ABSTRACT
Hepatitis C virus (HCV) infection often causes chronic hepatitis, liver cirrhosis, and ultimately hepatocellular carcinoma. However, the mechanisms underlying HCV-induced liver pathogenesis are still not fully understood. By transcriptome sequencing (RNA-Seq) analysis, we recently identified host genes that were significantly differentially expressed in cell culture-grown HCV (HCVcc)-infected cells. Of these, tribbles homolog 3 (TRIB3) was selected for further characterization. TRIB3 was initially identified as a binding partner of protein kinase B (also known as Akt). TRIB3 blocks the phosphorylation of Akt and induces apoptosis under endoplasmic reticulum (ER) stress conditions. HCV has been shown to enhance Akt phosphorylation for its own propagation. In the present study, we demonstrated that both mRNA and protein levels of TRIB3 were increased in the context of HCV replication. We further showed that promoter activity of TRIB3 was increased by HCV-induced ER stress. Silencing of TRIB3 resulted in increased RNA and protein levels of HCV, whereas overexpression of TRIB3 decreased HCV replication. By employing an HCV pseudoparticle entry assay, we further showed that TRIB3 was a negative host factor involved in HCV entry. Both in vitro binding and immunoprecipitation assays demonstrated that HCV NS3 specifically interacted with TRIB3. Consequently, the association of TRIB3 and Akt was disrupted by HCV NS3, and thus, TRIB3-Akt signaling was impaired in HCV-infected cells. Moreover, HCV modulated TRIB3 to promote extracellular signal-regulated kinase (ERK) phosphorylation, activator protein 1 (AP-1) activity, and cell migration. Collectively, these data indicate that HCV exploits the TRIB3-Akt signaling pathway to promote persistent viral infection and may contribute to HCV-mediated pathogenesis.
IMPORTANCE TRIB3 is a pseudokinase protein that acts as an adaptor in signaling pathways for important cellular processes. So far, the functional involvement of TRIB3 in virus-infected cells has not yet been demonstrated. We showed that both mRNA and protein expression levels of TRIB3 were increased in the context of HCV RNA replication. Gene silencing of TRIB3 increased HCV RNA and protein levels, and thus, overexpression of TRIB3 decreased HCV replication. TRIB3 is known to promote apoptosis by negatively regulating the Akt signaling pathway under ER stress conditions. Most importantly, we demonstrated that the TRIB3-Akt signaling pathway was disrupted by NS3 in HCV-infected cells. These data provide evidence that HCV modulates the TRIB3-Akt signaling pathway to establish persistent viral infection.
INTRODUCTION
Hepatitis C virus (HCV) is an enveloped virus with a positive-sense, single-stranded RNA genome. HCV causes both acute and persistent infection and often leads to liver cirrhosis and hepatocellular carcinoma. It is estimated that approximately 170 million people are chronically infected with HCV (1). HCV belongs to the genus Hepacivirus within the Flaviviridae family. The HCV genome consists of 9,600 nucleotides (nt) and harbors a single open reading frame. This polyprotein is processed by both viral and cellular proteases into 10 individual proteins, including structural (core, E1, and E2) and nonstructural (p7 and NS2 to NS5B) proteins (2). Nonstructural 3 (NS3) is a 70-kDa multifunctional protein that displays serine protease and RNA helicase activities. Its enzyme activities are essential for viral protein processing and HCV replication. In addition, NS3/4A protease suppresses the host innate immune response by targeting mitochondrial antiviral-signaling protein (MAVS) for cleavage (3). Moreover, NS3 is known to possess oncogenic potential and to induce cell proliferation (4).
HCV is highly dependent on cellular proteins for its own propagation. By transcriptome sequencing (RNA-Seq) analysis, we previously identified 30 host genes that were highly differentially expressed in cell culture-grown HCV (HCVcc)-infected cells (5). Among these, tribbles homolog 3 (TRIB3) was selected for further characterization. TRIB3 (also known as TRB3 or SKIP3) is a pseudokinase protein that belongs to tribbles family (6). The tribbles gene was first identified in Drosophila melanogaster to regulate cell division and migration. Functional loss of tribbles resulted in defects in Drosophila wing formation (6). There are three known mammalian homologs of the tribbles gene: TRIB1/C8FW/SKIP1, TRIB2/C5FW/SKIP2/SINK, and TRIB3/NIPK/SKIP3. The tribbles family structurally consists of an N-terminal region, a central pseudokinase domain, and a C-terminal region. While retaining some distinct typical features of a canonical kinase, the central pseudokinase domain of TRIB3 lacks crucial motifs for ATP anchoring and phosphate transfer, causing it noncatalytic activity (6). Despite its lack of kinase activity, TRIB3 has been shown to modulate various signaling pathways and cell fate. As a binding partner of Akt (also known as protein kinase B), TRIB3 can mask phosphorylation sites in Akt, leading to the suppression of its activity (7). Under conditions of endoplasmic reticulum (ER) stress, TRIB3 promotes apoptosis by negatively regulating the Akt signaling pathway (8, 9). In contrast, TRIB3 expression is highly upregulated in some cancer cells and promotes cell proliferation by positively regulating the mitogen-activated protein kinase (MAPK)–extracellular signal-regulated kinase (ERK) pathway (10). To date, the functional involvement of TRIB3 in virus-infected cells has never been demonstrated.
We recently performed RNA-Seq analysis to identify host factors involved in HCV propagation (5). In the present study, we selected TRIB3 and showed that HCV upregulated TRIB3 expression via ER stress. TRIB3 interrupted HCV propagation by suppressing the Akt signaling pathway. However, HCV has evolved to manipulate TRIB3-Akt signaling by using the NS3 protein. In addition, HCV modulated TRIB3 to promote MAPK-ERK signaling, thus providing a favorable environment for viral propagation.
MATERIALS AND METHODS
Plasmids and DNA transfection.
Total cellular RNAs were isolated from Huh7 cells by using RiboEx (GeneAll), and cDNA was synthesized by using a kit (Toyobo) according to the manufacturer's instructions. Full-length TRIB3 and Akt were amplified by using primer sets listed in Table 1. PCR products were inserted into the HindIII and XbaI sites of the p3xFlag-CMV10 vector (Sigma-Aldrich) to clone Flag-tagged TRIB3 and into the BamHI and XbaI sites of the pEF6-V5-His vector (Invitrogen) to clone the V5-tagged Akt expression plasmid. TRIB3 and Akt coding sequences were also subcloned into the pGEX-4T-1 vector (Amersham Biosciences) to generate glutathione S-transferase (GST)-tagged TRIB3 and GST-tagged Akt, respectively. The Flag-tagged TRIB3 ΔAkt mutant was constructed by PCR-based mutagenesis using a primer set described in Table 1. TRIB3 promoter constructs (from bp −2000 to +558) were cloned by using Huh7 genomic DNA and inserted into the pGL3 Basic vector (Promega). Myc-tagged HCV core, NS3, NS4B, NS5A, and NS5B plasmids were described previously (11). For DNA transfection, cells were transfected with the expression plasmid by using a polyethyleneimine reagent (Sigma-Aldrich) as we described previously (11).
TABLE 1.
Primers used for cloning in this study
| Construct | Primer sequencea | Enzyme |
|---|---|---|
| Flag-TRIB3 | TAAAGCTTATGCGAGCCACCCCTCT | HindIII |
| TTTCTAGACTAGCCATACAGAACCAC | XbaI | |
| Flag-TRIB3 ΔAkt | GAAGAGCAGGACAGGCGAGTATGAGGCCCGTGA | |
| CGGGCCTCATACTCG CCTGTCCTGCTCTTCGGC | ||
| GST-TRIB3 | TAGGATCCATGCGAGCCACCCCTC | BamHI |
| TTCTCGAGGCCATACAGAACCACT | XhoI | |
| Akt-V5 | ACGGATCCATGAGCGACGTGGCTA | BamHI |
| TATCTAGAGTGGCCGTGCCGCTGGCC | XbaI | |
| GST-Akt | ACGGATCCATGAGCGACGTGGCTA | BamHI |
| TACTCGAGGGCCGTGCCGCTGGCC | XhoI | |
| Full-length TRIB3 promoter | AGACGCGTTCCCCAGGGATTTGGAAACAGG | MluI |
| GACTCGAGCTCGCCCCGTCGTTCCGCGTGG | XhoI |
Enzyme sites in sequences are shown in boldface type.
Cell culture.
Both Huh7 and Huh7.5 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 1% nonessential amino acids. HEK293T cells were grown in DMEM containing 5% FBS. Huh7 cells harboring a subgenomic replicon derived from genotype 1b and interferon (IFN)-cured cells were grown as reported previously (12).
Generation of TRIB3 stable cells.
To make cell lines stably expressing Flag-tagged TRIB3 (TRIB3 stable cell lines), Huh7 cells were transfected with the p3xFlag-CMV10-TRIB3 expression plasmid and cultured for 3 weeks in the presence of 500 μg/ml of G418. Single positive clones were selected by immunoblot analysis using an anti-Flag monoclonal antibody. Huh7 cells transfected with an empty vector (p3xFlag-CMV10) were selected as described above and used as a control.
Preparation of infectious virus.
HCVcc was generated as described previously (13). Briefly, a monolayer of Huh7.5 cells was washed twice in phosphate-buffered saline (PBS), trypsinized, and resuspended at a concentration of 5 × 106 cells/ml in Opti-MEM (Invitrogen, Carlsbad, CA). After a brief centrifugation at 1,000 rpm, the cells were resuspended in 400 μl of Cytomix solution containing 2 mM ATP and 5 mM glutathione. The cells were then mixed with 10 μg of Jc1 viral RNA and electroporated by using a Gene Pulser Xcell instrument (Bio-Rad Laboratories, Hercules, CA) in a 4-mm-gap cuvette. Cell culture supernatants were collected 4 days after electroporation.
Immunoprecipitation.
HEK293T cells were cotransfected with Flag-tagged TRIB3 and Myc-tagged core, NS3, NS4B, NS5A, and NS5B. Total amounts of DNA were adjusted by adding an empty vector. Twenty-four hours after transfection, cells were harvested, and an immunoprecipitation assay was performed as we described previously (14). To verify endogenous protein interactions, either subgenomic replicon cells were transfected with Flag-tagged TRIB3 for 36 h or TRIB3 stable cells were electroporated with Jc1 RNA and incubated for 4 days. Cells were lysed in buffer containing 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 0.1% NP-40, and a protease inhibitor cocktail. After centrifugation at 13,500 rpm for 15 min, the supernatant was incubated with the appropriate antibody at 4°C overnight. The samples were further incubated with 40 μl of protein A beads for 1 h. The beads were washed five times in washing buffer, and bound protein was then detected by an immunoblot assay.
GST pulldown assay.
The GST-TRIB3 and GST-Akt fusion proteins were expressed in Escherichia coli BL21 and purified with glutathione-Sepharose 4B beads (Amersham Biosciences) according to the manufacturer's instructions. An in vitro binding assay was performed as we described previously (14).
RNA interference.
Small interfering RNAs (siRNAs) targeting two different 3′ nontranslated regions (NTRs) of TRIB3 (TRIB3#1 [5′-GACCAUAGGUCACUGUCUA-3′] and TRIB3#2 [5′-CCAGGUCCAUACUCUAGGU-3′]) and the universal negative-control siRNA were purchased from Bioneer (South Korea). siRNA transfection was performed by using the Lipofectamine RNAiMax reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol.
Luciferase reporter assay.
Huh7 cells were cotransfected with the luciferase gene and the plasmids indicated in the figures together with the pCH110 reference plasmid. Forty-eight hours after transfection, cells were harvested, and luciferase assays were then performed as we described previously (15). A dual-luciferase reporter assay was performed as we reported previously (12).
Immunoblot analysis.
Cells were washed twice with PBS; lysed in buffer containing 20 mM Tris HCl (pH 7.2), 150 mM NaCl, 10% glycerol, 1% NP-40, 10 mM NaF, 30 mM sodium pyrophosphate, 1 mM EDTA, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride (PMSF), and a protease inhibitor cocktail (16) for 15 min on ice; and centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was collected, and equal amounts of protein were subjected to SDS-PAGE and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane. Immunoblot analysis was performed as we described previously (11), using the following antibodies: mouse anti-Myc (Santa Cruz), mouse anti-TRIB3 (Santa Cruz), mouse anti-GST (Santa Cruz), rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz), rabbit anti-Akt (Cell Signaling), mouse anti-Akt phospho-Ser473 (Cell Signaling), rabbit anti-ERK (Cell Signaling), rabbit anti-ERK phospho-Thr202/Tyr204 (Cell Signaling), mouse anti-β-actin (Santa Cruz), mouse anti-Flag (Sigma-Aldrich), mouse anti-V5 (Invitrogen), rabbit anti-NS3, rabbit anti-NS5A, and rabbit anticore (Byung-Yoon Ahn, Korea University). Either horseradish peroxidase-conjugated goat anti-rabbit antibody or goat anti-mouse antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) was used as a secondary antibody. Following detection of phospho-Akt (p-Akt) and phospho-ERK, membranes were stripped by using stripping buffer (62.5 mM Tris [pH 6.8], 100 mM β-mercaptoethanol, 2% SDS) for 30 min at 50°C and then reprobed with either rabbit anti-Akt or rabbit anti-ERK antibody (16).
Immunofluorescence assay.
Huh7 cells seeded onto cover slides were either mock infected or infected with Jc1 for 4 days. Cells were washed twice with PBS and fixed with cold methanol at −20°C for 5 min. After two washes with PBS, the cells were then permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. After three washes with PBS, fixed cells were blocked with 0.25% bovine serum albumin (BSA) in PBS for 1 h at room temperature. The cells were then incubated with a mouse anti-TRIB3 antibody and a rabbit anti-NS3 antibody. After three washes with PBS, cells were incubated with tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-mouse IgG (Abnova) and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature. Cells were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to label nuclei. After three washes with PBS, cells were analyzed by using the Zeiss LSM 700 laser confocal microscopy system (Carl Zeiss, Inc., Thornwood, NY).
Quantification of RNA.
Quantitative real-time PCR (qRT-PCR) experiments were carried out by using an iQ5 multicolor real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA) as we reported previously (12).
HCV pseudoparticle production and entry assay.
HCV pseudoparticles (HCVpp) with E1 and E2 glycoproteins derived from genotype 1a (H77) or genotype 2a (JFH1) and vesicular stomatitis virus (VSV) pseudoparticles (VSVpp) were generated as we described previously (15). Approximately 2.5 × 106 HEK293T cells were transfected with 2.5 μg of an HCV E1E2 or VSV glycoprotein G envelope protein-expressing plasmid, 7.75 μg of a Gag-Pol (polymerase)-packaging plasmid, and 7.75 μg of a transfer vector encoding the firefly luciferase reporter protein by using polyethyleneimine. Supernatants containing HCVpp or VSVpp were collected 48 h after transfection. For the infection assay, Huh7 cells were transfected with siRNAs for 48 h and then infected with either HCVpp or VSVpp for 6 h. Cells were then replaced with fresh culture medium. At 48 h postinfection, cells were harvested, and luciferase activity was determined.
WST assay.
Cells seeded onto 24-well plates were transfected with either 20 nM negative-control siRNA or TRIB3-specific siRNAs. Cell viability was measured by using 30 μl of water-soluble tetrazolium salt (WST) in each well, and the plate was incubated for 1 h at 37°C. The plate was then shaken for 1 min, the aqueous layer in each well was transferred into a 96-well plate, and the absorbance was measured at 450 nm (15).
Cell proliferation assay.
Cell proliferation was determined by a WST assay. Huh7 cells were either mock infected or infected with Jc1 for 4 days and then transfected with either negative-control siRNA or TRIB3-specific siRNA. Cells were replated onto 96-well plates at a density of ∼2 × 103 cells per well. At the indicated time intervals after siRNA transfection, 5 μl of WST solution was added to each well to determine cell growth.
Cell death detection.
Cells were washed twice with PBS, and crystal violet was added to stain adherent cells. Stained cells were then lysed in 1% SDS and measured at an optical density (OD) at 595 nm (17).
Statistical analysis.
Data are presented as means ± standard deviations (SD). Student's t test was used for statistical analysis. The asterisks in the figures indicate significant differences, as noted in the legends.
RESULTS
HCV upregulates TRIB3 expression.
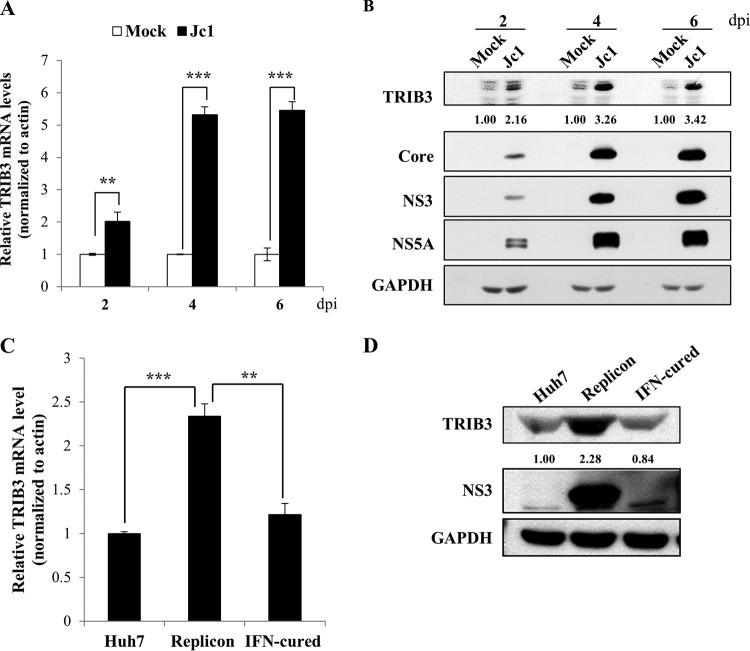
By employing RNA-Seq technology, we previously identified 30 genes that were highly differentially expressed in HCVcc-infected cells (5). Of these, we selected TRIB3 for further characterization. We showed that the mRNA level of TRIB3 was significantly increased in HCV-infected cells compared with mock-infected cells (Fig. 1A). Consistently, the protein level of TRIB3 gradually increased during the course of HCV infection (Fig. 1B). We further verified that both mRNA (Fig. 1C) and protein (Fig. 1D) levels of TRIB3 were markedly elevated in Huh7 cells harboring an HCV subgenomic replicon (genotype 1b) compared with those in IFN-cured and naive Huh7 cells. These data indicate that HCV upregulates TRIB3 expression.
FIG 1.
TRIB3 expression is increased in HCV-replicating cells. (A) Huh7 cells were either mock infected or infected with HCV Jc1. At the indicated time points postinfection, total cellular RNAs were extracted, and the TRIB3 mRNA level was analyzed by qRT-PCR. Experiments were carried out in triplicate. The asterisks indicate significant differences (**, P < 0.01; ***, P < 0.001). (B) Total cellular lysates harvested at various time points were immunoblotted with the indicated antibodies. Band intensities of the TRIB3 protein were normalized against those of GAPDH. dpi, days postinfection. (C) Total cellular RNAs were extracted from parental Huh7, HCV replicon, and IFN-cured cells, and the TRIB3 mRNA level was analyzed by qRT-PCR. (D) Total cellular lysates harvested from parental Huh7, HCV replicon, and IFN-cured cells were immunoblotted with the indicated antibodies.
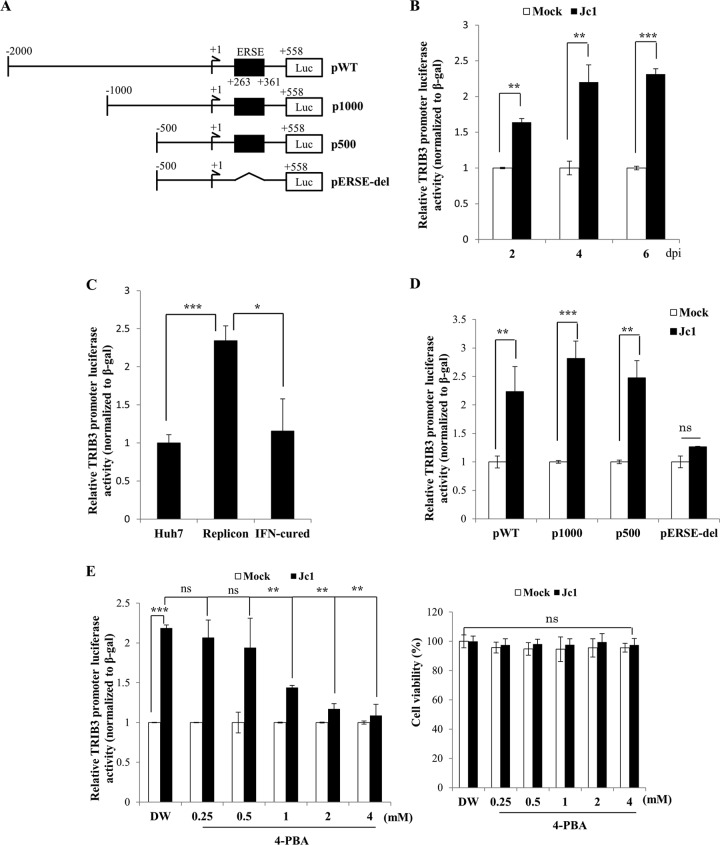
HCV upregulates TRIB3 promoter activity via ER stress.
To investigate if the transcriptional level of TRIB3 was also regulated by HCV, Huh7 cells were transfected with a luciferase reporter plasmid consisting of nt −2000 +558 of the TRIB3 promoter (17) (Fig. 2A) and then infected with Jc1. Figure 2B shows that TRIB3 promoter activities gradually increased during the course of HCV infection compared to mock-infected cells. We further demonstrated that the promoter activity of TRIB3 was significantly increased in Huh7 cells harboring the HCV subgenomic replicon compared to those in IFN-cured and naive Huh7 cells (Fig. 2C). We then investigated which region of the TRIB3 promoter was responsible for the upregulation of the transcriptional level of TRIB3. For this purpose, Huh7 cells were either mock infected or infected with Jc1 and then transfected with various constructs of the mutant TRIB3 promoter (Fig. 2A). As shown in Fig. 2D, luciferase activities were significantly increased in Jc1-infected cells transfected with either the p1000 or p500 mutant but not with the pERSE-del mutant (mutant lacking the ER stress response element [ERSE]), indicating that HCV upregulated TRIB3 promoter activity via ER stress. To corroborate this result, Huh7 cells that were mock infected or infected with Jc1 were treated with either H2O (vehicle) or increasing concentrations of 4-phenyl butyric acid (4-PBA), a chemical chaperone that alleviates ER stress by preventing misfolded-protein aggregation (18). Figure 2E shows that HCV-induced TRIB3 promoter activity was significantly reduced by treatment with 4-PBA in a dose-dependent manner (Fig. 2E, left) without causing any cell toxicity (Fig. 2E, right), confirming that HCV upregulates TRIB3 promoter activity via ER stress.
FIG 2.
TRIB3 promoter activity is upregulated by HCV-induced ER stress. (A) Schematic diagram of TRIB3 promoter constructs. pWT, full-length TRIB3 promoter; p1000, TRIB3 promoter mutant containing the region spanning nt −1000 to +558; p500, TRIB3 promoter mutant containing the region spanning nt −500 to +558; pERSE-del, TRIB3 promoter mutant lacking the ERSE sequence from p500. (B) Huh7 cells were either mock infected or infected with Jc1. At the indicated time points after HCV infection, the full-length TRIB3 promoter was transfected together with the β-galactosidase expression plasmid. Forty-eight hours after promoter transfection, luciferase reporter activities were determined. Data from three independent experiments were quantified. (C) Huh7 cells, HCV replicon cells, and IFN-cured cells were transfected with the TRIB3 promoter reporter construct together with the β-galactosidase expression plasmid. Forty-eight hours after transfection, luciferase activities were determined. (D) Huh7 cells were either mock infected or infected with Jc1. At 48 h postinfection, cells were transfected with either wild-type or mutant constructs of the TRIB3 promoter. Forty-eight hours after transfection, luciferase activities were determined. (E, left) Huh7 cells were either mock infected or infected with Jc1. At 4 days postinfection, cells were further transfected with the full-length TRIB3 promoter. Twenty-four hours after transfection, cells were either mock treated with double-distilled water (DW) or treated with increasing concentrations of 4-PBA. Twenty-four hours after inhibitor treatment, TRIB3 promoter activities were determined. The asterisks indicate significant differences (**, P < 0.01; ***, P < 0.001; ns, not determined). (Right) Huh7 cells were either mock infected or infected with Jc1. At 4 days postinfection, cells were either mock treated with double-distilled water or treated with increasing amounts of 4-PBA for 48 h. Cell viability was determined by a WST assay.
TRIB3 negatively regulates HCV propagation.
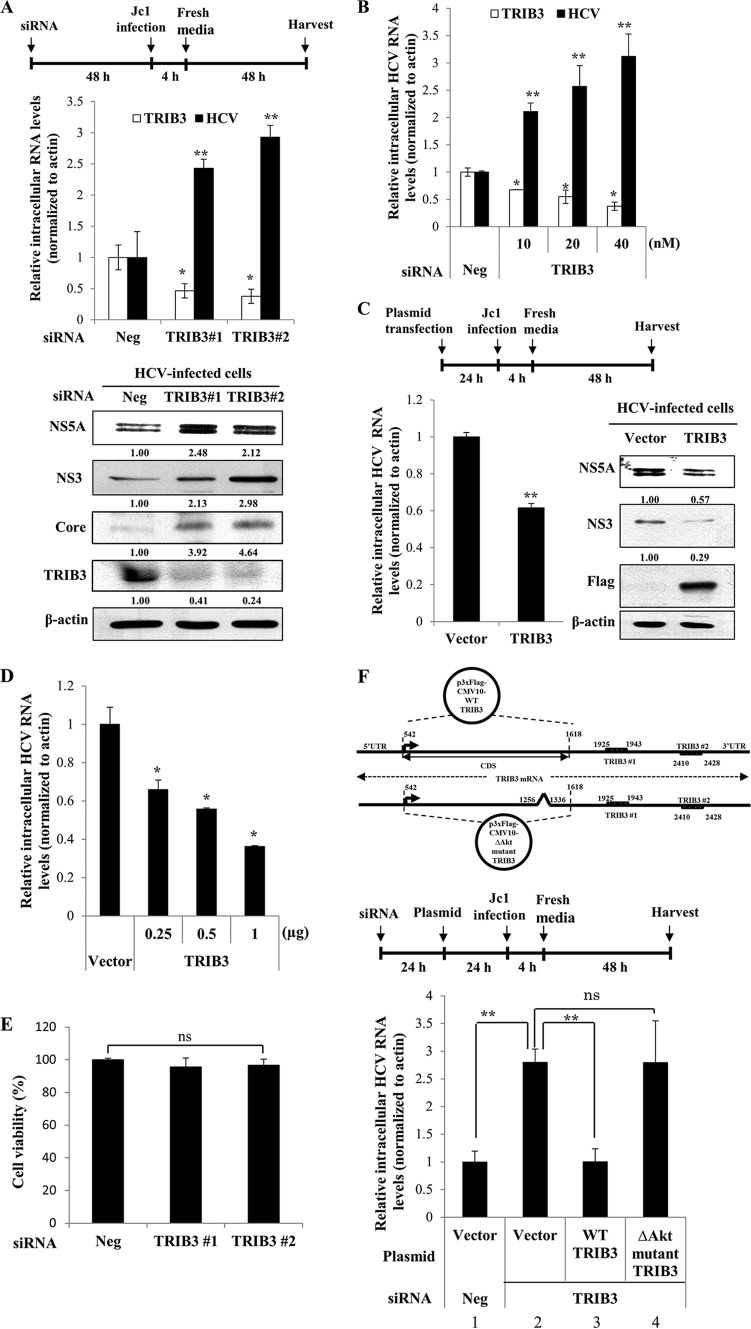
To investigate the functional involvement of TRIB3 in HCV propagation, Huh7 cells transfected with either negative-control siRNA or siRNAs targeting TRIB3 were infected with Jc1. At 48 h postinfection, both RNA and protein levels were determined, as depicted in the schematic illustration in Fig. 3A (top). Silencing of TRIB3 significantly impaired both intracellular mRNA and protein expressions of TRIB3 (Fig. 3A, middle and bottom). Surprisingly, both intracellular RNA and protein levels of HCV were significantly increased in TRIB3 knockdown cells. We further confirmed that the increase in the intracellular HCV RNA level occurred with TRIB3 siRNA in a dose-dependent manner (Fig. 3B). To corroborate this finding, we assessed HCV propagation in cells transiently expressing the TRIB3 protein, as depicted in Fig. 3C (top). Indeed, overexpression of TRIB3 resulted in a significant decrease of the HCV RNA level and marked reductions of viral protein expression levels (Fig. 3C, bottom). We further showed that the decrease of the intracellular HCV RNA level inversely correlated with the protein concentration of TRIB3 (Fig. 3D), confirming that TRIB3 negatively regulated HCV propagation. We also demonstrated that transfection of siRNAs displayed no cytotoxicity (Fig. 3E). In the present study, we used siRNAs targeting the outside of the coding sequence (CDS) region of the TRIB3 gene. To rule out an off-target effect of TRIB3 siRNAs, we analyzed the inhibitory effect of TRIB3 on HCV RNA expression using a Flag-tagged TRIB3 plasmid consisting of CDSs, as depicted in the schematic diagram in Fig. 3F (top). Huh7 cells were transfected with either negative-control siRNA or siRNA targeting TRIB3. Twenty-four hours after transfection, cells were further transfected with Flag-tagged TRIB3 for 24 h and then infected with Jc1. At 48 h postinfection, the intracellular HCV RNA level was determined, as depicted in the schematic illustration in Fig. 3F (middle). Figure 3F (bottom, lane 2) shows that the intracellular HCV RNA level was significantly elevated in TRIB3 knockdown cells. However, exogenous expression of wild-type TRIB3 effectively suppressed HCV replication in TRIB3 knockdown cells (Fig. 3F, bottom, lane 3), verifying that the effect of siRNA was specific to TRIB3. Because TRIB3 binds to Akt and negatively regulates Akt signaling (7–9) and Akt signaling has also been reported to be involved in HCV propagation (19, 20), we further investigated whether the inhibitory activity of TRIB3 on HCV propagation was mediated through the Akt signaling pathway. For this purpose, Huh7 cells transfected with the indicated siRNAs were further transfected with either the vector or the Akt binding-defective (ΔAkt) mutant TRIB3 expression plasmid (Fig. 3F, top), followed by Jc1 infection. As shown in Fig. 3F (bottom, lane 4), exogenous expression of the TRIB3 ΔAkt mutant could not suppress the HCV RNA level in TRIB3 knockdown cells, suggesting that the negative effect of TRIB3 on HCV propagation might be mediated through the Akt signaling pathway.
FIG 3.
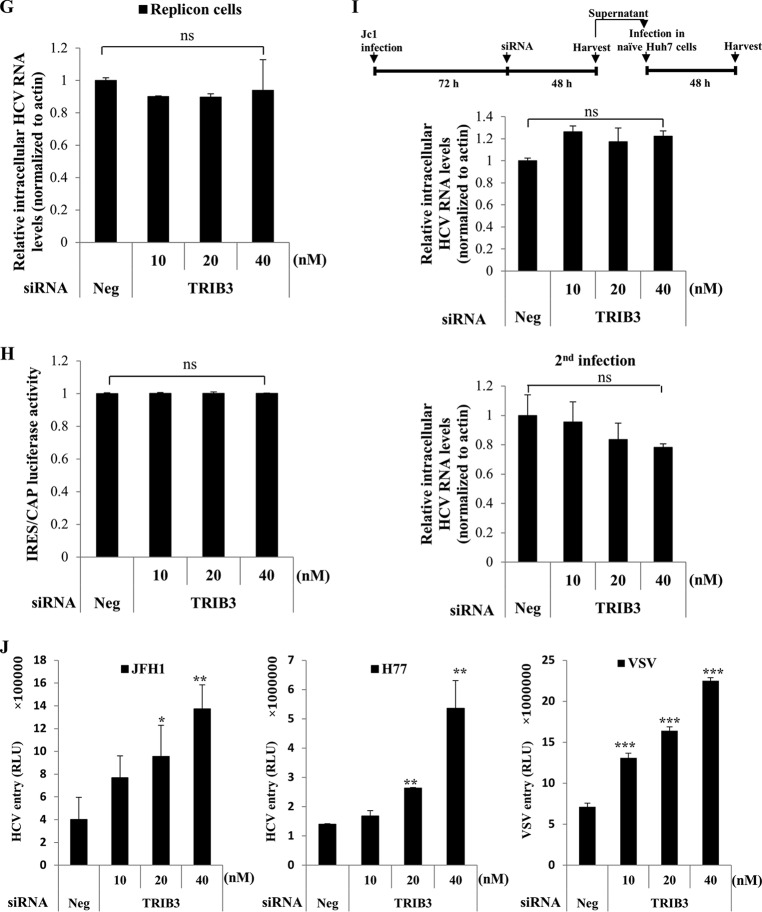
TRIB3 negatively regulates HCV propagation. (A, top) Schematic illustration of the experimental design. (Middle) Huh7 cells were transfected with 20 nM the indicated siRNAs and then infected with Jc1 48 h after transfection. At 2 days postinfection, both intracellular TRIB3 and HCV RNA levels were analyzed by qRT-PCR. (Bottom) Using the same cell lysates, protein levels were determined by immunoblot analysis with the indicated antibodies. Band intensities of normalized TRIB3 and HCV proteins were analyzed by using ImageJ. (B) Huh7 cells were transfected with either negative-control siRNA or increasing concentrations of TRIB3-specific siRNAs for 48 h and then infected with Jc1 for 48 h. Both intracellular TRIB3 and HCV RNA levels were analyzed by qRT-PCR. (C, top) Schematic illustration of the experimental design. Huh7 cells were transfected with either the vector or Flag-tagged TRIB3 for 24 h, followed by Jc1 infection. (Bottom) At 2 days postinfection, the intracellular HCV RNA level was analyzed by qRT-PCR (left), and protein levels were determined by immunoblot analysis using the indicated antibodies (right). Band intensities of normalized HCV proteins were analyzed by using ImageJ. (D) Huh7 cells were transfected with either the vector or increasing amounts of Flag-tagged TRIB3 for 24 h and then infected with Jc1. At 48 h postinfection, intracellular HCV RNA levels were determined by qRT-PCR. (E) Huh7 cells were transfected with 20 nM negative-control or TRIB3-specific siRNAs. Ninety-six hours after transfection, cell viability was determined by a WST assay. (F, top) Schematic diagram of plasmid constructs. UTR, untranslated region. (Middle) Schematic illustration of the experimental design. (Bottom) Huh7 cells were transfected with 20 nM the indicated siRNAs. Twenty-four hours after transfection, cells were further transfected with either the empty vector, wild-type (WT) TRIB3, or an Akt binding-defective TRIB3 mutant (ΔAkt mutant TRIB3) plasmid, respectively, for 24 h, followed by Jc1 infection. At 48 h postinfection, intracellular HCV RNA levels were analyzed by qRT-PCR. The asterisks indicate significant differences (**, P < 0.01). Experiments were performed in triplicate. (G) HCV subgenomic replicon cells were transfected with either negative-control siRNA or increasing amounts of TRIB3-specific siRNAs for 72 h. Intracellular HCV RNA levels were analyzed by qRT-PCR. (H) Huh7 cells were transfected with the indicated siRNAs. Forty-eight hours after transfection, cells were further cotransfected with the pRL-HL dual-reporter plasmid and the pCH110 β-galactosidase plasmid. Forty-eight hours after transfection, relative luciferase activities were determined. (I, top) Schematic illustration of the experimental design. (Middle) Huh7 cells were infected with Jc1 for 3 days and then transfected with the indicated siRNAs for 48 h. Intracellular HCV RNA levels were analyzed by qRT-PCR. (Bottom) Naive Huh7 cells were infected with virus-containing culture supernatants harvested from the cells described above. Intracellular HCV RNA levels were determined by qRT-PCR. (J) Huh7 cells were transfected with the indicated siRNAs for 48 h. Cells were then infected with either HCVpp derived from genotype 2a (JFH1) or genotype 1a (H77) or VSVpp. At 48 h postinfection, cells were harvested, and viral entry was determined by luciferase activity. The asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Experiments were performed in triplicate. Neg denotes universal control siRNA. RLU, relative light units.
TRIB3 is not involved in replication, translation, and virion production steps in the HCV life cycle.
To gain insight into the functional role of TRIB3 in the HCV life cycle, we determined which step of the HCV life cycle was regulated by TRIB3. As shown in Fig. 3G, silencing of TRIB3 displayed no effect on the HCV RNA level in HCV subgenomic replicon cells. We next explored the possible involvement of TRIB3 in HCV internal ribosome entry site (IRES)-mediated translation. For this purpose, Huh7 cells were transfected with either the negative-control siRNA or various concentrations of TRIB3-specific siRNAs and then transfected with pRL-HL and β-galactosidase plasmids as we reported previously (12), and luciferase activity was then determined. We demonstrated that knockdown of TRIB3 showed no effect on HCV IRES-dependent translation (Fig. 3H). To further investigate whether TRIB3 could regulate virion production, Huh7 cells were first infected with Jc1 for 3 days and then transfected with either the negative-control siRNA or various concentrations of TRIB3-specific siRNAs for 48 h, as depicted in the schematic illustration in Fig. 3I (top). Figure 3I (middle) shows that silencing of TRIB3 exerted no discernible effect on the intracellular HCV RNA level. To further confirm this result, naive Huh7 cells were infected with the virus-containing culture supernatant harvested from the above-described cells, and intracellular HCV RNAs were analyzed by qRT-PCR. As shown in Fig. 3I (bottom), silencing of TRIB3 had little effect on viral infectivity. These data suggest that TRIB3 may regulate other steps of the HCV life cycle.
TRIB3 negatively regulates HCV entry.
We therefore asked whether TRIB3 could regulate the entry step of the HCV life cycle. To address this question, Huh7 cells transfected with either the negative-control siRNA or various concentrations of TRIB3-specific siRNAs were infected with HCVpp derived from either JFH1 or H77. VSVpp were used as a control. As shown in Fig. 3J, not only HCVpp entry but also VSVpp entry was significantly increased in TRIB3 knockdown cells, and the increase occurred in a dose-dependent manner. These data suggest that TRIB3 may be a common negative host factor for the entry of various viruses.
The TRIB3-Akt pathway is abrogated in the context of HCV replication.
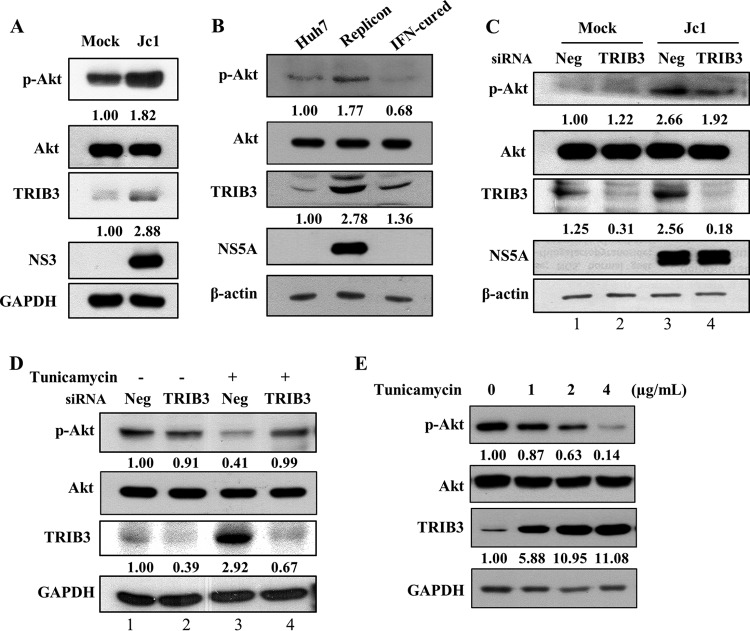
TRIB3 has been shown to suppress Akt phosphorylation under ER stress conditions (8, 9). Since HCV infection upregulated TRIB3 expression under ER stress conditions, we assumed that the p-Akt level should be decreased in HCV-infected cells. To our surprise, the p-Akt level was increased in Jc1-infected cells compared to the level in mock-infected cells (Fig. 4A). Consistently, the p-Akt level was higher in HCV replicon cells than in IFN-cured cells (Fig. 4B). Moreover, siRNA-mediated knockdown of TRIB3 expression displayed no further increase in the p-Akt level in HCV-infected cells (Fig. 4C, lane 3 versus lane 4). We therefore investigated whether the TRIB3-Akt pathway was distorted in Huh7 cells. As shown in Fig. 4D (lane 3), the TRIB3 expression level was increased by tunicamycin-induced ER stress, which in turn inhibited Akt phosphorylation. As expected, the p-Akt level bounced back to the basal level in TRIB3 knockdown cells (Fig. 4D, lane 4), indicating that the TRIB3-Akt pathway works normally in Huh7 cells. We further demonstrated that TRIB3 expression levels were increased by tunicamycin in a dose-dependent manner (Fig. 4E). Consistently, p-Akt protein levels were inversely correlated with TRIB3 protein levels in tunicamycin-treated cells. These data indicate that HCV interrupts the TRIB3-Akt pathway.
FIG 4.
HCV interrupts the TRIB3-Akt pathway. (A) Huh7 cells were either mock infected or infected with Jc1. At 4 days postinfection, protein expression levels were analyzed by immunoblot analysis using the indicated antibodies. Protein band intensities of p-Akt/Akt and TRIB3/GAPDH were analyzed by using ImageJ. (B) Total cellular lysates harvested from Huh7, replicon, and IFN-cured cells were immunoblotted with the indicated antibodies. Protein band intensities of p-Akt/Akt and TRIB3/β-actin were analyzed by using ImageJ. (C) Huh7 cells were either mock infected or infected with Jc1. At 4 days postinfection, cells were further transfected with a negative-control siRNA (Neg) or a TRIB3-specific siRNA. Forty-eight hours after transfection, protein expression levels were analyzed by immunoblotting with the indicated antibodies. Protein band intensities of p-Akt/Akt and TRIB3/β-actin were determined by using ImageJ. (D) Huh7 cells were transfected with 20 nM either the negative-control siRNA or TRIB3-specific siRNA. Forty-eight hours after transfection, cells were treated with either dimethyl sulfoxide (−) or 2 μg/ml of tunicamycin. Twenty-four hours after treatment, total cell lysates were immunoblotted with the indicated antibodies. Protein band intensities of p-Akt/Akt and TRIB3/GAPDH were analyzed by using ImageJ. (E) Huh7 cells were treated with either dimethyl sulfoxide (vehicle) or increasing amounts of tunicamycin for 18 h. Total cell lysates were immunoblotted with the indicated antibodies. Protein band intensities of p-Akt/Akt and TRIB3/GAPDH were analyzed by using ImageJ.
TRIB3 interacts with the protease domain of NS3.
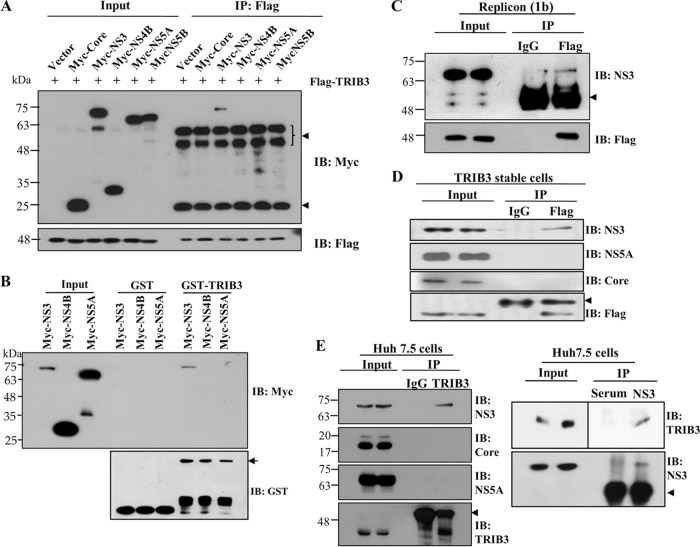
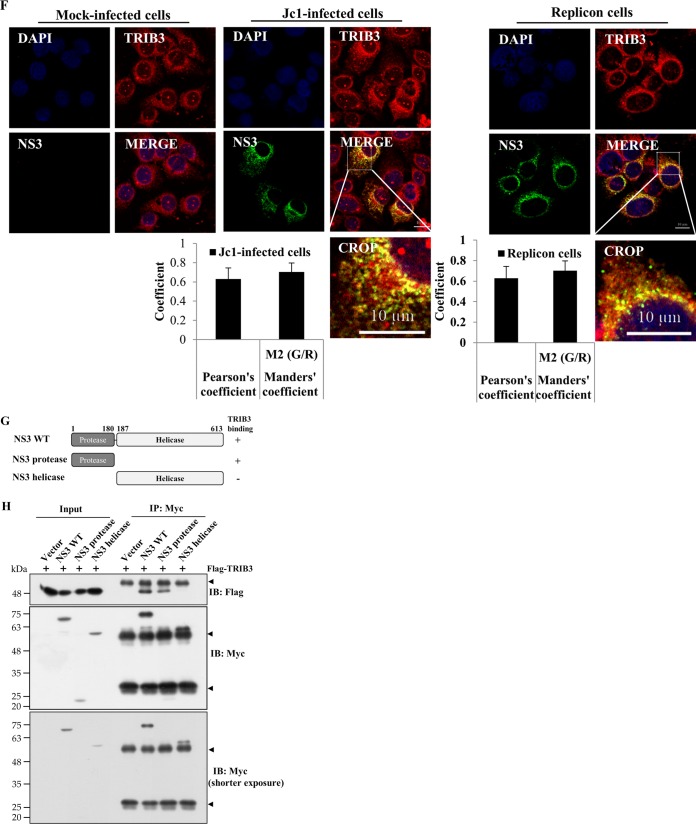
Since the cellular TRIB3-Akt pathway was disturbed by HCV, we hypothesized that this disturbance might be caused by a protein interaction between TRIB3 and viral proteins. For this purpose, HEK293T cells were cotransfected with Flag-tagged TRIB3 and each Myc-tagged viral protein expression plasmid. Protein interactions were determined by a coimmunoprecipitation assay. Figure 5A shows that TRIB3 interacted with NS3 but not with other viral proteins. We also verified that TRIB3 specifically interacted with NS3 in a GST pulldown assay (Fig. 5B). To further demonstrate this interaction, Huh7 cells harboring the HCV replicon derived from genotype 1b were transfected with Flag-tagged TRIB3, and total cell lysates were immunoprecipitated with either control IgG or an anti-Flag antibody. Bound protein was immunoblotted with an anti-NS3 antibody. As shown in Fig. 5C, TRIB3 specifically interacted with NS3. Using TRIB3 stable cells electroporated with in vitro-transcribed Jc1 RNA, we further demonstrated that NS3, but not other viral proteins, interacted with TRIB3 (Fig. 5D). Next, we investigated whether endogenous TRIB3 interacted with NS3. Cell lysates harvested 4 days after HCV RNA electroporation were immunoprecipitated with either control IgG or an anti-TRIB3 antibody, and bound protein was analyzed by immunoblotting with an anti-NS3 antibody. As shown in Fig. 5E (left), endogenous TRIB3 interacted with NS3 but not with core or NS5A. Reciprocally, the same cell lysates were immunoprecipitated with an anti-NS3 antibody, and bound protein was then detected by immunoblotting with an anti-TRIB3 antibody. We confirmed that endogenous TRIB3 interacted with HCV NS3 (Fig. 5E, right). These data suggest that TRIB3 might colocalize with NS3 in HCV-infected cells. To investigate this possibility, Huh7 cells were either mock infected or infected with Jc1, and an immunofluorescence assay was performed. Figure 5F (left) shows that TRIB3 and NS3 were colocalized in the cytoplasm of Jc1-infected cells, as indicated by yellow fluorescence. We also demonstrated the colocalization of TRIB3 and NS3 using HCV replicon cells (Fig. 5F, right). We further confirmed the colocalization of TRIB3 and NS3 by determining both Pearson's and Manders' coefficients using ImageJ (NIH) with the JACoP plug-in. These data clearly indicate that TRIB3 specifically interacts with HCV NS3 in vitro. To determine the region in NS3 responsible for TRIB3 binding, we constructed both protease- and helicase-truncated NS3 mutants (Fig. 5G), and interactions were determined by a transfection-based coimmunoprecipitation assay. As shown in Fig. 5H, TRIB3 interacted with both wild-type NS3 and the protease domain of NS3 but not with the helicase domain of NS3, indicating that the protease domain of NS3 was responsible for binding with TRIB3. Collectively, our data demonstrated that TRIB3 interacted with the protease domain of NS3.
FIG 5.
TRIB3 interacts with the protease domain of NS3. (A) HEK293T cells were cotransfected with Flag-tagged TRIB3 and the vector, Myc-tagged core, NS3, NS4B, NS5A, and NS5B, individually. Twenty-four hours after cotransfection, total cell lysates were immunoprecipitated (IP) with an anti-Flag antibody, and bound proteins were then analyzed by immunoblot (IB) analysis using an anti-Myc antibody. The arrowheads denote IgG. (B) HEK293T cells were transfected with Myc-tagged NS3, NS4B, and NS5A plasmids. Twenty-four hours after transfection, total cell lysates were harvested and incubated with either purified GST- or GST-TRIB3-conjugated glutathione beads. (Top) Bound proteins were detected by immunoblot analysis with an anti-Myc antibody. (Bottom) Protein expression levels of GST and GST-TRIB3 were verified by using an anti-GST antibody. The arrow indicates GST-TRIB3. (C) Huh7 cells harboring the HCV subgenomic replicon were transfected with a Flag-tagged TRIB3 expression plasmid. Thirty-six hours after transfection, total cellular extracts were immunoprecipitated with either mouse control IgG or mouse anti-Flag antibody. Bound proteins were analyzed by immunoblotting with an anti-NS3 antibody. The arrowhead indicates IgG. (D) Huh7 cells stably expressing the TRIB3 protein were electroporated with in vitro-transcribed Jc1 RNA. Four days after electroporation, total cell lysates were immunoprecipitated with either mouse control IgG or mouse anti-Flag antibody. Bound proteins were analyzed by immunoblot analysis using the indicated antibodies. The arrowhead denotes IgG. (E, left) Huh7.5 cells were electroporated with in vitro-transcribed HCV Jc1 RNA. Four days after electroporation, total cell lysates were immunoprecipitated with either mouse IgG or an anti-TRIB3 antibody, and bound proteins were then immunoblotted with the indicated antibodies. (Right) The same cell lysates were immunoprecipitated with either rabbit control serum or rabbit anti-HCV NS3 serum, and bound protein was then immunoblotted with an anti-TRIB3 antibody. The arrowheads denote IgG. (F, left) Huh7 cells seeded onto coverslips were either mock infected or infected with Jc1. At 4 days postinfection, cells were fixed in cold methanol at −20°C for 5 min, and immunofluorescence staining was performed by using an anti-TRIB3 monoclonal antibody and TRITC-conjugated goat anti-mouse IgG to detect TRIB3 (red) and a rabbit anti-NS3 antibody and FITC-conjugated goat anti-rabbit IgG to detect NS3 (green). (Right) Huh7 cells harboring the HCV subgenomic replicon were seeded onto coverslips and fixed in cold methanol at −20°C for 5 min. Immunofluorescence staining was performed as described above. Dual staining shows colocalization of TRIB3 and NS3 as yellow fluorescence in the merged images. Cells were counterstained with DAPI to label nuclei (blue). The enlarged selection marked by a white square is shown as a crop image. Colocalization of TRIB3 and HCV NS3 was verified by both Pearson's and Manders' overlap coefficients. More than 10 cells were applied to ImageJ analysis for quantification of the overlap coefficient, and error bars indicate the standard deviations of the means. G/R, ratio of green and red fluorophore colocalization. (G) Schematic illustration of the domain structure of the NS3 protein. (H, top) HEK293T cells were cotransfected with Flag-tagged TRIB3 and various constructs of Myc-tagged NS3. Twenty-four hours after transfection, cell lysates were immunoprecipitated with an anti-Myc antibody, and bound proteins were immunoblotted by using an anti-Flag antibody. (Middle and bottom) Immunoprecipitation efficiency was verified by immunoblot analysis using the same cell lysates with an anti-Myc antibody. The arrowheads denote IgG.
HCV NS3 disrupts the TRIB3-Akt association.
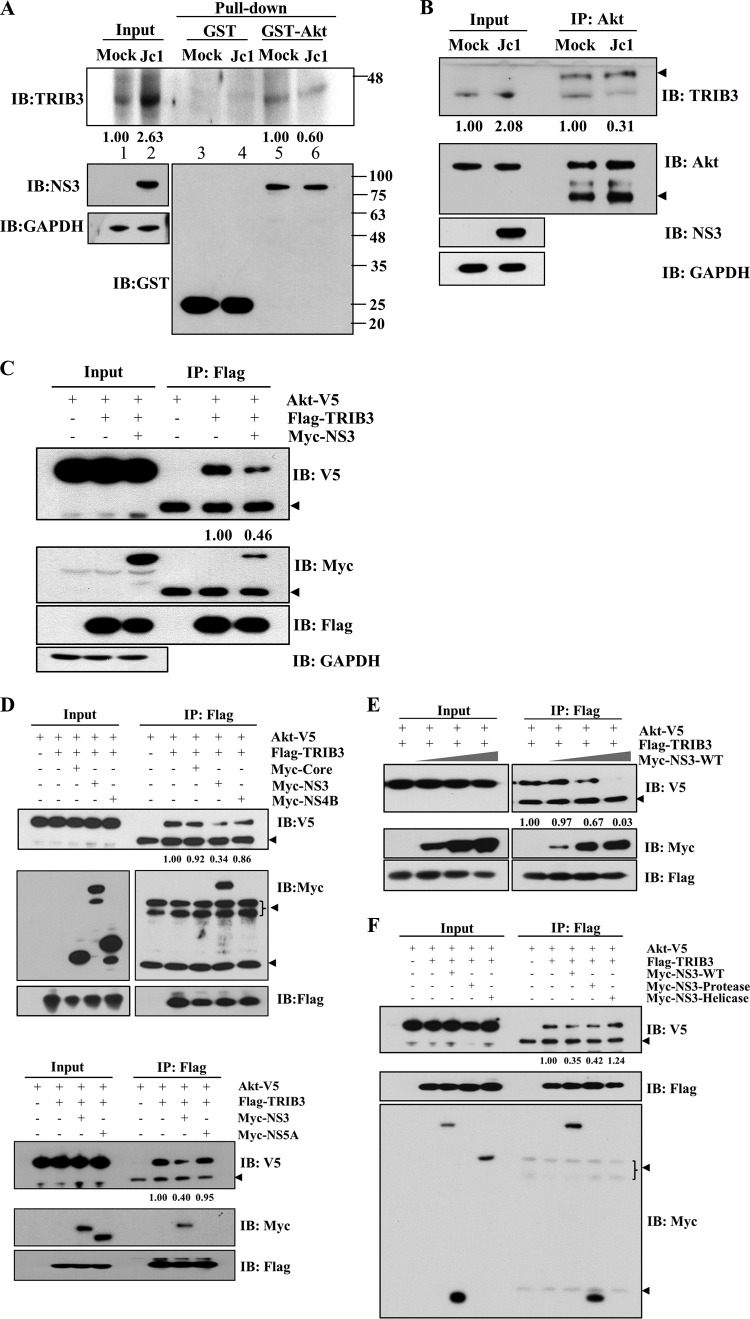
Although TRIB3 expression was upregulated by HCV, Akt phosphorylation was not suppressed in both HCV subgenomic replicon cells and HCV-infected cells. This suggests that HCV may interrupt protein interactions between TRIB3 and Akt. To explore this possibility, GST pulldown assays were performed by using either a GST control or GST-Akt incubated with cell lysates harvested from either mock- or Jc1-infected cells. As shown in Fig. 6A, The amount of TRIB3 protein pulled down by GST-Akt was considerably reduced in Jc1-infected cells compared with mock-infected cells. Because the protein level of TRIB3 was highly increased in Jc1-infected cells, the decreased level of TRIB3 protein pulled down by GST-Akt was remarkably high compared with input TRIB3 (Fig. 6A, lane 6 versus lane 2). We further verified that endogenous protein interactions between TRIB3 and Akt were markedly reduced in HCV-infected cells compared to mock-infected cells (Fig. 6B). Since NS3 interacted with TRIB3, we investigated whether the decrease of TRIB3-Akt binding was caused by NS3. For this purpose, HEK293T cells were cotransfected with Flag-tagged TRIB3 and V5-tagged Akt plasmids in the absence or presence of Myc-tagged NS3. For immunoprecipitation assays, total amounts of DNA were adjusted by adding an empty vector. Cell lysates harvested 36 h after transfection were immunoprecipitated with an anti-Flag antibody, and bound proteins were analyzed by immunoblot analysis. Indeed, the protein interaction between TRIB3 and Akt was prominently reduced by NS3 (Fig. 6C). We also showed that NS3 specifically disrupted the protein interaction between TRIB3 and Akt, whereas neither core, NS4B, nor NS5A could interrupt the protein interaction (Fig. 6D). We further verified that the protein interaction between TRIB3 and Akt was decreased by NS3 in a dose-dependent manner (Fig. 6E). To analyze precisely which domain of NS3 inhibited the protein interaction between TRIB3 and Akt, HEK293T cells were cotransfected with V5-tagged Akt and Flag-tagged TRIB3 using various constructs of NS3. Total cell lysates were immunoprecipitated with an anti-Flag antibody, and bound proteins were analyzed by immunoblot analysis using an anti-V5 antibody. Figure 6F shows that both wild-type NS3 and the protease domain of NS3 but not the helicase domain of NS3 suppressed the interaction between TRIB3 and Akt. Collectively, these data suggest that HCV disturbs the TRIB3-Akt association through the NS3 protein, and thus, the inhibitory effect of TRIB3 on the Akt signaling cascade may be impaired in HCV-replicating cells.
FIG 6.
HCV NS3 interrupts the interaction between TRIB3 and Akt. (A) Huh7 cells were either mock infected or infected with Jc1. At 6 days postinfection, total cell lysates were harvested and incubated with either GST- or GST-Akt-conjugated glutathione beads. Bound proteins were analyzed by immunoblot analysis using an anti-TRIB3 antibody. Protein band intensities of input TRIB3/GAPDH (lanes 1 and 2) and pulled-down TRIB3 normalized by Jc1/mock (lane 6 versus lane 5) were analyzed by using ImageJ. (B) Huh7 cells were either mock infected or infected with Jc1. At 6 days postinfection, total cell lysates were immunoprecipitated with an anti-Akt antibody, and bound protein was then immunoblotted with an anti-TRIB3 antibody. The arrowheads indicate IgG. Protein band intensities of input TRIB3/GAPDH (lanes 1 and 2) and immunoprecipitated TRIB3 normalized by Jc1/mock (lane 4 versus lane 3) were determined by using ImageJ. (C) HEK293T cells were cotransfected with V5-tagged Akt and vector- or Flag-tagged TRIB3 in the absence or presence of Myc-tagged NS3. Thirty-six hours after transfection, cell lysates were immunoprecipitated with an anti-Flag antibody, and bound proteins were analyzed by immunoblot analysis using either an anti-V5 or an anti-Myc antibody. The arrowheads denote IgG. Protein band intensities of normalized Akt-V5 were analyzed by using ImageJ. (D, top) HEK293T cells were cotransfected with V5-tagged Akt and Flag-tagged TRIB3 together with Myc-tagged core, NS3, and NS4B. Twenty-four hours after transfection, total cell lysates were immunoprecipitated with an anti-Flag antibody, and bound proteins were analyzed by immunoblot analysis using either an anti-V5 antibody or an anti-Myc antibody. The arrowheads indicate IgG. (Bottom) HEK293T cells were cotransfected with V5-tagged Akt and Flag-tagged TRIB3 together with Myc-tagged NS3 and NS5A. Total cell lysates were immunoprecipitated as described above. The arrowhead indicates IgG. Protein band intensities of normalized Akt-V5 were analyzed by using ImageJ. (E) HEK293T cells were cotransfected with Flag-tagged TRIB3 and V5-tagged Akt with increasing amounts (1, 3, and 5 μg) of Myc-tagged NS3. Total cell lysates harvested 36 h after cotransfection were immunoprecipitated with an anti-Flag antibody, and bound proteins were analyzed by immunoblot analysis using either an anti-V5 or an anti-Myc antibody. The arrowhead denotes IgG. Protein band intensities of normalized Akt-V5 were analyzed by using ImageJ. (F) HEK293T cells were cotransfected with V5-tagged Akt and Flag-tagged TRIB3 in the absence or presence of various mutant NS3 constructs. Total cell lysates harvested 36 h after cotransfection were immunoprecipitated with an anti-Flag antibody, and bound protein was analyzed by immunoblot analysis using an anti-V5 antibody. The arrowheads indicate IgG. Protein band intensities of normalized Akt-V5 were analyzed by using ImageJ.
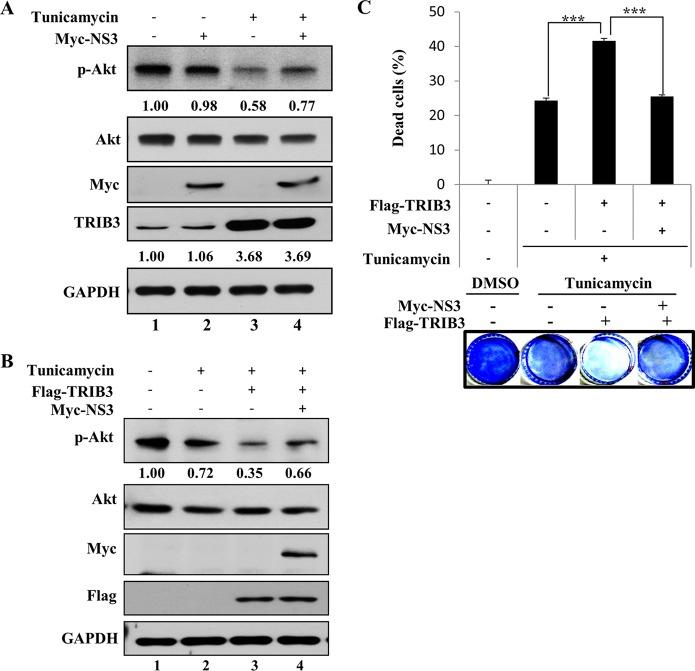
HCV NS3 attenuates the inhibitory activity of TRIB3 on Akt phosphorylation.
As TRIB3 exerts inhibitory activity on Akt phosphorylation under ER stress, we next investigated whether NS3 could inhibit the TRIB3-Akt signaling cascade. As shown in Fig. 7A (lane 3), treatment with tunicamycin upregulated TRIB3 expression, which led to inhibition of Akt phosphorylation. However, the inhibitory activity of TRIB3 on Akt phosphorylation was impaired by NS3 (Fig. 7A, lane 4). Consistent with data from previous reports (8, 9), overexpression of TRIB3 inhibited Akt phosphorylation to a greater extent in tunicamycin-treated cells (Fig. 7B, lane 3 versus lane 2). However, this inhibitory effect of TRIB3 on Akt activity was blocked by NS3 (Fig. 7B, lane 4), providing further evidence that the inhibitory activity of TRIB3 on Akt phosphorylation was attenuated by NS3. Since the inhibition of Akt signaling under conditions of ER stress causes cell apoptosis (8), we then investigated whether NS3 might interrupt TRIB3 function in ER stress-mediated cell death. For this purpose, Huh7 cells were transfected with either the vector or Flag-tagged TRIB3 in the absence or presence of Myc-tagged NS3. Cells were either left untreated or treated with tunicamycin, and cell death was then analyzed by a crystal violet staining assay. As shown in Fig. 7C, the percentage of dead cells was significantly increased by TRIB3 with tunicamycin treatment. It was noteworthy that the percentage of dead cells was substantially decreased by NS3 under the same conditions. Collectively, these data suggest that HCV may disturb the TRIB3-Akt signaling cascade to maintain host cell proliferation.
FIG 7.
HCV NS3 attenuates the inhibitory effect of TRIB3 on Akt phosphorylation and prevents ER stress-mediated apoptosis. (A) Huh7 cells were transfected with either vector- or Myc-tagged NS3. Twenty-four hours after transfection, cells were treated with either dimethyl sulfoxide (DMSO) (−) or 2 μg/ml of tunicamycin. Eighteen hours after treatment, total cell lysates were immunoblotted with the indicated antibodies. Protein band intensities of p-Akt/Akt and TRIB3/GAPDH were analyzed by using ImageJ. (B) Huh7 cells were transfected with either the vector or Flag-tagged TRIB3 in the absence or presence of Myc-tagged NS3. Twenty-four hours after transfection, cells were treated with either dimethyl sulfoxide (−) or tunicamycin, as indicated. Eighteen hours after treatment, total cell lysates were immunoblotted with the indicated antibodies. Protein band intensities of p-Akt/Akt were determined by using ImageJ. (C) Huh7 cells were transfected with Flag-tagged TRIB3 in the absence or presence of Myc-tagged NS3. Twenty-four hours after transfection, cells were either left untreated or treated with tunicamycin. Forty-eight hours after treatment, the percentage of adherent cells was measured by staining with crystal violet, and the percentage of dead cells was then determined.
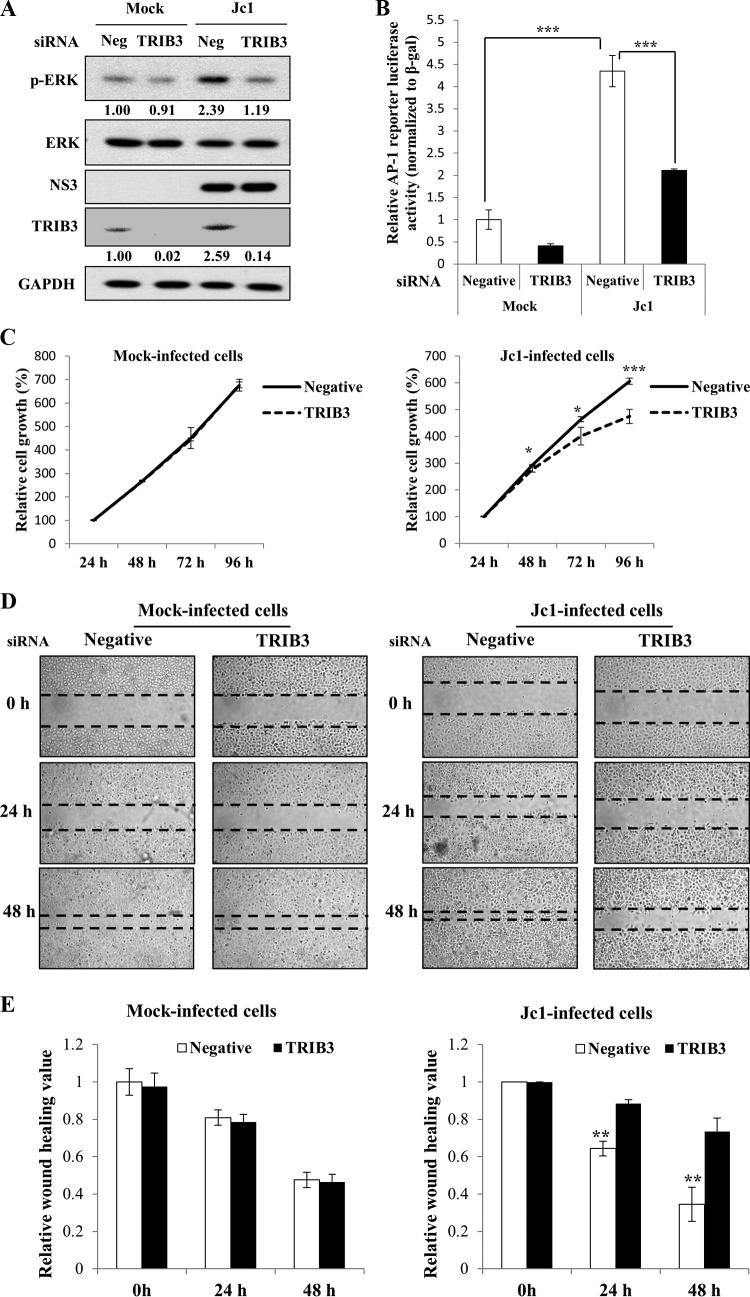
HCV regulates TRIB3 to promote cell proliferation and migration.
TRIB3 has also been implicated in the regulation of the ERK signaling pathway (10). To gain more insight into the role of TRIB3 in HCV-infected cells, Huh7 cells were either mock infected or infected with Jc1 and further transfected with either negative-control or TRIB3 siRNA, and ERK phosphorylation was analyzed by immunoblot analysis. As shown in Fig. 8A, the ERK phosphorylation level was markedly elevated in Jc1-infected cells. However, the ERK phosphorylation level was reduced to the basal level in TRIB3 knockdown cells. Using an activator protein 1 (AP-1) luciferase reporter construct containing three canonical AP-1 binding sites, we further investigated downstream AP-1 transcription factor activity (21). We showed that AP-1 reporter activity was increased 4-fold in Jc1-infected cells compared to mock-infected cells (Fig. 8B). Similarly to ERK phosphorylation, HCV-induced AP-1 transcriptional activity was impaired in TRIB3 knockdown cells, suggesting that HCV may regulate TRIB3 to ensure the cell survival signaling pathway. A cell proliferation assay further showed that silencing of TRIB3 significantly reduced cell growth in HCV-infected cells but not in mock-infected cells (Fig. 8C), implying that HCV appropriated host TRIB3 to maintain cell growth. To corroborate this finding, we performed a wound-healing assay. Huh7 cells were either mock infected or infected with Jc1 and then transfected with either negative-control or TRIB3-specific siRNA. Thirty-six hours after siRNA transfection, cells were serum starved, and a wound-healing assay was then performed by scratching across the cell monolayer, as previously reported (22). As shown in Fig. 8D and E, silencing of TRIB3 significantly impaired cell migration in HCV-infected cells compared to mock-infected cells. These data suggest that HCV may modulate TRIB3 to promote cell proliferation for ensuring persistent viral infection.
FIG 8.
HCV regulates TRIB3 to promote cell proliferation and migration. (A) Huh7 cells were either mock infected or infected with Jc1. At 4 days postinfection, cells were transfected with either negative-control siRNA or TRIB3-specific siRNA. Seventy-two hours after transfection, total cell lysates were immunoblotted with the indicated antibodies. Protein band intensities of p-ERK/ERK and TRIB3/GAPDH were analyzed by using ImageJ. (B) Huh7 cells were either mock infected or infected with Jc1. At 4 days postinfection, cells were transfected with either negative-control siRNA or TRIB3-specific siRNA. Twenty-four hours after transfection, cells were further transfected with an AP-1 luciferase reporter plasmid and the pCH110 reference plasmid. Forty-eight hours after plasmid transfection, luciferase reporter activity was determined. (C) Huh7 cells were either mock infected or infected with Jc1. At 4 days postinfection, cells were transfected with either negative-control siRNA or TRIB3-specific siRNA. Cells were then replated onto 96-well plates. At the indicated time intervals after siRNA transfection, WST assays were performed to determine cell growth. (D) Huh7 cells were either mock infected or infected with Jc1. At 4 days postinfection, cells were transfected with either negative-control siRNA or TRIB3-specific siRNA. Thirty-six hours after siRNA transfection, cells were serum starved overnight. A wound-healing assay was performed by scratching across monolayer cells by using a sterile 200-μl pipette tip. Cells were then washed twice with PBS and cultured in DMEM containing 10% FBS. Images were captured by using phase-contrast microscopy at 24-h intervals. (E) Relative wounds measured from three independent experiments are plotted in a bar diagram. The asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
DISCUSSION
By transcriptome analysis using RNA-Seq technology, we identified TRIB3, which was highly expressed in HCVcc-infected cells. TRIB3 is a mammalian homolog of Drosophila tribbles, which can be induced under ER stress. Despite the important role that TRIB3 plays in regulating cell fate under various conditions (23, 24), very little is known about the involvement of TRIB3 in viral infection. Here, we demonstrated that both mRNA and protein expressions of TRIB3 were upregulated by HCV. We further showed that TRIB3 promoter activity was upregulated by HCV-induced ER stress. Using an ER stress inhibitor, 4-PBA, we verified that the upregulation of TRIB3 promoter activity in HCV-infected cells was mediated by ER stress. It was previously reported that HCV also upregulates the expression of protein phosphatase 2A (PP2A) (25), wild-type peroxisome proliferator-activated receptor gamma coactivator 1 alpha (WT-PGC-1α), and liver-specific PGC-1α (L-PGC-1α) (26) via ER stress. The ERSE region of the TRIB3 promoter contains binding sites for certain transcription factors, including CHOP and ATF4 (17). Previous studies have shown that HCV infection upregulates the expression of CHOP and ATF4 (27, 28). CHOP was activated and translocated to the nuclei in HCV-infected cells (27). In our primary screening, the CHOP mRNA level but not the ATF4 mRNA level was increased 2-fold in HCV-infected cells (data not shown). Consistently, the CHOP mRNA level was also increased 2-fold in HCV replicon cells (genotype 1b) compared to IFN-cured cells (data not shown). It is thus possible that HCV may upregulate TRIB3 expression via the transcription factor CHOP.
ER stress-induced apoptosis has proven to be a host response to eliminate virus-infected cells (29). However, HCV has evolved mechanisms to evade the host response to establish persistent infection (30). TRIB3 is a key mediator in ER stress-mediated apoptosis (8, 9, 31), and thus, HCV could escape from apoptotic signals by modulating TRIB3. Indeed, TRIB3 expression was upregulated by HCV-induced ER stress, which led to the inhibition of HCV propagation. However, we demonstrated that HCV has evolved a strategy to manipulate this inhibitory function of TRIB3 by interrupting the TRIB3-Akt pathway through the NS3 protein. To our knowledge, this is the first study demonstrating that HCV modulates TRIB3 to evade the host cell's apoptotic response.
We then assessed the functional role of TRIB3 in HCV propagation. To our surprise, both HCV RNA and HCV protein levels were significantly increased in TRIB3 knockdown cells. Moreover, transient expression of TRIB3 led to significant decreases of HCV RNA and protein levels, implying that TRIB3 negatively regulates HCV propagation. TRIB3 has been shown to interact with Akt and inhibit Akt signaling (7–9, 32). Using an Akt binding-defective TRIB3 mutant, we demonstrated that the negative effect of TRIB3 on HCV propagation was mediated through the Akt signaling pathway. Using an HCVpp assay, we further showed that TRIB3 acted as a negative host factor in the entry step of the HCV life cycle. We also demonstrated that VSV entry was also negatively regulated by TRIB3. In this regard, further studies may be needed to determine whether TRIB3 functions as a common negative host factor for the entry of other viruses. Since TRIB3 expression was upregulated in HCV-replicating cells, we initially hypothesized that the p-Akt level should be decreased by HCV infection. Nevertheless, the p-Akt level was increased in both HCV replicon cells and HCV-infected cells. We further showed that the siRNA-mediated knockdown of TRIB3 expression displayed no increase in p-Akt levels in Jc1-infected cells, suggesting that HCV might manipulate the TRIB3-Akt pathway. To test this hypothesis, we assayed the protein interaction between HCV and TRIB3. Indeed, we demonstrated that TRIB3 interacted with NS3 by both in vitro pulldown and coimmunoprecipitation assays. We further showed that endogenous TRIB3 specifically interacted with NS3 in HCV-infected cells. The protein interaction between NS3 and TRIB3 was mediated through the protease domain of NS3. We verified that endogenous TRIB3 colocalized with NS3 in the cytoplasm of HCV replicon cells and Jc1-infected cells. All these data suggest that HCV may manipulate the TRIB3-Akt pathway via NS3 to facilitate persistent viral infection. As expected, the protein interaction between TRIB3 and Akt was interrupted by NS3 in a dose-dependent manner. We further demonstrated that this inhibition was mediated through the protease domain of NS3, further validating the functional significance of the protein interaction between NS3 and TRIB3. These data indicate that HCV disrupts the TRIB3-Akt association via the NS3 protein, and thereby, the TRIB3-Akt signaling pathway is impaired in HCV-replicating cells. Consequently, TRIB3 is no longer able to suppress Akt phosphorylation in HCV-infected cells.
Accumulating evidence shows that HCV modulates Akt signaling to support viral propagation and to inhibit the cell death response (19, 20, 33–35). In these reports, it was shown that HCV activates the phosphatidylinositol 3-kinase (PI3K)–Akt pathway to facilitate viral entry, replication, and RNA translation (19, 20, 35). In fact, HCV has evolved to develop multiple strategies to maintain Akt activation. HCV NS5A downregulates phosphatase and tensin homolog (PTEN) expression to relieve the inhibitory effect of PTEN on the PI3K-Akt pathway (33). In addition, HCV NS3/4A cleaves T cell protein tyrosine phosphatase (TC-PTP) to enhance Akt activity (34). In the present study, we further showed that HCV NS3 interacted with TRIB3 to dissociate Akt from TRIB3, verifying that HCV counteracts a key negative regulator of the Akt signaling pathway to maintain persistent viral propagation. Moreover, NS3 attenuated the effect of TRIB3 on ER stress-mediated cell death.
Besides Akt signaling, TRIB3 is also involved in ERK signaling to regulate cell proliferation and migration (10, 24). In this respect, viral modulation of TRIB3 might contribute to the malignant phenotype of HCV-infected liver cells. It was previously reported that HCV NS3 induces invasive activity of cancer cells via regulation of MAPK-ERK signaling (36). Furthermore, HCV NS3 associates with p53 and suppresses p53 transcriptional activity to exert oncogenic potential (37, 38). By analyzing both the ERK phosphorylation level and AP-1 reporter activity, we further show that HCV manipulates TRIB3 to ensure the cell survival signaling pathway. All these data indicate that HCV modulates TRIB3 signaling pathways to establish persistent viral infection.
ACKNOWLEDGMENTS
We thank Charles Rice (The Rockefeller University) for Huh7.5 cells and Ralf Bartenschlager (University of Heidelberg) for the Jc1 construct.
We have no conflicts of interest.
REFERENCES
- 1.Scheel TK, Rice CM. 2013. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med 19:837–849. doi: 10.1038/nm.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindenbach BD, Rice CM. 2005. Unravelling hepatitis C virus replication from genome to function. Nature 436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 3.Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. 2005. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein of the mitochondria to evade innate immunity. Proc Natl Acad Sci U S A 102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B, Li X, Li Y, Guo H, Sun SY, He QQ, Wang Y, Luo J, Wen JF, Zheng H, Feng DY. 2010. The effects of hepatitis C virus non-structural protein 3 on cell growth mediated by extracellular signal-related kinase cascades in human hepatocytes in vitro. Int J Mol Med 26:273–279. [PubMed] [Google Scholar]
- 5.Than TT, Tran GV, Son K, Park EM, Kim S, Lim YS, Hwang SB. 2016. Ankyrin repeat domain 1 is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry. Sci Rep 6:20819. doi: 10.1038/srep20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohan F, Keeshan K. 2013. The functionally diverse roles of tribbles. Biochem Soc Trans 41:1096–1100. doi: 10.1042/BST20130105. [DOI] [PubMed] [Google Scholar]
- 7.Du K, Herzig S, Kulkarni RN, Montminy M. 2003. TRB3: a tribbles homolog that inhibits AKT/PKB activation by insulin in liver. Science 300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 8.Yan W, Wang Y, Xiao Y, Wen J, Wu J, Du L, Cai W. 2014. Palmitate induces TRB3 expression and promotes apoptosis in human liver cells. Cell Physiol Biochem 33:823–834. doi: 10.1159/000358655. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Wen HJ, Guo ZM, Zeng MS, Li MZ, Jiang YE, He XG, Sun CZ. 2011. TRB3 overexpression due to endoplasmic reticulum stress inhibits AKT kinase activation of tongue squamous cell carcinoma. Oral Oncol 47:934–939. doi: 10.1016/j.oraloncology.2011.06.512. [DOI] [PubMed] [Google Scholar]
- 10.Izrailit J, Berman HK, Datti A, Wrana JL, Reedijk M. 2013. High throughput kinase inhibitor screens reveal TRB3 and MAPK-ERK/TGFβ pathways as fundamental Notch regulators in breast cancer. Proc Natl Acad Sci U S A 110:1714–1719. doi: 10.1073/pnas.1214014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen LN, Lim YS, Pham LV, Shin HY, Kim YS, Hwang SB. 2014. Stearoyl coenzyme A desaturase 1 is associated with hepatitis C virus replication complex and regulates viral replication. J Virol 88:12311–12325. doi: 10.1128/JVI.01678-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngo HT, Pham LV, Kim JW, Lim YS, Hwang SB. 2013. Modulation of mitogen-activated protein kinase-activated protein kinase 3 by hepatitis C virus core protein. J Virol 87:5718–5731. doi: 10.1128/JVI.03353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim YS, Hwang SB. 2011. Hepatitis C virus NS5A protein interacts with phosphatidylinositol 4-kinase type III and regulates viral propagation. J Biol Chem 286:11290–11298. doi: 10.1074/jbc.M110.194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim YS, Tran HT, Park SJ, Yim SA, Hwang SB. 2011. Peptidyl-prolyl isomerase Pin1 is a cellular factor required for hepatitis C virus propagation. J Virol 85:8777–8788. doi: 10.1128/JVI.02533-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park C, Min S, Park EM, Lim YS, Kang S, Suzuki T, Shin EC, Hwang SB. 2015. Pim kinase interacts with nonstructural 5A protein and regulates hepatitis C virus entry. J Virol 89:10073–10086. doi: 10.1128/JVI.01707-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H. 2000. Akt/Protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J Biol Chem 275:30934–30942. doi: 10.1074/jbc.M004112200. [DOI] [PubMed] [Google Scholar]
- 17.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. 2005. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K. 2015. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol 61:45–52. doi: 10.1016/j.biocel.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Tian Y, Machida K, Lai MM, Luo G, Foung SK, Ou JH. 2012. Transient activation of the PI3K-AKT pathway by hepatitis C virus to enhance viral entry. J Biol Chem 287:41922–41930. doi: 10.1074/jbc.M112.414789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, Kim KH, Kim HY, Cho HK, Sakamoto N, Cheong J. 2010. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett 584:707–712. doi: 10.1016/j.febslet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung DI, Hahn JH, Kim YM, Park SH, Lee H. 2006. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem 281:34833–34847. doi: 10.1074/jbc.M605483200. [DOI] [PubMed] [Google Scholar]
- 22.Dixit U, Pandey AK, Liu Z, Kumar S, Neiditch MB, Klein KM, Pandey VN. 2015. FUSE binding protein 1 facilitates persistent hepatitis C virus replication in hepatoma cells by regulating tumor suppressor p53. J Virol 89:7905–7921. doi: 10.1128/JVI.00729-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegedus Z, Czibula A, Kiss-Toth E. 2006. Tribbles: novel regulators of cell function; evolutionary aspects. Cell Mol Life Sci 63:1632–1641. doi: 10.1007/s00018-006-6007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoyama T, Nakamura T. 2011. Tribbles in disease: signaling pathways important for cellular function and neoplastic transformation. Cancer Sci 102:1115–1122. doi: 10.1111/j.1349-7006.2011.01914.x. [DOI] [PubMed] [Google Scholar]
- 25.Christen V, Treves S, Duong FH, Heim MH. 2007. Activation of endoplasmic reticulum stress response by hepatitis viruses up-regulates protein phosphatase 2A. Hepatology 46:558–565. doi: 10.1002/hep.21611. [DOI] [PubMed] [Google Scholar]
- 26.Yao W, Cai H, Li X, Li T, Hu L, Peng T. 2014. Endoplasmic reticulum stress links hepatitis C virus RNA replication to wild-type PGC-1α/liver-specific PGC-1α upregulation. J Virol 88:8361–8374. doi: 10.1128/JVI.01202-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merquiol E, Uzi D, Mueller T, Goldenberg D, Nahmias Y, Xavier RJ, Tirosh B, Shibolet O. 2011. HCV causes chronic endoplasmic reticulum stress leading to adaptation and interference with the unfolded protein response. PLoS One 6:e24660. doi: 10.1371/journal.pone.0024660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan SW, Egan PA. 2005. Hepatitis C virus envelope proteins regulate CHOP via induction of the unfolded protein response. FASEB J 19:1510–1512. [DOI] [PubMed] [Google Scholar]
- 29.Barber GN. 2001. Host defense, viruses and apoptosis. Cell Death Differ 8:113–126. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- 30.Fuentes-González AM, Contreras-Paredes A, Manzo-Merino J, Lizano M. 2013. The modulation of apoptosis by oncogenic viruses. Virol J 10:182–199. doi: 10.1186/1743-422X-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang N, Zhang W, Xu S, Lin H, Wang Z, Liu H, Fang Q, Li C, Peng L, Lou J. 2014. TRIB3 alters endoplasmic reticulum stress-induced β-cell apoptosis via the NF-κB pathway. Metabolism 63:822–830. doi: 10.1016/j.metabol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Salazar M, Lorente M, García-Taboada E, Pérez Gómez E, Dávila D, Zúñiga-García P, María Flores J, Rodríguez A, Hegedus Z, Mosén-Ansorena D, Aransay AM, Hernández-Tiedra S, López-Valero I, Quintanilla M, Sánchez C, Iovanna JL, Dusetti N, Guzmán M, Francis SE, Carracedo A, Kiss-Toth E, Velasco G. 2015. Loss of Tribbles pseudokinase-3 promotes Akt-driven tumorigenesis via FOXO inactivation. Cell Death Differ 22:131–144. doi: 10.1038/cdd.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng D, Zhang L, Yang G, Zhao L, Peng F, Tian Y, Xiao X, Chung RT, Gong G. 2015. Hepatitis C virus NS5A drives a PTEN-PI3K/Akt feedback loop to support cell survival. Liver Int 35:1682–1691. doi: 10.1111/liv.12733. [DOI] [PubMed] [Google Scholar]
- 34.Brenndörfer ED, Karthe J, Frelin L, Cebula P, Erhardt A, Schulte am Esch J, Hengel H, Bartenschlager R, Sällberg M, Häussinger D, Bode JG. 2009. Nonstructural 3/4A protease of hepatitis C virus activates epithelial growth factor-induced signal transduction by cleavage of the T-cell protein tyrosine phosphatase. Hepatology 49:1810–1820. doi: 10.1002/hep.22857. [DOI] [PubMed] [Google Scholar]
- 35.Shi Q, Hoffman B, Liu Q. 2016. PI3K-Akt signaling pathway upregulates hepatitis C virus RNA translation through the activation of SREBPs. Virology 490:99–108. doi: 10.1016/j.virol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Lu L, Zhang Q, Wu K, Chen X, Zheng Y, Zhu C, Wu J. 2015. Hepatitis C virus NS3 protein enhances cancer cell invasion by activating matrix metalloproteinase-9 and cyclooxygenase-2 through ERK/p38/NF-κB signal cascade. Cancer Lett 356:470–478. doi: 10.1016/j.canlet.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 37.Deng L, Nagano-Fujii M, Tanaka M, Nomura-Takigawa Y, Ikeda M, Kato N, Sada K, Hotta H. 2006. NS3 protein of hepatitis C virus associates with the tumour suppressor p53 and inhibits its function in an NS3 sequence-dependent manner. J Gen Virol 87:1703–1713. doi: 10.1099/vir.0.81735-0. [DOI] [PubMed] [Google Scholar]
- 38.Kwun HJ, Jung EY, Ahn JY, Lee MN, Jang KL. 2001. p53-dependent transcriptional repression of p21 (waf1) by hepatitis C virus NS3. J Gen Virol 82:2235–2241. doi: 10.1099/0022-1317-82-9-2235. [DOI] [PubMed] [Google Scholar]