. The major advantage of inhibitors removal by biological treatment is the cost-effectiveness and the minimization of filtration steps. Whereas, other detoxification methods required substantial investments. Also, biological treatment is an eco-friendly, economically-viable and can be used for the industrial applications.
Acknowledgments
Authors thank to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – BIOEN Project Grants: 2008/57926-4 and 2010/13828-9), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Process No. 150745/2015-0 and 401308/2014-6) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support.
Compliance with Ethical Standards
Conflict of interest
The authors also declare that they have no conflict of interest in the publication.
Contributor Information
Swapnil C. Gaikwad, Email: gaikwad.swapnil1@gmail.com
Silvio S. da Silva, Phone: +55 12 31595146, Email: silviosilverio@usp.br
References
- 1.Demirbas A. Bioethanol from cellulosic materials: a renewable motor fuel from biomass. Energy Sources. 2005;27:327–337. doi: 10.1080/00908310390266643. [DOI] [Google Scholar]
- 2.Gírio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Lukasik R. Hemicelluloses for fuel ethanol: a review. Bioresour Technol. 2010;101:4775–4800. doi: 10.1016/j.biortech.2010.01.088. [DOI] [PubMed] [Google Scholar]
- 3.Canilha L, Chandel AK, Milessi TSS, Antunes FAF, Freitas WLC, Felipe MGA, da Silva SS. Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification and ethanol fermentation. J Biomed Biotechnol. 2012;1:1–15. doi: 10.1155/2012/989572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamman AS, Lee JM, Kim YC, Hwang IT, No-J P, Hwang YK, Chang JS, Hwang JS. Furfural: hemicellulose/xylose derived biochemical. Biofuels Bioprod Biorefin. 2008;2:438–454. doi: 10.1002/bbb.95. [DOI] [Google Scholar]
- 5.Chandel AK, Kapoor RK, Singh A, Kuhad RC. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol. 2007;98:1947–1950. doi: 10.1016/j.biortech.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 6.Cardona CA, Quintero JA, Paz IC. Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour Technol. 2010;101:4754–4766. doi: 10.1016/j.biortech.2009.10.097. [DOI] [PubMed] [Google Scholar]
- 7.Larsson S, Reimann A, Nilvebrant N, Jönsson LJ. Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl Biochem Biotechnol. 1999;77:91–103. doi: 10.1385/ABAB:77:1-3:91. [DOI] [Google Scholar]
- 8.Melo WGP, Arcuri SL, Rodrigues A, Morais PB, Meirelles LA, Pagnocca FC. Starmerella aceti f.a., sp. nov., an ascomycetous yeast species isolated from fungus garden of the leafcutter ant Acromyrmex balzani. Int J Syst Evol Microbiol. 2014;64:1428–1433. doi: 10.1099/ijs.0.058818-0. [DOI] [PubMed] [Google Scholar]
- 9.Almeida JRM, Modig T, Petersson A, Hän-Hagerdal B, Lidén G, Gorwa-Grauslund MF. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol. 2007;82:340–349. doi: 10.1002/jctb.1676. [DOI] [Google Scholar]
- 10.Mussatto SI, Roberto IC. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol. 2004;93:1–10. doi: 10.1016/j.biortech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Chandel AK, da Silva SS, Singh OV. Detoxification of lignocellulose hydrolysates: biochemical and metabolic engineering towards white biotechnology. BioEnergy Res. 2013;6:388–401. doi: 10.1007/s12155-012-9241-z. [DOI] [Google Scholar]
- 12.Kurtzman CP, Robnett CJ, Basehoar-Powers E. Phylogenetic relationships among species of Pichia, Issatchenkia and Williopsis determined from multigene sequence analysis, and the proposal of Barnettozyma gen. Nov., Lindnera gen. nov. and Wickerhamomyces gen.nov. FEMS Yeast Res. 2008;8:939–954. doi: 10.1111/j.1567-1364.2008.00419.x. [DOI] [PubMed] [Google Scholar]
- 13.Gouveia ER, Nascimento RT, Souto-Maior AM, Rocha GJM. Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Quim Nova. 2009;32:1500–1503. doi: 10.1590/S0100-40422009000600026. [DOI] [Google Scholar]
- 14.Luo C, Brink DL, Blanch HW. Identification of potential fermentation inhibitors in conversion of hybrid poplar hydrolyzate to ethanol. Biomass Bioenergy. 2002;22:125–138. doi: 10.1016/S0961-9534(01)00061-7. [DOI] [Google Scholar]
- 15.Aguilar R, Ramírez JA, Garrote G, Vázquez M. Kinetic study of the acid hydrolysis of sugarcane bagasse. J Food Eng. 2002;55:309–318. doi: 10.1016/S0260-8774(02)00106-1. [DOI] [Google Scholar]
- 16.Brienzo M, Siqueira AF, Milagres AMF. Search for optimum conditions of sugarcane bagasse hemicellulose extraction. Biochem Eng J. 2009;46:199–204. doi: 10.1016/j.bej.2009.05.012. [DOI] [Google Scholar]
- 17.Martinez A, Rodriguez ME, York SW, Preston JF, Ingram LO. Effects of Ca(OH)2 treatments (“Overliming”) on the composition and toxicity of bagasse hemicellulose hydrolysates. Biotechnol Bioeng. 2000;69:526–536. doi: 10.1002/1097-0290(20000905)69:5<526::AID-BIT7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Martiniano SE, Philippini RR, Chandel AK, Soares LCR, Pagnocca FC, da Silva SS. Evaluation of new xylose fermenting yeast strains from Brazilian ecosystems for ethanol production from sugarcane bagasse hemicellulose hydrolysate. 3 Biotech. 2013;3:345–352. doi: 10.1007/s13205-013-0145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parajó JC, Dominguez H, Domínguez JM. Improved xylitol production with Debaryomyces hansenii Y-7426 from raw or detoxified wood hydrolysates. Enzyme Microb Technol. 1997;21:18–24. doi: 10.1016/S0141-0229(96)00210-4. [DOI] [Google Scholar]
- 20.Carvalho GBM, Mussato SI, Cândido EJ, Silva JBA. Comparison of different procedures for the detoxification of Eucalyptus hemicellulosic hydrolysate for use in fermentative processes. J Chem Technol Biotechnol. 2006;81:152–157. doi: 10.1002/jctb.1372. [DOI] [Google Scholar]
- 21.Fonseca BG, Moutta RO, Ferraz FO, Vieira ER, Nogueira AS, Baratella BF, Rodrigues LC, Hou-Rui Z, da Silva SS. Biological detoxification of different hemicellulosic hydrolysates using Issatchenkia occidentalis CCTCC M 2006097 yeast. J Ind Microbiol Biotechnol. 2011;38:199–207. doi: 10.1007/s10295-010-0845-z. [DOI] [PubMed] [Google Scholar]
- 22.Hou-Rui Z, Xiang-Xiang Q, da Silva SS, Sarrouh BF, Ai-Hua C, Yu-Heng Z, Ke J, Qiu X. Novel isolates for biological detoxification of lignocellulosic hydrolysate. Appl Biochem Biotechnol. 2009;152:199–212. doi: 10.1007/s12010-008-8249-5. [DOI] [PubMed] [Google Scholar]