1. Introduction
Syncope, defined as a self-limited transient loss of consciousness and postural tone due to global cerebral hypoperfusion, is a common reason for emergency room visits, with a third of these visits leading to an inpatient admission.[1] Syncope carries an estimated 2-year mortality rate of over 25% and is strongly associated with falls, a potentially devastating contributor to morbidity and mortality among the elderly. Given this association, it is not surprising that up to 35% of syncopal episodes result in injury.[2] Dizziness is similarly associated with poor outcomes in the elderly,[3] overlapping substantially with syncope and falls through commonly shared pathophysiologic mechanisms.[4] To optimize care of this vulnerable patient population, it is imperative to recognize the etiologies and associated risk factors for syncope in older adults, as well as appreciate their influence on diagnosis and management.
2. Epidemiology
Syncope accounts for up to 6% of all hospitalizations and 3% of all emergency room visits.[5],[6] The prevalence of syncope increases with age, exceeding 20% in those aged ≥ 75 years,[7] with an annual incidence that approaches 2% in persons over age 80 years. Older adults with syncope have an average of 3.5 chronic medical illnesses and are taking 3 times as many medications as the general population, factors that contribute to the complexity of assessing and managing syncope in older adults. In addition to multi-morbidity and polypharmacy, there are also several age-related changes in cardiovascular structure and function that contribute to the higher incidence and prevalence of syncope in the elderly. These most importantly include attenuated baroreceptor and autonomic reflexes, diastolic dysfunction, impaired adrenergic responsiveness, and impaired maintenance of intravascular volume related to decreased salt/water handling and reduced renin-aldosterone levels.[8]
Causes of syncope differ according to age groups, largely due to the varying prevalence of multi-morbidity and polypharmacy, and age-related cardiovascular changes. Etiologies may be grouped according to underlying pathophysiology—neurally-mediated, orthostatic/dysautonomic, and cardiac which include arrhythmic and structural etiologies (Table 1).[9] Neurally-mediated etiologies account for almost two-thirds of syncope, whereas arrhythmic and structural cardiovascular etiologies account for the minority. Although identifying a predominant etiology is important for management, syncope is often multi-factorial in the elderly, highlighting the importance of maintaining a broad differential during the evaluation period.
Table 1. Causes of syncope according to underlying pathophysiology.
| Neurally-mediated | Orthostatic/dysautonomic | Arrhythmic | Structural cardiovascular | Mimickers of syncope |
| Vasovagal | Hypovolemia | Ventricular tachycardia | Aortic stenosis | Stroke, transient ischemic attack |
| Vasodepressor | Medications | Torsades de pointes | Aortic dissection | Seizures |
| Carotid sinus hypersensitivity | Postprandial | Supraventricular tachycardia | Hypertrophic cardiomyopathy | Migraine |
| Post-micturition, defecation | Parkinson's Disease | Atrial fibrillation and flutter | Pulmonary hypertension | Narcolepsy |
| Post-cough | Diabetic/other neuropathies | Sick sinus syndrome | Atrial myxoma | Panic attacks |
| Post-laugh, | Amyloid | Atrioventricular block | Pulmonary embolism | Conversion disorder |
| Post-exercise | Spinal cord disease | Pacemaker syndrome | Subclavian steal | Concussion |
3. Etiologies
3.1. Neurally-mediated syncope
Neurally-mediated etiologies are the most common cause of syncope in older adults,[9] and include vasovagal syncope and carotid sinus syndrome. Vasovagal syncope is a reflex-mediated transient autonomic failure to maintain vascular tone,[10] classically described to result from sudden sympathetic withdrawal leading to a reduced cardiac output and cerebral hypoperfusion. This can occur as a consequence of bradycardia resulting from unopposed vagal tone, termed cardioinhibitory syncope; or as a consequence of smooth muscle relaxation causing peripheral and splanchnic bed vasodilation and a reduced preload, termed vasodepressor syncope. Among older adults in whom vagal tone is reduced,[11] vasodepressor syncope is much more common, often occurring without classic prodromal features such as nausea, pallor and diaphoresis. Vasovagal syncope can occur following any activity associated with a sudden change in sympathetic tone such as micturation or defecation, exercise, or even coughing or laughing. While obtaining a detailed history is the single most important element for diagnosis, tilt-table testing may be useful in the setting of diagnostic uncertainty.
Carotid sinus syndrome is an underappreciated cause of syncope in the elderly associated with underlying carotid sinus hypersensitivity (CSH). The pathophysiology of CSH is believed to relate to underuse hypersensitivity. Increased stiffness of the carotid vasculature, as occurs with atherosclerosis and aging, can impede normal transduction of pressure to the baroreflex receptors, thereby increasing receptor sensitivity over time through intrinsic feedback mechanisms. Consequently, exertion of pressure in the neck can inadvertently stretch and activate the carotid sinus, leading to an undesired decrease in sympathetic tone. In carotid sinus syndrome, or symptomatic CSH, this process results in reduced cardiac output via vasodilation and/or bradycardia.
Carotid sinus syndrome is increasingly common at older ages.[12] Prevalence estimates suggest a rate as high as 30% among the elderly,[4] but likely represent an overestimation as this rate does not account for the prevalence of asymptomatic CSH found in healthy older adults which may also be as high as 30%.[13] Diagnosis may be aided by performing a carotid sinus massage, which causes an asystolic period of ≥ 3 s in the cardioinhibitory variant of CSH and/or a fall in systolic blood pressure of ≥ 50 mmHg or to less than 80 mmHg in the vasodepressor variant of CSH. Carotid sinus massage is generally safe in those without a history of cerebrovascular accident and without audible carotid bruits. Although physicians may be hesitant to perform carotid sinus massage due to the theoretical risk of stroke, the risk of neurological complications was 0.17% to 0.45% in two studies assessing the safety of the maneuver after excluding patients with clinical history of stroke, or transient ischemic attack, and/or audible carotid artery bruits.[14],[15] Patients with a history of ventricular tachycardia and ventricular fibrillation should also be excluded from receiving carotid massage, as ventricular dysrhythmias during carotid sinus massage have been reported, albeit rarely, in this setting.
Carotid sinus massage should be performed on one side at a time by applying gentle digital pressure for 5–10 s at the bifurcation of the carotid artery, below the angle of the jaw at the level of the cricothyroid cartilage. While the procedure was initially performed only in the supine position, performance in the upright position (as performed during a tilt-table session) increases sensitivity.[16] Even when positive, it is important to consider alternative etiologies of syncope as well, given high rates of asymptomatic CSH among the elderly.
Preventive measures for neurally-mediated syncope include avoidance of clear triggers and amelioration of other contributing factors such as hypovolemia (by increasing fluid intake, ingesting salt tabs, and/or dose-reducing or eliminating diuretics and vasodilators) and orthostatic pooling of blood (by applying compression stockings).[17] Among pharmacologic interventions studied to date, midodrine may offer symptom relief but remains inadequately studied[18] as we await results from the Prevention of Syncope Trial IV (POST 4).[19] Pacemaker implantation has also been studied, but unfortunately has not improved outcomes, even when syncope appears to primarily be cardioinhibitory,[20],[21] and is therefore discouraged.
3.2. Orthostasis and dysautonomia
Orthostatic hypotension is another common cause of syncope in the elderly, with a prevalence reported as high as 30% among those aged > 75 years, and up to 50% among frail elderly adults living in nursing homes.[22] In contradistinction to neurally-mediated syncope in which autonomic reflexes are hyperactive, orthostatic hypotension occurs as a result of impaired autonomic reflexes. With standing, venous blood can pool leading to a reduced effective blood volume, which can subsequently produce hypotension, cerebral hypoperfusion, and syncope.[23] Orthostatic hypotension can occur in the setting of volume contraction (dehydration, poor oral intake) or medications (diuretics, anti-hypertensive therapy), especially when superimposed upon age-related changes in autonomic reflexes, adrenergic responsiveness, and intravascular volume.
Orthostatic hypotension is defined as a sustained decline of ≥ 20 mmHg in systolic or ≥ 10 mmHg in diastolic blood pressure upon standing.[24] The requisite inclusion of associated symptomatology in the definition of orthostatic hypotension is an area of debate, as many older adults experience an asymptomatic decline in blood pressure when assuming the upright position potentially leading to erroneous attribution of orthostatic hypotension as the cause of syncope.
Postprandial syncope is a particularly relevant subtype of orthostatic hypotension, defined as syncope that occurs following a meal, typically within 30–90 min.[25] With food consumption, especially of warm foods and carbohydrates, there is a release of vasodilatory gastrointestinal and pancreatic peptides leading to venous pooling in the splanchnic vascular beds, subsequently reducing effective blood volume and inciting hypotension and syncope. Notably, sequential stressors, such as standing after eating, appear additive to the risk of hypotension and syncope, with potential to increase rates of symptomatic hypotension even among functionally independent older adults.[26]
Treatment largely depends on identifying and treating contributing factors, such as withdrawal of offending medications. Preventive strategies include behavioral modification such as slowly rising from a supine position, physical countermeasures like crossing legs when standing, increasing salt and water intake, avoidance of straining and/or prolonged standing in warm weather, and changing dietary habits in the case of postprandial hypotension.[17] Additionally, compression stockings and/or abdominal binders with graded pressures of 30–40 mmHg may be beneficial by reducing venous pooling. Caffeine intake and cold water ingestion may also be useful for postprandial syncope by reducing splanchnic blood flow and transiently increasing blood pressure. If behavioral interventions fail to improve symptoms, pharmacologic therapies such as fludrocortisone, salt tabs, and midodrine may be considered when the risk/benefit ratio is favorable.[22]
Underlying autonomic insufficiency must also be considered in older adults, especially when syncope is recurrent. Autonomic insufficiency may be idiopathic, or stem from comorbid conditions such as diabetes or amyloidosis, or neurological disorders such as multiple system atrophy, Bradbuy-Eggleston syndrome, or Parkinsonism (Shy-Drager). If present, management of syncope should include treatment of the underlying disorder. Novel agents like atomoxetine (norepinephrine transport inhibitor), droxidopa (oral prodrug for norepinephrine), and pyridostigmine (cholinesterase inhibitor at the autonomic ganglia) may offer additional pharmacologic options for those with autonomic insufficiency,[27] but have not been well-studied in an older population with concurrent comorbidities and/or polypharmacy.
3.3. Cardiac syncope
Syncope in the setting of organic heart disease represents an important subtype of syncope, with a prognosis that is worse compared to those without heart disease. In addition to the usual non-cardiac etiologies of syncope, arrhythmic and structural cardiovascular causes must also be considered in those with heart disease.
The prevalence of bradyarrhythmias and tachyarrhythmias increase with age.[28] Bradycardia can result from medications, sick sinus syndrome from degenerative changes of the sinus node, and/or atrioventricular block, leading to syncope through impaired cardiac output caused by a reduced heart rate. Atrial and ventricular tachycardias can also cause syncope through a reduced cardiac output, typically mediated by impaired stroke volume that is due to incomplete relaxation and inadequate filling time.[29]
Diagnostic modalities for detecting arrhythmias range from electrocardiograms and in-hospital telemetry to event monitors and internal loop recorders. The utility of a given diagnostic modality for detecting arrhythmia is highly dependent on the frequency of the arrhythmia, with the duration of monitoring directly related to diagnostic yield. Accordingly, implantable loop recorders (ILR) have the highest diagnostic yield, more commonly revealing bradyarrhythmias than tachyarrhythmias. Treatment options can be temporizing or curative, and include atrioventricular-nodal blocking agents, anti-arrhythmic agents, and radiofrequency ablation for tachyarrhythmias; and discontinuation of inciting agent(s) and/or pacemaker implantation for bradyarrhythmias.
Syncope in organic heart disease can also directly result from any structural cardiovascular abnormalities that can impair cardiac output. The most common structural cardiovascular cause of syncope is aortic stenosis. Calcific degenerative aortic stenosis is the most common valvular lesion in the elderly, with a prevalence of about 6% by age 86 years.[30] Severe aortic stenosis is associated with effort syncope, which can result from an inadequate increase in cardiac output commensurate with increased demands, or from an associated arrhythmic event. Symptomatic aortic stenosis manifest as syncope, or alternatively as angina or heart failure, connotes a particularly poor prognosis, warranting a prompt evaluation and possible intervention to prevent morbidity and mortality.[31] Diagnosis is made by echocardiography, and may be aided by cardiac catheterization and/or dobutamine stress test in some clinical scenarios.
Curative treatment options include surgery for patients with low perioperative mortality, and percutaneous transcatheter aortic valve replacement (TAVR), which has emerged as an option for prohibitive and high-risk (and possibly intermediate-risk) surgical candidates.[32] Ameliorating factors include avoidance of hypovolemia and vasodilators, which reduce preload and can further impair cardiac output, thereby increasing risk of adverse events such as syncope and cardiac arrest.
Pulmonary hypertension, atrial myxoma, hypertrophic cardiomyopathy, aortic dissection, pulmonary embolism, and subclavian steal syndrome are additional structural cardiovascular abnormalities that can cause syncope, but are less common and beyond the scope of this review.
4. Diagnostic approach and testing
The initial work-up for syncope should include a thorough history, physical examination, and 12-lead electrocardiogram. A specific diagnosis can be identified in at least half of cases with this initial information.[10] Table 2 lists historical clues associated with a particular diagnosis.
Table 2. Historical clues for diagnosis.
| Historical clues | Possible diagnosis |
| Nausea, diaphoresis, long prodrome, absent history of cardiovascular disease, recurrent syncope for > 4 yrs | Neurally-mediated syncope |
| Unpleasant stimulus during/after, defecation, micturition, laughter, swallowing, coughing | Neurally-mediated syncope |
| During/after a meal | Postprandial hypotension |
| After assuming upright posture | Orthostatic hypotension |
| With neck pressure | Carotid sinus hypersensitivity |
| While supine, with history of heart failure and/or coronary artery disease | Arrhythmic |
| During exertion | Aortic stenosis, hypertrophic cardiomyopathy, myocardial ischemia |
| With arm exercise | Subclavian steal |
| Confusion, tongue biting, head turning | Seizure |
Not all patients who present with syncope require hospitalization. It is thus imperative to identify those who are vulnerable to life-threatening events and require an inpatient evaluation, and those who can be discharged safely. Risk stratification is primarily based on determining the likelihood that the cause of syncope is cardiac in nature, given its worse prognosis compared to non-cardiac or unknown causes.[7] Accordingly, several risk stratifications tools focusing on condition-related risk rather than age itself, have been developed to assist clinicians. These include the Boston Syncope criteria,[33] Risk Stratification of Syncope (ROSE) criteria,[34] and the San Francisco Rule,[35] which have excellent sensitivity (close to and above 90%) for identifying high-risk patients (those who develop adverse outcomes).
Syncope observational units offer an adjunctive strategy to risk-stratification for efficient cost-effective management of patients who present with syncope.[36] Developed to standardize the evaluation and management of syncope, observational units have the potential to increase the diagnostic yield of testing, reduce admission rates, and improve clinical outcomes at a reduced overall cost of care.[37] Such units are typically designed to be multidisciplinary in nature, involving emergency department staff, cardiologists, and physicians with expertise in syncope. Although promising, observational units have not yet been studied or implemented on a large-scale basis.
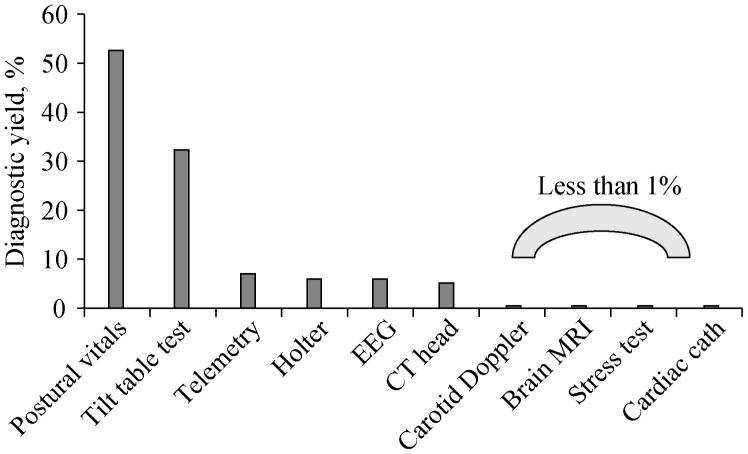
Despite their underutilization, provocative tests such as postural vital signs represent the most cost-effective test for syncope.[38] As shown in Figure 1, postural vitals signs are significantly more likely to yield a definitive diagnosis compared to electroencephalograms, head and neck imaging, cardiac enzymes, electrocardiograms, and telemetry;[39] and costs much less. Carotid massage is another inexpensive maneuver that can safely be performed at the bedside, and may yield a definitive diagnosis without expensive technology.
Figure 1. Diagnostic yields of commonly ordered tests for syncope.
(Adapted from Pires LA, et al.[39]). EEG: electroencephalogram.
The diagnostic yield of an echocardiogram is related to the probability of finding significant structural heart disease, which can often be determined from a careful initial history, physical examination, and electrocardiogram.[40] For example, for those with known or suspected aortic stenosis, echocardiography is an invaluable tool to confirm and classify severity of disease, and therefore risk-stratify the patient. In the absence of suggestive cardiovascular findings (such as a history of heart failure, signs and symptoms consistent with heart failure or angina, a murmur on exam, or an abnormal electrocardiogram), it may be reasonable to defer an echocardiogram in the early phase of the workup.
Other cardiovascular diagnostic studies may be appropriate when the diagnosis is unclear or if the diagnosis has important future clinical implications. For unexplained syncope that occurs in a high-risk setting (e.g., with potential for physical injury, or with occupational implications) or is recurrent, tilt table testing is a Class I indication.[10] Tilt-table testing is also indicated (Class II) if a diagnosis would alter therapy, but is not indicated if the cause of syncope is known or if identification of a cause will not alter therapy.
There are several useful studies when arrhythmia is suspected. Unless the frequency of the arrhythmia is high, the diagnostic yield of short-term electrocardiographic monitoring is low compared to long-term monitoring.[41] Holter monitors and event monitors may be helpful in detecting less frequent but clinically relevant arrhythmias. Newer wireless event monitors that utilize a transdermal patch have been studied in a population with a broad age range, and offer accuracy and improved adherence without the hassle of wearing wires and leads.[42] Among the monitoring strategies, ILR, whose battery can last up to three years, has the highest diagnostic utility, directly contributing to a diagnosis of over 75% of unexplained syncope cases in a recently studied cohort.[43] Whether ILR can ultimately prevent falls through early detection of arrhythmic causes of syncope is unclear, and warrants further investigation.[44]
In the presence of severe structural heart disease and/or electrocardiogram abnormalities, or in the setting of high-risk occupations where an arrhythmic cause of syncope requires exclusion, it may be reasonable to pursue invasive electrophysiologic testing (IEP) to identify malignant ventricular tachyarrhythmias.[10] However, given that tachyarrhythmias are less commonly implicated in syncope compared to bradyarrhythmias, the diagnostic yield for IEP is usually low.
Unless there is clinical suggestion (post-ictal state, tongue-biting, and/or focal neurological deficits) for mimickers of syncope such as seizures or stroke, neurological testing and consultation is not typically necessary for evaluation of syncope. Head computed tomography, carotid Doppler imaging, and electroencephalography have very limited utility due to low sensitivity and specificity.[39] Similarly, although frequently requested, neurology consultation is not necessary in most patients with true syncope, especially when the history and physical examination strongly suggest a cardiac etiology. Primary neurological events generally produce focal findings and are rarely the cause of true syncope which, by definition, is associated with transient loss of consciousness caused by global cerebral hypoperfusion without residual neurological deficits.
5. Implications on driving
Sudden loss of consciousness, as occurs with syncope, while operating machinery such as a motor vehicle may cause significant property damage as well as serious injury or death to the operator and/or others. International guidelines suggest abstinence from driving for up to 6 months following a syncopal episode,[45],[46] and grants permission to resume driving if syncope is not recurrent during the restricted time frame. Some countries and some states in the USA have taken these guidelines one step further, enacting laws to restrict driving, albeit with a great deal of variability with regard to the clinical scenario warranting restriction, and the duration for which the restriction remains. While such restrictions are probably intuitive in cases of severe untreated arrhythmias and recurrent neurally-mediated syncope, it is less clear for other types of syncope.
There is limited data with regards to risk factors for syncope recurrence. In a retrospective study of almost 4000 patients among whom 380 experienced syncope, risk factors for occurrence of syncope while driving included: male gender, age > 65 years, presence of prodromal symptoms, history of cardiovascular disease or stroke, and prior episodes of syncope while driving.[47] In a recent prospective study of a younger cohort (mean age 38 years) with vasovagal syncope, the rate of serious injury from syncope while driving was 0.0035%, raising doubt regarding the need for any restriction at all.[48] Clearly, guidelines and laws should be followed when present. In their absence, until better data becomes available, decisions on activity restrictions in patients with syncope should be made on an individualized basis, accounting for the risk of recurrence.
6. Future research
Future research should focus on the following areas: (1) continued development and study of pharmacologic therapies for syncope; (2) large-scale evaluation of syncope observation units especially among frail older adults, (3) improved risk stratification for driving after a syncopal episode; and (4) development of strategies to improve the management of syncope worldwide.
Although behavioral modifications can significantly improve symptoms and potentially prevent recurrent episodes of syncope, there remains a need for additional targeted therapies for those who fail conservative measures. Future study should focus on continued development and study of therapies that target the diverse underlying pathophysiology of syncope to prevent morbidity and mortality associated with syncope.
Syncope observation units may facilitate a cost-effective multidisciplinary approach to syncope, and have potential to improve clinical outcomes while also reducing admissions. Large-scale studies evaluating their impact on clinical outcomes, especially in the most vulnerable older adult population, are critical for the future of syncope observation units.
The risk of syncope while driving, and subsequent risk for injury, is poorly characterized with significant variation in recommendations across countries and even states within the USA. Unfortunately, studies to date have either been retrospective in nature or failed to include a large proportion of older adults, the populous that comprise the majority of those who experience syncope. Future studies should therefore prospectively examine the influence of patient-level factors on the risk of driving in the setting of different types of syncope among a broadly-aged cohort.
As the geriatric population continues to grow worldwide, syncope will remain an important complex clinical condition requiring medical providers to give thoughtful consideration to contributing factors, potential etiologies, and optimal therapeutic interventions. Research efforts should focus on developing strategies to improve awareness of present guidelines, encourage integration of the pre-existing evidence base with geographic and cultural differences in presentations and etiologies, and facilitate implementation of population-specific processes to ensure high quality care of this vulnerable population.
7. Clinical pearls
In summary, syncope is an important cause of morbidity and mortality in older adults. Numerous age-related changes in cardiovascular structure and function, multimorbidity, and polypharmacy contribute to the incidence and prevalence of syncope in older adults. The most common etiologies in older adults are neurally-mediated, which includes vasovagal syncope, and orthostasis/dysautonomia. Arrhythmia and cardiac structural disease, while less common causes of syncope in older adults, are associated with worse outcomes compared to neurally-mediated and orthostatic etiologies. Older adults can be risk-stratified based on the presence of high-risk features which most prominently include pre-existing or suspected cardiac disease. Higher risk patients warrant inpatient evaluation, whereas lower risk patients may not. A careful history and physical, electrocardiogram, and postural vitals signs are important for evaluation, and may be sufficient to make a diagnosis. Accordingly, the diagnostic yield of postural vital signs is high, the yield of echocardiography depends on the presence of underlying or suspected cardiac disease, and the yield of brain imaging, electroencephalogram, and carotid Doppler are low.
References
- 1.Sun BC, Emond JA, Camargo CA., Jr Characteristics and admission patterns of patients presenting with syncope to U.S. emergency departments, 1992–2000. Acad Emerg Med. 2004;11:1029–1034. doi: 10.1197/j.aem.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor W, Snustad D, Peterson J, et al. Syncope in the elderly. Am J Med. 1986;80:419–428. doi: 10.1016/0002-9343(86)90716-3. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med. 2000;132:337–344. doi: 10.7326/0003-4819-132-5-200003070-00002. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh S, Da Costa D, Kenny RA. Outcome of an integrated approach to the investigation of dizziness, falls and syncope in elderly patients referred to a ‘syncope’ clinic. Age Ageing. 1993;22:53–58. doi: 10.1093/ageing/22.1.53. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor WN. Evaluation and outcome of patients with syncope. Medicine (Baltimore) 1990;69:160–175. doi: 10.1097/00005792-199005000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sun BC, Emond JA, Camargo CA., Jr Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. 2005;95:668–671. doi: 10.1016/j.amjcard.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;347:878–885. doi: 10.1056/NEJMoa012407. [DOI] [PubMed] [Google Scholar]
- 8.Forman DE, Lipsitz LA. Syncope in the elderly. Cardiol Clin. 1997;15:295–311. doi: 10.1016/s0733-8651(05)70337-4. [DOI] [PubMed] [Google Scholar]
- 9.Brignole M, Menozzi C, Bartoletti A, et al. A new management of syncope: prospective systematic guideline-based evaluation of patients referred urgently to general hospitals. Eur Heart J. 2006;27:76–82. doi: 10.1093/eurheartj/ehi647. [DOI] [PubMed] [Google Scholar]
- 10.Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS) Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009) Eur Heart J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloomfield D, Maurer M, Bigger JT., Jr Effects of age on outcome of tilt-table testing. Am J Cardiol. 1999;83:1055–1058. doi: 10.1016/s0002-9149(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 12.Humm AM, Mathias CJ. Unexplained syncope—is screening for carotid sinus hypersensitivity indicated in all patients aged > 40 years? J Neurol Neurosurg Psychiatry. 2006;77:1267–1270. doi: 10.1136/jnnp.2006.093518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr SR, Pearce MS, Brayne C, et al. Carotid sinus hypersensitivity in asymptomatic older persons: implications for diagnosis of syncope and falls. Arch Intern Med. 2006;166:515–520. doi: 10.1001/archinte.166.5.515. [DOI] [PubMed] [Google Scholar]
- 14.Puggioni E, Guiducci V, Brignole M, et al. Results and complications of the carotid sinus massage performed according to the “method of symptoms”. Am J Cardiol. 2002;89:599–601. doi: 10.1016/s0002-9149(01)02303-7. [DOI] [PubMed] [Google Scholar]
- 15.Munro NC, McIntosh S, Lawson J, et al. Incidence of complications after carotid sinus massage in older patients with syncope. J Am Geriatr Soc. 1994;42:1248–1251. doi: 10.1111/j.1532-5415.1994.tb06505.x. [DOI] [PubMed] [Google Scholar]
- 16.Morillo CA, Camacho ME, Wood MA, et al. Diagnostic utility of mechanical, pharmacological and orthostatic stimulation of the carotid sinus in patients with unexplained syncope. J Am Coll Cardiol. 1999;34:1587–1594. doi: 10.1016/s0735-1097(99)00365-4. [DOI] [PubMed] [Google Scholar]
- 17.Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis. 2013;55:425–433. doi: 10.1016/j.pcad.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romme JJ, Reitsma JB, Black CN, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev. 2011:CD004194. doi: 10.1002/14651858.CD004194.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assessment of Midodrine in the Prevention of Vasovagal Syncope: The Prevention of Syncope Trial IV (POST 4) 2015. https://clinicaltrials.gov/ct2/show/NCT01456481.
- 20.Parry SW, Steen N, Bexton RS, et al. Pacing in elderly recurrent fallers with carotid sinus hypersensitivity: a randomised, double-blind, placebo controlled crossover trial. Heart. 2009;95:405–409. doi: 10.1136/hrt.2008.153189. [DOI] [PubMed] [Google Scholar]
- 21.Ryan DJ, Nick S, Colette SM, et al. Carotid sinus syndrome, should we pace? A multicentre, randomised control trial (Safepace 2) Heart. 2010;96:347–351. doi: 10.1136/hrt.2009.176206. [DOI] [PubMed] [Google Scholar]
- 22.Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 2007;120:841–847. doi: 10.1016/j.amjmed.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Shaw BH, Claydon VE. The relationship between orthostatic hypotension and falling in older adults. Clin Auton Res. 2014;24:3–13. doi: 10.1007/s10286-013-0219-5. [DOI] [PubMed] [Google Scholar]
- 24.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 25.Lipsitz LA, Nyquist RP., Jr Wei JY, Rowe JW. Postprandial reduction in blood pressure in the elderly. N Engl J Med. 1983;309:81–83. doi: 10.1056/NEJM198307143090205. [DOI] [PubMed] [Google Scholar]
- 26.Maurer MS, Karmally W, Rivadeneira H, et al. Upright posture and postprandial hypotension in elderly persons. Ann Intern Med. 2000;133:533–536. doi: 10.7326/0003-4819-133-7-200010030-00012. [DOI] [PubMed] [Google Scholar]
- 27.Arnold AC, Shibao C. Current concepts in orthostatic hypotension management. Curr Hypertens Rep. 2013;15:304–312. doi: 10.1007/s11906-013-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow GV, Marine JE, Fleg JL. Epidemiology of arrhythmias and conduction disorders in older adults. Clin Geriatr Med. 2012;28:539–553. doi: 10.1016/j.cger.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima JA, Weiss JL, Guzman PA, et al. Incomplete filling and incoordinate contraction as mechanisms of hypotension during ventricular tachycardia in man. Circulation. 1983;68:928–938. doi: 10.1161/01.cir.68.5.928. [DOI] [PubMed] [Google Scholar]
- 30.Iivanainen AM, Lindroos M, Tilvis R, et al. Natural history of aortic valve stenosis of varying severity in the elderly. Am J Cardiol. 1996;78:97–101. doi: 10.1016/s0002-9149(96)00235-4. [DOI] [PubMed] [Google Scholar]
- 31.Carabello BA. Evaluation and management of patients with aortic stenosis. Circulation. 2002;105:1746–1750. doi: 10.1161/01.cir.0000015343.76143.13. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:e521–643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 33.Grossman SA, Fischer C, Lipsitz LA, et al. Predicting adverse outcomes in syncope. J Emerg Med. 2007;33:233–239. doi: 10.1016/j.jemermed.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed MJ, Newby DE, Coull AJ, et al. The ROSE (risk stratification of syncope in the emergency department) study. J Am Coll Cardiol. 2010;55:713–721. doi: 10.1016/j.jacc.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 35.Quinn JV, Stiell IG, McDermott DA, et al. Derivation of the San Francisco Syncope rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43:224–232. doi: 10.1016/s0196-0644(03)00823-0. [DOI] [PubMed] [Google Scholar]
- 36.Shen WK, Decker WW, Smars PA, et al. Syncope Evaluation in the Emergency Department Study (SEEDS): a multidisciplinary approach to syncope management. Circulation. 2004;110:3636–3645. doi: 10.1161/01.CIR.0000149236.92822.07. [DOI] [PubMed] [Google Scholar]
- 37.Sun BC, McCreath H, Liang LJ, et al. Randomized clinical trial of an emergency department observation syncope protocol versus routine inpatient admission. Ann Emerg Med. 2014;64:167–175. doi: 10.1016/j.annemergmed.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299–1305. doi: 10.1001/archinternmed.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pires LA, Ganji JR, Jarandila R, Steele R. Diagnostic patterns and temporal trends in the evaluation of adult patients hospitalized with syncope. Arch Intern Med. 2001;161:1889–1895. doi: 10.1001/archinte.161.15.1889. [DOI] [PubMed] [Google Scholar]
- 40.Sarasin FP, Junod AF, Carballo D, et al. Role of echocardiography in the evaluation of syncope: a prospective study. Heart. 2002;88:363–367. doi: 10.1136/heart.88.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assar MD, Krahn AD, Klein GJ, et al. Optimal duration of monitoring in patients with unexplained syncope. Am J Cardiol. 2003;92:1231–1233. doi: 10.1016/j.amjcard.2003.07.042. [DOI] [PubMed] [Google Scholar]
- 42.Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127:95 e11–e97. doi: 10.1016/j.amjmed.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edvardsson N, Frykman V, van Mechelen R, et al. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace. 2011;13:262–269. doi: 10.1093/europace/euq418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhangu J, McMahon CG, Hall P, et al. Long-term cardiac monitoring in older adults with unexplained falls and syncope. Heart. 2016;102:681–686. doi: 10.1136/heartjnl-2015-308706. [DOI] [PubMed] [Google Scholar]
- 45.Epstein AE, Miles WM, Benditt DG, et al. Personal and public safety issues related to arrhythmias that may affect consciousness: implications for regulation and physician recommendations. A medical/scientific statement from the American Heart Association and the North American Society of Pacing and Electrophysiology. Circulation. 1996;94:1147–1166. doi: 10.1161/01.cir.94.5.1147. [DOI] [PubMed] [Google Scholar]
- 46.Brignole M, Alboni P, Benditt DG, et al. Guidelines on management (diagnosis and treatment) of syncope-update 2004. Executive Summary. Eur Heart J. 2004;25:2054–2072. doi: 10.1016/j.ehj.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Sorajja D, Nesbitt GC, Hodge DO, et al. Syncope while driving: clinical characteristics, causes, and prognosis. Circulation. 2009;120:928–934. doi: 10.1161/CIRCULATIONAHA.108.827626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan VHRD, Maxey C, Sheldon R. Prospective assessment of the risk of vasovagal syncope during driving. JACC Clin Electrophysiol. doi: 10.1016/j.jacep.2015.10.006. Published Online First: November 2015. [DOI] [Google Scholar]