Abstract
The present study was conducted to examine the ability of six promising indigenous isolates of Beauveria bassiana (NBAII-Bb-5a, 7, 14, 19, 23 and 45) as an endophyte in maize stem and leaf tissues. Maize seedlings (var. Nithyashree) were inoculated with conidial suspensions and were examined for endophytic establishment in leaf and stems at different intervals during 15–90 days after treatment. All six isolates showed colonization in stem and leaf tissues with varying abilities of colonization and persistence. The mean percent colonization ranged from 7.41 to 20.37 % in older stem tissues and 3.70 to 21.29 % in young stem tissues and in leaf, it ranged from 6.46 to 27.78 % in older leaf tissues and 11.11 to 26.85 % in young leaf tissues. Among six isolates tested, Bb-23 isolate recorded the maximum mean colonization in older stem (20.37 %), older leaf (27.78 %) and in young stem (21.29 %). Bb-5a isolate showed maximum mean colonization in young leaf tissues (26.85 %). Persistence of inoculated fungal isolates decreased with increase in age of the plant. No physical symptoms of damage were observed in any of the B. bassiana treated plants. No colonization of B. bassiana was observed in the untreated control maize plants. The results obtained in plating and PCR techniques were similar with regard to the confirmation of endophytic establishment of B. bassiana. This study indicated the possibility of using B. bassiana as an endophyte in maize for management of maize stem borer, Chilo partellus.
Keywords: Entomopathogenic fungi, Beauveria bassiana, Endophyte, Maize
Introduction
Maize is an important cereal crop cultivated all over the world and it is the third major crop in India [1]. Maize has great importance for grain and fodder purpose and it is also used for production of oil, alcohol, acetic, lactic acid, glucose, starches for edible and laundry purpose, adhesives and methanol. Around 140 insect pests infest maize, among them ten pest species cause severe yield loss. Chilo partellus (Swinhoe) is one of the most important pests causing yield loss in maize and it mainly attacks the crop during kharif season [2]. The pest management strategies against this borer pest were focused mainly on the use of chemical insecticides. Control of borer pests by chemical insecticides is extremely difficult because of its cryptic life cycle, expensive and has adverse effects on environment as well as human health. Hence there is a need for development of an alternate, safe protection technology using endophytic entomofungal pathogens. In recent years, endophytic strains of Beauveria bassiana have been used for management of insect pests like, European corn borer, Ostrinia nubilalis in maize [3] and banana weevil Cosmopolites sordidus [4]. Endophytic colonization of entomopathogenic fungi within the plant system has more advantageous than external application because it gives season long protection against pests and it is cost effective. In India, the entomopathogenic fungus, B. bassiana has not been exploited as an endophyte in maize for stem borer (C. partellus) management. Six indigenous isolates of B. bassiana were identified as promising against C. partellus in the laboratory bioassay studies [5]. The present study was undertaken to determine the ability of these six promising isolates of B. bassiana to establish as an endophytes in maize.
Materials and Methods
A glasshouse experiment was conducted at National Bureau of Agricultural Insect Resources (NBAIR), Bengaluru, India to examine the endophytic ability of different isolates of entomopathogenic fungus B. bassiana in stem and leaf tissues of maize.
Seeds of the maize (Zea may L.) cultivar Nithyashree were obtained from University of Agricultural Sciences, Bengaluru, Karnataka. Maize seeds were surface sterilized with sodium hypochlorite (3 %) for 2 min, then with ethanol (70 %) for 2 min followed by two washing with sterile distilled water. The surface sterilized seeds were dried and then sown in plastic pots (15 cm diameter) filled with sterile soil (autoclaved at 121 °C for 20 min). The pots were kept at 21–22 °C, 60–80 % RH in the glasshouse and watered frequently. Three replications (Ten plants per replication) were maintained for each treatment.
The six indigenous isolates of B. bassiana (NBAII-Bb-5a, 7, 14, 19, 23 and 45) used in this study were initially isolated from different insect cadavers and soil samples from different geographical regions of India (Table 1). These fungal cultures were maintained on Sabouraud’s Dextrose Yeast Agar (SDYA) (Dextrose 40 g, Mycological peptone 10 g, yeast extract 5 g, agar 20 g in 1000 ml of distilled water) slants at −20 °C at the culture repository of ICAR-NBAIR, Bengaluru until further use. Inoculums for succeeding studies were always prepared from the original culture.
Table 1.
Details of isolates of B. bassiana used in the study
| Sl no. | Isolates code | Source | Location | Genbank accession number |
|---|---|---|---|---|
| 1 | NBAII-Bb5a | Hypothenemus hampei (coffee berry borer) | Madikeri, Karnataka | JF837134 |
| 2 | NBAII-Bb7 | Plocaederus ferrugines (cashew root and stem borer) | Puttur, Karnataka | JF837097 |
| 3 | NBAII-Bb14 | Unknown insect | Doddaballapura, Karnataka | JF837092 |
| 4 | NBAII-Bb19 | Banana rhizosphere soil | Trichy, Tamil Nadu | KC121555 |
| 5 | NBAII-Bb23 | Maruca testulalis (legume pod borer) | Karaikal, Puducherry | JF837082 |
| 6 | NBAII-Bb45 | Carrot rhizosphere soil | Nedugula, Tamil Nadu | JF837094 |
Each isolate was grown on sterilized rice for 15 days at 25 ± 1 °C. The conidial suspension of each isolate required for the experiment was prepared by suspending 1 g of 15 days old conidiated rice in sterile distilled water with 0.1 % Tween 80. Conidial suspension was filtrated through three layered muslin cloth to get hyphal-free conidial suspension. The conidial concentration in the suspension was adjusted to 1 × 108 conidia/ml using Neubauer’s improved haemocytometer under light microscope.
Plant Inoculation
The conidial suspension of each isolate was sprayed on the maize seedling (1 × 108 conidia/ml; 5 ml/seedling) at 15 and 30 days after germination. The control plants were sprayed with sterile distilled water with 0.01 % Tween 80. Top of the each pot was covered with aluminum foil to avoid conidial contact with soil in the pot [6].
Studies on Endophytic Establishment
Endophytic colonization of the six isolates of B. bassiana in maize was studied at 15, 30, 45, 60, 75 and 90 days after treatment. Three plants were randomly selected at each sampling period from each isolate. Plants were uprooted from pots and washed thoroughly with running tap water. From each plant, two older leaves (emerged before spraying) and two younger leaves (emerged after spraying) and two pieces of older stem (emerged before spraying) and two pieces of growing tip of the stem (emerged after spraying) were collected for endophytic colonization studies.
By Plating Technique
The leaf and stem samples were first surface sterilized with sodium hypochlorite (1 %) for 5 min and then with ethanol (70 % v/v) for 30 s. The surface sterilized samples were washed three times in sterilized distilled water for a minute each. The samples then, were cut into 5 mm pieces and transferred aseptically into petri dishes containing SDYA medium with chloramphenicol (100 mg/ml). The final washed water was also plated (0.1 ml) on SDYA plates to check the effectiveness of surface sterilization. The plates were maintained at 25 ± 1 °C in a biological oxygen demand (BOD) incubator. The fungal growth from the plated bits of stem and leaf were examined under microscope for confirmation of B. bassiana growth. Percent colonization of each isolate in the older and young leaf/stem bits at different sampling times was calculated based on the number bits yielding B. bassiana and total number of bits plated. The percent colonization data were arcsine transformed and were analyzed by two-way ANOVA (analysis of variance) following completely randomized design [7].
By PCR Technique
Genomic DNA of B. bassiana was extracted from surface sterilized young and adult leaf and stem bits of both treated as well as untreated maize plants. DNA was extracted according to the instructor manual of CTAB (Cetyl Trimethyl Ammonium Bromide) method [7]. 300 mg of surface sterilized samples were grinded in pre-warmed CTAB extraction buffer and kept for incubation at 65 °C for 60–90 min in water bath with occasional stirring. After that allow the samples to cool, 5 ml of chloroform: isoamylalcohol (24:1 ratio) mixture was added. The samples were centrifuged at 7000 rpm for 15 min at 20 °C. The supernatant was carefully taken into new test tube containing 25 µl RNase (5 mg/ml) and kept for incubation at room temperature for 30 min. To this 6 ml of isopropanol was added and centrifuged at 7000 rpm for 10 min at 4 °C. Discard the supernatant, 8 ml of cold CTAB wash buffer was added into pellet and kept for 20 min at room temperature. The samples were centrifuged at 7000 rpm for 3 min at 4 °C. Discarded the supernatant, then washed the pellet with 70 % cold ethanol and centrifuged at 3000 rpm for 5 min at 4 °C. The supernatant was discarded, dried the pellet at room temperature. Finally the DNA pellet was dissolved in 100 μl of (10 mM Tris–HCl + 0.1 mM EDTA at pH 8.0) TE buffer and stored at −20 °C.
Endophytic B. bassiana DNA was amplified by PCR by using B. bassiana specific SCAR (sequence-characterized amplified region) primer (SCA15441 (F 5′ TTCCGAACCCGGTTAAGAGAC 3′, R 5′ TTCCGAACCCATCATCCTGC 3′) [7]. Final volume of PCR mixture (50 μl) consisting of 50 ng of fungal genomic DNA, 50 pmol each of SCAR primers, 1.25 mM for each of dATP, dGTP, dCTP, dTTP, 2.5 units of Taq DNA polymerase, 5 μl of polymerase buffer, 2.5 Mm MgCl2 and sterile water to makeup 50 μl of total volume. The PCR amplifications program was consisting of initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 52 °C for 1 min, extension at 72 °C for 3 min, and final extension at 72 °C for 10 min and store at 4 °C. Quantarus Thermal cycler was used to carry out the cycles. PCR product was visualized in 1.4 % agarose gel with ethidium bromide. The molecular weight of the amplified DNA fragment size was calculated according to Ling et al. [8].
Results
By Plating Technique
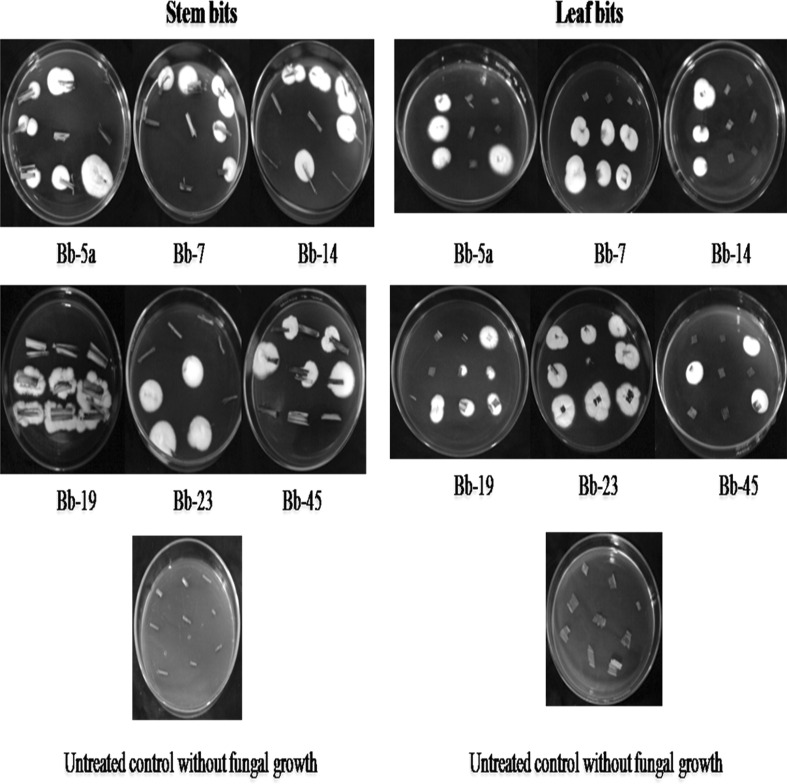
White hyphal growth was noticed from both older and young stem/leaf bits of the treated maize plants (Fig. 1). Microscopic examination of this white hyphal growth the bits showed typical conidiophores, phialides and conidia of B. bassiana indicating the endophytic colonization of the fungus in stem and leaf tissues of maize. But no such B. bassiana growth was found from the stem/leaf bits of the un-treated control maize plants (Fig. 1).
Fig. 1.
B. bassiana growth (white fugal growth) from the treated stem and leaf bits
The results of the plating technique of stem and leaf are presented in Tables 2 and 3 respectively. All six isolates showed colonization in older and young stem/leaf tissues of the maize with varying per cent colonization and persistence during 15–90 DAT. The mean percent colonization of the six isolates ranged from 7.41 to 20.37 % in older stem tissues and 3.70–21.29 % in young stem tissues (Table 2). In leaf, the mean percent colonization ranged from 6.46 to 27.78 % in older leaf tissues and 11.11–26.85 % in young leaf tissues (Table 3). The results indicated that the isolates of B. bassiana colonized both older and younger stem/leaf tissues irrespective of whether the plant part received the spraying of B. bassiana or not. Among all the isolates tested, Bb-23 isolate recorded the maximum mean colonization in older stem (20.37 %) and in young stem (21.29 %), followed by Bb-45 (12.96 and 11.11 %), Bb-7 (12.02 and 16.67), Bb-14 (11.11 and 3.70), Bb-5a (10.18 and 12.04) and Bb-19 (7.41 and 9.26) in older and young stem tissues respectively (Table 2). With regard leaf, Bb-23 isolate showed the mean colonization of 27.78 % in older leaf and 24.07 % in young leaf and other isolates, Bb-45, Bb-5a, Bb-7, Bb-14 and Bb-19 showed 23.14 and 18.52, 22.22 and 26.85, 20.37 and 13.89, 14.81 and 11.11 and 6.46 and 12.04 % colonization in older and young leaf tissues respectively (Table 3). The untreated control samples of leaf and stem did not show colonization of any of the B. bassiana isolates.
Table 2.
Percentage colonization of B. bassiana in older/young stem tissues of maize
| Isolates | Days after treatment stem | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Older stem tissues | Young stem tissues | |||||||||||||
| 15 | 30 | 45 | 60 | 75 | 90 | Mean | 15 | 30 | 45 | 60 | 75 | 90 | Mean | |
| Bb5a | 5.55b | 38.88a | 16.66b | 0.00 | 0.00 | 0.00 | 10.18A | 11.11b | 22.22b | 22.22b | 5.55b | 11.11b | 0.00 | 12.04A |
| Bb7 | 5.55b | 5.55b | 61.01a | 0.00 | 0.00 | 0.00 | 12.02A | 11.11b | 11.11b | 66.66a | 0.00 | 11.11b | 0.00 | 16.67A |
| Bb14 | 0.00 | 27.77a | 38.88a | 0.00 | 0.00 | 0.00 | 11.11A | 0.00 | 22.22b | 0.00 | 0.00 | 0.00 | 0.00 | 3.70B |
| Bb19 | 0.00 | 27.77a | 16.66b | 0.00 | 0.00 | 0.00 | 7.41A | 0.00 | 33.33a | 22.22b | 0.00 | 0.00 | 0.00 | 9.26A |
| Bb23 | 11.11b | 50.00a | 49.99a | 11.11b | 0.00 | 0.00 | 20.37A | 11.11b | 33.33a | 66.66a | 16.66b | 0.00 | 0.00 | 21.29A |
| Bb45 | 22.21b | 22.21b | 0.00 | 33.33a | 0.00 | 0.00 | 12.96A | 0.00 | 22.22b | 0.00 | 44.44a | 0.00 | 0.00 | 11.11A |
| Control | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Values in columns followed by the different letter (a, b, c) are significantly different with each other according to LSD (P < 0.01)
Values in columns followed by the different letter (A, B) are significantly different with each other according to LSD (P < 0.01)
Table 3.
Percentage colonization of B. bassiana in older/young leaf tissues of maize
| Isolates | Days after treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Older leaf tissues | Young leaf tissues | |||||||||||||
| 15 | 30 | 45 | 60 | 75 | 90 | Mean | 15 | 30 | 45 | 60 | 75 | 90 | Mean | |
| Bb5a | 55.55a | 38.88b | 38.88b | 0.00 | 0.00 | 0.00 | 22.22A | 55.55a | 22.22b | 77.77a | 5.55c | 0.00 | 0.00 | 26.85A |
| Bb7 | 16.66b | 44.44b | 16.66b | 44.44b | 0.00 | 0.00 | 20.37A | 0.00 | 22.22b | 11.11c | 49.99b | 0.00 | 0.00 | 13.89A |
| Bb14 | 16.66b | 55.55a | 16.66b | 0.00 | 0.00 | 0.00 | 14.81B | 0.00 | 44.44b | 11.11c | 11.10c | 0.00 | 0.00 | 11.11A |
| Bb19 | 0.00 | 27.77b | 11.01c | 0.00 | 0.00 | 0.00 | 6.46B | 0.00 | 22.22b | 44.44b | 5.55c | 0.00 | 0.00 | 12.04A |
| Bb23 | 0.00 | 77.77a | 77.77a | 11.11c | 0.00 | 0.00 | 27.78A | 0.00 | 44.44b | 88.88a | 11.11c | 0.00 | 0.00 | 24.07A |
| Bb45 | 33.33b | 38.88b | 22.21b | 44.44b | 0.00 | 0.00 | 23.14A | 22.22b | 33.33b | 33.33b | 0.00 | 11.11c | 11.11c | 18.52A |
| Control | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Values in columns followed by the different letter (a, b, c) are significantly different with each other according to LSD (P < 0.01)
Values in columns followed by the different letter (A, B) are significantly different with each other according to LSD (P < 0.01)
Bb-5a isolate showed continuous colonization in older stem and leaf tissues up to 45 DAT, whereas in young stem and leaf it showed colonization up to 75 and 60 DAT respectively. Bb-7 isolate showed colonization up to 45 and 75 DAT in older and young stem tissues respectively, whereas in older and young leaf tissues it showed colonization up to 60 DAT. Bb-14 isolate persisted up to 45 and 30 DAT in older and young stem tissues respectively, whereas in older and young leaf tissues it persistence up to 45 and 60 DAT, respectively. Bb-19 isolate persistence up to 45 DAT in older and young stem tissues, in older and young leaf tissues it persistence up to 45 and 60 DAT. Bb-23 isolates showed continuous colonization up to 60 DAT in both older and young stem and leaf tissues. Bb-45 showed colonization up to 60 DAT in older and young stem tissues, whereas in older and young leaf it showed colonization up to 60 and 90 DAT.
Bb-5a isolate has recorded significantly higher percent colonization in older stem tissues (38.88 %) at 30 DAT and in older leaf tissues (61.01 %) and in young leaf tissues (77.77 %) at 15 and 45 DAT. Bb-7 isolate showed higher percent colonization in older and young stem tissues (61.01 and 66.66 % respectively) at 45 DAT. Bb-14 isolate showed higher percent colonization in older stem tissues (27.77 and 38.88 %) at 30 and 45 DAT respectively, whereas in older leaf tissues it showed higher percent colonization (55.55 %) at 30 DAT. Bb-19 isolate showed higher percent colonization at 30 DAT in both older and young stem tissues (27.77 and 33.33 % respectively). Bb-23 isolate showed higher percent colonization at 30 and 45 DAT in older (50.00 and 49.99 % respectively) and young stem tissues (33.33 and 66.66 % respectively), whereas in leaf it showed higher percent colonization at 30 and 45 DAT in older leaf tissues (77.77 %) and at 45 DAT in young leaf tissues (88.88 %). Bb-45 isolate showed higher percent colonization at 60 DAT in both older stem tissues (33.33 %) and young stem tissues (44.44 %). It was found that, the persistence of inoculated fungi decreased with the increase in age of the plant (Tables 2, 3). The plants treated with B. bassiana isolates did not show any pathogenic symptoms.
By PCR Technique
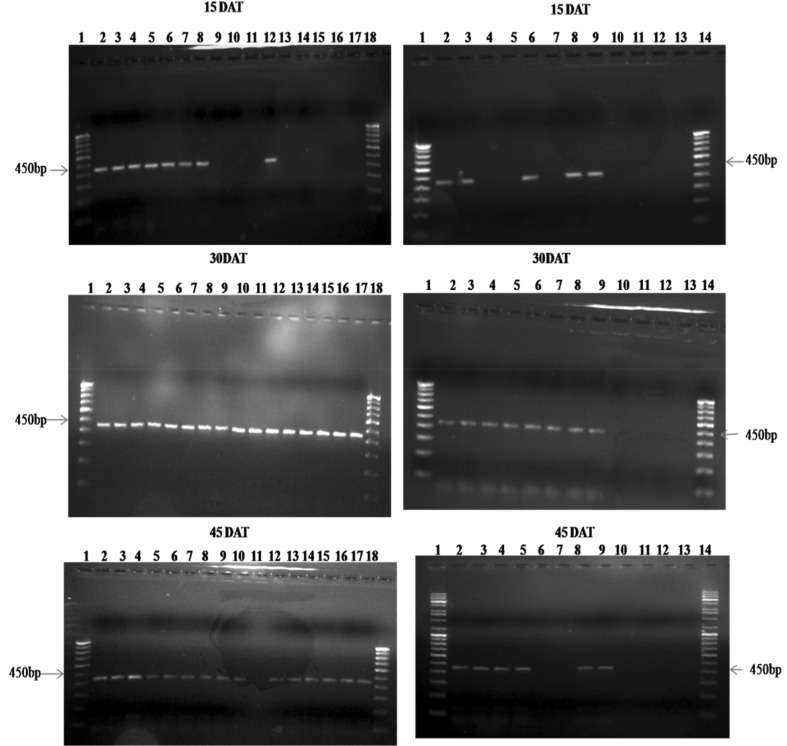
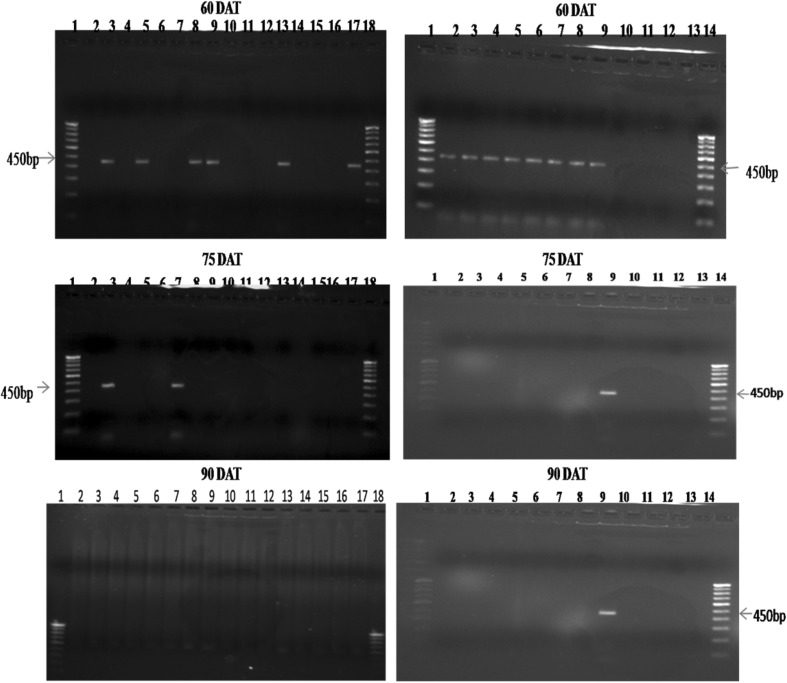
B. bassiana specific SCAR primer SCA15441 amplified the genomic DNA extracted from B. bassiana treated maize leaf and stem tissues (from both older and young tissue samples) and it failed to amplify the DNA extracted from untreated control leaf and stem tissues. This indicated that, the SCAR primer does not bind to any other genomic DNA except for the genomic DNA of the entomopathogenic fungus, B. bassiana. During the sampling periods (at 15, 30, 45, 60, 75 and 90 DAT), B. bassiana specific amplicon (450 pb) was obtained from leaf and stem tissues (from both older and young tissue samples) of treated plants using SCA15441 (Figs. 2, 3). The plant tissues which showed B. bassiana colonization in plating technique were confirmed by PCR amplification.
Fig. 2.
PCR amplification of genomic DNA extracted from older and young stem and leaf tissues of B. bassiana treated maize. Lanes 1–18: 1 100 bp ladder, 2 Bb5a older stem, 3 Bb5a young stem, 4 Bb5a older leaf, 5 Bb5a young leaf, 6 Bb7 older stem, 7 Bb7 young stem, 8 Bb7 older leaf, 9 Bb7 young leaf, 10 Bb14 older stem, 11 Bb14 young stem, 12 Bb14 older leaf, 13 Bb14 young leaf, 14 Bb19 older stem, 15 Bb19 young stem, 16 Bb19 older leaf, 17 Bb19 young leaf, 18 100 bp ladder. Lanes 1–14: 1 100 bp ladder, 2 Bb23 older stem, 3 Bb23 young stem, 4 Bb23 older leaf, 5 Bb23 young leaf, 6 Bb45 older stem, 7 Bb45 young stem, 8 Bb45 older leaf, 9 Bb45 young leaf, 10 control older stem, 11 control young stem, 12 control older leaf, 13 control young leaf, 14 100 bp ladder
Fig. 3.
PCR amplification of genomic DNA extracted from older and young stem and leaf tissues of B. bassiana treated maize. Lanes 1–18: 1 100 bp ladder, 2 Bb5a older stem, 3 Bb5a young stem, 4 Bb5a older leaf, 5 Bb5a young leaf, 6 Bb7 older stem, 7 Bb7 young stem, 8 Bb7 older leaf, 9 Bb7 young leaf, 10 Bb14 older stem, 11 Bb14 young stem, 12 Bb14 older leaf, 13 Bb14 young leaf, 14 Bb19 older stem, 15 Bb19 young stem, 16 Bb19 older leaf, 17 Bb19 young leaf, 18 100 bp ladder. Lanes 1–14: 1 100 bp ladder, 2 Bb23 older stem, 3 Bb23 young stem, 4 Bb23 older leaf, 5 Bb23 young leaf, 6 Bb45 older stem, 7 Bb45 young stem, 8 Bb45 older leaf, 9 Bb45 young leaf, 10 control older stem, 11 control young stem, 12 control older leaf, 13 control young leaf, 14 100 bp ladder
Discussion
The current study indicated that, all six indigenous isolates of B. bassiana were able to colonize in stem and leaf tissues of maize, when applied as spore suspension by foliar spray. Since the untreated control maize plants did not yield any B. bassiana growth in both plating and PCR methods, it is assumed that B. bassiana got established as endophyte in the treated maize plants by artificial inoculation. The present studies are congruent with Bing and Lewis, 1991 [9] who described endophytic colonization of B. bassiana in corn.
The six isolates tested showed considerable variations in their ability to colonize and persist in stem and leaf tissues of maize. Among six isolates tested, Bb-23 isolate recorded the maximum mean colonization in older stem (20.37 %), older leaf (27.78 %) and in young stem (21.29 %) and Bb-5a isolate in young leaf tissues (26.85 %). The maximum persistence of endophytic colonization in young leaf tissues was observed up to 90DAT with Bb-45 isolate and in the older leaf tissues up to 60DAT with Bb-7, Bb-23 and Bb-45 isolates. In young stem tissues, persistence up to 75 DAT was observed with Bb-7 and Bb-5a isolates and in older stem tissues, persistence up to 60 DAT was observed with Bb-23 and Bb-45 isolates. The endophytic colonization of the isolates may be depended on their ability to adjust to the nitch area of stem and leaf tissues of the host plant and the reason for higher colonization of particular isolate in particular plant part could be caused by diverse microbial and physiological environment present inside the plant parts. Several endophytic fungi show a certain degree of tissue specificity because they are adapted to particular conditions present inside the plant tissues [10].
In general, all isolates showed higher percent colonization in maize stem and leaf at 30–45 days of crop age which coincides with stem borer (C. partellus) infestation [11]. This coincidence could be a key factor for management of borer pest by endophytically established B. bassiana. Persistence of inoculated fungal isolates decreased with increase in age of the plant. It might be due to non availability of nutrients to the fungus at maturation period, competition between the inoculated fungus and other natural endophytes and varying host response at different stages of plant [7].
Our study showed that in the fungal treated maize plants, B. bassiana can be detected in both old and young stem/leaf tissues, irrespective of the plant part receiving B. bassiana spray or not. This indicates the endophytic spread of the B. bassiana isolates in the maize plant. Wagner and Lewis, 2000 [12] reported passive movement of B. bassiana in the corn plant through xylem vessels. Such B. bassiana isolates with systemic properties can fit into the Integrated Pest Management (IPM) of maize stem borer. Further studies are required in this direction.
Acknowledgments
The authors are thankful to Director, National Bureau of Agricultural Insect Resources, Bangalore, Karnataka, India for the constant support provided throughout the research.
Contributor Information
S. Renuka, Email: renuraj17@gmail.com
B. Ramanujam, Email: bonamramanujam58@gmail.com
B. Poornesha, Email: poorniintbiotech@gmail.com
References
- 1.Ali N, Singh G, Singh SP, Dhaka SS, Ram M, Tawar KB (2014) Efficacy of different management practices against Chilo partellus (Swinhoe) in Kharif maize in Western Uttar Pradesh. Int J Adv Res 2: 952–956. ISSN 2320-5407
- 2.Sk Jalali, Sihgh SP. Bio-ecology of Chilo partellus (Swinhoe) (Lepibbptera; Pyralioae) and evaluation of its natural enemies: a review. Agric. Rev. 2003;24:79–100. [Google Scholar]
- 3.Lewis L, Bruck D, Gunnarson R. On-farm evaluation of Beauveria bassiana for control of Ostrinia nubilalis in Iowa, USA. BioControl. 2002;47:167–176. doi: 10.1023/A:1014574526071. [DOI] [Google Scholar]
- 4.Akello J, Dubois T, Gold CS, Coyne D, Nakavuma J, Paparu P. Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp.) J Invertebr Pathol. 2007;96:34–42. doi: 10.1016/j.jip.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Renuka S, Ramanujam B, Poornesha B. Screening of Beauveria bassiana (Balsamo) Vuillemin isolates against maize Stem Borer, Chilo partellus (Lepidoptera: Pyralidae) and the effect of solid substrates on conidial production and virulence. J Pure App Microbiol. 2015;9:2979–2986. [Google Scholar]
- 6.Tefera T, Vidal S. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. BioControl. 2009;54:663–669. doi: 10.1007/s10526-009-9216-y. [DOI] [Google Scholar]
- 7.Biswas C, Dey P, Satpathy S, Satya P. Establishment of the fungal entomopathogen Beauveria bassiana as a season long endophyte in jute (Corchorus olitorius) and its rapid detection using SCAR marker. BioControl. 2012;57:565–571. doi: 10.1007/s10526-011-9424-0. [DOI] [Google Scholar]
- 8.Ling J, Ling KLE, Chan KW, French GL. Computer programs for accurate determination of size of DNA fragments in agarose gels. J Clin Pathol. 1987;40:692–695. doi: 10.1136/jcp.40.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bing LA, Lewis LC. Suppression of Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) vuillemin. Environ Entomol. 1991;20:1207–1211. doi: 10.1093/ee/20.4.1207. [DOI] [Google Scholar]
- 10.Carroll FE, Muller E, Sutton BC. Preliminary studies on the incidence of needle endophytes in some European conifers. Sydowia. 1977;29:89–103. [Google Scholar]
- 11.Kumar VK, Sharma HC, Reddy KD. Antibiosis mechanism of resistance to spotted stem borer, Chilo partellus in sorghum, Sorghum bicolor. Crop Prot. 2006;25:66–72. doi: 10.1016/j.cropro.2005.04.001. [DOI] [Google Scholar]
- 12.Wagner BL, Lewis LC. Colonization of corn, Zea mays, by the entomopathogenic fungus Beauveria bassiana. Appl Environ Microbiol. 2000;66:3468–3473. doi: 10.1128/AEM.66.8.3468-3473.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]