Abstract
Objective
Surveillance of patients identified with small abdominal aortic aneurysm (AAA) from an AAA screening program poses a challenge for health systems due to numerous patient follow-ups. This study evaluates the surveillance outcomes of patients identified with small AAA from a large screening program.
Methods
A retrospective chart review of all patients screened for small AAA (3.0 – 5.4 cm) from 2007–2011 was conducted. Patients with small AAA and no previous history of repair were tracked for follow-up using the 2013 RESCAN follow-up guidelines according to aortic diameter: (3.0 – 3.9 cm, 3 years; 4.0 –4.4 cm, 2 years; 4.5 – 5.4 cm, 1 year). Socioeconomic factors including marital status, distance to hospital from residence, estimated household income, and employment disability status that may influence the follow-up rate and all-cause mortality after screening were also evaluated.
Results
A total of 568 patients (mean±stdev: 73.4±7.2 years old) with small AAA (3.6±0.6 cm) were analyzed. Patient follow-up rate was 65.1% (n=370/568). Reasons for follow-up failure were: lack of physician ordering scan (n=139, 70.2%), delayed ordering of scans (n=36, 18.2%), patient no-show (n=18, 9.1%), or patient death prior to follow-up (n=5, 2.5%). Of all patient-specific factors, patients with smaller diameters were unlikely to achieve follow-up scans (p<.001). A significantly higher risk of all-cause mortality was found for patients with no ultrasound follow-up scan (hazard ratio, p-value: 0.369, p<0.001), assisted living (0.381, p<0.001), older age (1.04, p=0.001), and lower household incomes (0.989, p=0.01).
Conclusions
The follow-up rate of small AAA patients was poor at 65.1%. The data indicate that socioeconomic factors do not significantly affect follow-up success. Therefore, physician ordering of scans may exert the greatest influence on follow-up rates in patients with small AAA. Automatic ordering of follow-up scans for small AAA patients is proposed to improve follow-up rates.
Introduction
The knowledge gained from the major abdominal aortic aneurysm (AAA) screening clinical trials1–4 has led to a substantial reduction in AAA-related mortality in the older male population. Overall, AAA screening programs have yielded AAA detection rates of around 4–8% of all screened patients.5 Ultrasound screening is dependable at identifying patients with AAA ≥5.5 cm in maximum aortic diameter, but the vast majority of diagnosed AAA patients have aneurysms that range from 3.0 – 5.4 cm.6 Surveillance imaging, risk factor modification, and drug therapy are the recommended regimen for patients with such small AAA.7 Surveillance guidelines created by the Society of Vascular Surgeons (SVS) in 2009 were developed to assist clinicians with tracking patients with small AAA8. The 2013 RESCAN Collaborators meta-analysis study further refined the surveillance intervals by determining when the risk of AAA rupture reaches 1% by the next follow-up scan.9 However, an influx of many newly diagnosed AAA patients from a population screening program can pose burdens in any health care system.
Tracking patients with small AAA remains a challenge to clinicians. The RESCAN study revealed that time intervals vary globally on performing follow-up imaging scans of patients with small AAA.9 An analysis of the Veterans Affairs (VA) Northern California Health Care System (VANCHCS) AAA screening program previously determined that a large number of inappropriate screens were ordered during the first 5 years of implementation and some detected aneurysms were not given an appropriate follow-up imaging study.10 We suspect that some physicians are unsure of the current United States Preventative Services Task Force (USPSTF) AAA screening criteria1l and may be unfamiliar with current AAA surveillance guidelines. The purpose of this study was to evaluate the surveillance outcomes of patients identified with small AAA from a large screening program.
Methods
This study was conducted under an approved, waived informed consent protocol by the VANCHCS institutional review board. An electronic medical record (EMR) retrospective chart review of all veterans screened for AAA between January 1, 2007 and December 31, 2011. A billing code specific to AAA screening was utilized to obtain a list of all patients screened for AAA. Patients identified from this list with small AAA (3.0 – 5.4 cm in maximum aortic diameter) with no history of AAA repair were analyzed in this study for follow-up adherence. Patients with previous history of AAA repair were removed from the final analysis.
Clinical follow-up was evaluated using the recommended surveillance time intervals based on aneurysm size, from the 2013 RESCAN guidelines9. Briefly, imaging surveillance intervals should be: 3 years (3.0– 3.9 cm), 2 years (4.0– 4.4 cm), and 1 year (4.5 – 5.4 cm). These intervals were established based upon the rationale on maintaining the risk of AAA rupture less than 1% from initial screening diameter diagnosis to the next follow-up scan. A follow-up for AAA was achieved if the abdominal aorta was visualized and documented by the radiologist at least once within the defined RESCAN surveillance interval, after the initial AAA screening study. A follow-up imaging study consisted of either an ultrasound or computerized tomography (CT) scan of the aorta. Incidental aortic imaging (scans not ordered by a physician specifically for AAA) also counted towards follow-up if this met surveillance time-intervals and visualization of the aorta was documented by a radiologist in the medical record.
Follow-up for small AAA is initiated when an EMIR clinical reminder is sent to the primary care physician that becomes active at an outpatient clinic appointment, indicating that AAA screening may be appropriate for the patient. This clinical reminder cites age 65 or greater, male gender, and smoking history as indications for such screening. At this point, initial AAA screening may be ordered at the discretion of the primary care physician in consultation with the patient during the clinic visit. Results of the initial AAA screening exam are reported via an EMIR alert back to the primary care physician, at whose discretion further follow-up imaging is ordered or not. If a follow-up scan is ordered, then a reminder is sent to radiology to perform the scan upon the ordered date. For incidental scans, if the radiologist alerts the primary care physician that a patient’s AAA diameter requires attention from an incidental finding, then follow-up scan ordering is at the choice of the primary care physician. Any vascular consultation for AAA from screening is also at the discretion of the primary care physician.
The effect on follow-up imaging of socioeconomic factors such as race, marital status, distance to hospital from patient residence, estimated household income of zip code residence, and employment disability status were also evaluated. Marital status consisted of single (never married), married, divorced, or widowed. Distance to hospital (Sacramento VA Medical Center, Mather, CA) from a patient’s residence in miles was measured using Google Map Distance Calculator (Google, Inc. Mountain View, CA). Estimated household income was ascertained by zip code of residence using “American FactFinder” from the 2009–2013 U.S. Census Bureau American Community Survey 5-year Estimates database.12 Employment disability status was defined as being “unemployable” in the medical record; a veteran that is unable to work due to their physical or mental disability, and is compensated at the 100% VA disability rate, even though their service-connected disabilities may not be rated by the VA at the 100% level.
Univariate tests were conducted to test associations between patient socioeconomic or clinical characteristics and follow-up rates. Chi square tests were conducted for categorical covariates and the Kruskall-Wallis test was used for continuous covariates. Covariates significantly associated at the 0.1 level with failure to follow-up would then be included in a multivariate logistic regression model to simultaneously test for associations. A Cox proportional hazards model was fit to test for effects on all-cause mortality. A backward selection procedure of patient clinical and socioeconomic characteristics was used to obtain the final model. The full model was fit with all candidate patient characteristics and then the covariate with the highest p-value was removed one at a time until all remaining covariates were significant at the 0.05 level. Survival probability was then determined from the final hazard function. All values were considered censored by December 31, 2014, if death was not observed for that subject before this date. Since the minimum time required for follow-up adherence for an AAA patient is 3 years, and the final AAA screening could have occurred on December 31, 2011, the analysis period for this study concluded on December 31, 2014. All statistical analyses were performed using SAS® software version 9.4 (SAS Institute, Cary, NC). A p-value < 0.05 was considered statistically significant.
Results
A total of 568 patients were enrolled in the study, which consisted of 564 males (99.3%) and 4 females (0.7%), with (mean ± standard deviation) 73.4 ± 7.2 years of age. The race distribution of subjects was: 392 (69.0%) white, 50 (8.8%) black/African-American, 14 (2.4%) Asian/Pacific Islander, 2 (0.4%) American Indian, and 110 (19.4%) unknown/declined to state. The marital status distribution was: 27 (4.8%) patients were single/never married, 288 (50.7%) patients were married, 166 (29.2%) patients were divorced, 71(12.5%) patients were widowed, and 16 (2.8%) patients were separated. The average distance to the hospital from a patient’s residence was 113.9±239.8 miles. The average estimated household income for each patient was $56,938±$19,656. There were 515 (90.7%) patients that rented/owned a home independently and 53 (9.3%) patients that required assisted living or resided in a nursing home. A total of 57 (10.0%) patients were not employable and 511(90.0%) patients were employable (Table I).
Table I.
Summary of Patient Data
| Total (N=568) | Follow-Up (N=370) | No Follow-Up (N=198) | ||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | P-Value | |
| Age (years) | 73.4±7.2 | 73.6±7.3 | 73.1 ± 7 | 0.23 |
| Distance to Hospital From Residence (mi) |
114 ± 240 | 100 ± 182 | 139 ± 320 | 0.08 |
| Estimated Total Median Household Income ($) |
56938 ± 19656 |
56451 ± 19963 | 57857 ± 19080 | 0.46 |
| AAA Screening Size (cm) | 3.6 ± 0.59 | 3.66 ± 0.58 | 3.48 ± 0.57 | <.001 |
| N Total) | N(%) | N(%) | P-Value | |
| Race | 0.85 | |||
| White | 392 (69) | 255 (65.1) | 137 (34.9) | |
| African American | 50 (8.8) | 32 (64) | 18 (36) | |
| Asian/Pacific Islander | 14 (2.5) | 10 (71.4) | 4 (28.6) | |
| American Indian | 2 (0.4) | 2 (100) | 0 (0) | |
| Unknown/Undeclared | 110 (19.4) | 71 (64.6) | 39 (35.5) | |
| Marital Status | 0.92 | |||
| Never Married | 27 (4.8) | 18 (66.7) | 9 (33.3) | |
| Married | 288 (50.7) | 186 (64.6) | 102 (35.4) | |
| Divorced | 166 (29.2) | 111 (66.9) | 55 (33.1) | |
| Widowed | 71 (12.5) | 45 (63.4) | 26 (36.6) | |
| Separated | 16 (2.8) | 10 (62.5) | 6 (37.5) | |
| Housing Status | 0.29 | |||
| Homeowner/Renter | 515 (90.7) | 339 (65.8) | 176 (34.2) | |
| Assisted Living | 53 (9.3) | 31 (58.5) | 22 (41.5) | |
| Employment Disability | 0.07 | |||
| Eligible | 57 (10) | 31 (54.5) | 26 (4.5) | |
| Not Eligible | 511 (90) | 339 (66.3) | 172 (33.7) | |
| Living Status | 0.5 | |||
| Alive | 421 (74.1) | 280 (66.5) | 141 (33.5) | |
| Deceased | 147 (25.9) | 90 (61.2) | 57 (38.8) | |
| Died From Rupture | 3 (0.5) | 2 (66.7) | 1 (33.3) | |
| Gender | 0.52 | |||
| Male | 564 (99.3) | 368 (65.3) | 196 (34.7) | |
| Female | 4 (0.7) | 2 (50) | 2 (50) | |
AAA, Abdominal Aortic Aneurysm; SD, Standard Deviation
There were 428 patients (75.4%) with AAA diameters 3.0 – 3.9 cm, 66 patients (11.6%) with AAA diameters 4.0 – 4.4 cm, and 74 patients (13.0%) with AAA diameters 4.5 – 5.4 cm. A total of 147 patients (25.9%) died within the analysis period. Although absolute cause of death was not established, AAA rupture cannot be ruled out as the cause of death of three patients. Among these, one refused surgical repair after vascular consultation, another was evaluated but assessed to be unfit for surgery, and another died unexpectedly after surgical consultation.
The total follow-up rate of AAA patients meeting RESCAN guidelines was 65.1% (n=370). Of these patients, only 124 (33.5%) received vascular consultation for AAA. There were 307 (83%) follow-up scans ordered specifically for AAA and 63 (17%) were incidental follow-up scans. A total of 198 (34.9%) patients did not receive follow-up. The reasons for failure to follow-up, in order of prevalence, were as follows: failure of physician to order scan (n=124, 70.2%), delayed ordering of scan by physician (n=36, 18.2%), patient non-attendance (n=18, 9.1%), or death of patient prior to follow-up (n=5, 2.5%). The 3.0– 3.9 cm patient cohort for both follow-up and no follow-up groups had the lowest follow-up rate (61.9%) and highest percentage (76.1%) of images not ordered, respectively, among all other patient cohorts. For late follow-ups, 16 patients received vascular consultation for AAA. In nearly all instances (88.4%), failure of timely follow-up (imaging not ordered or late exams performed) was unrelated to patient behavior. Table II summarizes the follow-up data.
Table II.
Summary of Follow-Up Data Using RESCAN AAA Surveillance Recommendations
| 3.0 – 3.9 cm | 4.0 – 4.4 cm | 4.5 – 5.4 cm | Overall | |
|---|---|---|---|---|
| (n=428) | (n=66) | (n=74) | (n=568) | |
| Follow-Up Rate by AAA Size | N(%) | |||
| Follow-Up Rate | 265 (61.9) | 53 (80.3) | 52 (70.3) | 370 (65.1) |
| Follow-Up Consulted for AAA | 54 (20.4) | 37 (69.8) | 33 (63.5) | 124 (33.5) |
| Ultrasound | 193 (72.8) | 38 (71.7) | 28 (53.8) | 259 (70) |
| CT Scan | 72 (27.2) | 15 (28.3) | 24 (46.2) | 111 (30) |
| Reasons for No Follow-Up | N(%) | |||
| Image Not Ordered | 124 (76.1) | 6 (46.1) | 9 (40.9) | 139 (70.2) |
| Late Image | 23 (14.1) | 5 (38.5) | 8 (36.4) | 36 (18.2) |
| Patient No-Show | 13 (8) | 1 (7.7) | 4 (18.2) | 18 (9.1) |
| Patient Died Before Scan | 3 (1.8) | 1 (7.7) | 1 (4.5) | 5 (2.5) |
AAA, abdominal aortic aneurysm; CT, computerized tomography; RESCAN, RESCAN Collaboration Group;
None of the statistical tests for the socioeconomic and clinical variables between “follow-up” and “no follow-up” groups were significant at the 0.1 level, except for initial AAA diameter (p<.001), as patients with smaller diameters were unlikely to achieve follow-up scans (Table I). Therefore, the data revealed that out of any of the various patient characteristics analyzed, socioeconomic or clinical, only initial AAA diameter from screening influenced whether or not AAA patients received follow-up.
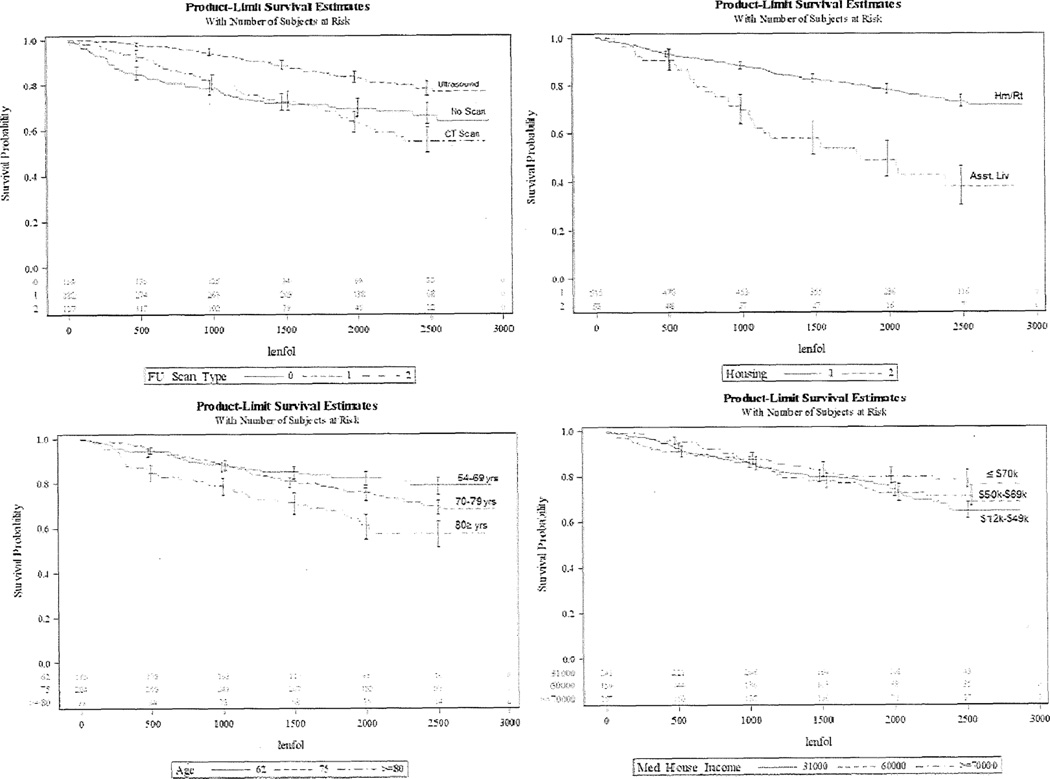
The final hazard model for all-cause mortality (Table III), based on all measured patient characteristics, revealed significant associations between all-cause mortality and follow-up scan type (p<0.001), assisted living status (p<0.001), older age (p=0.001), and lower estimated household income (p=0.01). The total censoring of patients for this hazard analysis was 74.3%. The hazard ratios for scan type revealed that patients who received an ultrasound follow-up had the highest survival probability (hazard ratio=0.369 compared to CT scan), followed by patients that received no follow-up scan (0.876 compared to CT scan), while patients receiving CT scans had the lowest survival probability. Patients designated as residing in assisted living had lower survival probability than renters or homeowners (hazard ratio=0.381). The hazard ratio for median household income was 0.989, indicating that for every $1,000 increase in average median household income, the hazard function decreased by 1.1%. The hazard ratio for age was 1.040, indicating that for every year increase in average age, the hazard function increased by 4%. Figure 1 shows the survival curves of the resulting data.
Table III.
Hazard Analysis Final Model Estimates
| Parameter | Parameter Estimate |
Standard Error | P-Value | Hazard Ratio |
|---|---|---|---|---|
| Age (per change in year) | 0.03946 | 0.01232 | 0.001 | 1.04 |
| Estimated Total Median | ||||
| Household Income (per change in $1000) |
−0.01147 | 0.00469 | 0.01 | 0.989 |
| Housing Status | −0.96383 | 0.21868 | <.001 | 0.381 |
| Follow-Up Scan Type | ||||
| Ultrasound vs. No Scan | −0.86507 | 0.20459 | <.001 | 0.421 |
| Ultrasound vs. CT Scan | 0.13252 | 0.20864 | <.001 | 0.369 |
| CT Scan vs. No Scan | −0.13252 | 0.20651 | 0.52 | 0.876 |
CT, Computerized Tomography
Figure 1. Patient Factors Significantly Associated with Survival Probability.
Figure displays the survival curves between survival probability and follow-up days after screening among follow-up scan type (top left), housing status (top right), age (bottom left), and estimated total median household income (bottom right). The number of patients for per follow-up interval is shown at the bottom of each graph. Age and incomes (in $ thousands) are divided each into three ranges to better visualize distribution of data. Asst Liv, Assisted Living; FU, Follow-Up; Hm/Rt, Homeowner/Renter; lenfol, length of follow-up in days; Med House Income, estimated median household income.
FU Scan Type Graph: 0=No scan, 1=Ultrasound, 2=CT Scan
Housing Status Graph: 1=Homeowner/Renter, 2=Assisted Living
Age Graph: 62=54–69 years, 75=70–79 years
Median Household Income Graph: 31000412,000–$49,000, 60000450,000–$69,000
Discussion
This study analyzes the surveillance outcomes of patients identified with small AAA from a screening program in a large health system. The study is novel because it represents the first comprehensive analysis looking for associations among successful small AAA follow-up evaluation, as well as several socioeconomic and clinical factors that might influence patient compliance with small AAA surveillance. Based on the data, only initial AAA screening diameter seemed to significantly influence the follow-up rate of small AAA surveillance. For example, we have found no other such significant associations between such follow-up and age, gender, race, marital status, distance from home to hospital, estimated household income, or employability. In the absence of such “patient-centric” associations and data on initial AAA screening diameter, we believe that primary care physicians assume the most important role in affecting successful follow-up imaging after initial identification of small AAA from screening.
In addition to analysis for associations between clinical and socioeconomic factors with follow-up imaging after the initial identification of small AAA, a hazard analysis was conducted to determine whether patients who failed to get such follow-up evaluation were at higher risk of all-cause mortality. The final model showed that patients who did not obtain a follow-up ultrasound exam were in fact more likely to die. Other factors that were also significantly correlated with death included older age, lower household incomes, and assisted living dependence. Interestingly, CT follow-up imaging associated with all-cause death to a greater degree than either ultrasound follow-up imaging or no follow-up imaging. This could possibly reflect the fact that CT imaging is used more frequently than ultrasound in the evaluation of other serious conditions, such as cancer. Thereby, this may identify a “sicker” patient cohort. Our data suggests that ultrasound surveillance benefits the patient the most, with decreasing mortality risk over time.
The current study examined the extent to which appropriate follow-up imaging (after initial identification of small AAA) was obtained over a 5-year period at VANCHCS. The VANCHCS AAA screening program was initiated under the SAAAVE Act in January 200713 and continues to enroll eligible veterans to identify patients at risk for AAA rupture. As currently constructed, this EMIR-based screening program is primary care initiated and relies on a clinical alerts system to obtain appropriate follow-up for small AAAs. In theory, this program can elicit appropriate ongoing follow-up of small AAA only if primary care physicians submit timely orders for such follow-up imaging when alerts are received. However, the poor follow-up rate of 65.1% was surprising for patients diagnosed with a potentially serious health condition such as AAA, and reveals needed improvement for the current system in place. A much higher follow-up rate would be hoped for at a large integrated health system.
We believe that there are two potential reasons why primary care physicians do not submit timely orders for follow-up imaging. First, they may not be familiar with AAA surveillance guidelines. In our previous 5-year study10 we found that a large number of inappropriate screenings that did not meet USPSTF criteria for AAA screening were ordered by primary care physicians. We speculate that this may translate to unfamiliarity with RESCAN AAA surveillance guidelines. Also, EMR clinical alert systems might play a role in low AAA follow-up rates. Primary care physicians receive numerous electronic alerts for patients that include diagnostic tests, lab tests, clinical care reminders, drug prescription refills, addendums to notes, co-signing/signing reminders, and pending procedures. Once the alert is opened in the EMR, it could be permanently lost within the system if not acted on or purposefully renewed. Such electronic alerts can be extremely numerous for primary care physicians who follow large patient panels, possibly leading to missed alerts concerning initial AAA screening results.
These potential obstacles (lack of physician knowledge of AAA screening and surveillance guidelines, potential loss of initial AAA screening and surveillance imaging reports secondary within burdensome EMR alert systems) may be surmounted if a health system were to implement an algorithm in the EMR to automatically invite patients back for follow-up imaging and alert primary care physicians and radiology of this automatic order after identification of small AAA. This is potentially a zero cost solution that could further improve patient access to care. Whether such an automatic invitation method helps large health care systems improve follow-up rates warrants further study.
Our study had several limitations. First, this was a retrospective analysis in which we could only determine associations between patient characteristics and successful follow-up imaging for small AAA. Second, the subjects in the study were a homogenous cohort of mostly male veterans of a single large institution that is potentially not representative of the general population of AAA patients. For example, we previously found the VANCHCS population has higher AAA detection rates10 and veterans are generally known to be more at risk for cardiovascular disease than the general population.14 Third, the actual follow-up rate may be lower than the 65.1% found in this study. The VANCHCS EMR system does not have a specific AAA follow-up scan code for physicians to utilize upon initial AAA detection, or a “checklist” protocol for AAA surveillance, as ordering a follow-up scan was left to the discretion of primary care physicians. The 307 follow-up scans ordered specifically for AAA surveillance could make the actual follow-up rate at 54%. However, this study focuses on overall follow-up of small AAA, not how these patients were followed. Incidental scans were included in this study because clinicians would view the radiology report of a subsequent abdominal diagnostic scan to visualize the aorta as a cost-saving measure, rather than order a new AAA follow-up scan in the absence of a scan specific for AAA surveillance. We also did not include homeless veterans with AAA in the study because we required an address to help perform a thorough socioeconomic analysis, thereby excluding additional patients for this study. Lastly, the VANCHCS has several attributes that may negate the effects of distance to hospital and household incomes on successful small AAA follow-up imaging. For instance, the Sacramento VA Medical Center is centrally located geographically within VANCHCS, perhaps making it strategically located so that its intended region of Northern California is particularly well-served. VANCHCS also has instituted outreach programs, including telehealth programs and rural outpatient clinics that could possibly minimize the effects of socioeconomic differences between patients.
Conclusions
The total follow-up rate for the surveillance of small AAA patients is 65.1%. Other than initial AAA screening diameter, the data provide no evidence that patient socioeconomic and clinical characteristics affect the rate of follow-up imaging. Therefore, the primary care physician who orders scans is most likely to be responsible for such low follow-up rates in patients with small AAA. Given the large patient panels and related large associated ordering burdens for most primary care providers, automatic ordering of follow-up scans by the EMR constitutes a logical alternative to improve follow-up rates. Our study points to a need for improved institutional protocols for AAA surveillance, including systems to provide timely notification to vascular surgeons regarding imaging results that indicate elevated AAA rupture risk.
Acknowledgments
The authors thank Peggi Stover, MBA, Narges Zazi, BS, Arlene Gonzalves, RN, and Tanmayee Yenumula, BS for assisting with data collection. This work is supported by VA Merit Career Development Award (CDA-2) IK2CX000521, The National Center for Advancing Translational Sciences (NCATS), The National Institutes of Health (NIH) UL1TR000002, and a Medtronic, Inc. Extramural Research Grant.
Footnotes
Disclaimer
The contents represented in this work do not represent the official views of the Department of Veterans Affairs or the United States Government.
Contributor Information
Kevin C. Chun, Department of Surgery, Sacramento VA Medical Center, Mather, CA
Ashley S. Schmidt, Department of Surgery, Sacramento VA Medical Center, Mather, CA
Sukhmine Bains, Department of Surgery, Sacramento VA Medical Center, Mather, CA
Anthony T. Nguyen, Department of Research, Sacramento VA Medical Center, Mather, CA
Kiana M. Samadzadeh, Department of Research, Sacramento VA Medical Center, Mather, CA
Machelle D. Wilson, Department of Public Health Sciences, Division of Biostatistics, University of California, Davis, Sacramento, CA.
John H. Peters, Department of Medicine, Sacramento VA Medical Center, Mather, CA Department of Medicine, University of California, Davis, Sacramento, CA.
Eugene S. Lee, Department of Surgery, Sacramento VA Medical Center, Mather, CA Department of Surgery, University of California, Davis, Sacramento, CA.
References
- 1.Ashton HA, Buxton MJ, Day NE, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 2.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. The British journal of surgery. 1995;82:1066–1070. doi: 10.1002/bjs.1800820821. [DOI] [PubMed] [Google Scholar]
- 3.Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ (Clinical research ed) 2005;330:750. doi: 10.1136/bmj.38369.620162.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ (Clinical research ed) 2004;329:1259. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nature reviews Cardiology. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 6.Von Allmen RS, Powell JT. The management of ruptured abdominal aortic aneurysms: screening for abdominal aortic aneurysm and incidence of rupture. The Journal of cardiovascular surgery. 2012;53:69–76. [PubMed] [Google Scholar]
- 7.Golledge J, Norman PE. Current status of medical management for abdominal aortic aneurysm. Atherosclerosis. 2011;217:57–63. doi: 10.1016/j.atherosclerosis.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Chaikof EL, Brewster DC, Dalman RL, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. Journal of vascular surgery. 2009;50:880–896. doi: 10.1016/j.jvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Bown MJ, Sweeting MJ, Brown LC, Powell JT, Thompson SG. Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA : the journal of the American Medical Association. 2013;309:806–813. doi: 10.1001/jama.2013.950. [DOI] [PubMed] [Google Scholar]
- 10.Chun KC, Teng KY, Van Spyk EN, Carson JG, Lee ES. Outcomes of an abdominal aortic aneurysm screening program. Journal of vascular surgery. 2013;57:376–381. doi: 10.1016/j.jvs.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 11.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Annals of internal medicine. 2005;142:203–211. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 12.American FactFinder. 2014 ed, editor. Bureau USC. 2009–2013 American Community Survey 5-year Estimates. American Community Survey: United States Census Bureau; 2014. [Google Scholar]
- 13.Lee ES, Pickett E, Hedayati N, Dawson DL, Pevec WC. Implementation of an aortic screening program in clinical practice: implications for the Screen For Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act. Journal of vascular surgery. 2009;49:1107–1111. doi: 10.1016/j.jvs.2008.12.008. [DOI] [PubMed] [Google Scholar]