Abstract
Background
The management of children with pediatric mastocytosis poses a challenge. This is because there is limited information as to the application of clinical and laboratory findings and bone marrow histopathology as they relate to medical intervention and communication.
Objective
We sought to examine clinical aspects of pediatric mastocytosis in relationship to serum tryptase levels and bone marrow pathology to provide practical guidance for management.
Methods
Between 1986 and 2012, 105 children were evaluated at the National Institutes of Health. Organomegaly was confirmed by means of ultrasound. Baseline tryptase levels and at least 1 subsequent tryptase measurement was available in 84 and 37 of these children, respectively. Fifty-three children underwent a bone marrow examination. These data were used to examine relationships between clinical findings, tryptase levels, and marrow histopathology.
Results
In patients with high tryptase levels and severe mediator symptoms, all with organomegaly had systemic disease, and none without organomegaly had systemic disease. Serum tryptase levels differed significantly between patients with urticaria pigmentosa and those with diffuse cutaneous (P < .0001) and systemic mastocytosis (P < .0001) and in all 3 categories versus control subjects (P < .0001). Tryptase levels and symptoms decreased over time in most patients, and tryptase levels correlated with bone marrow mast cell burden in patients with systemic mastocytosis (P < .0001). There was a significant relationship between clinical resolution and the percentage decrease in tryptase levels (P = .0014).
Conclusions
The majority of children experienced major or complete disease resolution (57%), whereas These remainder exhibited partial improvement. Organomegaly was a strong indicator of systemic disease. Serum tryptase levels furthered classification and reflected clinicopathologic findings, while sequential tryptase measurements were useful in supplementing clinical judgment as to disease course.
Keywords: Mast cells, tryptase, urticaria pigmentosa, cutaneous mastocytosis, diffuse cutaneous mastocytosis, mastocytosis, bone marrow examination
Mastocytosis is characterized by an abnormal accumulation of mast cells in tissues, including the skin and bone marrow.1 The signs and symptoms of mastocytosis, such as flushing, blistering, pruritus, and diarrhea, are a result of mast cell mediator release and infiltration of tissues with mast cells. In children, the disease is typically limited to the skin; however, indolent systemic mastocytosis (ISM) is also observed. The most common cutaneous variant is urticaria pigmentosa (UP; also known as maculopapular cutaneous mastocytosis2), followed by diffuse cutaneous mastocytosis (DCM) and mastocytoma. The literature supports a 30% to 65% improvement rate for pediatric-onset mastocytosis.3-6
A baseline serum tryptase level is routinely obtained during initial diagnostic evaluation. A value of 20 ng/mL or greater is a minor diagnostic criterion for systemic disease based on guidelines developed in the adult population, and an increased tryptase level is reported to help predict children at risk for episodes of severe mediator release.5,7 We routinely obtain tryptase levels, and this information provided a unique resource to assess whether the serum tryptase level as an objective surrogate marker of mast cell burden over time would help with reinforcing the clinical impression of disease evolution, as well as the relationship of a serum tryptase level to other indicators of systemic disease, including organomegaly.
As will be shown, within our cohort of 105 children, disease was clinically stable or improved with time. Serum tryptase levels remained the same or trended downward, reinforcing the clinical impression. The presence of organomegaly was a strong predictor of systemic disease among patients with increased tryptase levels and severe mediator symptoms. These observations and others to be detailed support the value of repeated tryptase level determinations in the management of mastocytosis in the pediatric age group.
METHODS
Patients
One hundred five patients from 6 months to 21 years of age with a diagnosis of cutaneous mastocytosis were enrolled in institutional review board–approved protocols (03-I-0041, 90-I-0120, and 93-I-0136) at the National Institutes of Health (NIH) after obtaining informed consent. The population consisted of 67 male (63.8%) and 38 female (36.2%) subjects. All subjects were managed with conservative therapy, and none received cytoreductive agents. The diagnostic evaluation included a CBC and differential and measurement of platelet counts, blood chemistries, liver enzymes, serum IgE levels, prothrombin time, partial thromboplastin time (PTT), lupus antibodies, and serum tryptase levels. Based on existing recommendations,8,9 53 patients with severe mediator symptoms and/or a palpable liver or spleen below the costal margin who also had an abdominal ultrasound to confirm organomegaly, according to age-appropriate radiologic values,10 underwent a bone marrow biopsy and aspirate. Based on these findings, patients were categorized by disease variant according to World Health Organization (WHO) criteria.11 Sixty-six (62.8%) children were categorized with UP, 2 (3%) of whom had the nodular form subtype; 14 (13.3%) had DCM; 6 (5.7%) had a mastocytoma; and 19 (18.2%) had ISM (Table I). In the majority of patients (n = 89 [84.7%]), disease onset was in the first year of life. Ninety-five percent of patients were white. The discussion of serum tryptase levels as they relate to clinical manifestations, pathology, and prognosis reflects those patients with 1 or more tryptase determinations evaluated at our center after tryptase determinations became available (n = 84 [80%]). The 6 patients with mastocytomas are included in the demographic data as a representation of disease incidence and in the analyses related to bone marrow evaluations but not in other analyses.
TABLE I.
Disease variant based on WHO criteria
| Diagnosis | Total | M/F | Age of onset | Ethnicity |
|---|---|---|---|---|
| UP (MPCM) | 66 | 40/26 | Birth to 2 y | 62 W/3 AA/1 A |
| DCM | 14 | 11/3 | Birth to 8 mo | 13 W/1 H |
| Mastocytoma | 6 | 5/1 | 3 mo to 12 y | 6 W |
| ISM | 19 | 11/8 | Birth to 6 mo | 19 W |
Two patients within the UP variant had the nodular form subtype (1 white and 1 Asian patient).
A, Asian; AA, African American; H, Hispanic; MPCM, maculopapular cutaneous mastocytosis; W, white.
Serum tryptase and IgE determinations
Total serum tryptase levels were determined during routine visits. None were obtained during systemic reactions. Samples were assayed by using a commercial assay (ImmunoCAP; Quest Diagnostics, Madison, NJ) with the normal reference range of 0.0 to 11.5 ng/mL (Mayo Clinic Laboratories, Rochester, Minn). In 16 patients a serum tryptase level was not determined because a Clinical Laboratory Improvement Amendments–approved assay was not available at the time of the NIH visit. The serum IgE level was determined by using the Immulite XPI (Siemens Medical Solutions, Malvern, Pa), solid-phase chemiluminescence assay.
Bone marrow preparation and analysis
Bone marrow trephine biopsy specimens were fixed either in B-5 fixative or B-Plus fixative, embedded in paraffin, and processed by using standard procedures.12 Immunohistochemical studies with anti-tryptase, anti-CD117 (Cell Marque, Hot Springs, Ark), and anti-CD25 (Vision BioSystems, Norwell, Mass) antibodies were performed by using immunoperoxidase staining procedures and an automated immunostainer (Ventana Medical System, Tucson, Ariz), according to the manufacturer's instructions. Bone marrow biopsy specimens were scored in a blinded fashion by a hematopathologist (I.M.). Multiparameter flow cytometry was performed on aspirates for CD2, CD25, CD45, and CD117. The CellQuest program was used for data analysis.12 Detection of the KIT D816V mutation was performed by using RT-PCR/RFLP.12
Symptom resolution score
Resolution was scored over the time interval between the first and last tryptase measurement. Complete resolution was defined by means of complete regression of skin lesions, as well as skin-specific and constitutional symptoms and need for symptomatic therapy.8,13 Major resolution was determined by a greater than 50% regression of skin lesions and only periodic need for medications. Partial resolution was demonstrated by 10% to 50% regression of skin lesions and a similar reduction in skin-specific and constitutional symptoms, as well as a decreased need for medications.
Statistical analysis
A Wilcoxon rank sum test was used to compare tryptase levels between different patients of different disease categories and control subjects. Linear regression depicting the relationship between age and tryptase level was performed within each patient and displayed along with all patients within a disease category. The association between disease resolution and percentage decrease in tryptase level was analyzed with Spearman correlation. To illustrate the relationship between symptom prevalence and age, we show the symptom prevalence for each age category, with corresponding 95% CIs. The estimates at different age categories are not independent because patients contribute once to the estimate for all age categories for which they were followed. When patients had multiple values within an age category, the data point that fell closest to the midpoint of the age category was used. A loess smoothed curve was used to provide a description of the symptom prevalence across age categories. Scatterplots illustrating relationships between disease categories, tryptase levels, bone marrow mast cells, and IgE levels are presented, and corresponding Spearman rank correlation coefficients were computed. Statistical graphics were created and analysis was performed with SAS (SAS Institute, Cary, NC) and PRISM software (GraphPad Software, La Jolla, Calif).
RESULTS
Initial serum tryptase measurement and routine laboratory studies
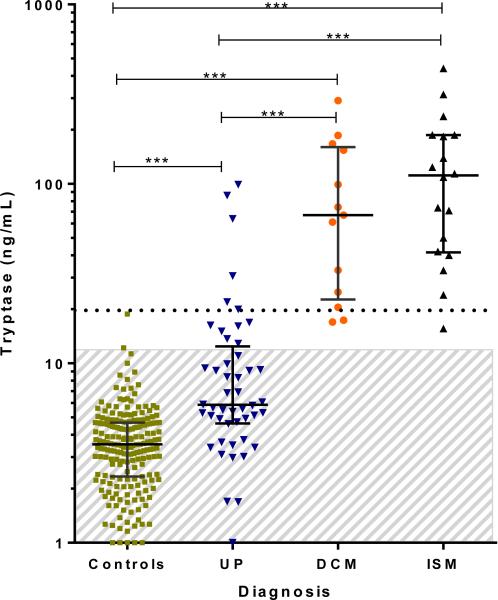
In the initial analysis, we examined tryptase levels obtained during the first visit in children subsequently categorized as having UP, DCM, or ISM (Fig 1). Patients’ values were compared with control samples obtained from children evaluated in a pediatric clinic at NIH.14 Children with UP had median, 25th, and 75th percentile tryptase levels of 5.9, 4.7, and 12.0 ng/mL, respectively, compared with the control population (3.5, 2.3, and 4.7 ng/mL; P ≤ .0001). Twelve children with UP had a tryptase level that exceeded the normal range, whereas 6 children had a value greater than the WHO minor diagnostic cutoff of 20 ng/mL. Thus serum tryptase levels in children with UP were generally less than 20 ng/mL, if not within the normal range. Patients with DCM had significantly greater tryptase levels than those with UP (median, 25th, and 75th percentile tryptase levels: 67.0, 24.9, and 154.0 ng/mL, respectively; P < .0001). The median, 25th, and 75th percentile tryptase levels in children with systemic disease were 111.5, 42.0, and 187.0 ng/mL, respectively, and were significantly increased when compared with those of control subjects, patients with UP, or both (P < .0001 for both). No child in this study with a tryptase level within the normal range had a final diagnosis of systemic disease.
FIG 1.
Baseline serum tryptase levels in children with mastocytosis and control subjects. Age-matched control subjects include atopic and nonatopic children. Symbols represent mastocytosis variants (control subjects, n = 199; patients with UP, n = 48; patients with DCM, n = 13; patients with SM, n = 18). Median bars and 25th and 75th percentiles are shown. Two patients with the nodular UP form are included within the UP graph (values of 63.9 and 86.2 ng/mL). One patient each with ISM and DCM who had marrow biopsies performed did not have tryptase measurements. Patients with mastocytomas are not shown. The shaded area represents normal tryptase range (<11.4 mg/mL). ***P < .0001.
Results of routine laboratory studies were generally within the normal range, and those with increased values were without clinical consequence. Patients with increased indices and greater than 1 determination had values return to normal (3 with monocytosis, 22 with lymphocytosis, and 12 with thrombocytosis). Seven patients with an increased PTT (4 with lupus antibodies, 1 with Factor VII deficiency, and 2 of unknown cause) had their PTT values return to normal. Three patients (2 with DCM and 1 with UP) had mild anemia caused by iron deficiency. One patient had a markedly increased alkaline phosphatase level diagnosed as transient hyperphosphatemia of infancy. We also noted an inverse correlation between serum tryptase and IgE levels previously reported in patients with mastocytosis (see Fig E1 in this article's Online Repository at www.jacionline.org).15,16
Organomegaly and bone marrow findings
Of the 53 patients who underwent bone marrow biopsies, all 19 with organomegaly had systemic mastocytosis (SM), whereas all 34 without organomegaly did not (Table II). Thus even among patients with severe mediator symptoms8,13 and serum tryptase levels of greater than 20 ng/mL, only patients with organomegaly (16/25) had systemic disease, whereas none of the patients without organomegaly (9/25) had systemic disease. Sixteen of 19 of the children with SM had the D816V mutation in the bone marrow aspirate. In contrast, 8 children with UP (serum tryptase levels of 99, 12, 9, 8, 6, 5, 4, and 3 ng/mL) had a bone marrow biopsy performed solely on the basis of severe mediator symptoms. All had normal biopsy specimens, and the KIT D816V mutation was not found. Eight patients with DCM (serum tryptase levels of 167, 126, 74, 61, 33, 26, and 25 ng/mL; 1 patient no value), and severe cutaneous symptoms had bone marrow biopsies, and all biopsy specimens were negative for systemic disease and the KIT D816V mutation.
TABLE II.
Organomegaly and bone marrow findings
| Patient counts |
|||||
|---|---|---|---|---|---|
| Tryptase level* | Severe mediator symptoms, yes or no | Organomegaly, yes or no | No bone marrow biopsy performed | Bone marrow biopsy performed | No. with + bone narrow result†/no. with bone marrow biopsy performed |
| <20 | Yes | Yes | 0 | 1 | 1/1 |
| ≥20 | Yes | Yes | 0 | 16 | 16/16 |
| Not done | Yes | Yes | 0 | 1 | 1/1 |
| <20 | No | Yes | 0 | 0 | 0 |
| ≥20 | No | Yes | 0 | 1 | 1/1 |
| Not done | No | Yes | 0 | 0 | 0 |
| <20 | Yes | No | 2 | 8 | 0/8 |
| ≥20 | Yes | No | 6 | 9 | 0/9 |
| Not done | Yes | No | 0 | 17 | 0/17 |
| <20 | No | No | 31 | 0 | 0 |
| ≥20 | No | No | 5 | 0 | 0 |
| Not done | No | No | 8 | 0 | 0 |
| Totals | 52 | 53 | 19/53 | ||
Measurement in proximity to the time a decision about performing a bone marrow was made.
Bone marrow biopsy revealed systemic disease. The bone marrow procedure was performed at an age range of 1 to 18 years, with a mean of 4.76 years. The time of performance of the bone marrow biopsy after age of onset was a mean of 4.57 years.
Symptom and disease improvement over time
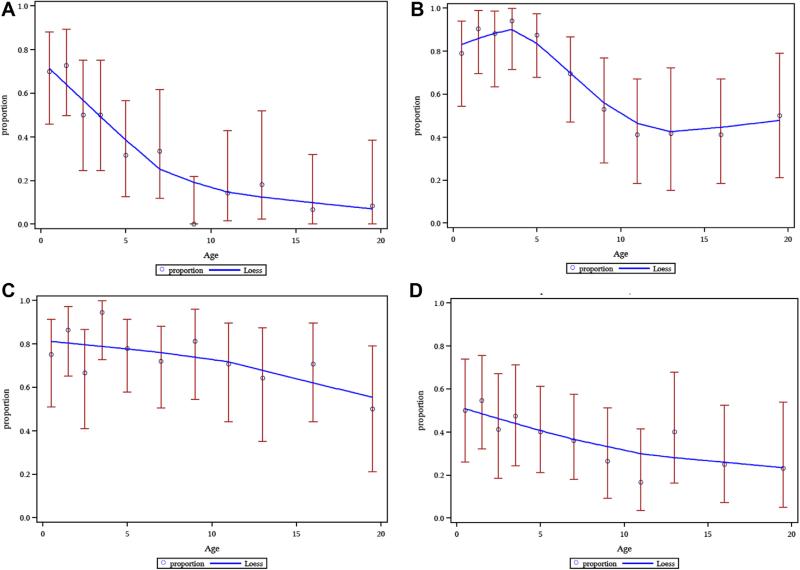
The literature supports the conclusion that symptoms and disease in patients with UP and DCM lessen with time overall,1,3,16,17 and this decreasing prevalence of symptoms with age is reflected in our data (Fig 2). The trend appears to be especially strong for blistering (Fig 2, A). Pruritus and flushing tended to be more persistent (Fig 2, B and C). Patients with DCM accounted for the most severe and prolonged duration of cutaneous symptoms. Gastrointestinal symptoms, including diarrhea, improve over time (Fig 2, D).
FIG 2.
Mast cell mediator symptom prevalence by age category. A, Blistering. B, Pruritus. C, Flushing. D, Diarrhea. Within each panel, each dot represents the prevalence for specific age categories (0-1, 1-2, 2-3, 3-4, 4-6, 6-8, 10-12, 12-14, 14-18, and ≥ 18 years), and the red bar is the 95% CI for that prevalence. The blue loess curves illustrate prevalence across age categories when all mastocytosis variants are analyzed collectively. Symptoms were scored as present (1) or not present (0).
Patients with at least 18 months of follow-up were classified with respect to the degree of clinical resolution. A major resolution was more common in our patients with UP or DCM and less common in our patients with ISM (Table III). Table III also displays the mean percentage decrease in serum tryptase levels within each disease category and the percentage of children within that category who experienced a complete, major, or partial resolution of disease, showing that patients with greater disease resolution tended to have a larger percentage decrease in tryptase levels. This association is significant (P < .0014, Spearman rank correlation) and illustrated by examples of skin resolution with decreasing serum tryptase levels (see Fig E2 A, B, and C, in this article's Online Repository at www.jacionline.org for UP, DCM, and ISM, respectively).
TABLE III.
Symptom resolution score and change in serum tryptase levels over time
| Follow-up time (y) by resolution status, mean (no.) |
Percentage decrease in serum tryptase levels by resolution status, mean (no.) |
|||||
|---|---|---|---|---|---|---|
| Disease | Complete | Major | Partial | Complete | Major | Partial |
| UP | 1.8 (1) | 6.1 (12) | 7.3 (6) | 86.3 (1) | 43.7 (12) | 32.8 (6) |
| DCM | — | 7.4 (4) | 3.1 (3) | — | 74.6 (4) | 34.4 (3) |
| ISM | 10.4 (1) | 5.8 (3) | 6.5 (7) | 66.3 (1) | 43.9 (3) | 23.9 (7) |
| All | 6.1 (2) | 6.3 (19) | 6.1 (16) | 76.3 (2) | 50.3 (19) | 29.2 (16) |
One child with the nodular form of maculopapular cutaneous mastocytosis had a major remission from the nodular subtype to maculopapular cutaneous mastocytosis and a decrease in serum tryptase level from 86.2 to 30.6 ng/mL.
Sequential tryptase determinations
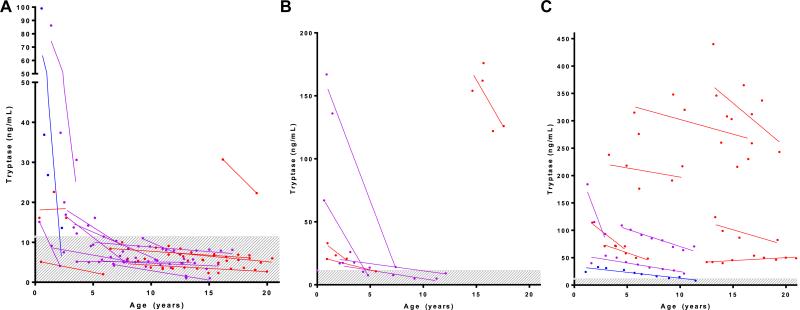
We plotted the trajectory of sequential tryptase levels after the initial evaluation and diagnosis in 37 patients who were followed for 1.5 to 12 years. In all children with UP, tryptase levels decreased or remained stable (Fig 3, A). The decrease was particularly pronounced in those patients with UP (followed for an average of 6.5 years) in whom the initial tryptase level was well above the normal range and decreased to within the normal range by the time the most recent serum tryptase level was recorded. Serum tryptase levels in most children with DCM were greater than 20 ng/mL at diagnosis and also decreased when these patients were followed for an average of 6 years (Fig 3, B). Serum tryptase determinations also decreased in almost all patients with SM, although tryptase levels generally remained greater than the normal range (Fig 3, C). Thus in children with all variants of mastocytosis, serum tryptase levels decreased or remained stable with time. The patients are color coded by disease resolution status in these figures, illustrating that patients with the greatest percentage decrease in tryptase level tended to have greater clinical resolution.
FIG 3.
Serum tryptase levels over time. Sequential serum tryptase levels in patients with UP (n = 18; nodular maculopapular cutaneous mastocytosis, n = 1; A), DCM (n = 7; B), or ISM (n = 11; C) with at least 18 months of follow-up. Linear regression lines are presented for each patient, along with each observation. Blue lines denote values from patients with complete disease resolution, purple lines denote major resolution, and red lines denote partial resolution. Serum tryptase levels decreased or remained stable over time for all variants. The shaded area represents the tryptase normal range (<11.4 mg/mL).
Bone marrow mast cell burden correlated with serum tryptase level
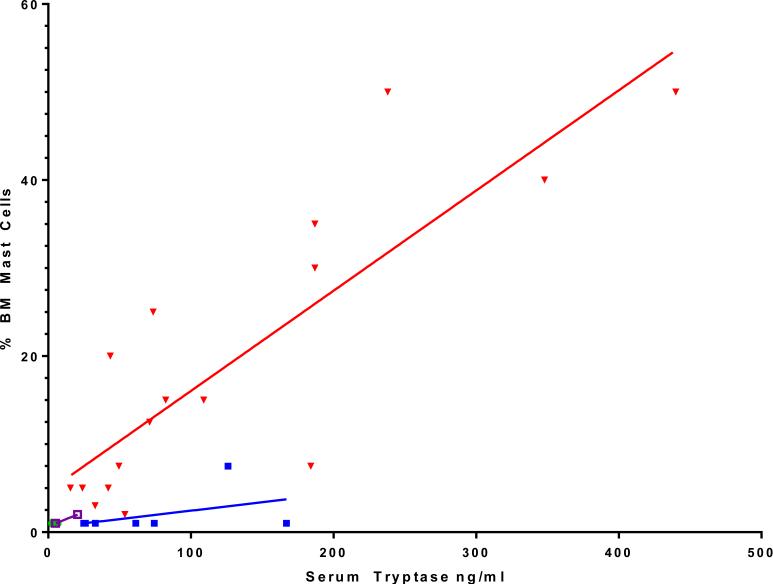
In addition, we examined whether there was a relationship between serum tryptase level and marrow mast cell burden. We found that children with UP and tryptase levels within the normal range had few mast cells observed within bone marrow (Fig 4). In contrast, those patients with bone marrow pathology consistent with systemic disease had increases in serum tryptase levels, which correlated with the percentage of mast cell cellularity within bone marrow (Spearman rho = 0.84 and P < .0001 for ISM). These observations are illustrated with representative biopsy specimens. Fig E3, A, in this article's Online Repository at www.jacionline.org presents findings in a child with UP with a serum tryptase level of 6.1 ng/mL. The corresponding bone marrow was normocellular, with typical morphology and number of mast cells. Fig E3, B, shows a child with DCM and a serum tryptase level of 26.0 ng/mL with marrow that revealed normal mast cell morphology and low mast cell burden. Two children with ISM and serum tryptase levels of 53.7 and 238.0 ng/mL had marrow studies that revealed both abnormal mast cell morphology and increased mast cell numbers (see Fig E3, C and D).
FIG 4.
Percentage of mast cell cellularity observed on bone marrow biopsy specimens correlated with serum tryptase levels for ISM. Data shown are for bone marrow biopsy specimens in which there was a tryptase level obtained at the time of biopsy (n = 32). Green circles indicate UP (n = 6), filled squares indicate DCM (n = 7), open squares indicate mastocytomas (n = 2), and triangles indicate ISM (n = 17). The 2 children with a nodular subtype did not have a bone marrow biopsy performed. Note that some data points superimpose. Correlation coefficient Spearman rho = 0.84 (P < .0001) for SM and Spearman rho = 0.41 (P = .363) for DCM. UP values are all 1% or less, with no correlation.
DISCUSSION
The management of children with cutaneous mastocytosis, SM, or both is a challenge for the physician. We analyzed 105 children followed at NIH to determine the value of serum tryptase in conjunction with symptoms and physical examinations in managing disease. Reassuringly, children within all categories of disease either remained stable or improved. We found that organomegaly was a strong indicator of who might need a bone marrow biopsy among patients with high tryptase levels and severe mediator symptoms. Tryptase determinations tended to decrease with time in concert with improving symptoms. Finally, tryptase levels correlated with the percentage of mast cells found on the bone marrow biopsy specimens from patients with ISM.
In 2001, the WHO criteria for the diagnosis of SM were formulated by using adult data. The current discussions centering on the evaluation for systemic disease in pediatric-onset disease suggest that consideration be given to performing a bone marrow examination when the serum tryptase level is greater than 20 ng/mL, severe mediator symptoms are present, or organomegaly or hematologic abnormalities are noted.8,9 In our patients with UP limited to cutaneous symptoms and minor gastrointestinal symptoms, the majority had a serum tryptase level in the normal range, and a tryptase level within the normal range indicated that the child did not have systemic disease. We did not pursue a bone marrow procedure in children with UP and a serum tryptase level of greater than the normal range unless severe mediator symptoms were present. We found that all patients with UP selected for a bone marrow biopsy on the basis of severe mast cell mediator symptoms with a normal or increased serum tryptase level (n = 8) had normal bone marrow findings and were negative for the D816V mutation. In contrast, all patients selected for a bone marrow biopsy on the basis of hepatosplenomegaly had findings on bone marrow examination diagnostic of systemic disease (Table II). Additionally, these patients were positive for the KIT D816V mutation, which has been reported to indicate that disease is more likely to persist into adulthood.17 We also noted that although in adults it is not unusual for systemic disease to progress and be accompanied by increasing serum tryptase levels,18,19 we observed the opposite clinical course in children, with symptoms improving in general with time, and this improvement was accompanied by a decrease in serum tryptase levels (Fig 3 and Table III). Finally, we found that in those patients with bone marrow pathology consistent with systemic disease, increases in serum tryptase levels correlated with percentages of mast cells in the marrow (Fig 4).
These data led us to several observations. First, none of the 9 patients with serum tryptase levels of greater than 20 ng/mL and severe mediator symptoms but no organomegaly showed systemic disease on bone marrow evaluation. This suggests isolated increased tryptase levels might not be a criterion on which to base the decision to perform a bone marrow biopsy. This is consistent with an earlier report that an isolated increased tryptase level is not a sufficient reason for a marrow biopsy, although in that report only 3 bone marrow biopsies were performed in 101 cases and only when the tryptase level was greater than 100 ng/mL.5 Our data also suggest that the decision to perform a marrow biopsy only with a tryptase level of greater than 100 ng/mL is too restrictive a criterion because 10 children in our study with a positive marrow result had tryptase levels of less than 100 ng/mL. We did find that those children with hepatosplenomegaly are more likely to have systemic disease, although in our study marrow findings did not alter disease management. Thus we recommend a bone marrow examination be performed only in situations in which there is significant concern for the health of the child and the findings might change management and evolve over time (Table II).
Second, serum tryptase levels generally decrease with time in the pediatric group (Fig 3). An upward trend would be unusual and thus suggest the need for monitoring over concern about disease progression.
Finally, these observations form a basis on which to begin a discussion of what clinical and laboratory findings reveal within the pediatric population and to examine whether existing disease classifications based largely on adult data can be applied to pediatric disease. Note that if evidence of severe disease persists into the adult years, a bone marrow biopsy might be warranted.
On the basis of our data, we would argue that there is value to determining serum tryptase levels at 6- to 12-month intervals. Other studies have noted that children with cutaneous and systemic disease tend to improve with time (maximum follow-up, 5.1 years).20-23 As shown in Fig 3, in patients with sequential tryptase data, tryptase levels decreased or remained stable over a period of 1.5 to 12 years. In no case was there progression to systemic disease in those with cutaneous disease only, even in those children with tryptase levels of greater than 20 ng/mL. Additionally, the percentage decrease in serum tryptase levels tended to be associated with symptom improvement (Table III).
Children with DCM provide a particular challenge, including when to make the decision to perform a bone marrow biopsy. In this study the majority of patients with DCM (approximately 85%) had tryptase levels of greater than 20 ng/mL at diagnosis, and none had organomegaly. Bone marrow examinations were performed in 7 of 14 patients with DCM, and the WHO criteria for systemic disease were not fulfilled. Moreover, of the patients with DCM followed for an average of 6 years, all improved clinically and experienced a decrease in serum tryptase levels.
We were also able to address the question of disease improvement. When all data were combined, there was a consistent pattern of clinical symptom improvement (Fig 3 and Table III). The associated regression of lesions and resolution of mast cell mediator symptoms were more pronounced in children with a marked decrease in serum tryptase levels over several years (Fig 3). Decreasing tryptase levels occurred under conservative therapy and did not reflect use of cytoreductive strategies. We also again verified that IgE levels are generally not increased (see Fig E1).24
We did not perform a genetic analysis on skin biopsy specimens. What is known is that mutations and polymorphisms in KIT other than D816V can be identified in the skin. Little is known about the presence or persistence of these genetic findings in adults, and the presence or absence of these mutations has not been correlated with disease severity, bone marrow findings, or clinical course.17,25 This is an area of investigation that we plan to pursue. However, our data do show a striking correlation between systemic disease and the absence or presence of the D816V mutation in bone marrow.
In summary, we found the determination of serum tryptase levels over time to be a reassuring correlate of clinical improvement. We did not find evidence that serum tryptase levels alone were useful in selecting children for a marrow examination to evaluate for systemic disease. Rather, organomegaly served as the most useful predictor of those likely to have systemic disease documented by a bone marrow biopsy. These findings provide further guidance in the management of children with mastocytosis, including when to perform a bone marrow biopsy, and highlight the need for tailoring the WHO criteria in the pediatric population.
Supplementary Material
Key messages.
Organomegaly was the best predictor of who needed a bone marrow biopsy.
Sequential tryptase determinations were useful in supplementing clinical judgment.
Acknowledgments
We thank Sarah J. Austin, MS, for her critical review of the manuscript.
Supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations used
- DCM
Diffuse cutaneous mastocytosis
- ISM
Indolent systemic mastocytosis
- NIH
National Institutes of Health
- PTT
Partial thromboplastin time
- SM
Systemic mastocytosis
- UP
Urticaria pigmentosa
- WHO
World Health Organization
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259–70. doi: 10.2165/11588890-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amon U, Hartmann K, Horny HP, Nowak A. Mastocytosis—an update. J Dtsch Dermatol Ges. 2010;8:695–712. doi: 10.1111/j.1610-0387.2010.07482.x. [DOI] [PubMed] [Google Scholar]
- 3.Caplan R. The natural course of urticaria pigmentosa. Arch Dermatol. 1963;87:146–57. doi: 10.1001/archderm.1963.01590140008002. [DOI] [PubMed] [Google Scholar]
- 4.Brockow K, Akin C, Huber M, Metcalfe DD. Assessment of the extent of cutaneous involvement in children and adults with mastocytosis: relationship to symptomatology, tryptase levels, and bone marrow pathology. J Am Acad Dermatol. 2003;48:508–16. doi: 10.1067/mjd.2003.98. [DOI] [PubMed] [Google Scholar]
- 5.Lange M, Niedoszytko M, Renke J, Glen J, Nedoszytko B. Clinical aspects of paediatric mastocytosis: a review of 101 cases. J Eur Acad Dermatol Venereol. 2013;27:97–102. doi: 10.1111/j.1468-3083.2011.04365.x. [DOI] [PubMed] [Google Scholar]
- 6.Middelkamp Hup MA, Heide R, Tank B, Mulder PG, Oranje AP. Comparison of mastocytosis with onset in children and adults. J Eur Acad Dermatol Venereol. 2002;16:115–20. doi: 10.1046/j.1468-3083.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Twose I, Vano-Galvan S, Sanchez-Munoz L, Morgado JM, Matito A, Torrelo A, et al. Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy. 2012;67:813–21. doi: 10.1111/j.1398-9995.2012.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435–53. doi: 10.1111/j.1365-2362.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 9.Valent P, Sperr WR, Schwartz LB, Horny HP. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114:3–12. doi: 10.1016/j.jaci.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg HK, Markowitz RI, Kolberg H, Park C, Hubbard A, Bellah RD. Normal splenic size in infants and children: sonographic measurements. AJR Am J Roentgenol. 1991;157:119–21. doi: 10.2214/ajr.157.1.2048509. [DOI] [PubMed] [Google Scholar]
- 11.Horny HP, Akin C, Metcalfe DD, Escribano L, Bennett JM, Valent P, et al. In: World Health Organization (WHO) classification of tumours. Pathology & genetics: tumours of haematopoietic and lymphoid tissues. Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. IARC Press; Lyon (France): 2008. pp. 54–63. [Google Scholar]
- 12.Maric I, Robyn J, Metcalfe DD, Fay MP, Carter M, Wilson T, et al. KIT D816V-associated systemic mastocytosis with eosinophilia and FIP1L1/PDGFRA-associated chronic eosinophilic leukemia are distinct entities. J Allergy Clin Immunol. 2007;120:680–7. doi: 10.1016/j.jaci.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Uzzaman A, Maric I, Noel P, Kettelhut BV, Metcalfe DD, Carter MC. Pediatric-onset mastocytosis: a long term clinical follow-up and correlation with bone marrow histopathology. Pediatr Blood Cancer. 2009;53:629–34. doi: 10.1002/pbc.22125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komarow HD, Hu Z, Brittain E, Uzzaman A, Gaskins D, Metcalfe DD. Serum tryptase levels in atopic and nonatopic children. J Allergy Clin Immunol. 2009;124:845–8. doi: 10.1016/j.jaci.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller U, Helbling A, Hunziker T, Wuthrich B, Pecoud A, Gilardi S, et al. Mastocytosis and atopy: a study of 33 patients with urticaria pigmentosa. Allergy. 1990;45:597–603. doi: 10.1111/j.1398-9995.1990.tb00945.x. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez de Olano D, de la Hoz Caballer B, Nunez Lopez R, Sanchez Munoz L, Cuevas Agustin M, Dieguez MC, et al. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish network on mastocytosis (REMA). Clin Exp Allergy. 2007;37:1547–55. doi: 10.1111/j.1365-2222.2007.02804.x. [DOI] [PubMed] [Google Scholar]
- 17.Lanternier F, Cohen-Akenine A, Palmerini F, Feger F, Yang Y, Zermati Y, et al. Phenotypic and genotypic characteristics of mastocytosis according to the age of onset. PLoS One. 2008;3:e1906. doi: 10.1371/journal.pone.0001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–36. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 19.Sperr WR, Jordan JH, Fiegl M, Escribano L, Bellas C, Dirnhofer S, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002;128:136–41. doi: 10.1159/000059404. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Amitai D, Metzker A, Cohen HA. Pediatric cutaneous mastocytosis: a review of 180 patients. Isr Med Assoc J. 2005;7:320–2. [PubMed] [Google Scholar]
- 21.Akoglu G, Erkin G, Cakir B, Boztepe G, Sahin S, Karaduman A, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969–73. doi: 10.1111/j.1468-3083.2006.01696.x. [DOI] [PubMed] [Google Scholar]
- 22.Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, Gutierrez-Castrellon P, Ruiz-Maldonado R. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285–90. doi: 10.1111/j.1468-3083.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- 23.Verzijl A, Heide R, Oranje AP, van Schaik RH. C-kit Asp-816-Val mutation analysis in patients with mastocytosis. Dermatology. 2007;214:15–20. doi: 10.1159/000096907. [DOI] [PubMed] [Google Scholar]
- 24.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2010. Vital Health Stat. 2011;250:1–80. [PubMed] [Google Scholar]
- 25.Bodemer C, Hermine O, Palmerini F, Yang Y, Grandpeix-Guyodo C, Leventhal PS, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804–15. doi: 10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.