Abstract
Objective
To identify the factors associated with the development of postoperative atrial fibrillation (POAF) after coronary artery bypass graft (CABG) in elderly patients with coronary artery disease (CAD).
Methods
A total of 81 patients with CAD who underwent CABG were enrolled in the study. Patients were divided into two groups: Group 1, without postoperative atrial fibrillation (59 patients, 74.6% men, mean age 65.8 ± 4.0 years); Group 2, with early new-onset atrial fibrillation after CABG (22 patients, 90.9% men, mean age 67.7 ± 5.4 years). Interleukin (IL)-6, IL-8, IL-10, C-reactive protein (CRP), fibrinogen, superoxide dismutase (SOD), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and troponin I were studied.
Results
During the observation period, atrial fibrillation occurred in 27.2% cases, an average of 4.9 ± 3.8 days after surgery. In group 2, the left atrium (LA) dimension was larger than in group 1 (43.9 ± 3.4 mm vs. 37.6 ± 3.9 mm, P < 0.001). Patients with POAF had significantly higher IL-6 (72.7 ± 60.8 pg/mL vs. 38.0 ± 34.6 pg/mL, P = 0.04), IL-8 (11.9 ± 6.0 pg/mL vs. 7.7 ± 5.4 pg/mL, P = 0.01) and SOD (2462.0 ± 2029.3 units/g vs. 1515.0 ± 1292.9 units/g, P = 0.04) compared with group without POAF. The multivariate analysis showed that the odds ratio (OR) for POAF development in patients with left atrium more than 39 mm was 2.1 [95% confidence interval (CI): 1.2−3.8, P = 0.0004], IL-6 levels more than 65.18 pg/mL—1.4 (95% CI: 1.1−2.7, P = 0.009), IL-8 levels more than 9.67 pg/mL—1.2 (95% CI: 1.1−3.7, P = 0.009), SOD more than 2948 units/g—1.1 (95% CI: 1.01−2.9, P = 0.04).
Conclusions
In our study, the independent predictors of postoperative atrial fibrillation after CABG in elderly patients were left atrium dimension and the increased postoperative concentration of IL-6, IL-8 and superoxide dismutase.
Keywords: Antioxidant, Atrial fibrillation, Coronary artery bypass graft, Interleukins, Troponin
1. Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality of the elderly population in economic developed countries. Coronary artery disease (CAD) is one of the main manifestations of CVD. Revascularization is indicated in patients with stable CAD and progressive or refractory symptoms despite medical treatment. Elderly patients with multivessel disease undergoing cardiac surgery represent a high mortality and complication risk especially in on-pump coronary artery bypass graft (CABG) with aortic cross clamping.[1] The main factors of post-CABG complications usually include anesthesia, cardiopulmonary bypass, hypo-perfusion, aortic cross clamping, hypoxia-induced inflammation and others. Also elderly patients after CABG usually have a longer mechanical ventilation period and intensive care unit stay.[2]
Off-pump coronary artery bypass provides good quality graft without exposing the elderly patient to cardiopulmonary bypass, and may be the optimal choice in patients with multiple comorbidities not suitable for on-pump procedures.[3]
Atrial fibrillation (AF) is the most common arrhythmia in early post-CABG period and occurs in 20% to 35% patients.[3],[5] In old patients, AF may be a life-threatening complication. Postoperative AF (POAF) has been associated with an increase in early and late mortality rates, hospital adverse events, particularly hemodynamic instability, thromboembolic events and heart failure progression.[4] According to the literature, it is necessary to derive an effective bedside tool in order to predict POAF and its related complications.[5]
The aim of the study was to identify the factors associated with the new-onset POAF after CABG in elderly patients with CAD.
2. Methods
A total of 81 patients with CAD who underwent CABG were enrolled in this prospective study in Samara Regional Cardiology Centre (Russia) from January to June 2015. Inclusion criteria: stable CAD and signed informed consent. Exclusion criteria: mechanical heart valve prosthesis or valve disease which is expected to require valve replacement intervention, liver and renal diseases, oncology diseases, stroke or transient ischemic attack, coagulopathy, history of AF, thyroid disease, and age over 80 years.
All patients underwent standard instrumental and laboratory tests. Transthoracic echocardiography was performed on ultrasound scanners Logiq 5 and 7 (General Electric company, USA) in M, B, D-modes. CABG was perfomed on-pump or off-pump. After median sternotomy, routine unicaval two stage venous cannulation and aortic cannulation was performed for cardiopulmonary bypass. After ante- and retrograde cardioplegia protocol, a cross-clamp was placed. The primary study end point was new onset of AF after CABG.
Patients were divided into two groups: Group 1, without POAF (59 patients, 74.6% men, mean age 65.8 ± 4.0 years); Group 2, with early new-onset AF after CABG (22 patients, 90.9% men, mean age 67.7 ± 5.4 years). POAF was registered during monitoring in intensive care unit and using ECG.
Interleukin (IL)-6, IL-8, IL-10, C-reactive protein (CRP), fibrinogen, superoxide dismutase (SOD), N-terminal pro-B-type natriuretic peptide (NT-proBNP), troponin I levels were studied on admission and 3.8 ± 1.4 days after surgery. The levels of cytokines, CRP, SOD were assessed using the immunoassay technique on Thermo Scientific Multiscan FC microplate photometer (China) with the following test-systems: IL-6, IL-8, IL-10, NT-proBNP and CRP enzyme-linked immunosorbent assay (ELISA kit)—BEST (“Vector-BEST”, Novosibirsk, Russian Federation), ELISA— SOD (“Cytokin”, Saint-Petersburg, Russian Federation). Fibrinogen levels were measured with coagulometer STA - COMPACT (Roche, Switzerland) according to standard Clauss method (1957). Troponin levels were measured with immunoassay system UNICEL® DXI 600 ACCESS (Beckman Coulter, USA).
Descriptive statistics was carried out using Statistica 7.0 software (StatSoft Inc., USA). Quantitative variables are shown as mean ± SD. The preoperative risk models EuroSCORE were used to predict morbidity and mortality. Univariate and multivariate logistic regression analysis were used to identify factors associated with POAF. Differences were considered significant at P < 0.05.
3. Results
During the observation period AF occurred in 27.2% cases on average 4.9 ± 3.8 days after surgery. The clinical and instrumental indicators of patients are demonstrated in Table 1.
Table 1. Clinical and instrumental indicators of studied patients.
| Group 1 (n = 59) | Group 2 (n = 22) | Р | |
| Male | 44 (74.6%) | 20 (90.9%) | 0.1 |
| Age, yrs | 65.9 ± 4.0 | 67.7 ± 5.4 | 0.13 |
| Smokers | 24 (40.7%) | 5 (22.7%) | 0.13 |
| Body mass index > 30 kg/m2 | 37 (62.7%) | 15 (68.2%) | 0.6 |
| Stable angina | |||
| I | − | − | |
| II | 16 (27.2%) | 4 (18.2%) | 0.4 |
| III | 40 (67.8%) | 17 (77.3%) | 0.4 |
| IV | 1 (1.7%) | − | 0.8 |
| History of myocardial infarction | 31 (52.5%) | 13 (59.1%) | 0.59 |
| History of CAD, months | 65.4 ± 48.5 | 107.4 ± 75.9 | 0.004 |
| Hypertension | 59 (100.0%) | 22 (100.0%) | 0.8 |
| NYHA | |||
| I | − | − | |
| II | 47 (79.7%) | 15 (68.2%) | 0.28 |
| III | 12 (20.3%) | 7 (31.8%) | 0.28 |
| IV | − | − | |
| Diabetes melitus | 13 (22.0%) | 5 (22.7%) | 0.9 |
| Transient ischemic attack/stroke | 9 (15.3%) | 3 (13.6%) | 0.86 |
| Peripheral artery disease | 52 (88.1%) | 16 (72.7%) | 0.09 |
| Chronic pulmonary disease | 6 (10.2%) | 1 (4.5%) | 0.42 |
| Chronic kidney disease | 17 (28.8%) | 9 (40.9%) | 0.3 |
| Medical treatment before surgery | |||
| β-blockers | 50 (84.7%) | 18 (81.8%) | 0.75 |
| ACEI/ARB | 48 (81.4%) | 17 (77.3%) | 0.68 |
| Calcium channel blockers | 11 (18.6%) | 3 (13.6%) | 0.59 |
| Nitrates | 21 (35.6%) | 12 (54.5%) | 0.12 |
| Diuretics | 5 (8.5%) | 3 (13.6%) | 0.49 |
| Statins | 50 (74.6%) | 19 (65.5%) | 0.86 |
| Aspirin | 51 (86.4%) | 17 (77.3%) | 0.3 |
| Clopidogrel | 48 (81.4%) | 16 (72.7%) | 0.4 |
| Left atrium, mm | 37.6 ± 3.9 | 43.9 ± 3.4 | < 0.001 |
| End-systolic dimension of left ventricle, mm | 35.2 ± 7.1 | 37.5 ± 7.1 | 0.23 |
| End diastolic dimension of left ventricle, mm | 52.5 ± 6.4 | 54.4 ± 7.4 | 0.31 |
| End-systolic volume of left ventricle, mL | 53.6 ± 27.5 | 55.7 ± 12.0 | 0.81 |
| End diastolic volume of left ventricle, mL | 121.0 ± 32.9 | 127.4 ± 19.9 | 0.55 |
| Ejection fraction of left ventricle, % | 59.1 ± 9.5 | 55.2 ± 9.6 | 0.13 |
| GFR (CKD-EPI), mL/min per 1.73 m2 | 66.4 ± 16.5 | 74.4 ± 19.7 | 0.09 |
| EuroScore risk | 1.78 ± 1.4 | 1.75 ± 1.5 | 0.27 |
| Left coronary artery ≥ 50% | 10 (16.9%) | 6 (27.3%) | 0.3 |
| Number of grafts | 2.5 ± 0.8 | 2.8 ± 0.7 | 0.13 |
| Off-pump | 9 (15.3%) | 2 (9.1%) | 0.07 |
Data are presented as mean ± SD or n (%) unless other indicated. ACEI/ARB: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; CAD: coronary artery disease; CKD-EPI: chronic kidney disease epidemiology collaboration; GFR: glomerular filtration rate; NYHA: New York Heart Association.
Patients of group 2 had longer history of CAD (107.4 ± 75.9 vs. 65.4 ± 48.5 months, P = 0.004), and a larger left atrium (LA) dimension (43.9 ± 3.4 vs. 37.6 ± 3.9 mm, P < 0.001) compared with group 1. There were no significant differences between patients of both groups according to other indicators.
In patients with POAF in the postoperative period IL-6 (72.7 ± 60.8 pg/mL vs. 38.0 ± 34.6 pg/mL, P = 0.04), IL-8 (11.9 ± 6.0 pg/mL vs. 7.7 ± 5.4 pg/mL, P = 0.01) and SOD (2462.0 ± 2029.3 units/g vs. 1515.0 ± 1292.9 units/g, P = 0.04) levels were higher, compared with patients without POAF. No significant differences between patients of the two groups according to others indicators were found. Data are shown in Table 2.
Table 2. Laboratory findings.
| Group 1 (n = 59) | Group 2 (n = 22) | Р | |
| WBC before surgery, × 109/L | 7.0 ± 1.8 | 6.7 ± 2.0 | 0.69 |
| WBC after surgery, × 109/L | 13.4 ± 3.3 | 13.5 ± 3.4 | 0.78 |
| Segmented neutrophils, % | 74.9 ± 8.7 | 74.7 ± 9.1 | 0.86 |
| Lymphocytes, % | 11.8 ± 7.4 | 12.3 ± 7.2 | 0.44 |
| Monocytes, % | 3.6 ± 2.2 | 3.5 ± 3.0 | 0.42 |
| Eosinophils, % | 1.6 ± 1.0 | 1.9 ± 1.3 | 0.65 |
| Fibrinogen before surgery, g/L | 3.4 ± 0.9 | 3.5 ± 1.1 | 0.69 |
| Fibrinogen after surgery, g/L | 4.2 ± 1.2 | 4.4 ± 1.0 | 0.42 |
| IL-6 before surgery, pg/mL | 25.7 ± 13.2 | 30.1 ± 26.5 | 0.07 |
| IL-6 after surgery, pg/mL | 38.0 ± 34.6 | 72.7 ± 60.8 | 0.04 |
| IL-8 before surgery, pg/mL | 2.2 ± 1.3 | 2.7 ± 2.4 | 0.26 |
| IL-8 after surgery, pg/mL | 7.7 ± 5.4 | 11.9 ± 6.0 | 0.01 |
| IL-10 before surgery, pg/mL | 7.4 ± 4.7 | 6.3 ± 3.3 | 0.33 |
| IL-10 after surgery, pg/mL | 11.6 ± 5.7 | 11.9 ± 6.4 | 0.86 |
| CRP before surgery, mg/L | 1.2 ± 0.93 | 1.4 ± 1.3 | 0.29 |
| CRP after surgery, mg/L | 4,5 ± 0,8 | 4.7 ± 0.7 | 0.46 |
| SOD before surgery, units/g | 2523.2 ± 2187.9 | 3230.2 ± 2223.7 | 0.13 |
| SOD after surgery, units/g | 1515.0 ± 1292.9 | 2462.0 ± 2029.3 | 0.04 |
| NT-proBNP before surgery, pg/mL | 239.9 ± 138.1 | 309.7 ± 295.1 | 0.56 |
| NT-proBNP after surgery, pg/mL | 748.1 ± 697.4 | 882.4 ± 783.2 | 0.4 |
| Troponin I after surgery, mg/L | 2.4 ± 2.1 | 2.4 ± 1.8 | 0.69 |
Data are presented as mean ± SD. CRP: C-reactive protein; IL: interleukin; NT-proBNP: N-terminal pro-B-type natriuretic peptide; SOD: superoxide dismutase; WBC: white blood cell.
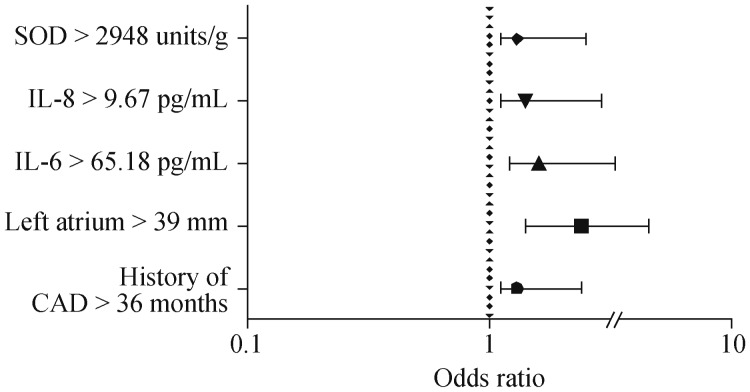
The univariate analysis (Figure 1A) showed that the odds ratio (OR) for POAF development in patients with a history of CAD more than 36 months was 1.3 [95% confidence interval (CI) 1.1−2.4, Р = 0.009], a left atrium dimension of more than 39 mm—2.4 (95% CI: 1.4−4.5, P = 0.0001), IL-6 levels more than 65.18 pg/mL—1.6 (95% CI: 1.2−3.3, P = 0.01), IL-8 levels more than 9.67 pg/mL—1.4 (95% CI: 1.1−2.9, P = 0.001), SOD more than 2948 units/g—1.3 (95% CI: 1.1−2.5, P = 0.04).
Figure 1. Factors, associated with POAF (univariate analysis).
CAD: coronary artery disease; IL: interleukin; POAF: postoperative atrial fibrillation; SOD: superoxide dismutase.
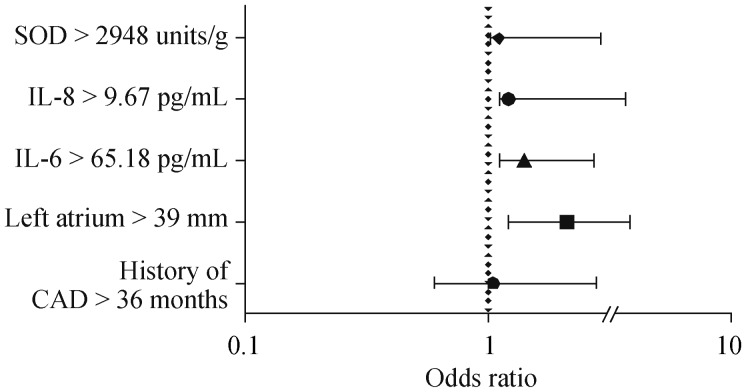
The multivariate analysis (Figure 1B) showed that the OR for POAF development in elderly patients with left atrium more than 39 mm was 2.1 (95% CI: 1.2−3.8, P = 0.0004), IL-6 levels more than 65.18 pg/mL—1.4 (95% CI: 1.1−2.7, P = 0,009), IL-8 levels more than 9.67 pg/mL—1.2 (95% CI: 1.1−3.7, P = 0.009), SOD more than 2948 units/g –1.1 (95% CI: 1.01−2.9, P = 0.04).
4. Discussion
AF is prevalent arrhythmia after CABG. In our study, the incidence of POAF has been reported in 27.2% of elderly patients following cardiac surgery. According to literature, 20%−40% of POAF occurs on the days 2−3 after operation.[3] According to our data, the new onset of POAF occurred on 4.9 ± 3.8 days.
We have demonstrated that patients with POAF had history of CAD longer than patients without arrhythmia. History of CAD was the only clinical factor that associated with POAF in univariate regression analysis but lost statistical significance in multivariable analysis. No significant association with POAF was observed for the other clinical indicators. In contrast to our data, Ivanovic, et al.[6] showed that the metabolic syndrome, irrespective of hypertension, diabetes, and obesity was associated with POAF. In another study,[7] the mean age of patients with POAF was higher and they more often had hypertension and chronic kidney disease compared with our research.
Figure 2. Factors, associated with POAF (multivariate analysis).
CAD: coronary artery disease; IL: interleukin; POAF: postoperative atrial fibrillation; SOD: superoxide dismutase.
In this study, we showed that LA is potentially associated with POAF, which is comparable to the data of Onk, et al.[8] but he also showed that the left ventricular ejection fraction was also a predictor of POAF. Ashes, et al.[9] demonstrated that new-onset or worsened left ventricle diastolic dysfunction after CABG surgery is associated with an increased incidence of POAF which was not confirmed in our study perhaps due to the small number of patients enrolled in the study.
Different biological pathways have been related with POAF. Novel biomarkers of inflammation, oxidative stress, myocardial ischemia and neurohumoral activation have been studied in pathogenesis of arrhythmia after CABG.[10]–[12] Inflammatory factors such as the increase in white blood cell count and CRP levels were implicated in the pathogenesis of POAF.
Nonspecific indicators of systemic inflammation (white blood cells) in our study were increased in both groups, but no significant differences between groups were obtained. According to our data, such conventional tests potentially cannot be used to stratify the risk of AF after CABG.
Cardiopulmonary bypass related hemodynamic changes may induce intraoperative atrial ischemia that has been hypothesized to play an important role in the development of POAF. The generation of reactive oxygen species, inflammatory factors, such as CRP, fibrinogen and ILs during the atrial cannulation and also the ischemia-reperfusion phase of cardiac surgery promotes the development of postoperative complications.[11],[12]
Our study showed no significant difference in CRP levels between the groups that is potentially related to antibiotic administration to all the patients in early postoperative period. The data is different from some other studies who revealed the influence of high concentration of CRP in increasing of the new onset POAF after CABG.[13]
IL levels as main markers of the inflammatory process usually increase in patients with POAF. This inflammatory process may contribute to the development of postoperative complications such as myocardial injury and multiple organ dysfunctions. We found a potential significant increase in the level of IL-6 and IL-8 after cardiac surgery in patients with POAF. In our study, the results of multivariate logistic regression showed significant association of high concentration of IL-6 and IL-8 with POAF. These cytokines are pro-inflammatory factors. The literature data about the role of these markers in the incidence of arrhythmia is contradictory. Erdem, et al.[14] demonstrated that the CRP level was significantly higher in the AF group, and multivariate logistic regression analysis showed that CRP was an independent predictor of POAF together with mean platelet volume, which suggested the participation of inflammation in the pathogenesis of POAF after CABG. But Narducci, et al.[12] showed that neither peripheral nor tissue preoperative CRP levels correlated with occurrence of arrhythmia.
Oktay, et al.[11] studied the role of oxidative stress following cardiac surgery and its contribution to POAF development. Placement and removal of the aortic cross clamp during CABG induces ischemia-reperfusion damage and leads to increased oxidative stress. According to this data, postoperative increased levels of oxidative stress indicators support the impact of oxidative stress on the pathogenesis of POAF.[11] They also investigated the relationship between POAF and oxidative stress associated with ischemia reperfusion damage during aortic cross clamp manipulation in CABG surgery by measuring plasma total oxidative stress (TOS) levels,[11] and showed that plasma TOS levels after placement and removal of an aortic cross clamp were statistically significantly different in patients who developed POAF compared with those who remained in sinus rhythm. In multivariate logistic regression analysis age, hematocrit level, pump temperature, and plasma TOS levels were found to be independent predictors of POAF.
We demonstrated that the concentration of SOD was high in the preoperative period in both groups and was associated with multi-vessel coronary disease in elderly patients with CAD, which is accompanied by the activation of oxidative stress markers and antioxidants. In the post-operative period, we observed potential significant lowering of SOD levels in both groups which may be related to the consumption of antioxidants that is similar to literature data.[15] In our study, in patients with POAF, the level of SOD after cardiac surgery remains higher compared to the patients without POAF which indicates the activation of oxidative stress in the group during myocardial revascularization. Our findings differ from the work of Stevanovic, et al.,[16] where the oxidative stress biomarkers and antioxidants was comparable in patients with and without POAF.
The concentration of troponin in our study increased after CABG in both groups but the difference between patients was not significant. Hernández-Romero, et al.[17] found that a high preoperative level of high-sensitivity troponin T was an independent predictor of AF after CABG. The author suggested that peri-operative myocardial damage is not associated with an arrhythmia. Narducci, et al.[12] found that only post-operative high-sensitivity troponin T level correlated with POAF, suggesting the primary role of an ischemic trigger of atrial fibrillation.
We studied plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels as a potential predictor of heart failure in patients with CAD after CABG. We showed the increase of this biomarker after cardiac surgery in both groups but the difference between patients with and without POAF was not significant. Our data varies from Pilatis, et al.,[18] who showed an association of increased preoperative BNP level with the development of POAF. On the other hand, Hernández-Romero D, et al.,[17] demonstrated that NT-proBNP could not serve as a predictor of AF after CABG. However, our study has limitation due to the small number of the enrolled patients that potentially affects the strength of our results.
In conclusion, in our study the independent predictors of postoperative atrial fibrillation after coronary artery bypass graft in elderly patients were left atrium dimension and the increased postoperative concentration of IL-6, IL-8 and superoxide dismutase.
References
- 1.Fink HA, Hemmy LS, MacDonald R, et al. Cognitive Outcomes After Cardiovascular Procedures in Older Adults: A Systematic Review. Ann Intern Med. 2015;163:107–117. doi: 10.7326/M14-2793. [DOI] [PubMed] [Google Scholar]
- 2.Ozen A, Unal EU, Songur M, et al. Coronary artery bypass grafting in the octogenarians: should we intervene, or leave them be? J Geriatr Cardiol. 2015;12:147–152. doi: 10.11909/j.issn.1671-5411.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhurandhar V, Saxena A, Parikh R, et al. Outcomes of on-pump versus off-pump coronary artery bypass graft surgery in the high risk (AusSCORE > 5) Heart Lung Circ. 2015;24:1216–1224. doi: 10.1016/j.hlc.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Altarabsheh SE, Deo SV, Rababa'h AM, et al. Off-pump coronary artery bypass reduces early stroke in octogenarians: a meta-analysis of 18,000 patients. Ann Thorac Surg. 2015;99:1568–1575. doi: 10.1016/j.athoracsur.2014.12.057. [DOI] [PubMed] [Google Scholar]
- 5.Mariscalco G, Biancari F, Zanobini M, et al. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: The POAF Score. J Am Heart Assoc. 2014;3:e000752. doi: 10.1161/JAHA.113.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanovic B, Tadic M, Bradic Z, et al. The influence of the metabolic syndrome on atrial fibrillation occurrence and outcome after coronary bypass surgery: a 3-year follow-up study. Thorac Cardiovasc Surg. 2014;62:561–568. doi: 10.1055/s-0034-1372349. [DOI] [PubMed] [Google Scholar]
- 7.Shimony A, Afilalo J, Flynn AW, et al. Usefulness of right ventricular dysfunction to predict new-onset atrial fibrillation following coronary artery bypass grafting. Am J Cardiol. 2014;113:913–918. doi: 10.1016/j.amjcard.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 8.Onk OA, Erkut B. Is the Preoperative administration of amiodarone or metoprolol more effective in reducing atrial fibrillation: after coronary bypass surgery? Medicine (Baltimore) 2015;94:e1576. doi: 10.1097/MD.0000000000001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashes CM, Yu M, Meineri M, et al. Diastolic dysfunction, cardiopulmonary bypass, and atrial fibrillation after coronary artery bypass graft surgery. Br J Anaesth. 2014;113:815–821. doi: 10.1093/bja/aeu208. [DOI] [PubMed] [Google Scholar]
- 10.Bouchot O, Guenancia C, Kahli A, et al. Low circulating levels of growth differentiation factor-15 before coronary artery bypass surgery may predict postoperative atrial fibrillation. J Cardiothorac Vasc Anesth. 2015;29:1131–1139. doi: 10.1053/j.jvca.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Oktay V, Baydar O, Sinan UY. The effect of oxidative stress related with ischemia-reperfusion damage on the pathogenesis of atrial fibrillation developing after coronary artery bypass graft surgery. Arch Turk Soc Cardiol. 2014;42:419–425. doi: 10.5543/tkda.2014.84032. [DOI] [PubMed] [Google Scholar]
- 12.Narducci ML, Pelargonio G, Rio T, et al. Predictors of postoperative atrial fibrillation in patients with coronary artery disease undergoing cardiopulmonary bypass: a possible role for myocardial ischemia and atrial inflammation. J Cardiothorac Vasc Anesth. 2014;28:512–519. doi: 10.1053/j.jvca.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Bidar E, Maesen B, Nieman F, et al. A prospective randomized controlled trial on the incidence and predictors of late-phase postoperative atrial fibrillation up to 30 days and the preventive value of biatrial pacing. Heart Rhythm. 2014;11:1156–1162. doi: 10.1016/j.hrthm.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 14.Erdem K, Ayhan S, Ozturk S, et al. Usefulness of the mean platelet volume for predicting new-onset atrial fibrillation after isolated coronary artery bypass grafting. Platelets. 2014;25:23–26. doi: 10.3109/09537104.2013.767443. [DOI] [PubMed] [Google Scholar]
- 15.Stanger O, Aigner I, Schimetta W, et al. Antioxidant supplementation attenuates oxidative stress in patients undergoing coronary artery bypass graft surgery. Tohoku J Exp Med. 2014;232:145–154. doi: 10.1620/tjem.232.145. [DOI] [PubMed] [Google Scholar]
- 16.Stevanovic A, Coburn M, Menon A, et al. The importance of intraoperative selenium blood levels on organ dysfunction in patients undergoing off-pump cardiac surgery: a randomised controlled trial. PLoS One. 2014;9:e104222. doi: 10.1371/journal.pone.0104222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Romero D, Vílchez JA, Lahoz Á, et al. High-sensitivity troponin T as a biomarker for the development of atrial fibrillation after cardiac surgery. Eur J Cardiothorac Surg. 2014;45:733–738. doi: 10.1093/ejcts/ezt488. [DOI] [PubMed] [Google Scholar]
- 18.Pilatis ND, Anyfantakis ZA, Spiliopoulos K, et al. The role of BNP and CRP in predicting the development of atrial fibrillation in patients undergoing isolated coronary artery bypass surgery. ISRN Cardiol. 2013:235018. doi: 10.1155/2013/235018. [DOI] [PMC free article] [PubMed] [Google Scholar]