Abstract
Objective
To investigate the associations of placental telomere length with placental abruption (PA) risk and interactions between placental telomere length and placental mtDNA copy number on PA risk.
Materials and methods
Relative telomere length and mitochondrial DNA (mtDNA) copy number in placental samples collected from 105 cases and 73 controls were measured in two batches using qRT-PCR. Mean differences in relative telomere length between PA cases and controls were examined. After creating batch-specific median cutoffs for relative telomere length (84.92 and 102.53) and mtDNA copy number (2.32 and 1.42), interaction between the two variables was examined using stratified logistic regression models.
Results
Adjusted mean difference in relative telomere length between PA cases and controls was −0.07 (p>0.05). Among participants with low mtDNA copy number, participants with short relative telomere length had a 3.07-fold higher odds (95%CI:1.13–8.38) of PA as compared with participants with long relative telomere length (the reference group). Among participants with high mtDNA copy number, participants with short relative telomere length had a 0.71-fold lower odds (95%CI:0.28–1.83) of PA as compared with the reference group (interaction p-value=0.03).
Conclusion
Findings suggest complex relationships between placental telomere length, mtDNA copy number, and PA risk which warrants further larger studies.
Keywords: Placental telomere length, placental mtDNA copy number, placental abruption, pregnancy, oxidative stress
Introduction
The placenta is a highly complex organ that serves as a source and target of hormones that direct the course of pregnancy. It also plays a key role in fetal growth as a maternal-fetal interface. Therefore, pregnancy complications of placental origin have major maternal and offspring health impact. Placental abruption (PA), the premature separation of the placenta from the uterus, is a life threatening condition that complicates up to 1% of pregnancies (1, 2). Investigators have identified risk factors that have been associated with PA including young or advanced maternal age and smoking (3, 4). While available evidence supports the role of uteroplacental ischemia and chronic hypoxia as pathologies underlying PA, specific mechanisms and related biomarkers are not fully described (5).
Telomeres are multiple tandem repeats of the base sequence 5′-TTAGGG-3′ located at chromosome terminals of most eukaryotic organisms (6). As eukaryotic tissues age or are damaged, telomeres shorten due to “end replication” problem related to DNA replication, marking cellular senescence. Telomere shortening has been associated with chronic diseases such as diabetes, hypertension, and cancer (7, 8). Findings from a large Mendelian randomization study support a causal role of telomere length variation in age-related diseases (9). Investigators have also implicated shortened placental and leukocyte telomeres in the pathogenesis of pregnancy complications including preeclampsia, intrauterine growth retardation and gestational diabetes (10, 11). However, to our knowledge, telomere length shortening, particularly placental telomere length shortening, which can chronicle antepartum stress from oxidative stress, has not been investigated in relation to risk of PA.
The guanine triplet repeat in telomeres is highly sensitive to oxidative stress, therefore, oxidative stress may lead to accelerated telomere shortening and cell death (12, 13). Recent studies indicate potential differences in adverse perinatal outcomes (e.g. preterm premature rupture of membrane and still birth) among women with shortened placental telomeres, dependent on oxidative stress (14, 15). Cells under oxidative stress have been shown to have higher replication of the mitochondria, critical component of the aerobic metabolic pathway (15). This may be reflected by higher mitochondrial DNA (mtDNA) copy number, commonly, or by lower mtDNA copy number in cases of failing organ/tissues or patients with underlying lipid disorders (16, 17). Investigators have also reported U-shaped relationships between mtDNA copy number and adverse outcomes (e.g. colorectal cancer risk) (18). In disorders that have oxidative stress as a common pathophysiological mechanism, such as placental abruption or preeclampsia, mtDNA copy number abundance in various tissues (including whole blood and placenta) has emerged as a possible biomarker of oxidative stress (16, 17). In a sample of 105 PA cases and 75 controls, we investigated associations of placental telomere length with risk of PA. Further, given that the relationship between placental telomere length and PA may differ by placental mtDNA copy number, we also examined interactions between placental telomere length and placental mtDNA copy number on PA risk.
Methods
Study Setting and Study Population
This study was conducted as part of the Peruvian Abruptio Placentae Epidemiology (PAPE) study, a case-control study (424 PA cases and 369 controls) designed to investigate risk factors of PA (19). Briefly, participants were women who delivered at the Hospital Nacional dos de Mayo, Instituto Especializado Materno Perinatal, and Hospital Madre Niño San Bartolomè in Lima, Peru, during the period from September 2006 through September 2008. PA status was ascertained through routine clinical examination performed by an attending physician. PA was diagnosed if there was evidence of blood clot behind the placenta accompanied by at least two of the following signs and symptoms: (1) vaginal bleeding in late pregnancy that was not associated with placenta previa or cervical lesions; (2) uterine tenderness and/or abdominal pain; and (3) fetal distress or death. Controls were selected from eligible women who delivered at the participating institutions during the study period. Eligible controls were women who did not have a diagnosis of PA and whose medical record review later confirmed this fact. Non-singleton births were excluded. A total of 105 PA case and 73 control PAPE study participants with available DNA samples were included in the present study. All study participants provided written informed consent in accordance with procedures approved by the human subjects review committees of participating study hospitals and the Swedish Medical Center, Seattle WA.
Data Collection
Information regarding maternal socio-demographic characteristics, medical history, reproductive history, and lifestyle characteristics before and during the current pregnancy was collected during in-person interviews administered by trained research interviewers using a standardized, structured questionnaire. A brief physical examination was conducted to measure maternal height, weight, and mid-arm circumference. Maternal and infant records were reviewed to collect information on course of pregnancy, antepartum, labor, and delivery characteristics. Gestational age was based on the date of the last menstrual period and was confirmed by an ultrasound examination performed before 20 weeks gestation. Pre-pregnancy body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Placental Sample Collection and DNA Extraction
Placentas were collected immediately after delivery. Placentas were weighed, double bagged and transported in coolers. The chorionic plate and overlying membranes were removed, and tissue biopsies (approximately 0.5 cm3 each) were obtained from 8 sites (4 maternal and 4 fetal). Care was taken to avoid blood and chorioamniotic membrane contamination. Biopsies were placed in cryotubes, snap frozen in liquid nitrogen, and stored at −80°C until analysis. For the current study, a pooled sample (~20 grams) from four fetal placental samplings was homogenized using a Tissue Tearor (Biospec Products Inc., Bartlesville, OK) in a lysis buffer from the Qiamp DNA Mini Kit (Qiagen Inc, Valencia, CA) with added Proteinase Kt. DNA from placenta was extracted using a standardized protocol adapted from Qiamp DNA Mini Kit (Qiagen Inc., Valencia, CA). Placental DNA quality was determined using PicoGreen dsDNA quantitation assay on a SpectraMax Plus 384 Microplate Reader (Molecular Devices, Sunnyvale, CA).
Placental Telomere Length and Mitochondrial DNA (mtDNA) Copy Number Measurement
Telomere length was measured by using real-time quantitative PCR method (qRT-PCR), described by Cawthon (17, 19). Briefly, two master mixes of PCR reagents were prepared, one with the telomere (T) primer pair, the other one with the single copy gene (S) primer pair (17, 20). A fresh standard curve, from a pooled control samples, ranging from 70ng/μl to 1,94ng/μl (serial dilutions 1:2), was included in every “T” and “S” PCR run. 30ng of DNA sample was added to each reaction. Each sample was run in triplicate. A high-precision MICROLAB STARlet Robot (Hamilton Life Science Robotics, Bonaduz AG, Switzerland) was used for transferring in a 384-well format plate a volume of 7μl reaction mix and 3μl DNA (10ng/μl). All PCRs were performed on a 7900HT Fast Real Time PCR System (Applied Biosystems). The thermal cycling profile for both amplicons began with 50°C for 2 minutes followed by incubation at 95°C for 2 minutes to activate the AmpliTaq DNA polymerase. For telomere PCR, these were followed by 2 cycles of 15 s at 95°C, 15 s at 49°C; and 35 cycles of 15 s at 95°C, 10 s at 62°C, 15 s at 74°C. At the end of each real-time PCR reaction a melting curve was added for both T and S PCR. The average of the three T measurements was divided by the average of the three S measurements to calculate the average T:S ratio or relative telomere length.
Real-time DNA PCR analyses was performed to measure mtDNA copy number using the NovaQUANT™ Human Mitochondrial to Nuclear DNA Ratio Kit (catalog #72620 EMD Millipore, Billerica, MA) according to the manufacturer’s instructions (21). This kit provides plates pre-aliquoted with the primers to compare the levels of two nuclear genes (BECN1 and NEB) to two mitochondrial genes (ND1 and ND6) in a RT-qPCR. 2ng of DNA was added to 80uL of SYBR Select Master Mix (catalog #4472908 Life Technologies, Carlsbad, CA) and 20uL of the mixture is applied to each of the four wells of pre-aliquoted primers. Plates were run in the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) with the cycling conditions: 95°C for 10 minutes followed by 40 Cycles of: 95°C for 15 seconds 64°C for 1 minute. Analyses were s done using the ABI Prism 7000 SDS software RQ Study Application version 1.1. Relative copy number method was used to calculate the mtDNA copy number. Ct values were defined as the cycle number in which fluorescence first crosses the threshold, obtained for each of the two target genes and two reference genes. This is accomplished by averaging the copy numbers calculated from the ND1/BECN1 pair and the ND6/NEB pair. To calculate copy number, we determined N = 2−ΔCt where ΔCt1 = CtND1 − CtBECN1 and ΔCt2 = CtND6 − CtNEB, then averaged 2−ΔCt1 and 2−ΔCt2. All assays were performed without knowledge of pregnancy outcome.
Statistical Analysis
Continuous variables were described using mean ± standard deviation (SD), and Student’s t-test was used to compare the means between cases and controls. Categorical variables were described using counts and proportions. Chi-square test or Fisher’s exact test was used to compare proportions. Mean differences in relative placental telomere length between PA cases and controls were evaluated, adjusting for gestational age and maternal age at delivery, mode of delivery, and pre-pregnancy body mass index using linear regression analysis. Partial Spearman correlations adjusted for maternal age at delivery were used to estimate the associations of relative placental telomere length with other risk factors, among controls. To examine interactions, we conducted stratified analyses and examined independent and joint effects of relative placental telomere length and placental mtDNA copy number on risk of PA. For these analyses, we categorized each participant according to batch-specific median relative placental telomere length (long/short) and placental mtDNA copy number (low/high) cutoffs. First, we examined whether associations of relative placental telomere length with PA risk differ among strata defined by placental mtDNA copy number. We also examined whether the joint effect of relative placental telomere length and placental mtDNA copy number on PA risk was greater than expected given their independent effects. For these analyses, we created a variable that categorized women as (1) long relative placental telomere length and low placental mtDNA copy number, (2) long relative placental telomere length and low placental mtDNA copy number, (3) long relative placental telomere length and low placental mtDNA copy number, and (4) long relative placental telomere length and low placental mtDNA copy number. The long relative placental telomere length and low placental mtDNA copy number group constituted the reference group in these analyses. The interaction p-value was used to examine statistical significance of the interactions. Statistical analyses were conducted using SAS and R software.
Results
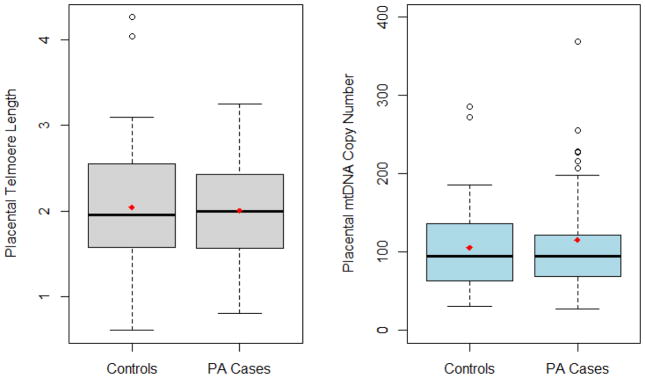
Study participant characteristics are summarized in Table 1. PA cases, compared to controls, had higher proportions of preeclampsia and C-section delivery. Cases also delivered earlier in gestation. PA cases had lower mean relative placental telomere length (mean: 2.00; SD: 0.56) and higher placental mtDNA copy number (mean: 115.20; SD: 144.90), compared with controls (2.04 [0.69] and 105.60 [53.1]), although these differences were not statistically significant. Figure 1 describes means and medians of placental telomere length and mtDNA copy number by PA case control status. The multi-variable adjusted mean difference in relative placental telomere length between PA cases and controls was −0.07 (95%CI: −0.32, 0.15). We observed significant correlations between relative placental telomere length and placental mtDNA copy number (r = −0.30, p-value=0.04) among controls.
Table 1.
Selected Characteristic of Placental Abruption (PA) Cases and Controls
| Characteristics | PA Cases (N=105) | Controls (N=73) | P-value2 |
|---|---|---|---|
| Maternal age at delivery (years)1 | 27.6 ± 6.3 | 28.4 ± 6.6 | 0.40 |
| Nulliparous | 48 (46%) | 29 (40%) | 0.43 |
| Maternal education ≤ high school | 86 (82%) | 57 (78%) | 0.53 |
| Single marital status | 20 (19%) | 10 (14%) | 0.35 |
| Received prenatal care | 87 (83%) | 66 (90%) | 0.07 |
| Smoked during pregnancy | 7 (6.7%) | 1 (1.4%) | 0.14 |
| Chronic hypertension | 7 (6.7%) | 1 (1.4%) | 0.18 |
| Preeclampsia | 25 (24%) | 6 (8.2%) | <0.00 |
| Pre-pregnancy body mass index (kg/m2)1 | 23.4 ± 3.0 | 23.5 ± 3.7 | 0.80 |
| Gestational age at delivery | 34.9 ± 4.7 | 37.1 ± 3.8 | <0.00 |
| Placental mtDNA copy number | 115.2 ± 144.9 | 105.6 ± 53.1 | 0.54 |
| Placental Telomere Length 1 | 2.00 ± 0.56 | 2.04 ± 0.69 | 0.67 |
mean ± standard deviation (SD), otherwise N (%)
P-value from Student’s t test for continuous variables and Chi-square test / Fisher’s exact test for categorical variables.
Figure 1.
Box plot displaying mean and median for placental telomere length and mtDNA copy number by placental abruption (PA) case control status (means are indicated in diamond)
Among participants with low mtDNA copy number, participants with short relative telomere length had a 3.07-fold higher odds (95%CI: 1.13–8.38) of PA as compared with participants with long relative telomere length (the reference group) (Table 2). Among participants with high mtDNA copy number, participants with short relative telomere length had a 0.71-fold lower odds (95%CI: 0.28–1.83) of PA as compared with the reference group (interaction p-value=0.03). Participants who had both long relative placental telomere length and high placental mtDNA copy number had a 2.25-fold higher odds (95%CI: 0.84–6.00) of PA as compared with the reference group participants with both long relative placental telomere length and low mtDNA copy number. However, participants who had short relative placental telomere length and high placental mtDNA copy number had a 1.61-fold higher odds (95%CI: 0.64–4.05) of PA as compared with the reference group (interaction p-value=0.032).
Table 2.
Interaction between Placental Telomere Length (PTL) and Mitochondrial DNA Copy Number (mtDNA CN) on Risk of Placental Abruption (PA)
| PA Cases (N=105) | Controls (N=73) | Stratified Analysis | Joint Analysis | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)* | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* | |||
|
| ||||||
| Below median mtDNA CN, Above median placental telomere length | 18 | 22 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Below median mtDNA CN, Below median placental telomere length | 31 | 14 | 2.71 (1.12, 6.57) | 3.07 (1.13, 8.38) | 2.71 (1.12, 6.57) | 3.11 (1.17, 8.30) |
| Above median mtDNA CN, Above median placental telomere length | 26 | 15 | 1.00 (reference) | 1.00 (reference) | 2.12 (0.87, 5.16) | 2.25 (0.84, 6.00) |
| Above median mtDNA CN, Below median placental telomere length | 30 | 22 | 0.79 (0.34, 1.82) | 0.71 (0.28, 1.83) | 1.67 (0.73, 3.83) | 1.61 (0.64, 4.05) |
| P-value for interaction | 0.048 | 0.032 | ||||
Adjusted for maternal age, gestational age at delivery, mode of delivery, and pre-pregnancy body mass index.
Placental mtDNA copy number: Low: < median (< 84.92) in primary period of collection and (<102.53) in secondary period of collection; high: ≥ median (≥84.92) in primary period of collection and (≥102.53) in secondary period of collection. Placental telomere length: high: ≥ median (≥2.32) in batch 1 and (≥1.42) in batch 2; low: < median (<2.32) in batch 1 and (<1.42) in batch 2.
Discussion
In the current study, we found that PA cases, on average, had lower relative placental telomere length compared with controls, although the association was not statistically significant. Among controls, significant correlations were observed between placental telomere length and placental mtDNA copy number. We also observed significant interactions between placental telomere length and placental mtDNA copy number on PA risk. Inverse association of placental telomere length with PA risk was observed only among women who had low placental mtDNA copy number.
To date, no study investigated the relationships between placental telomere length and risk of PA. Investigators have previously made consistent observations of shortened placental telomeres in pregnancies complicated by preeclampsia as well as severe intra-uterine growth restriction (IUGR) secondary to placental insufficiency (11, 22, 23). Other investigators have also shown a decrease in telomerase activity during placental maturation leading to suggestions that dysfunctional telomeres might be part of the mechanisms of placental aging during pregnancy (24, 25). Oxygen is a known regulator of placental development and function (26). Both oxidative stress and impaired placental function are pathways implicated in the pathogenesis of PA (17). Therefore, placental telomerase activity and shorter placental telomere length could indicate a state of oxidative stress in placenta, along with other antepartal stress, and characterize placental aging related pathophysiologic processes that may lead to PA. Our findings of shorter placental telomere length among PA cases, compared with controls, support this thesis.
Our study is also the first to examine interactions between placental telomere length and placental mtDNA copy number on PA risk. Placental mtDNA copy number is a novel biomarker of oxidative stress and systemic mitochondrial dysfunction. Mitochondria is susceptible to oxidative damage (27), and oxidative stress may contribute to increased mtDNA copy numbers through mechanisms that involve reactive oxygen species (ROS)-induced damage to cellular structural elements (including the lipid membranes of mitochondria) and promote mitochondrial replication (27). Increased mtDNA copy number has been associated with perinatal complications characterized by oxidative stress. We have previously reported that the odds of PA was elevated among women with higher maternal blood mtDNA copy number (≥ 336.9) as compared with those with lower values (<336.9) (adjusted OR = 1.60: 95% CI 1.04–2.46) (17).
Recent studies show that association of placental telomere length shortening with adverse perinatal outcomes may be dependent on the level of oxidative stress (14). This is in line with findings of investigations in other research areas. Shen et al. reported that the association between telomere length and breast cancer risk may be modified with dietary intake of antioxidants or antioxidant supplements (28). Since placental telomere length may be a causally related biomarker or an end-point of several adverse mechanisms that could end in PA, it is important to evaluate potential interactions between placental telomere length and these mechanisms, such as oxidative stress. Our observation that the inverse association between placental telomere length and PA risk existed only among women with low placental mtDNA copy number is intriguing. Lower mtDNA copy number has been reported in failing organ/tissues (e.g. heart failure) (29) or in patients with underlying lipid disorders (e.g. hyperlipidemia patients) (30). Investigators have also suggested U-shaped relationships between mtDNA copy number and colorectal cancer risk (18). One or more of these explanations may have played a role in our observation and our findings warrant further investigations.
Some limitations of the current study deserve mention. The temporal relationship between shortened placental telomere length and the onset of PA is not clear due to the cross sectional nature of the assessment. The generalizability of our findings needs confirmation by replication studies that are conducted among other populations. Caution is needed while interpreting our study findings because of the small size of the study. In addition, the small sample size limited further stratified analysis by factors such as preeclampsia that may confound the relationships.
In sum, we found significant interaction between relative placental telomere length and placental mtDNA copy number on risk of PA. Studies that help unravel the underlying mechanisms of telomere shortening and oxidative stress can facilitate understanding of placental cellular senescence that may lead to PA and other adverse perinatal complications. Further, such studies may help in identification, preventative and therapeutic targets to mitigate adverse perinatal complications of placental origin.
Acknowledgments
This study was supported by grants from the National Institute of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD059827 and T32HD052462), the National Heart Lung and Blood Institute (K01HL10374), and support by the National Cancer Institute to Roswell Park Cancer Institute Genomics Shared Resource (P30CA016056).
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Macdonald PC, Gant NF, Cunnigham FG, Williams JW. Williams Obstetrics. Appelton and Lange. 1989 [Google Scholar]
- 2.Oyelese Y, Ananth CV. Placental abruption. Obstetrics & Gynecology. 2006;108(4):1005–16. doi: 10.1097/01.AOG.0000239439.04364.9a. [DOI] [PubMed] [Google Scholar]
- 3.Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. American Journal of Epidemiology. 1996;144(9):881–9. doi: 10.1093/oxfordjournals.aje.a009022. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez SE, Pacora PN, Farfan JH, Fernandez A, Qiu C, Ananth CV, et al. Risk factors of abruptio placentae among Peruvian women. American journal of obstetrics and gynecology. 2006;194(1):225–30. doi: 10.1016/j.ajog.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Ananth CV, Peltier MR, Kinzler WL, Smulian JC, Vintzileos AM. Chronic hypertension and risk of placental abruption: is the association modified by ischemic placental disease? American journal of obstetrics and gynecology. 2007;197(3):273e1–e7. doi: 10.1016/j.ajog.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 6.Torbergsen T, øian P, Mathiesen E, Borud O. Pre-eclampsia-a mitochondrial disease? Acta obstetricia et gynecologica Scandinavica. 1989;68(2):145–8. doi: 10.3109/00016348909009902. [DOI] [PubMed] [Google Scholar]
- 7.Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36(2):195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 8.Uziel O, Singer JA, Danicek V, Sahar G, Berkov E, Luchansky M, et al. Telomere dynamics in arteries and mononuclear cells of diabetic patients: effect of diabetes and of glycemic control. Experimental gerontology. 2007;42(10):971–8. doi: 10.1016/j.exger.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nature genetics. 2013;45(4):422–7. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risques RA, Vaughan TL, Li X, Odze RD, Blount PL, Ayub K, et al. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiology Biomarkers & Prevention. 2007;16(12):2649–55. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 11.Toutain J, Prochazkova-Carlotti M, Cappellen D, Jarne A, Chevret E, Ferrer J, et al. Reduced placental telomere length during pregnancies complicated by intrauterine growth restriction. PloS one. 2013;8(1):e54013. doi: 10.1371/journal.pone.0054013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee DB, Ghosh A, Lu J, Bohr VA, Liu Y. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA repair. 2011;10(1):34–44. doi: 10.1016/j.dnarep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Zglinicki T, Pilger R, Sitte N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radical Biology and Medicine. 2000;28(1):64–74. doi: 10.1016/s0891-5849(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari F, Facchinetti F, Saade G, Menon R. Placental telomere shortening in stillbirth: a sign of premature senescence? The Journal of Maternal-Fetal & Neonatal Medicine. 2015:1–6. doi: 10.3109/14767058.2015.1046045. [DOI] [PubMed] [Google Scholar]
- 15.Polettini J, Dutta E, Behnia F, Saade G, Torloni M, Menon R. Aging of intrauterine tissues in spontaneous preterm birth and preterm premature rupture of the membranes: A systematic review of the literature. Placenta. 2015 doi: 10.1016/j.placenta.2015.05.003. http://dx.doi.org/10.1016/j.placenta.2015.05.003. [DOI] [PubMed]
- 16.Tan D, Goerlitz DS, Dumitrescu RG, Han D, Seillier-Moiseiwitsch F, Spernak SM, et al. Associations between cigarette smoking and mitochondrial DNA abnormalities in buccal cells. Carcinogenesis. 2008;29(6):1170–7. doi: 10.1093/carcin/bgn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams MA, Sanchez SE, Ananth CV, Hevner K, Qiu C, Enquobahrie DA. Maternal blood mitochondrial DNA copy number and placental abruption risk: results from a preliminary study. International journal of molecular epidemiology and genetics. 2013;4(2):120. [PMC free article] [PubMed] [Google Scholar]
- 18.Thyagarajan B, Wang R, Barcelo H, Koh W-P, Yuan J-M. Mitochondrial copy number is associated with colorectal cancer risk. Cancer Epidemiology Biomarkers & Prevention. 2012;21(9):1574–81. doi: 10.1158/1055-9965.EPI-12-0138-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Paz NC, Sanchez SE, Huaman LE, Chang GD, Pacora PN, Garcia PJ, et al. Risk of placental abruption in relation to maternal depressive, anxiety and stress symptoms. Journal of affective disorders. 2011;130(1):280–4. doi: 10.1016/j.jad.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic acids research. 2002;30(10):e47-e. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKernan KJ, Spangler J, Zhang L, Tadigotla V, McLaughlin S, Warner J, et al. Expanded genetic codes in next generation sequencing enable decontamination and mitochondrial enrichment. PloS one. 2014;9(5):e96492. doi: 10.1371/journal.pone.0096492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biron-Shental T, Sukenik-Halevy R, Sharon Y, Goldberg-Bittman L, Kidron D, Fejgin MD, et al. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. American journal of obstetrics and gynecology. 2010;202(4):381, e1–e7. doi: 10.1016/j.ajog.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Davy P, Nagata M, Bullard P, Fogelson N, Allsopp R. Fetal growth restriction is associated with accelerated telomere shortening and increased expression of cell senescence markers in the placenta. Placenta. 2009;30(6):539–42. doi: 10.1016/j.placenta.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izutsu T, Kudo T, Sato T, Nishiya I, Ohyashiki K, Mori M, et al. Telomerase activity in human chorionic villi and placenta determined by TRAP and in situ TRAP assay. Placenta. 1998;19(8):613–8. doi: 10.1016/s0143-4004(98)90022-4. [DOI] [PubMed] [Google Scholar]
- 25.Kyo S, Takakura M, Tanaka M, Kanaya T, Sagawa T, Kohama T, et al. Expression of telomerase activity in human chorion. Biochemical and biophysical research communications. 1997;241(2):498–503. doi: 10.1006/bbrc.1997.7767. [DOI] [PubMed] [Google Scholar]
- 26.Tuuli M, Longtine M, Nelson D. Review: Oxygen and trophoblast biology–a source of controversy. Placenta. 2011;32:S109–S18. doi: 10.1016/j.placenta.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James AM, Murphy MP. How mitochondrial damage affects cell function. Journal of biomedical science. 2002;9(6):475–87. doi: 10.1159/000064721. [DOI] [PubMed] [Google Scholar]
- 28.Shen J, Gammon M, Terry MB, Wang Q, Teitelbaum S, Neugut A, et al. Telomere length, oxidative damage, antioxidants and breast cancer risk. Cancer Research. 2008;68(9 Supplement):1876–1876. doi: 10.1002/ijc.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K-i, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circulation research. 2001;88(5):529–35. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 30.Liu CS, Kuo CL, Cheng WL, Huang CS, Lee CF, Wei YH. Alteration of the copy number of mitochondrial DNA in leukocytes of patients with hyperlipidemia. Annals of the New York Academy of Sciences. 2005;1042(1):70–5. doi: 10.1196/annals.1338.008. [DOI] [PubMed] [Google Scholar]