ABSTRACT
Although genes encoding enzymes and proteins related to ethanolamine catabolism are widely distributed in the genomes of Pseudomonas spp., ethanolamine catabolism has received little attention among this metabolically versatile group of bacteria. In an attempt to shed light on this subject, this study focused on defining the key regulatory factors that govern the expression of the central ethanolamine catabolic pathway in Pseudomonas aeruginosa PAO1. This pathway is encoded by the PA4022-eat-eutBC operon and consists of a transport protein (Eat), an ethanolamine-ammonia lyase (EutBC), and an acetaldehyde dehydrogenase (PA4022). EutBC is an essential enzyme in ethanolamine catabolism because it hydrolyzes this amino alcohol into ammonia and acetaldehyde. The acetaldehyde intermediate is then converted into acetate in a reaction catalyzed by acetaldehyde dehydrogenase. Using a combination of growth analyses and β-galactosidase fusions, the enhancer-binding protein PA4021 and the sigma factor RpoN were shown to be positive regulators of the PA4022-eat-eutBC operon in P. aeruginosa PAO1. PA4021 and RpoN were required for growth on ethanolamine, and both of these regulatory proteins were essential for induction of the PA4022-eat-eutBC operon. Unexpectedly, the results indicate that acetaldehyde (and not ethanolamine) serves as the inducer molecule that is sensed by PA4021 and leads to the transcriptional activation of the PA4022-eat-eutBC operon. Due to its regulatory role in ethanolamine catabolism, PA4021 was given the name EatR. Both EatR and its target genes are conserved in several other Pseudomonas spp., suggesting that these bacteria share a mechanism for regulating ethanolamine catabolism.
IMPORTANCE The results of this study provide a basis for understanding ethanolamine catabolism and its regulation in Pseudomonas aeruginosa PAO1. Interestingly, expression of the ethanolamine-catabolic genes in this bacterium was found to be under the control of a positive-feedback regulatory loop in a manner dependent on the transcriptional regulator PA4021, the sigma factor RpoN, and the metabolite acetaldehyde. Previously characterized regulators of ethanolamine catabolism are known to sense and respond directly to ethanolamine. In contrast, PA4021 (EatR) appears to monitor the intracellular levels of free acetaldehyde and responds through transcriptional activation of the ethanolamine-catabolic genes. This regulatory mechanism is unique and represents an alternative strategy used by bacteria to govern the acquisition of ethanolamine from their surroundings.
INTRODUCTION
Ethanolamine serves as a source of carbon and nitrogen for a variety of bacteria, including members of the Enterobacteriaceae, Pseudomonadaceae, and Firmicutes (1–5). From studies mostly centered on Salmonella enterica subsp. enterica serovar Typhimurium and Escherichia coli, there is now a basic understanding of the catabolic steps involved in ethanolamine utilization. Extracellular ethanolamine enters the bacterial cell through simple diffusion or carrier-mediated transport (6). Upon entry, ethanolamine is cleaved into acetaldehyde and ammonia through the actions of an ethanolamine-ammonia lyase (EutBC), which uses adenosylcobalamin (AdoCbl) as a cofactor (3, 7, 8). An acetaldehyde dehydrogenase then converts acetaldehyde into acetate (9, 10). In addition to this set of core enzymes, some bacteria possess several other auxiliary proteins that aid in the catabolism of ethanolamine (11). For example, members of the Enterobacteriaceae often utilize an alcohol dehydrogenase (EutG) that reduces acetaldehyde to ethanol and shell proteins (EutSMNLK), which form a microcompartment to encapsulate the ethanolamine-degrading enzymes (10, 12–14).
Two types of regulatory systems have been described regarding ethanolamine catabolism. EutR is an AraC-like transcriptional regulator that positively regulates expression of the eut genes in S. Typhimurium and E. coli (15–17). Induction of the eut genes by EutR involves ethanolamine and AdoCbl (15, 16). EutR is thought to be the regulator of ethanolamine catabolism for most Enterobacteriaceae (11). The other known regulatory system is found among Firmicutes and consists of the sensor kinase EutW and its cognate response regulator EutV (4). The presence of ethanolamine leads to the autophosphorylation of EutW, which subsequently phosphorylates EutV (4). Phosphorylated EutV positively regulates transcription of the eut genes through an antitermination mechanism (18).
In the current study, we describe a third regulatory system for ethanolamine catabolism that was found to function in the opportunistic pathogen Pseudomonas aeruginosa PAO1. The eutBC genes are in an operon with genes encoding an acetaldehyde dehydrogenase (PA4022) and an ethanolamine transporter (PA4023 or eat). The PA4022-eat-eutBC operon is preceded by a conserved −24/−12 promoter recognized by the alternative sigma factor σ54 or RpoN (19–21). Unlike sigma factors of the σ70 class, the RpoN RNA polymerase (RNAP) holoenzyme cannot spontaneously isomerize from a closed configuration to an open complex (19, 22). Instead, this transition requires a unique group of transcriptional regulators known as enhancer-binding proteins (EBPs) (21). EBPs interact specifically with RpoN and couple the energy of nucleotide hydrolysis to the opening of the RpoN-RNAP complex (21).
The presence of an RpoN promoter upstream of PA4022-eat-eutBC suggested that transcription of this operon is most likely regulated by an EBP. Accordingly, we searched for and identified the adjacent PA4021 gene encoding the EBP that positively regulates expression of the PA4022-eat-eutBC genes in response to acetaldehyde, an intermediate of ethanolamine catabolism. Growth on ethanolamine and acetaldehyde-induced expression of the PA4022-eat-eutBC operon were dependent on both PA4021 and RpoN. This is the first description of an acetaldehyde-responsive EBP, and based on conservation, many other Pseudomonas spp. are expected to regulate the catabolism of ethanolamine through a PA4021-RpoN mechanism.
MATERIALS AND METHODS
Bacteria and media.
The P. aeruginosa and Escherichia coli strains used in this study are given in Table S1 in the supplemental material. The ΔPA4021, ΔPA4022, Δeat, and ΔeutB deletion mutants of P. aeruginosa PAO1 were constructed using established methods that have been described (23–25). Bacteria were grown in Lennox broth (LB) or minimal medium (22 mM KH2PO4, 42 mM Na2HPO4, 8.6 mM NaCl, 1.0 mM MgSO4, 5.0 μM FeSO4, and 2 mg liter−1 cyanocobalamin [pH 7.0]). Minimal media were supplemented with carbon and nitrogen sources to final concentrations of 20 mM, unless otherwise stated. Solid bacteriological medium was prepared with the addition of Difco Bacto agar at 15 g liter−1. For plasmid maintenance in E. coli, the medium was supplemented with carbenicillin (100 μg ml−1), kanamycin (50 μg ml−1), and/or gentamicin (20 μg ml−1). For plasmid and marker selection in P. aeruginosa, carbenicillin (200 μg ml−1) or gentamicin (30 μg ml−1) was used as needed.
Plasmids and general molecular biology methods.
Plasmids and primers (oligonucleotides) used in this study are given in Tables S2 and S3 in the supplemental material, respectively. Restriction endonucleases, T4 ligase, and Phusion polymerase used for cloning purposes were purchased from New England BioLabs. PCR was performed according to the recommended conditions for the Phusion polymerase. DNA was isolated using Promega nucleic acid purification kits.
Cloning of the exaC, PA4021, PA4022, eat, and eutBC genes.
The exaC, PA4021, PA4022, eat, eat-eutBC, and PA4022-eat-eutBC genes were PCR amplified from genomic DNA of P. aeruginosa PAO1. The desired PCR products were gel purified, cloned into pCR-Blunt (Invitrogen), and verified through DNA sequencing (Genewiz). The exaC, eat-eutBC, and PA4022-eat-eutBC genes were subcloned into the XbaI/SacI sites of pBBR1MCS-5 (26) to yield the plasmids pBRL644, pBRL669, and pBRL668, respectively. The PA4022 gene was subcloned into the KpnI/XhoI sites of pBBR1MCS-5 to give plasmid pBRL579. The eat gene was subcloned into the HindIII/EcoRI sites of pBBR1MCS-5 to give plasmid pBRL581. The PA4021 gene was subcloned into two different plasmids. In the first cloning event, PA4021 was subcloned into the XbaI/SacI sites of ΔPlac-pBBR1MCS-5 (the pBBR1MCS-5 plasmid lacking the Plac promoter) (24) to give pBRL667. For the second cloning event, the PA4021 gene was subcloned into the XbaI sites of pTrc99a with a forward orientation relative to the trc promoter to yield pBRL602.
Cloning of exaC::lacZ, PA4022::lacZ, eat::lacZ, and truncated eat::lacZ fusions.
PCR was used to amplify the 5′-regulatory regions adjacent to the open reading frames (ORFs) for exaC, PA4022, and eat. The amplified 5′-regulatory regions (850 bp for exaC, 986 bp for PA4022, 2,716 bp for eat, and 928 bp for truncated eat) were then fused to the β-galactosidase (lacZ) ORF of E. coli through PCR (24, 27). The PA4022::lacZ, eat::lacZ, and truncated eat::lacZ fusions were subcloned into the XbaI site of ΔPlac-pBBR1MCS-5 (24) to yield the plasmids pBRL601, pBRL678, and pBRL597, respectively. The exaC::lacZ fusion was subcloned into the BamHI site of ΔPlac-pBBR1MCS-5 to give plasmid pBRL647. The GG dinucleotide (underlined) of −24 element of the putative RpoN promoter (TGGCCCGGCCCTTGCT) upstream of the PA4022 gene was changed to AA in plasmids pBRL601 and pBRL678 using the Q5 site-directed mutagenesis kit from New England BioLabs. The resulting plasmids pBRL679 and pBRL681 were sequenced to verify the presence of the desired mutations.
Synthesis of hydrazone.
The synthesis of valeric acid ethylidene hydrazide, referred simply to as hydrazone, was done as described previously, with minor modifications (28, 29). Briefly, in a round-bottom flask, valeric acid hydrazide (250 mg, 2.2 mmol) was dissolved in tetrahydrofuran and cooled in an ice bath. Acetaldehyde (5.4 ml, 96.1 mmol, 44 eq) was added dropwise, and the solution was stirred for 2 h. The reaction mixture was concentrated in vacuo. The hydrazone was then purified by flash column chromatography and concentrated in vacuo to yield a mixture composed of 84 mol% hydrazone (as a 1:1 mixture of diastereomers) and 16 mol% hydrazide as a white solid (275 mg). Nuclear magnetic resonance (NMR) data for the purified hydrazone are provided in Fig. S1 in the supplemental material.
Growth on ethanolamine.
Analysis was done in triplicate for each strain and condition. Strains were grown in LB for 18 h at 200 rpm at 37°C. Minimal medium supplemented with the desired carbon and nitrogen source was inoculated with 1% (vol/vol) of the LB-grown culture. The inoculated cultures were grown for 4.5 h (nitrogen source testing) or 24 h (carbon source testing) at 200 rpm and 37°C. The absorbance at 600 nm (OD600) was measured for each culture. For carbon source testing, minimal media were supplemented with 20 mM NH4Cl and 20 mM carbon source (ethanolamine, acetate, acetoin or ethanol) or 10 mM carbon source (hydrazide or hydrazone). For nitrogen source testing, minimal media were supplemented with 20 mM succinate and 20 mM nitrogen source (ethanolamine, acetamide, glycine, NH4Cl, KNO3−, or urea). Growth complementation experiments were done in a similar manner, except that medium was supplemented with 30 μg ml−1 gentamicin.
Mapping the PA4022-eat-eutBC operon.
The operon arrangement of the PA4022-eat-eutBC genes was examined using reverse transcriptase PCR (RT-PCR) (25). P. aeruginosa PAO1 was grown in minimal medium supplemented with 20 mM ethanolamine and 20 mM NH4Cl. At an OD600 of 0.3, 0.5 ml of culture was treated with 1.0 ml of RNAprotect bacterial reagent (Qiagen), and RNA was then purified from the treated cells using the RNeasy kit (Qiagen) (25). Prior to cDNA synthesis, the purified RNA was checked for genomic DNA contamination by PCR designed to amplify the rplU gene (24, 30, 31). Following this quality check, reverse transcriptase reactions were conducted using 500 ng of purified RNA and the iScript cDNA synthesis kit (Bio-Rad). The resulting cDNA served as a template in PCRs aimed to amplify ∼500-bp regions between the PA4022-eat, eat-eutB, and eutB-eutC genes with primers ZS466.f/ZS466.r, ZS467.f/ZS467.r, and ZS468.f/ZS468.r, respectively. PCR was also conducted using the purified RNA (negative control) and isolated genomic DNA of P. aeruginosa PAO1 (positive control). PCRs were analyzed by agarose gel electrophoresis.
β-Galactosidase (LacZ) assays.
Analysis was done in triplicate for each strain and condition. LacZ activity was measured using the Miller assay and is reported in Miller units (32). P. aeruginosa strains harboring PA4022::lacZ (pBRL601), eat::lacZ (pBRL678), exaC::lacZ (pBRL647), truncated eat::lacZ (pBRL597), mutated PA4022::lacZ (pBRL679), and mutated eat::lacZ (pBRL681) were grown in minimal medium supplemented with 20 mM acetate, 20 mM NH4Cl, and 30 μg liter−1 gentamicin to an OD600 of 0.3. Substrates, which were prepared as 2.0 M solutions in dimethyl sulfoxide (DMSO), were added to a final concentration of 2.0 mM, and LacZ activity was measured at 90 min postaddition of substrate. For experiments involving E. coli, the ΔlacZ mutant strain BW25113 (33) was cotransformed with (i) PA4022::lacZ (pBRL601), eat::lacZ (pBRL678), or ΔPlac-pBBR1MCS-5 and (ii) plasmid-borne PA4021 (pBRL602) or pTrc99a. The recombinant E. coli strains were grown at 200 rpm and 37°C in minimal medium supplemented with 40 mM glycerol, 20 mM NH4Cl, 100 μg ml−1 carbenicillin, and 20 μg ml−1 gentamicin. At an OD600 of 0.3, ethanolamine or acetaldehyde was added to a final concentration of 10 mM, and LacZ activity was measured at 2 h postaddition of substrate.
RESULTS
PA4021 gene is required for growth on ethanolamine in P. aeruginosa PAO1.
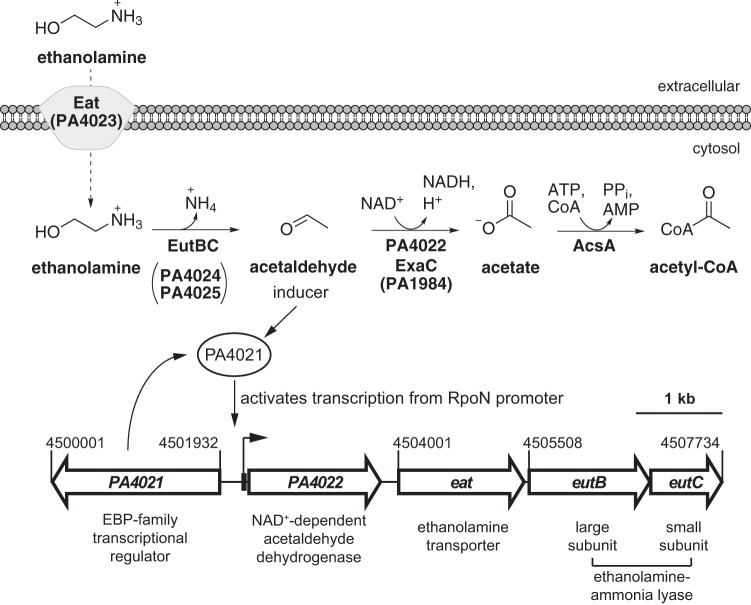
The PA4021 gene encodes an EBP that shares 60% homology (46% identity) with AcoR (PA4147), an EBP that regulates acetoin catabolism (34). Adjacent to PA4021 is a gene cluster encoding enzymes and proteins related to ethanolamine catabolism (Fig. 1). PA4022 encodes a NAD+-dependent acetaldehyde dehydrogenase that has no significant homology to the EutE acetaldehyde dehydrogenases found in Enterobacteriaceae (29). PA4023 encodes a homolog of the ethanolamine transporter Eat, a member of the amino acid-polyamine-organocation family (35). The large and small subunits of ethanolamine-ammonia lyase are putatively encoded by the PA4024 (eutB) and PA4025 (eutC) genes, respectively. The chromosomal clustering or arrangement of the eat-eutBC genes in P. aeruginosa PAO1 is similar to that of many other Proteobacteria (11). Located 68 bp upstream of the PA4022 ORF is a putative −24/−12 promoter that is recognized by the sigma factor RpoN. EBPs are regulatory proteins that activate transcription from RpoN promoters. Therefore, based on proximity, PA4021 was considered to be a potential activator of the PA4022-eat-eutBC genes in response to ethanolamine.
FIG 1.
Proposed catabolic pathway for ethanolamine in P. aeruginosa PAO1. The PA4022-eat-eutBC operon encodes a central catabolic pathway for ethanolamine. Ethanolamine enters the bacterium through simple diffusion and/or transport mediated by Eat (PA4023). Intracellular ethanolamine is hydrolyzed into ammonia and acetaldehyde by ethanolamine-ammonia lyase, which is composed of large EutB (PA4024) and small EutC (PA4025) subunits. The acetaldehyde intermediate is converted into acetate via an NAD+-dependent acetaldehyde dehydrogenase, PA4022 and/or ExaC (PA1984). Acetyl coenzyme A (acetyl-CoA) synthetase (AcsA) is required for acetate utilization in P. aeruginosa (58) and therefore is predicted to be responsible for the activation of acetate into acetyl-CoA during ethanolamine catabolism. The results of the current study suggest that the EBP PA4021 activates transcription of the PA4022-eat-eutBC operon from an RpoN promoter in response to acetaldehyde; a mechanism that is essential for ethanolamine catabolism.
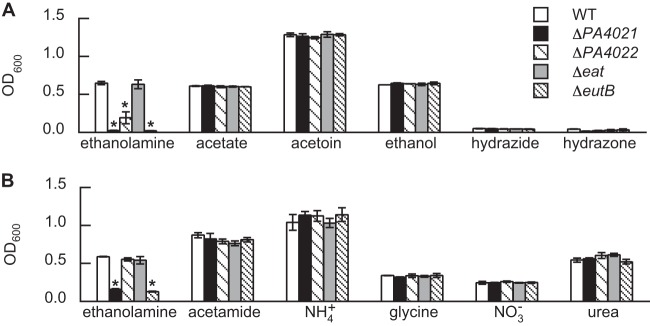
Consistent with PA4021 having a role in ethanolamine catabolism, a ΔPA4021 mutant of P. aeruginosa PAO1 did not grow on ethanolamine as either a sole source of carbon (Fig. 2A) or nitrogen (Fig. 2B). The growth deficiency of the ΔPA4021 mutant was similar to that of the ΔeutB mutant (Fig. 2), indicating the essentiality of PA4021 for ethanolamine utilization. The growth of the Δeat mutant on ethanolamine was identical to that of wild-type (WT) P. aeruginosa PAO1. This finding is in agreement with earlier studies suggesting that carrier-mediated transport is not essential for ethanolamine utilization (6, 12). Deletion of the PA4022 gene reduced the growth of P. aeruginosa PAO1 on ethanolamine as a sole source of carbon (Fig. 2A) but not nitrogen (Fig. 2B). In addition to PA4022, the PA1984 (exaC) gene also encodes an acetaldehyde dehydrogenase that was originally identified as a possible component of the ethanol oxidation system in P. aeruginosa (29, 36). PA4022 and ExaC are nearly identical proteins (98% identity), and both have been biochemically characterized as acetaldehyde dehydrogenases (29). This genetic redundancy would explain why PA4022 was nonessential for growth on ethanolamine, i.e., ExaC was expressed or present under the conditions used. Interestingly, in addition to accepting acetaldehyde as a substrate, PA4022 was shown to catalyze oxidative hydrolysis of the carbon-nitrogen double bond of hydrazones to generate hydrazides and acetate as products (29). Consumption of the acetate product was believed to be responsible for the growth of P. aeruginosa PAO1 on hydrazones (29). As shown in Fig. 2A, we did not observe growth on hydrazone for any of the P. aeruginosa strains used in this study. The discrepancy between our result and that of the earlier study is unclear.
FIG 2.
Growth on ethanolamine requires the PA4021 gene in P. aeruginosa PAO1. Ethanolamine was provided as the sole source of either carbon (A) or nitrogen (B) for growing WT P. aeruginosa PAO1 and its isogenic mutants: the ΔPA4021, ΔPA4022, Δeat, and ΔeutB mutants. (A) As a carbon source, the ΔPA4021 and ΔeutB mutants exhibited no growth on ethanolamine, whereas the ΔPA4022 mutant displayed reduced growth. In comparison, the ΔPA4021 and ΔPA4022 mutants generated cell densities identical to that of the WT on carbon sources that intercept with ethanolamine at key catabolic intermediates, such as acetaldehyde and acetate. These results indicate that the PA4021 and PA4022 genes are necessary for catabolism of ethanolamine and not acetaldehyde in general. (B) As a nitrogen source in the presence of succinate, the ΔPA4021 and ΔeutB mutants exhibited no growth on ethanolamine. In contrast, the growth of the ΔPA4022 mutant was identical to that of the WT, indicating that PA4022 is not required for the assimilation of ethanolamine as a nitrogen source. Strains were grown for 24 and 4.5 h on ethanolamine as a source of carbon and nitrogen, respectively. Significant differences in OD600 values were determined using an analysis of variance (ANOVA) with Dunnett's post hoc test (α-value, 0.05) and are indicated with asterisks. Data points represent mean values (n = 3) ± standard deviations (SD).
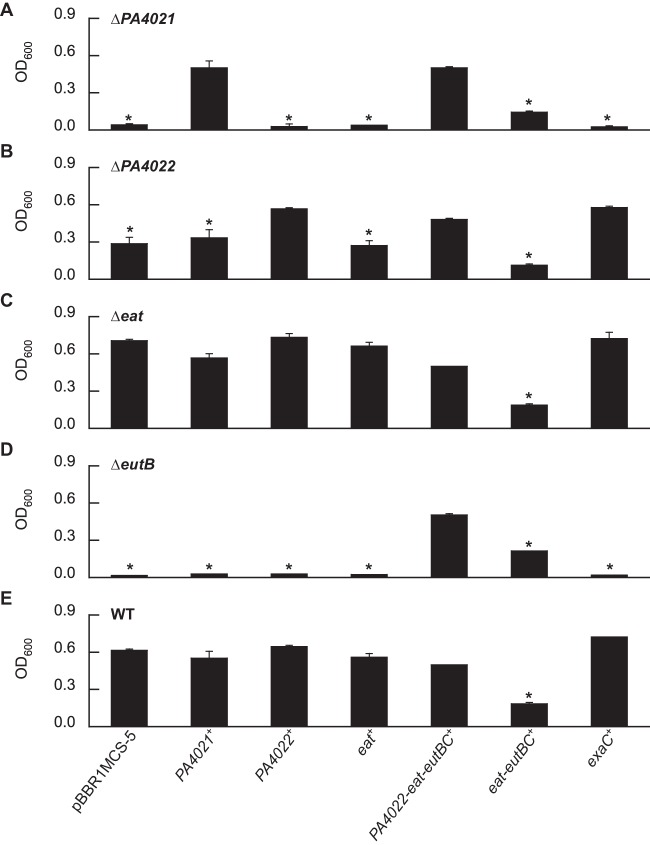
Plasmid-based expression of the PA4022-eat-eutBC genes rescues the growth of the ΔPA4021 mutant on ethanolamine.
It was reasoned that the inability of the ΔPA4021 mutant to grow on ethanolamine was due to insufficient expression of the PA4022-eat-eutBC genes. Indeed, expression of the entire PA4022-eat-eutBC operon from the lac promoter on the broad-host-range plasmid pBBR1MCS-5 rescued the growth of the ΔPA4021 mutant on ethanolamine as a carbon source (Fig. 3A). Growth of the ΔPA4021 mutant reached ∼25% of WT levels when only the eat-eutBC genes were expressed. In contrast, plasmid-based expression of only an acetaldehyde dehydrogenase (PA4022 or exaC) or the Eat transporter did not restore the growth of the ΔPA4021 mutant on ethanolamine.
FIG 3.
Expression of the PA4022-eat-eutBC genes from the lac promoter on the plasmid pBBR1MCS-5 restores the growth of the ΔPA4021 mutant on ethanolamine. The ΔPA4021 (A), ΔPA4022 (B), Δeat (C), and ΔeutB (D) mutants, in addition to the WT (E), were transformed with derivatives of pBBR1MCS-5 that carried either PA4021, PA4022, eat, PA4022-eat-eutBC, eat-eutBC, or exaC. Genetic complementation of the mutants was then determined by growing the recombinant strains on ethanolamine as the sole carbon source for 24 h. Significant differences in OD600 values were determined using an ANOVA with Dunnett's post hoc test (α-value, 0.05) and are indicated with asterisks. Data points represent mean values (n = 3) ± SD.
Growth on ethanolamine was measured for plasmid-carrying strains of the ΔPA4022 mutant (Fig. 3B), Δeat mutant (Fig. 3C), ΔeutB mutant (Fig. 3D), and WT (Fig. 3E). As expected, plasmid-based expression of PA4022 or exaC was sufficient to genetically complement the ΔPA4022 mutant (Fig. 3B). The cell densities observed for the plasmid-carrying strains of the Δeat mutant (Fig. 3D) and WT (Fig. 3E) were similar in value. Plasmid-based expression of the PA4022-eat-eutBC genes fully restored the growth of the ΔeutB mutant, whereas the expression of eat-eutBC resulted in partial recovery, at ∼25% of the WT levels (Fig. 3D). It is worth noting that while plasmid-based expression of the eat-eutBC genes partially compensated for the growth deficiencies of the ΔPA4021 and ΔeutB mutants, the presence of this plasmid actually reduced the overall growth of the ΔPA4022 mutant, Δeat mutant, and WT strains on ethanolamine. It is feasible that overexpression of the eat-eutBC genes generated high levels of EutBC activity, which led to abnormally large amounts of acetaldehyde, resulting in toxicity and growth inhibition of the strains.
PA4022-eat-eutBC genes are synthesized as a single transcript in the presence of ethanolamine.
Although the PA4022-eat-eutBC genes are clustered on the chromosome, it was not experimentally known whether these genes formed a single transcription unit. To address this, P. aeruginosa PAO1 was grown on ethanolamine as a carbon source to an OD600 of 0.3. RNA was purified and subsequently used for cDNA synthesis. PCR was performed on the synthesized cDNA using primers designed to specifically amplify regions between the PA4022-eat, eat-eutB, and eutB-eutC genes. Electrophoresis of the PCRs revealed the presence of only DNA fragments having the sizes of the predicted PA4022-eat, eat-eutB, and eutB-eutC amplicons (Fig. 4). This result indicates that the PA4022-eat-eutBC genes are arranged as an operon in P. aeruginosa PAO1.
FIG 4.
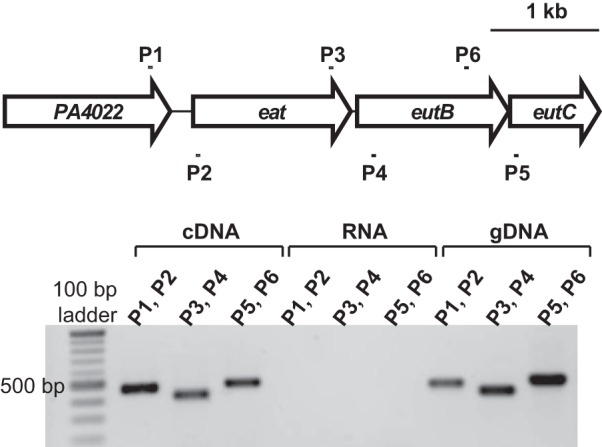
The PA4022-eat-eutBC genes are arranged in an operon in P. aeruginosa PAO1. RT-PCR was used to detect the PA4022-eat-eutBC transcript in P. aeruginosa PAO1 grown on ethanolamine. RNA was purified from ethanolamine-grown cells and subsequently used for cDNA synthesis. Regions (∼500 bp) between PA4022-eat, eat-eutB, and eutB-eutC were PCR amplified from synthesized cDNA using primers P1 and P2, P3 and P4, and P5 and P6, respectively. As expected, PCR products corresponding to PA4022-eat, eat-eutB, and eutB-eutC were observed in reactions that used cDNA or genomic DNA (gDNA) as the template. These products were not observed from PCRs using the purified RNA as the template.
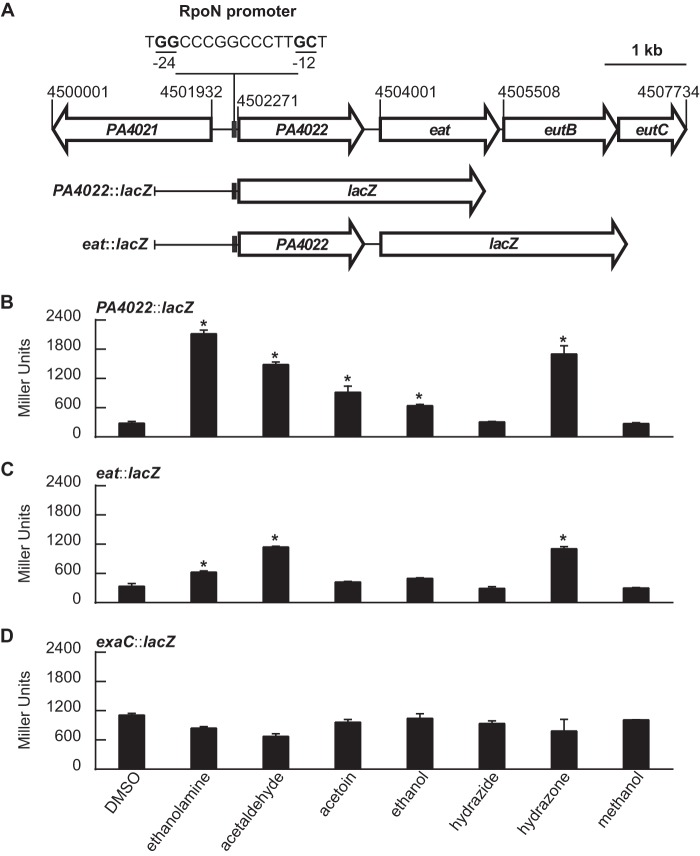
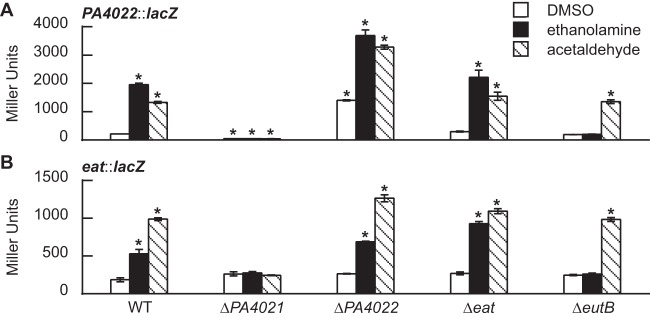
Expression of PA4022::lacZ and eat::lacZ is induced by acetaldehyde.
Plasmid-based LacZ fusions were constructed to assess expression of the PA4022-eat-eutBC locus. Additionally, because acetaldehyde is an intermediate of ethanolamine catabolism, it was considered necessary to measure the expression of exaC. The lacZ ORF of E. coli was fused to the 5′-regulatory region of either PA4022, eat, or exaC to generate the PA4022::lacZ, eat::lacZ, and exaC::lacZ, respectively (Fig. 5A). For the PA4022::lacZ and exaC::lacZ fusions, the 5′-regulatory region consisted of the ∼1,000-bp sequence upstream of the PA4022 or exaC ORF, respectively. For the eat::lacZ fusion, the 5′-regulatory region (∼2,700 bp) consisted of three key features: (i) the ∼1,000-bp 5′-regulatory region upstream of PA4022 ORF, (ii) the PA4022 ORF, and (iii) the 209-bp intergenic region between the PA4022 and eat ORFs.
FIG 5.
Acetaldehyde is an inducer of PA4022::lacZ and eat::lacZ in P. aeruginosa PAO1. (A) PA4022::lacZ and eat::lacZ were constructed by fusing the 5′-regulatory regions of each of these genes with lacZ. Fusions were individually cloned into a promoter-less plasmid ΔPlac-pBBR1MCS-5. (B) Expression of PA4022::lacZ increased with the addition of ethanolamine, acetaldehyde, acetoin, ethanol, or hydrazone. (C) Expression of eat::lacZ increased with the addition of ethanolamine, acetaldehyde or hydrazone. (D) Expression of the exaC::lacZ fusion did not significantly change with the addition of any substrate. P. aeruginosa PAO1 carrying the LacZ fusions was grown in acetate-minimal medium to an OD600 of 0.3 and then challenged with a 2.0 mM concentration of the indicated substrate. LacZ activities were measured 90 min postaddition of substrate. Significant differences in LacZ activities were determined using an ANOVA with Dunnett's post hoc test (α-value, 0.05) and are indicated with asterisks. Data points represent mean values (n = 3) ± SD.
The LacZ fusions were initially used to identify the inducers of the PA4022-eat-eutBC genes in P. aeruginosa PAO1. P. aeruginosa PAO1 harboring the PA4022::lacZ, eat::lacZ, or exaC::lacZ was grown in acetate-minimal medium to an OD600 of 0.3 and then challenged with an array of substrates provided at a final concentration of 2.0 mM. As anticipated, ethanolamine induced LacZ activity 7-fold for PA4022::lacZ (Fig. 5B) and 2-fold for eat::lacZ (Fig. 5C). Interestingly, the addition of acetaldehyde also induced LacZ activity, i.e., 7-fold for PA4022::lacZ and 5-fold for eat::lacZ. The addition of acetoin and ethanol caused a 2-fold increase in the expression of PA4022::lacZ. Acetaldehyde is an intermediate of acetoin and ethanol catabolism (37, 38), which might account for the inducing affects these substrates have on PA4022::lacZ. The expression of eat::lacZ, however, was not significantly affected by acetoin or ethanol. In the presence of hydrazone, LacZ activity increased 6-fold for PA4022::lacZ and 4-fold for eat::lacZ (Fig. 5B and C). As mentioned earlier, PA4022 was reported to be involved in the breakdown of hydrazones (29), and it was interesting to find that a hydrazone did positively regulate expression of the PA4022 gene. Hydrazones are compounds having a basic structure of R1R2C=NNR3R4 (39). The similarities in electronic properties shared between acetaldehyde and acyl hydrazones (40) might be the reason why the hydrazone used in this study induced the expression of the PA4022::lacZ and eat::lacZ.
Last, expression of exaC::lacZ did not significantly change with the addition of any the tested substrates (Fig. 5D). The LacZ activity of exaC::lacZ (∼900 MU) was >3-fold higher than the noninduced (background) LacZ activity measured for PA4022::lacZ (∼250 MU) and eat::lacZ (∼250 MU). Little is known on the expression of exaC in P. aeruginosa PAO1 (36, 41), but these results clearly show that the expression of exaC is not regulated in the same acetaldehyde-dependent manner as PA4022.
PA4021 is essential for induction of PA4022::lacZ and eat::lacZ.
Ethanolamine and acetaldehyde were identified as potential inducers of the PA4022-eat-eutBC genes. To investigate this further, expression of the PA4022::lacZ and eat::lacZ was measured in the ΔPA4021, ΔPA4022, Δeat, and ΔeutB mutants. As shown in Fig. 6, the addition of ethanolamine or acetaldehyde did not induce expression of PA4022::lacZ (Fig. 6A) or eat::lacZ (Fig. 6B) in the ΔPA4021 mutant. This result is consistent with PA4021 being a positive regulator of the PA4022-eat-eutBC genes.
FIG 6.
Induction of PA4022::lacZ and eat::lacZ are dependent on PA4021. Expression of PA4022::lacZ (A) and eat::lacZ (B) were measured in the ΔPA4021, ΔPA4022, Δeat, and ΔeutB mutants. Strains carrying the LacZ fusions were grown in acetate-minimal medium to an OD600 of 0.3 and then challenged with a 2.0 mM concentration of the indicated substrate. LacZ activities were measured 90 min postaddition of substrate. Significant differences in LacZ activities were determined using an ANOVA with Dunnett's post hoc test (α-value, 0.05) and are indicated with asterisks. Data points represent mean values (n = 3) ± SD.
In the case of the ΔPA4022 mutant, ethanolamine and acetaldehyde induced the expression of PA4022::lacZ by 2-fold (Fig. 6A). Furthermore, the noninduced (background) expression levels of PA4022::lacZ were 5-fold higher in the ΔPA4022 mutant than those observed in the WT. Acetaldehyde is an intermediate in both the biosynthesis and catabolism of ethanol, which is a by-product of P. aeruginosa PAO1 metabolism (38). An inability to catabolize or remove acetaldehyde generated from cellular metabolism might account for the elevated background expression of PA4022::lacZ in the ΔPA4022 mutant. In comparison, the expression of eat::lacZ in the ΔPA4022 mutant increased 2- and 5-fold with the addition of ethanolamine and acetaldehyde, respectively (Fig. 6B), but the background levels of eat::lacZ in the ΔPA4022 mutant were similar in value to those measured in WT. Unlike PA4022::lacZ, the eat::lacZ fusion possesses an intact PA4022 gene (see Fig. 5). The expression of the eat::lacZ fusion, which is carried on a low-copy-number plasmid, could generate sufficient PA4022 protein to counter the ΔPA4022 mutation. This would explain why eat::lacZ behaved in the same way for both the ΔPA4022 mutant and WT strains.
The fold changes observed for PA4022::lacZ and eat::lacZ in the Δeat mutant were similar in magnitude to the fold changes measured in the WT (Fig. 6). This result held true for the ΔeutB mutant as well but only regarding acetaldehyde, which induced the expression of PA4022::lacZ by 7-fold and eat::lacZ by 4-fold. In contrast, ethanolamine did not induce expression of PA4022::lacZ or eat::lacZ in the ΔeutB mutant. These results are consistent with the idea that acetaldehyde (the product of the EutBC reaction) is the inducer of the PA4022-eat-eutBC genes in P. aeruginosa PAO1.
PA4022::lacZ and eat::lacZ are induced by acetaldehyde in E. coli expressing the PA4021 gene.
The PA4021 gene was cloned under the trc promoter of the expression plasmid pTrc99a. The resulting plasmid was then introduced into the E. coli ΔlacZ mutant that harbored either PA4022::lacZ or eat::lacZ. The recombinant E. coli strains were grown in glycerol-minimal medium to an OD600 of 0.3 and then challenged with either ethanolamine or acetaldehyde. While the addition of ethanolamine did not induce expression of either LacZ fusion, acetaldehyde caused LacZ activity to increase 8-fold for PA4022::lacZ and 4-fold for eat::lacZ (Fig. 7). PA4021 was essential for this increase in expression, because replacement of the plasmid-borne PA4021 with empty pTrc99a resulted in background levels of induction of PA4022::lacZ and eat::lacZ.
FIG 7.
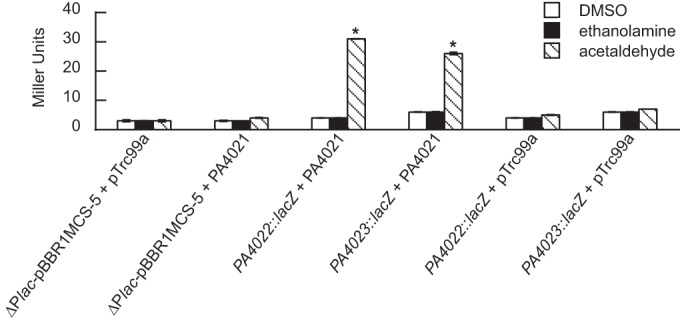
Heterologous expression of PA4021 allows for acetaldehyde induction of PA4022::lacZ and eat::lacZ in E. coli. The PA4021 gene was cloned into pTrc99a, and the resulting plasmid was transformed into an E. coliΔlacZ mutant, which harbored PA4022::lacZ, eat::lacZ, or empty plasmid control (ΔPlac-pBBR1MCS-5). The recombinant E. coli strains were assayed for LacZ activity in the presence of acetaldehyde, ethanolamine, and no substrate. The addition of acetaldehyde caused a >4-fold increase in LacZ activity for the PA4022::lacZ and eat::lacZ. The addition of ethanolamine had no effect. Substitution of the plasmid-borne PA4021 with empty plasmid pTrc99a resulted in no induction of either LacZ fusion in the presence of acetaldehyde. (Strains carrying the LacZ fusions were grown in glycerol-minimal medium to an OD600) of 0.3 and then challenged with a 2.0 mM concentration of the indicated substrate. LacZ activities were measured 2 h postaddition of substrate. Significant differences in LacZ activities were determined using an ANOVA with Dunnett's post hoc test (α-value, 0.05) and are indicated with asterisks. Data points represent mean values (n = 3) ± SD.
Growth on acetaldehyde does not require PA4021 or PA4022.
It was clear that expression of PA4022::lacZ was induced by acetaldehyde, but it was uncertain as to whether PA4022 and its regulator PA4021 are essential in the utilization of acetaldehyde. Due to the toxicity and volatile nature of this compound, P. aeruginosa strains were grown in sealed tubes completely filled with minimal medium containing 10 mM acetaldehyde and 20 mM KNO3. After an incubation period of 48 h at 37°C, the ΔPA4021 and ΔPA4022 mutants were found to have cell densities identical to that of the WT in the presence of acetaldehyde (see Fig. S2 in the supplemental material). However, under these oxygen-limiting conditions, growth on ethanolamine as a carbon source was absent for the ΔPA4021 mutant and reduced by ∼50% for the ΔPA4022 mutant. Even though these results indicate that the PA4021 and PA4022 genes are not needed for growth on acetaldehyde, they do reaffirm that these genes are crucial in the catabolism of ethanolamine in P. aeruginosa PAO1.
Growth on ethanolamine and induction of PA4022::lacZ and eat::lacZ is dependent on RpoN.
The results suggested that PA4021 was necessary for the activation of the PA4022-eat-eutBC genes in response to acetaldehyde. Because EBPs activate transcription from RpoN promoters, we next sought to verify that RpoN and its cognate promoter upstream of PA4022 were key factors in the regulation of ethanolamine catabolism in P. aeruginosa PAO1. As shown in Fig. 8, ethanolamine did not support the growth of an rpoN mutant (42) unless the PA4022-eat-eutBC genes were expressed from the lac promoter on the plasmid pBBR1MCS-5 (Fig. 8A). In addition, neither ethanolamine nor acetaldehyde induced expression of PA4022::lacZ and eat::lacZ in an rpoN mutant (Fig. 8B).
FIG 8.
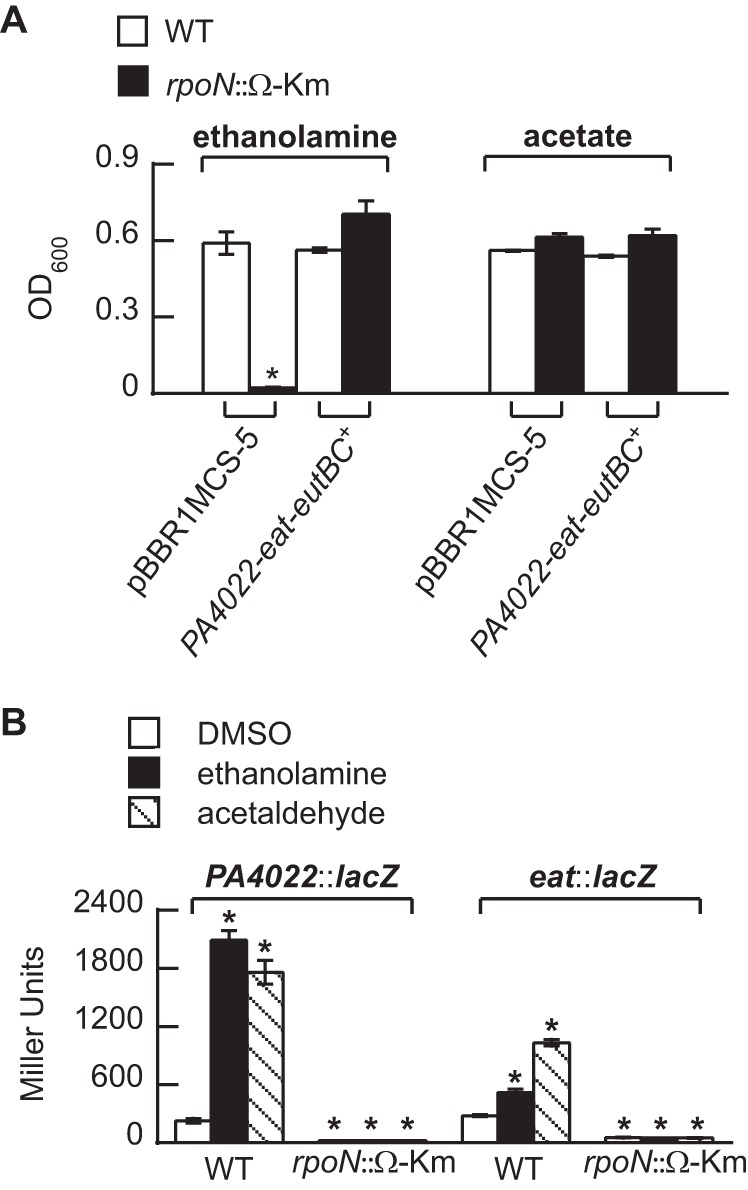
Ethanolamine catabolism is dependent on RpoN. (A) No growth on ethanolamine was observed for an rpoN mutant (rpoN::Ω-Km) (42). This growth deficiency was alleviated via plasmid-based expression of the PA4022-eat-eutBC genes. (B) There was no induction of PA4022::lacZ and eat::lacZ in the rpoN mutant. Significant differences in measured values were determined using an ANOVA with Dunnett's post hoc test (α-value, 0.05) and are indicated with asterisks. Data points represent mean values (n = 3) ± SD.
RpoN recognizes a distinct and highly conserved −24/−12 promoter with the consensus sequence TGGCAC-N6-TGCT (20). The GG and GC dinucleotides of the −24 and −12 elements (underlined), respectively, are critical for promoter activity. Positioned 68 bp upstream of the PA4022 ORF is a putative RpoN promoter with the sequence TGGCCCGGCCCTTGCT (Sigma 54 Promoter Database [http://www.sigma54.ca]). To validate the importance of this promoter, the conserved GG dinucleotide of the −24 element was changed to AA in PA4022::lacZ and eat::lacZ, which afterwards were assayed for LacZ activity in P. aeruginosa PAO1. As shown in Fig. 9, expression of the mutated PA4022::lacZ and eat::lacZ did not increase with the addition of ethanolamine or acetaldehyde. In parallel to these experiments, a truncated version of eat::lacZ was constructed by fusing the ∼1,000-bp upstream region of eat with the E. coli lacZ ORF. Notably, this truncated eat::lacZ fusion does not possess the 5′-regulatory region upstream of PA4022 and therefore is not under the control of the RpoN promoter in question. The background expression of the truncated eat::lacZ fusion was similar in value to that of the full-length or untruncated eat::lacZ (Fig. 9). However, expression of the truncated eat::lacZ fusion did not change with the addition of ethanolamine or acetaldehyde. This highlights the necessity of the RpoN promoter for the induction of the PA4022-eat-eutBC operon in response to acetaldehyde.
FIG 9.
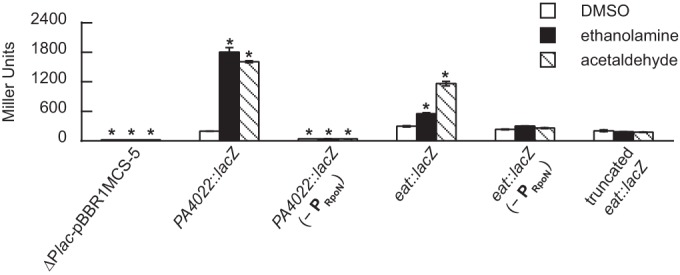
Induction of PA4022::lacZ and eat::lacZ is controlled by an RpoN promoter. Transcription of the PA4022-eat-eutBC operon in response to acetaldehyde was thought to be driven by an RpoN promoter located 68 bp upstream of PA4022. To address the functionality of this RpoN promoter, the conserved GG dinucleotide of the −24 element was changed to AA in both PA4022::lacZ and eat::lacZ. This mutation (−PRpoN) resulted in no induction of either lacZ fusion in the presence of ethanolamine or acetaldehyde. Additionally, when the 5′-regulatory region of eat::lacZ was truncated so as to include only the ∼1,000-bp sequence immediately upstream of eat (truncated eat::lacZ), LacZ activity did not increase with the addition of ethanolamine or acetaldehyde. (P. aeruginosa PAO1 carrying the LacZ fusions was grown in acetate-minimal medium to an OD600) of 0.3 and then challenged with a 2.0 mM concentration of the indicated substrate. LacZ activities were measured 90 min postaddition of substrate. Significant differences in LacZ activities were determined using an ANOVA with Dunnett's post hoc test (α-value, 0.05) and are indicated with asterisks. Data points represent mean values (n = 3) ± SD.
DISCUSSION
Prior to this study, there was little information regarding ethanolamine catabolism in Pseudomonas. In fact, the only things known about this subject were that (i) the eutBC genes are found in the genome sequences of several Pseudomonas spp. and (ii) EutBC activity had been reported for a few species, such as P. denitrificans and P. putida (1, 11). This limited information does indicate that under certain conditions, ethanolamine might be a necessary or desirable nutrient for a variety of Pseudomonas species. For enteric bacteria, the human intestine has been suggested to be a rich source of ethanolamine, which is primarily available in the form of the phospholipid phosphatidylethanolamine (43–45). In addition to phosphatidylethanolamine, Pseudomonas spp. could potentially have access to free or non-lipid-bound ethanolamine, because plants biosynthesize ethanolamine through direct decarboxylation of serine (46). It is interesting to note that ethanolamine utilization has been connected with virulence in pathogenic strains of S. Typhimurium and E. coli (17, 44, 45, 47). This raises questions about the physiological relevance or biological importance of ethanolamine catabolism among Pseudomonas species. As a first step in addressing this complex problem, this study identified and evaluated several key elements, e.g., genes and small molecules, surrounding ethanolamine catabolism in P. aeruginosa PAO1.
The primary focus of this study was the PA4022-eat-eutBC operon, because it encodes proteins at the core of ethanolamine catabolism, including an acetaldehyde dehydrogenase (PA4022), the ethanolamine transporter Eat (PA4023), and ethanolamine-ammonia lyase EutBC (PA4024 and PA4025). Taken together, these proteins comprise an entire catabolic pathway that hypothetically enables P. aeruginosa PAO1 to transport and degrade ethanolamine into the metabolite acetate. However, growth analysis of the ΔPA4022, Δeat, and ΔeutB mutants revealed that these genes were not equally important with regard to P. aeruginosa PAO1 using ethanolamine as a source of carbon and/or nitrogen. EutBC is known as an essential enzyme in ethanolamine catabolism, and as expected, deletion of the eutB gene completely abolished the growth of P. aeruginosa PAO1 on ethanolamine. Eat is a predicted transport protein thought to be involved in the uptake of extracellular ethanolamine (5, 11). Deletion of the eat gene in P. aeruginosa PAO1 did not impact the growth of this bacterium on ethanolamine. Published data indicate that carrier-mediated transport has an observable effect only when ethanolamine is present at micromolar concentrations or in an acidic environment (6). Overall, the observed phenotypes for the Δeat and ΔeutB mutants of P. aeruginosa PAO1 are consistent with previous findings describing the roles of these genes in other ethanolamine-degrading bacteria (5, 12).
Deletion of the PA4022 gene affected the growth of P. aeruginosa PAO1 differently, depending on whether ethanolamine was a carbon or nitrogen source. When ethanolamine served as the sole carbon source, deletion of the PA4022 gene reduced the growth of P. aeruginosa PAO1 by ∼50%. The catabolic breakdown of acetaldehyde into acetate is expected to be hindered in the ΔPA4022 mutant, which would account for its reduced growth on ethanolamine as a carbon source. In contrast, the ΔPA4022 mutant displayed wild-type growth on a preferred carbon source, with ethanolamine as the sole nitrogen source. This is understandable, because EutBC catalyzes the hydrolysis of ethanolamine to release ammonia, thereby fulfilling the nitrogen demands of the cell. These results suggest that PA4022 participates in the catabolism of ethanolamine, but its presence is not absolutely essential. As stated earlier, P. aeruginosa PAO1 possesses two genes, exaC (PA1984) and PA4022, encoding acetaldehyde dehydrogenases that are 98% identical in amino acid sequence and have similar enzymatic properties (29). The genetic redundancy or dual function of the exaC and PA4022 genes has been previously demonstrated for P. aeruginosa PAO1 when grown on ethanol, which is also catabolized through an acetaldehyde intermediate (29). Therefore, activity from ExaC most likely compensates for the ΔPA4022 mutation regarding ethanolamine catabolism as well.
The results of this study suggest a role for PA4022 in the catabolism of ethanolamine. However, a search of the literature revealed that PA4022 was previously characterized as a hydrazone dehydrogenase, an enzyme that hydrolyzes the carbon-nitrogen double bond of hydrazones to yield acetate and hydrazides as products. Hydrazones are not known or considered to be abundant in nature (39). Some examples of naturally occurring hydrazones include gyromitrin (48) of the mushroom Gyromitra esculenta and yoropyrazone (49), an alkaloid isolated from Streptomyces. As it pertains to P. aeruginosa, growth on a relatively simple hydrazone (valeric acid ethylidene hydrazide) was dependent on PA4022 and ExaC (29). It was thought that the acetate product liberated from the hydrolysis of hydrazone is a source of carbon and energy for bacterial growth. Contrary to this earlier finding, we did not observe any significant growth of P. aeruginosa PAO1 on hydrazone. Various types of minimal media, conditions (shaking versus static), and different strains (P. aeruginosa PAO1, PA14, and PAK) were used, but none proved successful for growing P. aeruginosa on hydrazone. Nonetheless, PA4022 was shown to catalyze the hydrolysis of hydrazones in vitro (29), and we found that the expression of a PA4022::lacZ fusion increased 6-fold in response to hydrazone. Both results implicate PA4022 in the degradation of hydrazones even though the growth data presented in this study question the validity of hydrazone as a viable carbon source for all strains of P. aeruginosa. More studies are needed to understand hydrazone utilization among various strains of P. aeruginosa.
Analysis of the PA4022::lacZ and eat::lacZ fusions revealed that expression of the PA4022-eat-eutBC operon is under the control of positive-feedback regulation exerted through the EBP PA4021, the sigma factor RpoN, and the metabolite acetaldehyde. The identification of acetaldehyde as the inducer of the PA4022-eat-eutBC operon was an unanticipated result, because ethanolamine has been shown to directly regulate expression of the eut genes in bacteria of the Enterobacteriaceae and Firmicutes (4, 15, 17). However, several lines of evidence clearly point to acetaldehyde as being the inducer of the PA4022-eat-eutBC operon and not ethanolamine as expected. First, the expression of PA4022::lacZ and eat::lacZ was repeatedly found to be induced with the addition of acetaldehyde. Second, the addition of ethanolamine failed to induce the expression of PA4022::lacZ and eat::lacZ in the ΔeutB mutant, suggesting that EutBC is required for induction. Third, background expression of PA4022::lacZ was elevated 5-fold in the absence of the acetaldehyde dehydrogenase PA4022. Last, acetaldehyde (but not ethanolamine) induced the expression of PA4022::lacZ and eat::lacZ in E. coli cells that heterologously expressed the PA4021 gene.
One possible explanation for acetaldehyde serving as the inducer of the PA4022-eat-eutBC operon instead of ethanolamine is the fact that P. aeruginosa PAO1 does not have a microcompartment dedicated to ethanolamine catabolism. For Enterobacteriaceae, ethanolamine catabolism occurs within an intracellular microcompartment consisting of the shell proteins EutSMNLK (12). The microcompartment is believed to contain or capture the toxic and volatile acetaldehyde, which allows for a more efficient utilization of this compound (50, 51). Homologs of the EutSMNLK shell proteins are not encoded in the genome of P. aeruginosa PAO1. To counter the absence of a microcompartment and maximize its growth potential on ethanolamine, P. aeruginosa PAO1 uses an acetaldehyde-responsive mechanism to dictate transcription of the PA4022-eat-eutBC operon. The outcome of this positive-feedback regulation is the production of adequate and balanced enzymatic activity that readily degrades ethanolamine into acetate while keeping acetaldehyde levels at a minimum to avoid toxicity and loss of carbon due to volatility. The results from the genetic complementation experiments illustrate the importance of balance in the Eat-EutBC-PA4022 pathway. When only the eat-eutBC genes were overexpressed in P. aeruginosa PAO1, its growth was significantly reduced on ethanolamine. This growth inhibition was likely a direct result of toxic concentrations of intracellular acetaldehyde generated by the excess activities of Eat-EutBC.
The induction of the PA4022-eat-eutBC operon by acetaldehyde is achieved through an EBP-RpoN mechanism. Previously, PA4021 was one of several uncharacterized EBPs in P. aeruginosa PAO1 (52). However, the proximity of the PA4021 gene to the PA4022-eat-eutBC operon in addition to the presence of an RpoN promoter upstream of the PA4022 gene were strong indicators that PA4021 might be an EBP that regulates transcription of the PA4022-eat-eutBC operon in relation to ethanolamine catabolism. This prediction appears to be correct because (i) deletion of the PA4021 gene eliminated the growth of P. aeruginosa PAO1 on ethanolamine, (ii) there was no induction of PA4022::lacZ or eat::lacZ in the ΔPA4021 mutant, and (iii) expression of only the PA4021 gene was essential and sufficient for induction of PA4022::lacZ and eat::lacZ in nonnative E. coli. Similar results were observed regarding the regulatory role of RpoN: the rpoN gene was required for growth on ethanolamine, there was no induction of PA4022::lacZ and eat::lacZ in an rpoN mutant, and mutation of the RpoN promoter in the 5′-regulatory regions of PA4022::lacZ and eat::lacZ rendered them unresponsive to induction by acetaldehyde.
Importantly, these findings provide a working model for the regulation of the PA4022-eat-eutBC operon in P. aeruginosa PAO1. In this model, PA4021 is expected to sense and bind to acetaldehyde. The binding of acetaldehyde would then stimulate PA4021 to adopt an active conformation that leads to the transcriptional activation of the PA4022-eat-eutBC operon in an RpoN-dependent manner. The transcript levels of PA4022-eat-eutBC would ultimately vary depending on the concentration of intracellular acetaldehyde. When grown in acetate-minimal medium, the LacZ activity of PA4022::lacZ was 5-fold higher in the ΔPA4022 mutant than in the wild-type P. aeruginosa PAO1. This result not only indicates that acetaldehyde catabolism is inhibited in the ΔPA4022 mutant, but more significantly, transcriptional activation of the PA4022 locus by PA4021 is not strictly limited to ethanolamine catabolism but also occurs during basic cellular metabolism to aid in the breakdown of intracellular acetaldehyde. However, the PA4021 and PA4022 genes were not required for growth on acetaldehyde, ethanol, or acetoin (with ethanol and acetoin being catabolized through an acetaldehyde intermediate). It would appear that even though PA4021 and its target gene PA4022 comprise a system involved in the sensing and breakdown of acetaldehyde, this system is not required for growth on all compounds catabolized through an acetaldehyde intermediate.
The regulatory function of PA4021 is reminiscent of the transcriptional regulator AlcR of Aspergillus nidulans (53). In this microorganism, AlcR is a regulator of ethanol catabolism that responds directly to acetaldehyde and upregulates the transcription of genes encoding alcohol (alcA) and aldehyde (aldA) dehydrogenases (53, 54). Similar to the situation described herein for P. aeruginosa PAO1, growth on compounds that are degraded into acetaldehyde induce expression of the alcA and aldA genes in Aspergillus nidulans (55, 56). Additionally, the aldehyde intermediates generated from the catabolism of other compounds, such as d-galacturonic acid and putrescine, also induce expression of the alcA and aldA genes (56). It would be interesting to determine what other aldehydes are sensed by PA4021 and induce expression of the PA4022 locus in P. aeruginosa PAO1.
PA4021 is a founding member of a class of EBPs in which transcriptional activity is directly controlled by acetaldehyde. Like other transcriptional regulators, PA4021 has a modular structure (663 amino acid residues) consisting of an N-terminal region possessing both GAF (residues 68 to 199) and PAS (residues 235 to 311) domains, a central RpoN-interaction domain (residues 334 to 500), and a C-terminal DNA-binding region possessing an FIS-type helix-turn-helix motif (residues 595 to 636). GAF and PAS domains serve as sensory input sites for transcriptional regulators (52) and are probably involved in relaying the presence of acetaldehyde to the transcriptional activity of PA4021. The FIS-type helix-turn-helix motif in the C-terminal region does suggest that PA4021 binds to specific DNA sequences upstream of the RpoN promoter in the 5′-regulatory region of the PA4022-eat-eutBC operon. A more in-depth study on the biochemical characteristics of the PA4021 protein is needed to identify both its DNA binding sequence and other genes that are regulated by this EBP.
Based on the results of the current study, we recommend PA4021 be given the name EatR to signify its regulatory function in ethanolamine catabolism in P. aeruginosa PAO1. Homologs of the eatR (PA4021) gene and the PA4022-eat-eutBC operon are clustered in the genomes for many Pseudomonas spp., including common strains of P. putida, P. denitrificans, and P. fluorescens (57). This suggests that a variety of Pseudomonas strains use an EatR-RpoN mechanism for regulating ethanolamine catabolism. However, regulation of ethanolamine catabolism through EatR-RpoN does not appear to be universal among Pseudomonas species. For example, the regulatory eutR gene is adjacent to the eat-eutBC operon in the genomes of some strains of P. denitrificans, P. oleovorans, and P. stutzeri, indicating that these specific strains use EutR to regulate ethanolamine catabolism. Homologs of the PA4022-eat-eutBC genes are distributed in the genomes of P. syringae, but the presence of a regulatory gene (eutR or eatR) is inconsistent or variable among strains. Hopefully, this study will create interest in the microbiology community to explore ethanolamine catabolism further in Pseudomonas, with the ultimate goal being a clarification of this catabolic process and its biological importance for this group of bacteria.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge support from the Undergraduate Honors Program of SUNY-ESF and the Center for Applied Microbiology at SUNY-ESF for this study.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00357-16.
REFERENCES
- 1.Blackwell CM, Scarlett FA, Turner JM. 1976. Ethanolamine catabolism by bacteria, including Escherichia coli. Biochem Soc Trans 4:495–497. doi: 10.1042/bst0040495. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell CM, Turner JM. 1978. Microbial metabolism of amino alcohols. Formation of coenzyme B12-dependent ethanolamine ammonia-lyase and its concerted induction in Escherichia coli. Biochem J 176:751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roof DM, Roth JR. 1988. Ethanolamine utilization in Salmonella Typhimurium. J Bacteriol 170:3855–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Papa MF, Perego M. 2008. Ethanolamine activates a sensor histidine kinase regulating its utilization in Enterococcus faecalis. J Bacteriol 190:7147–7156. doi: 10.1128/JB.00952-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garsin DA. 2010. Ethanolamine utilization in bacterial pathogens: roles and regulation. Nat Rev Microbiol 8:290–295. doi: 10.1038/nrmicro2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penrod JT, Mace CC, Roth JR. 2004. A pH-sensitive function and phenotype: evidence that EutH facilitates diffusion of uncharged ethanolamine in Salmonella enterica. J Bacteriol 186:6885–6890. doi: 10.1128/JB.186.20.6885-6890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang GW, Chang JT. 1975. Evidence for the B12-dependent enzyme ethanolamine deaminase in Salmonella. Nature 254:150–151. doi: 10.1038/254150a0. [DOI] [PubMed] [Google Scholar]
- 8.Scarlett FA, Turner JM. 1976. Microbial metabolism of amino alcohols. Ethanolamine catabolism mediated by coenzyme B12-dependent ethanolamine ammonia-lyase in Escherichia coli and Klebsiella aerogenes. J Gen Microbiol 95:173–176. [DOI] [PubMed] [Google Scholar]
- 9.Jones PW, Turner JM. 1984. Interrelationships between the enzymes of ethanolamine metabolism in Escherichia coli. J Gen Microbiol 130:299–308. [DOI] [PubMed] [Google Scholar]
- 10.Stojiljkovic I, Bäumler AJ, Heffron F. 1995. Ethanolamine utilization in Salmonella Typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J Bacteriol 177:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsoy O, Ravcheev D, Mushegian A. 2009. Comparative genomics of ethanolamine utilization. J Bacteriol 191:7157–7164. doi: 10.1128/JB.00838-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kofoid E, Rappleye C, Stojiljkovic I, Roth J. 1999. The 17-gene ethanolamine (eut) operon of Salmonella Typhimurium encodes five homologues of carboxysome shell proteins. J Bacteriol 181:5317–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka S, Sawaya MR, Yeates TO. 2010. Structure and mechanisms of a protein-based organelle in Escherichia coli. Science 327:81–84. doi: 10.1126/science.1179513. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury C, Sinha S, Chun S, Yeates TO, Bobik TA. 2014. Diverse bacterial microcompartment organelles. Microbiol Mol Biol Rev 78:438–468. doi: 10.1128/MMBR.00009-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roof DM, Roth JR. 1992. Autogenous regulation of ethanolamine utilization by a transcriptional activator of the eut operon in Salmonella Typhimurium. J Bacteriol 174:6634–6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheppard DE, Roth JR. 1994. A rationale for autoinduction of a transcriptional activator: ethanolamine ammonia-lyase (EutBC) and the operon activator (EutR) compete for adenosyl-cobalamin in Salmonella Typhimurium. J Bacteriol 176:1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luzader DH, Clark DE, Gonyar LA, Kendall MM. 2013. EutR is a direct regulator of genes that contribute to metabolism and virulence in enterohemorrhagic Escherichia coli O157:H7. J Bacteriol 195:4947–4953. doi: 10.1128/JB.00937-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker KA, Perego M. 2011. Transcription antitermination by a phosphorylated response regulator and cobalamin-dependent termination at a B12 riboswitch contribute to ethanolamine utilization in Enterococcus faecalis. J Bacteriol 193:2575–2586. doi: 10.1128/JB.00217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buck M, Cannon W. 1992. Specific binding of the transcription factor sigma-54 to promoter DNA. Nature 358:422–424. doi: 10.1038/358422a0. [DOI] [PubMed] [Google Scholar]
- 20.Barrios H, Valderrama B, Morett E. 1999. Compilation and analysis of sigma(54)-dependent promoter sequences. Nucleic Acids Res 27:4305–4313. doi: 10.1093/nar/27.22.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buck M, Gallegos MT, Studholme DJ, Guo Y, Gralla JD. 2000. The bacterial enhancer-dependent sigma(54) (sigma(N)) transcription factor. J Bacteriol 182:4129–4136. doi: 10.1128/JB.182.15.4129-4136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merrick MJ. 1993. In a class of its own–the RNA polymerase sigma factor sigma 54 (sigma N). Mol Microbiol 10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi KH, Schweizer HP. 2005. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol 5:30. doi: 10.1186/1471-2180-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundgren BR, Villegas-Peñaranda LR, Harris JR, Mottern AM, Dunn DM, Boddy CN, Nomura CT. 2014. Genetic analysis of the assimilation of C5-dicarboxylic acids in Pseudomonas aeruginosa PAO1. J Bacteriol 196:2543–2551. doi: 10.1128/JB.01615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarwar Z, Lundgren BR, Grassa MT, Wang MX, Gribble M, Moffat JF, Nomura CT. 2016. GcsR, a TyrR-like enhancer-binding protein, regulates expression of the glycine cleavage system in Pseudomonas aeruginosa PAO1. mSphere 1:e00020-16. doi: 10.1128/mSphere.00020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM Jr, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 27.Lundgren BR, Harris JR, Sarwar Z, Scheel RA, Nomura CT. 2015. The metabolism of (R)-3-hydroxybutyrate is regulated by the enhancer-binding protein PA2005 and the alternative sigma factor RpoN in Pseudomonas aeruginosa PAO1. Microbiology 161:2232–2242. doi: 10.1099/mic.0.000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh H, Suzuta T, Hoshino T, Takaya N. 2008. Novel dehydrogenase catalyzes oxidative hydrolysis of carbon-nitrogen double bonds for hydrazone degradation. J Biol Chem 283:5790–5800. [DOI] [PubMed] [Google Scholar]
- 29.Taniyama K, Itoh H, Takuwa A, Sasaki Y, Yajima S, Toyofuku M, Nomura N, Takaya N. 2012. Group X aldehyde dehydrogenases of Pseudomonas aeruginosa PAO1 degrade hydrazones. J Bacteriol 194:1447–1456. doi: 10.1128/JB.06590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundgren BR, Thornton W, Dornan MH, Villegas-Peñaranda LR, Boddy CN, Nomura CT. 2013. Gene PA2449 is essential for glycine metabolism and pyocyanin biosynthesis in Pseudomonas aeruginosa PAO1. J Bacteriol 195:2087–2100. doi: 10.1128/JB.02205-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacEachran DP, Ye S, Bomberger JM, Hogan DA, Swiatecka-Urban A, Stanton BA, O'Toole GA. 2007. The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator. Infect Immun 75:3902–3912. doi: 10.1128/IAI.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Bremer H. 1995. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem 270:11181–11189. doi: 10.1074/jbc.270.19.11181. [DOI] [PubMed] [Google Scholar]
- 33.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krüger N, Steinbüchel A. 1992. Identification of acoR, a regulatory gene for the expression of genes essential for acetoin catabolism in Alcaligenes eutrophus H16. J Bacteriol 174:4391–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jack DL, Paulsen IT, Saier MH. 2000. The amino acid/polyamine/organocation (APC) superfamily of transporters specific for amino acids, polyamines and organocations. Microbiology 146:1797–1814. [DOI] [PubMed] [Google Scholar]
- 36.Schobert M, Görisch H. 1999. Cytochrome c550 is an essential component of the quinoprotein ethanol oxidation system in Pseudomonas aeruginosa: cloning and sequencing of the genes encoding cytochrome c550 and an adjacent acetaldehyde dehydrogenase. Microbiology 145:471–481. [DOI] [PubMed] [Google Scholar]
- 37.Huang M, Oppermann FB, Steinbüchel A. 1994. Molecular characterization of the Pseudomonas putida 2,3-butanediol catabolic pathway. FEMS Microbiol Lett 124:141–150. doi: 10.1111/j.1574-6968.1994.tb07276.x. [DOI] [PubMed] [Google Scholar]
- 38.Filipiak W, Sponring A, Baur MM, Filipiak A, Ager C, Wiesenhofer H, Nagl M, Troppmair J, Amann A. 2012. Molecular analysis of volatile metabolites released specifically by Staphylococcus aureus and Pseudomonas aeruginosa. BMC Microbiol 12:113. doi: 10.1186/1471-2180-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blair LM, Sperry J. 2013. Natural products containing a nitrogen-nitrogen bond. J Nat Prod 76:794–812. doi: 10.1021/np400124n. [DOI] [PubMed] [Google Scholar]
- 40.Sugiura M, Kobayashi S. 2005. N-acylhydrazones as versatile electrophiles for the synthesis of nitrogen-containing compounds. Angew Chem Int Ed Engl 44:5176–5186. doi: 10.1002/anie.200500691. [DOI] [PubMed] [Google Scholar]
- 41.Görisch H. 2003. The ethanol oxidation system and its regulation in Pseudomonas aeruginosa. Biochim Biophys Acta 1647:98–102. doi: 10.1016/S1570-9639(03)00066-9. [DOI] [PubMed] [Google Scholar]
- 42.Heurlier K, Dénervaud V, Pessi G, Reimmann C, Haas D. 2003. Negative control of quorum sensing by RpoN (sigma54) in Pseudomonas aeruginosa PAO1. J Bacteriol 185:2227–2235. doi: 10.1128/JB.185.7.2227-2235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krivan HC, Franklin DP, Wang W, Laux DC, Cohen PS. 1992. Phosphatidylserine found in intestinal mucus serves as a sole source of carbon and nitrogen for Salmonellae and Escherichia coli. Infect Immun 60:3943–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Bäumler AJ. 2011. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A 108:17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertin Y, Girardeau JP, Chaucheyras-Durand F, Lyan B, Pujos-Guillot E, Harel J, Martin C. 2011. Enterohaemorrhagic Escherichia coli gains a competitive advantage by using ethanolamine as a nitrogen source in the bovine intestinal content. Environ Microbiol 13:365–377. doi: 10.1111/j.1462-2920.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 46.Rontein D, Nishida I, Tashiro G, Yoshioka K, Wu WI, Voelker DR, Basset G, Hanson AD. 2001. Plants synthesize ethanolamine by direct decarboxylation of serine using a pyridoxal phosphate enzyme. J Biol Chem 276:35523–35529. doi: 10.1074/jbc.M106038200. [DOI] [PubMed] [Google Scholar]
- 47.Kendall MM, Gruber CC, Parker CT, Sperandio V. 2012. Ethanolamine controls expression of genes encoding components involved in interkingdom signaling and virulence in enterohemorrhagic Escherichia coli O157:H7. mBio 3(3):e00050-12. doi: 10.1128/mBio.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michelot D, Toth B. 1991. Poisoning by Gyromitra esculenta–a review. J Appl Toxicol 11:235–243. doi: 10.1002/jat.2550110403. [DOI] [PubMed] [Google Scholar]
- 49.Abdelfattah MS, Toume K, Ishibashi M. 2012. Yoropyrazone, a new naphthopyridazone alkaloid isolated from Streptomyces sp. IFM 11307 and evaluation of its TRAIL resistance-overcoming activity. J Antibiot (Tokyo) 65:245–248. [DOI] [PubMed] [Google Scholar]
- 50.Brinsmade SR, Paldon T, Escalante-Semerena JC. 2005. Minimal functions and physiological conditions required for growth of Salmonella enterica on ethanolamine in the absence of the metabolosome. J Bacteriol 187:8039–8046. doi: 10.1128/JB.187.23.8039-8046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penrod JT, Roth JR. 2006. Conserving a volatile metabolite: a role for carboxysome-like organelles in Salmonella enterica. J Bacteriol 188:2865–2874. doi: 10.1128/JB.188.8.2865-2874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Studholme DJ, Dixon R. 2003. Domain architectures of sigma54-dependent transcriptional activators. J Bacteriol 185:1757–1767. doi: 10.1128/JB.185.6.1757-1767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Felenbok B, Flipphi M, Nikolaev I. 2001. Ethanol catabolism in Aspergillus nidulans: a model system for studying gene regulation. Prog Nucleic Acid Res Mol Biol 69:149–204. doi: 10.1016/S0079-6603(01)69047-0. [DOI] [PubMed] [Google Scholar]
- 54.Flipphi M, Mathieu M, Cirpus I, Panozzo C, Felenbok B. 2001. Regulation of the aldehyde dehydrogenase gene (aldA) and its role in the control of the coinducer level necessary for induction of the ethanol utilization pathway in Aspergillus nidulans. J Biol Chem 276:6950–6958. doi: 10.1074/jbc.M005769200. [DOI] [PubMed] [Google Scholar]
- 55.Flipphi M, Kocialkowska J, Felenbok B. 2002. Characteristics of physiological inducers of the ethanol utilization (alc) pathway in Aspergillus nidulans. Biochem J 364:25–31. doi: 10.1042/bj3640025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flipphi M, Kocialkowska J, Felenbok B. 2003. Relationships between the ethanol utilization (alc) pathway and unrelated catabolic pathways in Aspergillus nidulans. Eur J Biochem 270:3555–3564. doi: 10.1046/j.1432-1033.2003.03738.x. [DOI] [PubMed] [Google Scholar]
- 57.Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FSL. 2016. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res 44:D646–D653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kretzschmar U, Khodaverdi V, Adrian L. 2010. Transcriptional regulation of the acetyl-CoA synthetase gene acsA in Pseudomonas aeruginosa. Arch Microbiol 192:685–690. doi: 10.1007/s00203-010-0593-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.