ABSTRACT
Dengue, due to its global burden, is the most important arthropod-borne flavivirus disease, and early detection lowers fatality rates to below 1%. Since the metabolic resources crucial for viral replication are provided by host cells, detection of changes in the metabolic profile associated with disease pathogenesis could help with the identification of markers of prognostic and diagnostic importance. We applied 1H nuclear magnetic resonance exploratory metabolomics to study longitudinal changes in plasma metabolites in a cohort in Recife, Brazil. To gain statistical power, we used innovative paired multivariate analyses to discriminate individuals with primary and secondary infection presenting as dengue fever (DF; mild) and dengue hemorrhagic fever (DHF; severe) and subjects with a nonspecific nondengue (ND) illness (ND subjects). Our results showed that a decrease in plasma low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL) discriminated dengue virus (DENV)-infected subjects from ND subjects, and also, subjects with severe infection even presented a decrease in lipoprotein concentrations compared to the concentrations in subjects with mild infection. These results add to the ongoing discussion that the manipulation of lipid metabolism is crucial for DENV replication and infection. In addition, a decrease in plasma glutamine content was characteristic of DENV infection and disease severity, and an increase in plasma acetate levels discriminated subjects with DF and DHF from ND subjects. Several other metabolites shown to be altered in DENV infection and the implications of these alterations are discussed. We hypothesize that these changes in the plasma metabolome are suggestive of liver dysfunction, could provide insights into the underlying molecular mechanisms of dengue virus pathogenesis, and could help to discriminate individuals at risk of the development of severe infection and predict disease outcome.
IMPORTANCE Dengue, due to its global burden, is the most important mosquito-borne viral disease. There is no specific treatment for dengue disease, and early detection lowers fatality rates to below 1%. In this study, we observed the effects of dengue virus infection on the profile of small molecules in the blood of patients with mild and severe infection. Variations in the profiles of these small molecules reflected the replication of dengue virus in different tissues and the extent of tissue damage during infection. The results of this study showed that the molecules that changed the most were VLDL, LDL, and amino acids. We propose that these changes reflect liver dysfunction and also that they can be used to discriminate subjects with mild dengue from those with severe dengue.
INTRODUCTION
Dengue, due to its global burden, is the most important arthropod-borne disease nowadays. Dengue is caused by dengue virus (DENV), a positive-strand RNA virus with four distinct serotypes: DENV serotype 1 (DENV1) to DENV4. Estimates of the DENV infection burden at global levels indicate approximately 400 million cases per year and 500,000 cases of severe infection, with death occurring in about 2.5% cases of severe infection (1). Dengue disease is endemic in over 100 countries, and Latin America, Southeast Asia, and the Western Pacific are the most seriously affected regions. However, the threat of an outbreak of dengue fever now exists in Europe (2). Brazil is one of the most affected countries in Latin America, and in 2015, DENV epidemiology reached epidemic proportions, since more than 1,500,000 cases and a total of 839 deaths, which corresponded to an 80% increase compared to the incidence in 2014, were reported (3). The majority of dengue cases are asymptomatic, but in those with symptoms the clinical manifestations of DENV infection range from a self-limiting febrile illness to severe life-threatening disease. According to the new WHO criteria, DENV infection is classified as dengue without warning signs, dengue with warning signs, and severe dengue (4). This classification is aimed at facilitating clinical management of the disease and the triage of patients. However, the former classification into dengue fever (DF), dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS) (5) is more useful when it comes to comparison with previous studies regarding the pathogenesis of the disease. Vascular alterations, such as thrombocytopenia and fluid accumulation, as well as liver enlargement are clinical signs of DHF, and systemic plasma leakage is characteristic of DSS. Clinical and laboratory data are rather complex for characterization of the severity of dengue disease, and several other factors have been associated with it, such as age, host immunity, and viral genetics (6). The underlying molecular mechanisms of dengue pathogenesis, however, are not fully understood. There is no specific treatment for dengue disease, but early diagnosis lowers the fatality rate to below 1% (2). However, a tetravalent vaccine in a phase 3 efficacy trial is being tested in children in 5 Latin American countries. Recent results indicate that the vaccine is efficacious and that immunization with the vaccine leads to fewer hospitalizations (7).
Genomics (8, 9) and proteomics (10–12) have been used by different research groups in an attempt to identify early markers of dengue disease and/or its severity. More recently, metabolomics, the study of low-molecular-weight metabolites of physiological relevance, has proved an important tool for studying the changes in host metabolism during the course of viral infections (13–17). Since the metabolic resources crucial for viral replication are provided by host cells, the detection of changes in the metabolic profile associated with the pathological condition could help with the identification of markers of prognostic and diagnostic importance as well as the development of specific treatment strategies. In this sense, it has been demonstrated that infection of endothelial cells with the four DENV serotypes provoked significant alterations in the levels of extracellular metabolites, including amino acids and tricarboxylic acid (TCA) cycle intermediates (18). Different responses to the four serotypes were observed, which could be related to the broad range of manifestations and different DENV infection outcomes observed in the clinical setting. In addition, it was recently shown that gender discriminates the urine metabolic profile in subjects with DENV infection, and, like the in vitro study, changes in the levels of TCA cycle intermediates and amino acids contributed to the discrimination of groups according to infection outcomes (19).
Metabolomic data from human studies are often characterized by large variations among subjects, as opposed to the changes observed in animal and cultured cell studies (20). Therefore, small changes may be overlooked, and both the response and the impact may differ among subjects (21). Considering the broad variation in responses to DENV infection among individuals, which makes it difficult to identify markers of severity, one strategy is to evaluate longitudinal changes in metabolite levels. Indeed, it has been reported that longitudinal changes in the plasma metabolome and the levels of lipid mediators resolved with time in individuals with primary infection presenting as DF (22). The authors proposed that these changes are compatible with a self-limiting febrile illness.
Despite these reports, a comprehensive exploratory analysis of the metabolic changes occurring during human DENV infection is required, especially when it comes to identifying markers of dengue severity and intraindividual responses.
In the current study, we applied nuclear magnetic resonance (NMR) exploratory metabolomics to study the overall longitudinal changes in plasma metabolites in a well-characterized cohort in Recife, Brazil, from whom samples were collected during an outbreak of DENV3 infection. In order to gain statistical power, we used innovative statistical analysis of paired multivariate data to discriminate individuals with primary and secondary infection presenting as DF and DHF. In addition, results for DENV-infected subjects were compared to those for subjects presenting with a nonspecific fever, which highlights the specificity of the results to DENV infection and dengue disease.
MATERIALS AND METHODS
Dengue cohort.
The cohort of subjects with clinical dengue was established in Recife, Brazil, from 2004 to 2006 and has been described in detail elsewhere (23). In brief, patients ≥5 years old with suspected DENV infection were invited to enroll in the study. Sequential blood samples were collected within 30 days from the start of the symptoms. DENV infection was confirmed by serology (IgM and IgG), reverse transcription-PCR (RT-PCR), and/or virus isolation. Laboratory and clinical information was used to classify dengue cases into those with DF and those with DHF, according to previous WHO criteria (5). Classification of primary and secondary infection was performed by serology and RT-PCR, taking into account the IgM and IgG results for both acute- and convalescent-phase serum samples.
All subjects or their guardians signed an informed written consent. This cohort was approved by the ethics committee of the Brazilian Ministry of Health (CONEP: 4909, process no. 25000.119007/2002-03, CEP 68/02). The study was also reviewed by the Johns Hopkins University Institutional Review Board and approved under protocol JHM-IRB-3:03-08-27-01.
Patient sample selection.
In the present study, 96 samples from 48 DENV-infected subjects were selected. For each subject, we analyzed 2 samples obtained at different times, corresponding to (i) the onset of symptoms, usually fever, and (ii) the defervescence phase, i.e., days 3 to 5 after the onset of symptoms.
Thirty-four samples from 17 volunteers presenting with nonspecific nondengue (ND) illness, i.e., subjects with a febrile illness of unknown etiology and with negative results for DENV, were included in this study. By working with samples from subjects with ND-related illness as a control group, we preserved the characteristics of the cohort study conducted from 2004 to 2006, according to the classification criteria used during the period of the study (23). Additionally, the use of samples from the ND group was crucial for the metabolomic approach, since samples were collected during the same period and handled in exactly the same way as samples from subjects with DENV infection until NMR analysis. The importance of standardized sample handling and processing for metabolomic analysis is highlighted elsewhere (24).
Therefore, five groups of different subjects were compared in this study: patients with DF during primary infection (DFp), patients with DF during secondary infection (DFs), patients with DHF during primary infection (DHFp), patients with DHF during secondary infection (DHFs), and subjects with a ND-related illness.
Plasma samples for NMR metabolomics.
Frozen plasma samples were quickly thawed and diluted 2-fold with isotonic saline solution (0.9%, wt/vol) in water and deuterium oxide (D2O; 20%, vol/vol). Samples were centrifuged at 10,000 × g and 4°C for 10 min. The supernatant (550 μl) was used for analysis in 5-mm NMR tubes.
Sample processing and NMR analysis were conducted according to standardized protocols (25). All samples were randomly assayed.
1H NMR spectroscopy of plasma samples.
1H NMR spectra were acquired on a Bruker 400-MHz Avance DRX spectrometer (Bruker Biospin, Germany), operating at 400.13 MHz with a probe temperature of 300 K. One-dimensional spin echo spectra were acquired using the Carr-Purcell-Meiboom-Gill (CPMG) sequence to selectively highlight the signals from low-molecular-weight metabolites by attenuating the signals from macromolecules through a T2 filter. Each plasma spectrum was acquired using 16 dummy scans and 1,024 scans, 32,000 time domain points, a spectral width of 20,000 ppm, a relaxation delay of 2 s, an acquisition time of 1.36 s, and a spin echo delay of 400 μs. Suppression of the water peak was achieved by water irradiation during the relaxation delay.
Prior to Fourier transformation, free induction decays were multiplied by an exponential function corresponding to a line broadening of 0.3 Hz and zero filling.
Multivariate data analysis.
The data used for multivariate statistical analysis by supervised multilevel sparse partial least-square discriminant analysis (sPLS-DA) were derived from the NMR spectrum data points. NMR spectra were manually phased, baseline corrected, referenced to the α-glucose anomeric doublet at δ 5.23 ppm, and aligned using icoshift software. The signals for the water region (δ 4.45 to 5.15 ppm), the peaks for the ethanol (δ 1.18 and 3.66 ppm) used for antiseptic swabbing that contaminated samples during collection, and the N-acetyl signals of acetaminophen (δ 2.2 to 2.3 ppm) from the common medication taken by patients were excluded before postprocessing of the spectra.
The spectra were normalized by probabilistic quotient normalization (26) to reduce the effects of sample dilution or concentration of the compounds and were binned at 0.005 ppm (8 data points). A generalized log transformation was applied prior to multivariate statistical analysis (27). Data were built into a matrix where each row corresponded to an observation (subjects) and each column corresponded to a variable (frequency). The data matrix was complete for all plasma samples analyzed in this study and for all variables assessed; thus, there were no missing values.
NMR data were processed using NMRLab (28) in MATLAB (The MathWorks, Inc., Natick, MA). All subgroups of samples from patients with DFp, DFs, DHFp, and DHFs and ND subjects were analyzed using sPLS-DA (21). By fitting this statistical model, we aimed at the identification of the dimensions composed by the spectra that best discriminate the morbidity classes. This approach takes into account the correlated structure of our observations within individuals, since samples obtained at 2 different times were analyzed for each subject, and describes separately the effects of infection within and among subjects. Therefore, possible variations within each subject from the onset of symptoms to the defervescence phase are taken into account by fitting this statistical model.
The model-fitting exercise relied on the function multilevel of the mixOmics package (29, 30) in the R software environment (31). This function requires as input the specification of the number of components (ncomp) to be included in the model (ncomp = 3) and the number of variables kept on each component [keepX = c(50, 50, 50), where keepX is the input number of variables on each component and 50 is the number of variables]. We chose these input parameters after a performance-tuning exercise. We also identified the metabolites associated with the frequencies that contributed the most (provided the highest loads) to the discrimination of the groups. It is important to mention that even though 50 input variables were used, there were no restraints for the algorithm to consider in the fitting of all available variables. We assessed model uncertainty and validity by plotting the classification error rate as a function of the number of components based on the leave-one-out validation strategy. This validation method results in the use of 1 observation as the validation set and the remaining observations as the training set (n − 1, where n is the number of observations). Under this strategy, one observation at a time is left out of the model-fitting exercise and used for validation purposes. During the model fitting, the input was the training set, comprising all remaining observations except the one left out. The process was repeated for all observations in our sample. The average error across all trials was computed and used to evaluate the model. Potential candidate metabolites were selected according to the loading factors, which correspond to the relative weight of each original variable when calculating the components.
Additionally, according to the loading factors for sPLS-DA, the differences in the concentrations of the relevant metabolites were assessed at the univariate level by using the nonparametric Kruskal-Wallis test (P < 0.05). Significance was evaluated by Dunn's multiple-comparison test.
A word of caution is required when interpreting the P values associated with the significance tests reported in our work. Since the null hypotheses being tested were suggested by the same data used as input to calculate the test statistics, our P values do not express the nominal values indicated and should be regarded as informal approximations of the actual values. As a positive side, however, these exercises elicit potential null hypotheses worth testing against fresh new samples obtained in future studies. Null hypotheses formulated aprioristically in this way could then be formally tested and lead to P values that express their proper nominal values.
RESULTS
Subjects' demographic characteristics and metabolite profiles from 1H NMR spectra.
Table 1 shows the demographics of the ND subjects and subjects with DENV infection from whom the samples used in the present study were collected. It can be seen that the age of the DENV-infected subjects ranged from 6 to 84 years. Adults, i.e., individuals ≥18 years old, corresponded to the majority of the DENV-infected patients (73%). In addition, 66% of the samples from DENV-infected patients analyzed were from female subjects, whereas males accounted for 34% of the DENV-infected patients. Female subjects represented the majority of DHF cases. These demographic characteristics of the subjects are in agreement with DENV infection statistics reported in Brazil.
TABLE 1.
Demographic characteristics of ND subjects and DENV-infected subjectsa
| Characteristic | Value for the following group: |
|
|---|---|---|
| ND subjects (n = 17) | DENV-infected subjects (n = 48) | |
| Mean (range) age (yr) | 32.4 (5–63) | 32 (6–84) |
| No. (%) of subjects by gender | ||
| Female | 12 (70) | 29 (66) |
| Male | 5 (30) | 19 (34) |
| No. (%) of subjects with DENV3 infection | — | 48 (100) |
| No. (%) of subjects with the following dengue disease classification: | ||
| DF | — | 25 (52) |
| DHF | — | 23 (48) |
| No. (%) of subjects with primary infection presenting as: | ||
| DF | — | 14 (29) |
| DHF | — | 11 (23) |
| No. (%) of subjects with secondary infection presenting as: | ||
| DF | — | 11 (23) |
| DHF | — | 12 (25) |
—, not applicable.
According to disease classification, 52 and 48% of the samples from patients with DENV infection that were used for these analyses were from individuals with DF and DHF, respectively. Among the samples from patients with DF, 29% were from patients with primary infections and 23% were from patients with secondary infections. Among the samples from patients with DHF, 23 and 25% were from patients with primary and secondary infections, respectively.
Typical NMR spectra of samples from DFs, DHFs, and ND subjects are shown in Fig. 1. Visual differences in the acetate, glutamine, and alanine peaks and the very-low-density lipoprotein (VLDL)/low-density lipoprotein (LDL) and N-acetyl glycoprotein residual signals can be seen. Representative NMR spectra of samples from all subjects at the onset of symptoms and at the defervescence phase of the disease are shown in Fig. S2 to S6 in the supplemental material.
FIG 1.
Aliphatic region (δ 1H 0.5 to 3.0 ppm) of representative NMR spectra of plasma acquired using the Carr-Purcell-Meiboom-Gill sequence from samples from ND subjects (light blue), DFs patients (dark blue), and DHFs patients (black). All representative samples were obtained at the onset of symptoms. *, peaks for ethanol used for antiseptic swabbing that contaminated samples during collection; N-acetyl acetaminophen, signals for acetaminophen derived from common medication taken by the patients.
To characterize the differences in the metabolic profiles among all subgroups, multilevel sPLS-DA was used for overall group comparisons (Fig. 2), for discriminating ND-related illness and primary and secondary infections presenting as DF and DHF (Fig. 3A to D), for identifying differences among patients with primary and secondary infection presenting as DF and DHF (Fig. 4A to F), and for investigating longitudinal variations among the individuals in each subgroup separately (Fig. 5A to D).
FIG 2.
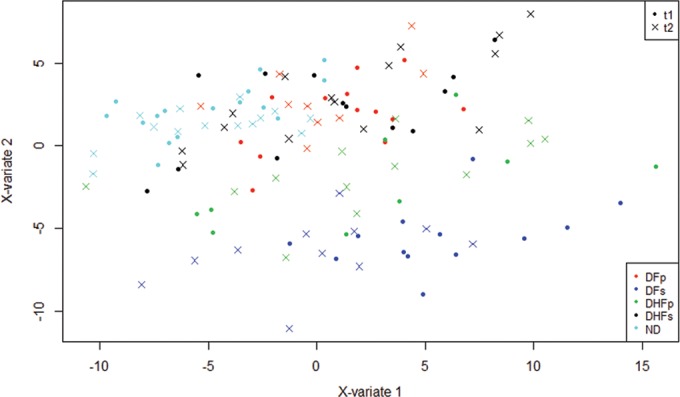
Multilevel sPLS-DA score plots for plasma samples from DENV-infected and ND subjects. sPLS-DA models were constructed using NMR metabolomics data for ND subjects and DENV-infected subjects with DFp, DFs, DHFp, and DHFs. t1, samples obtained at the onset of symptoms; t2, samples obtained at the defervescence phase; X-variate 1, component 1; X-variate 2, component 2. Error rates were below 10%.
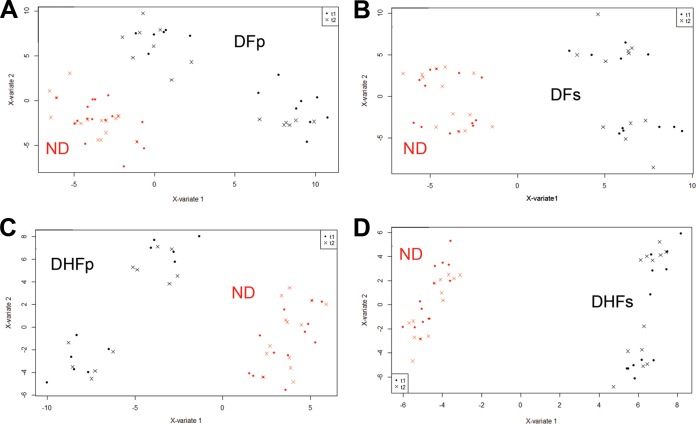
FIG 3.
Multilevel sPLS-DA score plots for plasma samples from subjects with dengue virus infection compared to samples from ND subjects. (A) DFp patients; (B) DFs patients; (C) DHFp patients; (D) DHFs patients. t1, samples obtained at the onset of symptoms; t2, samples obtained at the defervescence phase; X-variate 1, component 1; X-variate 2, component 2. For all paired analyses, error rates were below 10%.
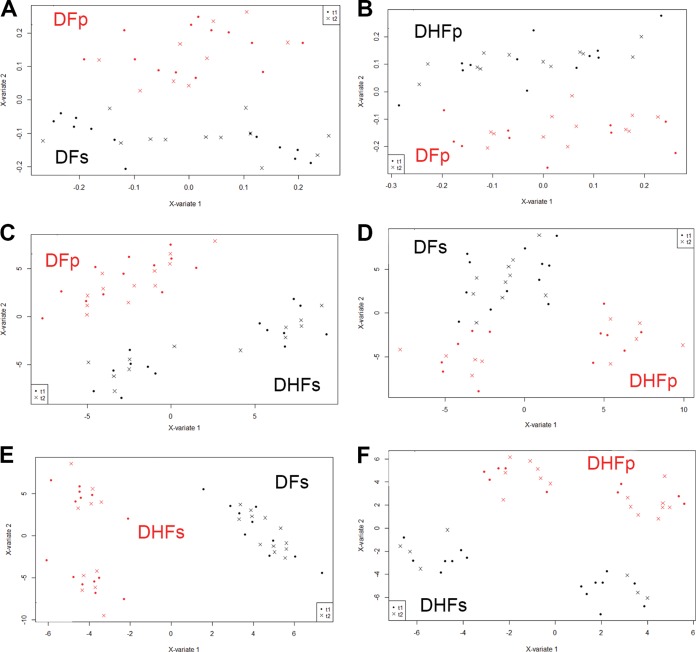
FIG 4.
Multilevel sPLS-DA score plots for plasma samples from subjects with dengue fever compared to subjects with dengue hemorrhagic fever. (A) DFp versus DFs patients; (B) DFp versus DHFp patients; (C) DFp versus DHFs patients; (D) DFs versus DHFp patients; (E) DFs versus DHFs patients; (F) DHFp versus DHFs patients. t1, samples obtained at the onset of symptoms; t2, samples obtained at the defervescence phase; X-variate 1, component 1; X-variate 2, component 2. For all paired analyses, error rates were below 10%.
FIG 5.
Multilevel sPLS-DA score plots for plasma samples obtained at two times from ND subjects (A), DFp patients (B), DFs patients (C), DHFp patients (D), and DHFs patients (E). t1, samples obtained at the onset of symptoms; t2, samples obtained at the defervescence phase; X-variate 1, component 1; X-variate 2, component 2. For all paired analyses, error rates were below 10%.
Multilevel sPLS-DA for overall group comparisons.
In order to have an overall picture of the discrimination pattern according to the plasma metabolite profiles for all 5 subgroups and the samples obtained at 2 different times, we performed an overall group multilevel sPLS-DA (Fig. 2). Score plots of overall group comparisons showed that all 5 subgroups could be discriminated according to plasma metabolite contents. The DFs group (Fig. 2, dark blue) was more strongly discriminated from all other groups. The ND group (Fig. 2, light blue) was also discriminated from all other groups. There was no clear separation between the DFp and DHFs groups when all groups were evaluated simultaneously. Variables that contributed to the discrimination of the groups were VLDL and LDL, and valine, lactate, glutamine, citrate, glycerophosphocholine, tyrosine, and betaine.
In addition to discriminating among the subgroups according to metabolic profile, Fig. 2 also shows that the variations in plasma metabolite levels within individuals over time did not contribute to the overall separation of the groups. The loadings for the overall group sPLS-DA are presented in Data Set S1 in the supplemental material.
According to these results, the application of multilevel sPLS-DA was appropriate for investigation of the metabolic profiles by 1H NMR analysis since model uncertainty and validity were less than 10% for all three components according to the classification error rate (see Fig. S1 in the supplemental material).
Multilevel sPLS-DA for comparison of DENV-infected subjects and ND subjects.
The overall discriminatory metabolic pattern shown in Fig. 2 was the starting point for more detailed analyses of the metabolic profiles of DENV-infected subjects. The plasma metabolites of the ND subjects (Fig. 2, light blue) could be discriminated from the plasma metabolites of DENV-infected subjects. In order to better investigate the metabolites with the strongest power to discriminate between the groups of subjects with DENV infection and ND subjects, a multilevel sPLS-DA was performed by considering two groups per analysis, and the score plots for all analyses are presented in Fig. 3. sPLS-DA statistics took into account the variations (between the time of onset of symptoms and the defervescence phase of the disease) within each group. Therefore, in these analyses, we were able to observe separately the effects of infection within and among subjects.
It can be seen that the metabolic profiles of samples from DFp (Fig. 3a), DFs (Fig. 3b), DHFp (Fig. 3c), and DHFs (Fig. 3d) patients could be strongly discriminated from the metabolic profile of ND subjects. Error rates for all comparisons were less than 10% (see Fig. S1 in the supplemental material). Additionally, according to the score plots, it can be observed that the metabolic profile of each group at the onset of symptoms and in the defervescence phase did not contribute to the discrimination of the ND and DENV groups.
Loading factors for the comparison of the samples from DFp and ND subjects indicated that an increase in alanine and tyrosine content and a decrease in acetate, glutamine, and lactate content in samples from DFp patients contributed to the separation from the ND group. Additionally, a decrease in the residual signals of mobile VLDL and the N-acetyl glycoprotein also contributed to the separation of the samples from DFp and ND subjects. Comparison of the samples from DFs and ND patients showed that an increase in the valine, alanine, histidine, tyrosine, lactate, and choline metabolite content and a decrease in acetate, citrate, VLDL, and N-acetyl glycoprotein content in samples from DFs patients contributed to the difference in the metabolic profile from that for the ND group.
When samples from DHFp and ND subjects were compared, a decrease in valine, glutamine, tyrosine, and betaine content and an increase in lactate, alanine, acetate, and formate content as well as a decrease in VLDL content and an increase in the choline metabolite content in DHFp patients contributed to the separation of the groups. On the basis of loading factor analyses, samples from DHFs patients could be discriminated from samples from ND subjects by a decrease in valine, leucine, and isoleucine (branched chain amino acid [BCAA]) content, a decrease in alanine, glutamine, tyrosine, histidine, lactate, and choline metabolite content, and an increase in acetate, pyruvate, and citrate content. Loading factor results for all analyses are presented in Data Set S1 in the supplemental material.
These results clearly show that several potential compounds may have contributed to the discrimination of dengue patient samples from ND patient samples. The overall changes in the concentrations of metabolites with the most discriminatory power according to the sPLS-DA pairwise comparison of the ND and DENV groups are presented in Table 2.
TABLE 2.
Metabolites with the most power to discriminate ND subjects and DENV-infected groups according to sPLS-DA pairwise multivariate statisticsa
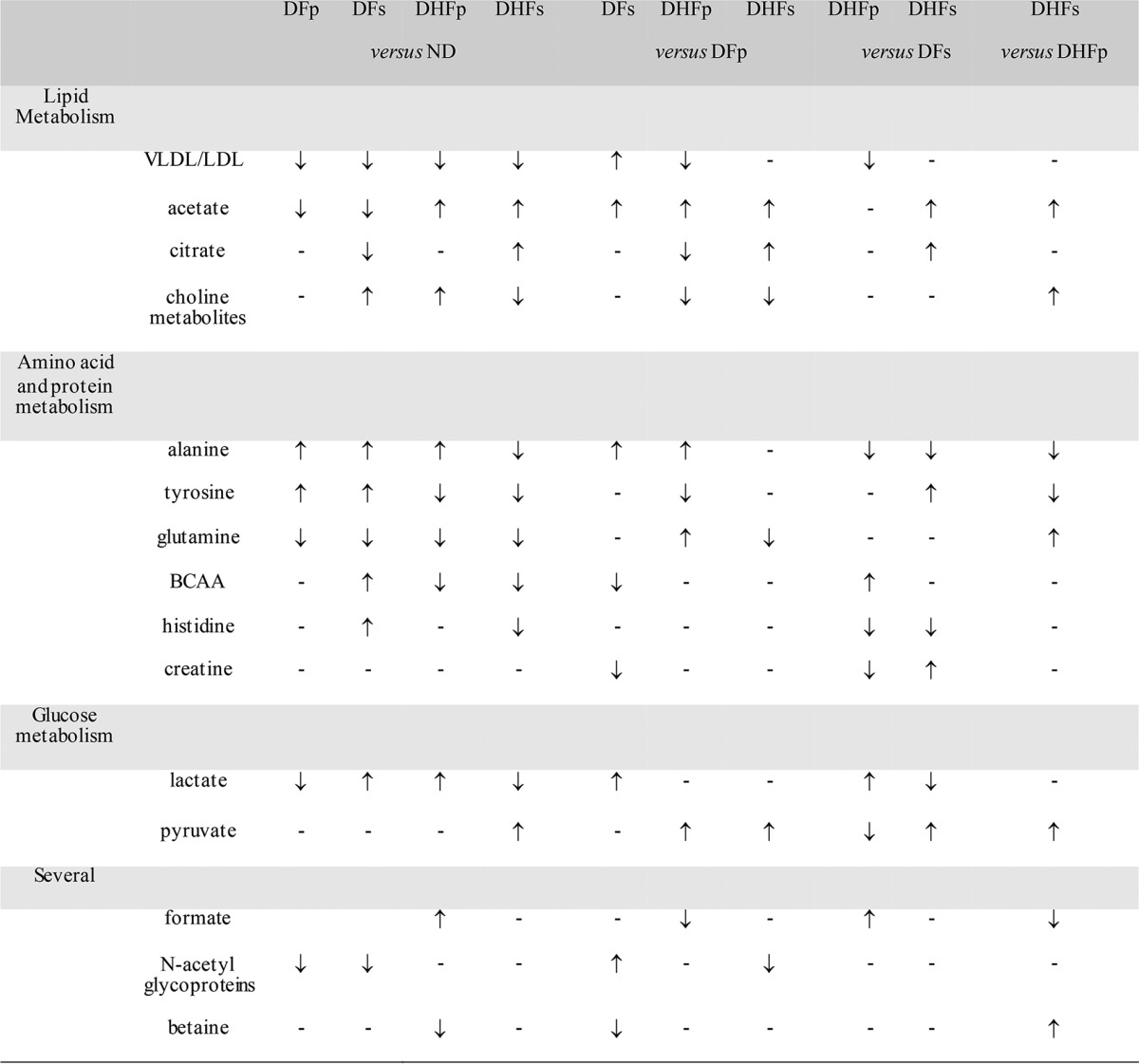
sPLS-DA pairwise models were built by taking into account the correlated structure of the samples (samples obtained at the onset of symptoms and the defervescence phase). Model uncertainty and validity were assessed by plotting the classification error rate as a function of the number of components on the basis of the leave-one-out validation strategy. For all models, error rates were below 10% (see Fig. S1 in the supplemental material). ↑, an increase in the plasma metabolite concentration in the first group compared to that in the second group; ↓, a decrease in the plasma metabolite concentration in the first group compared to that in the second group; –, metabolites that did not contribute to the discrimination of groups; BCAA, branched-chain amino acids; ND, nondengue patients; DFp, patients with primary infection presenting as dengue fever; DFs, patients with secondary infection presenting as dengue fever; DHFp, patients with primary infection presenting as dengue hemorrhagic fever; DHFs, patients with secondary infection presenting as dengue hemorrhagic fever.
Multilevel sPLS-DA for comparison of dengue virus-infected subject groups.
We next sought to compare the groups with DF and DHF in order to better investigate the metabolites with the strongest power to discriminate each DENV-infected group. Multilevel sPLS-DAs were performed by considering two groups per analysis, and the score plots for all analyses are shown in Fig. 4.
It can be seen that the multilevel sPLS-DA was able to discriminate subjects with DFp and DFs (Fig. 4A), DFp and DHFp (Fig. 4B), DFp and DHFs (Fig. 4C), DFs and DHFp (Fig. 4D), DFs and DHFs (Fig. 4E), and DHFp and DHFs (Fig. 4F). Error rates for all analyses were less than 10% (see Fig. S1 in the supplemental material). As pointed out for the comparison of ND subjects and the DENV-infected groups, the score plots presented in Fig. 4 showed that the metabolic profile of each group at the onset of symptoms and in the defervescence phase did not contribute to the discrimination of individuals with DENV infection.
According to the loading factors, several potential metabolites contributed to the ability to discriminate between DENV-infected subjects by paired analysis (see Data Set S1 in the supplemental material). DFs patients could be discriminated from DFp patients due to an increase in the residual signal of VLDL/LDL and that of N-acetyl glycoprotein and to an increase in plasma valine, acetate, and lactate concentrations and a decrease in plasma creatine concentrations. Multilevel sPLS-DA showed that DHFp patients could be discriminated from DFp patients by a decrease in the VLDL/LDL content and in choline metabolite content. Additionally, an increase in alanine, acetate, pyruvate, and glutamine concentrations and a decrease in citrate, tyrosine, and formate concentrations contributed to the ability to discriminate DHFp from DFp subjects. DHFs patients could be discriminated from DFp patients due to decreases in VLDL/LDL and N-acetyl glycoprotein concentrations and choline metabolite broad resonances. Variations in the levels of low-molecular-weight metabolites that contributed to the pattern of discrimination of DHFs and DFp subjects were an increase in acetate, pyruvate, and citrate concentrations and a decrease in glutamine content. DHFp subjects could be discriminated from DFs subjects due to a decrease in VLDL/LDL resonances, an increase in plasma valine, lactate, and formate concentrations, and a decrease in plasma alanine, histidine, pyruvate, and creatine concentrations. DHFs patients could be discriminated from DFs patients as a function of a decrease in alanine, lactate, and histidine concentrations and an increase in acetate, pyruvate, citrate, creatine, and tyrosine concentrations. DHFs patients could be discriminated from DHFp patients due to a decrease in alanine, tyrosine, and formate concentrations and to an increase in acetate, pyruvate, glutamine, and betaine concentrations and a broad signal of choline metabolites.
According to these results, several potential compounds may have contributed to the discrimination of the different groups of DENV-infected subjects according to disease severity and primary or secondary infection. The overall changes in the levels of metabolites with the most power to discriminate each DENV group according to the sPLS-DA pairwise comparison are presented in Table 2.
Multilevel sPLS-DA for comparing the time course of variations in plasma metabolite levels in all subject groups.
Finally, we applied sPLS-DA to observe fluctuations in plasma metabolite content within subjects as a function of disease progression (Fig. 5). In these analyses, the correlated structure of our observations within individuals, i.e., the plasma metabolite concentrations at the onset of symptoms and in the defervescence phase of disease, was taken into account. By fitting this statistical model, we aimed to identify the dimensions composed by the spectra that best discriminate, within subjects, the effects of infection.
The multilevel analysis indeed showed differences in plasma metabolite concentrations as infection progressed. The score plots of the time course analysis are presented in Fig. 5A for ND subjects, in Fig. 5B for DFp subjects, in Fig. 5C for DFs subjects, in Fig. 5D for DHFp subjects, and in Fig. 5E for DHFs subjects. It is important to mention that these differences were seen in all groups, including the ND group. Error rates for all analyses were less than 10% (see Fig. S1 in the supplemental material), and loading factor results are presented in Data Set S1 in the supplemental material.
In the case of ND subjects, loading factor results showed that an increase in valine, alanine, and histidine content and a decrease in acetate content from the first day of symptoms to the defervescence phase discriminated the subjects. Metabolites with differences in content from the first day of symptoms to the defervescence phase that contributed to the discrimination of DFp subjects were an increase in plasma VLDL/LDL, N-acetyl glycoprotein, choline metabolite, and tyrosine concentrations and a decrease in the plasma creatine concentration.
Loading factor results showed that DFs subjects presented an increase in the content of the BCAA isoleucine and valine as well as that of lactate, acetate, citrate, and tyrosine and a decrease in the content of pyruvate, glutamine, and formate. Additionally, an increase in VLDL/LDL and choline metabolite concentration between the first day of symptoms and the defervescence phase contributed to the discrimination of DFs subjects. Metabolites with different content between the first day of symptoms and the defervescence phase that contributed to the discrimination of DHFp subjects were a decrease in plasma lactate, pyruvate, citrate, and N-acetyl glycoprotein concentrations and an increase in plasma tyrosine concentrations. An increase in lactate and tyrosine concentrations and a decrease in glutamine and choline metabolite concentrations between the first day of symptoms and the defervescence phase were discriminatory in the case of DHFs subjects.
The overall changes in the content of metabolites with the most discriminating power from the onset of symptoms to the defervescence phase of the disease for each morbidity group according to sPLS-DA are presented in Table 3.
TABLE 3.
Metabolites with differences in levels between the onset of symptoms and the defervescence phase with the most power to discriminate ND subjects and DENV-infected subjects according to sPLS-DA pairwise multivariate statisticsa
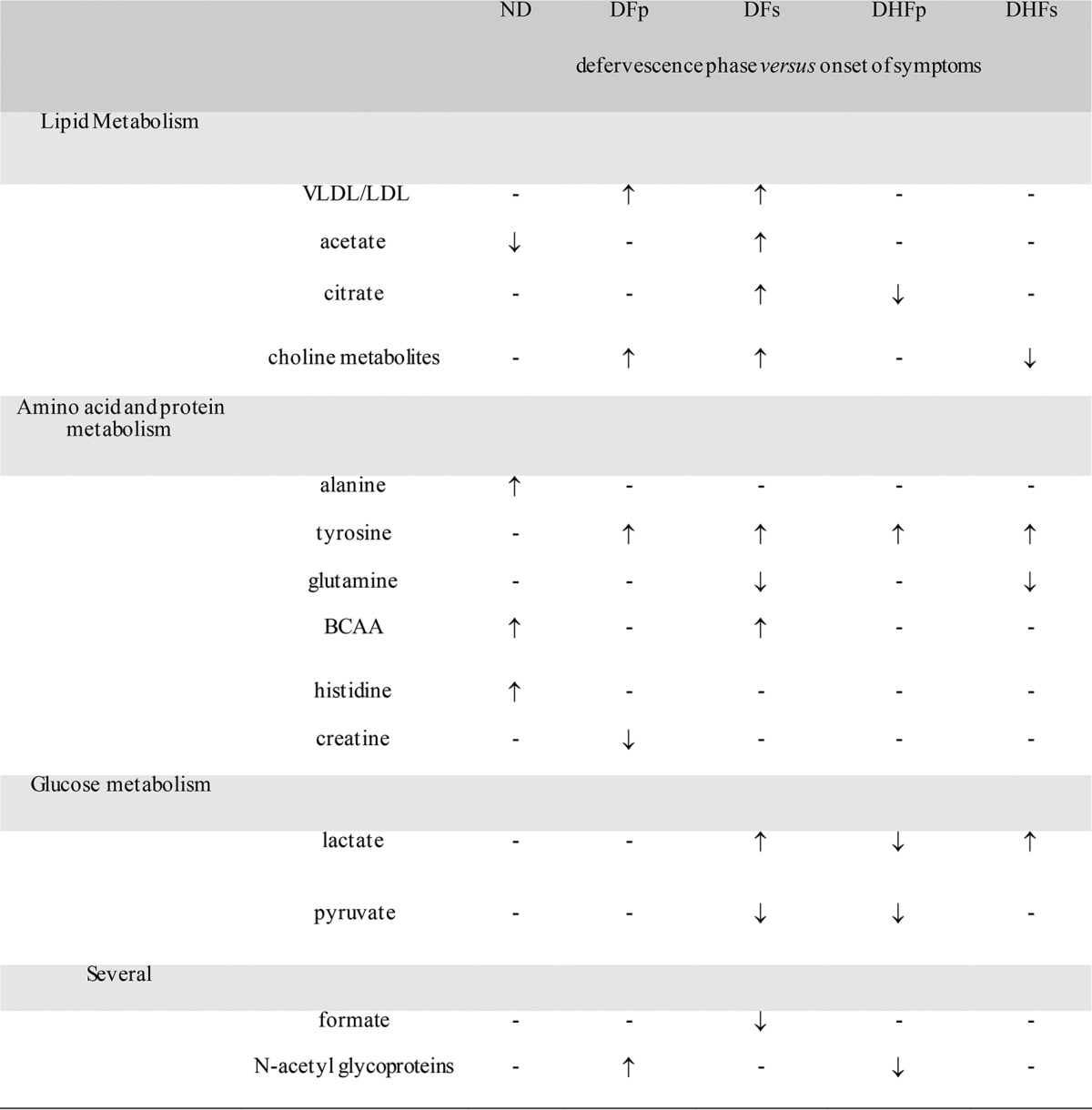
sPLS-DA pairwise models of uncertainty and validity were assessed by plotting the classification error rate as a function of the number of components based on the leave-one-out validation strategy. For all models, error rates were below 10% (see Fig. S1 in the supplemental material). ↑, an increase in the plasma metabolite concentration in the defervescence phase of the disease compared to that at the onset of symptoms for each group; ↓, a decrease in the plasma metabolite concentration in the defervescence phase of the disease compared to that at the onset of symptoms for each group; –, metabolites that did not contribute to the discrimination of groups; BCAA, branched-chain amino acids; ND, nondengue patients; DFp, patients with primary infection presenting as dengue fever; DFs, patients with secondary infection presenting as dengue fever; DHFp, patients with primary infection presenting as dengue hemorrhagic fever; DHFs, patients with secondary infection presenting as dengue hemorrhagic fever.
Univariate statistics for comparison of metabolite levels in ND and DENV-infected subjects.
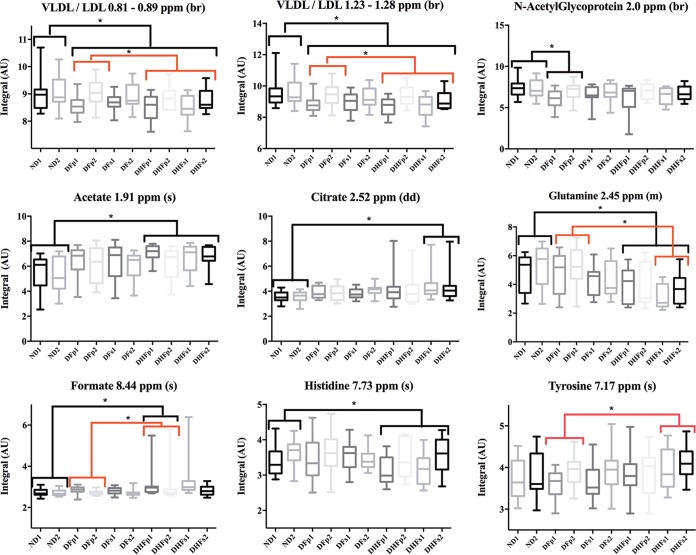
The potential differences in plasma metabolite and lipid content in DENV-infected and ND subjects observed in the multivariate analysis were further investigated at the univariate level (Fig. 6). Confirming the results presented in Table 2, VLDL/LDL content was significantly decreased in all samples from DENV-infected subjects compared to those in samples from ND subjects. Additionally, lipoprotein concentrations in samples from DHF subjects presented a significant decrease compared to the content in samples from DFp subjects, indicating that lipid metabolism is altered during DENV infection and that it might be associated with disease pathogenesis.
FIG 6.
Box plots of the variations in plasma metabolite concentrations observed in ND, DF, and DHF subjects. The variations in the CPMG spectra for all compounds were measured. For all groups, numbers correspond to samples collected at the onset of symptoms (suffix 1) and at the defervescence phase (suffix 2). br, broad resonance; s, singlet; m, multiplet; dd, double doublet. *, P < 0.05 by comparison of the DENV-infected and ND groups (black brackets) and the different DENV-infected groups (red brackets), as assessed by the Kruskal-Wallis test. Significance was evaluated by Dunn's multiple-comparison test. AU, absorbance units.
The plasma glutamine concentration was also significantly decreased in samples from DHF subjects compared to that in samples from ND subjects. Interestingly, a decrease in the plasma glutamine concentration also discriminated samples from DHFs subjects from those of DFp subjects. As suggested for the plasma lipoprotein concentration, the plasma glutamine concentration might be associated with disease severity.
Additionally, univariate analysis showed an increase in acetate, citrate, and formate concentrations and a decrease in the histidine concentration in DHF subjects compared to their concentrations in ND subjects. An increase in the formate content in samples from DHFp subjects compared to that in samples from DFp subjects was also observed. DFp subjects presented a significant decrease in N-acetyl glycoprotein levels compared to those in ND subjects. The increase in the tyrosine concentration in samples from DHFs subjects compared to that in samples from DFp subjects was also significant.
A decrease in the glutamine concentration and an increase in choline metabolite and tyrosine concentrations discriminated DHFs from DFp subjects. Additionally, as was observed in the DHFp group, a decrease in the LDL/VLDL concentration discriminated DHFs subjects from DFp subjects.
Univariate statistics for comparing time course variations in metabolite levels in ND and DENV-infected subjects.
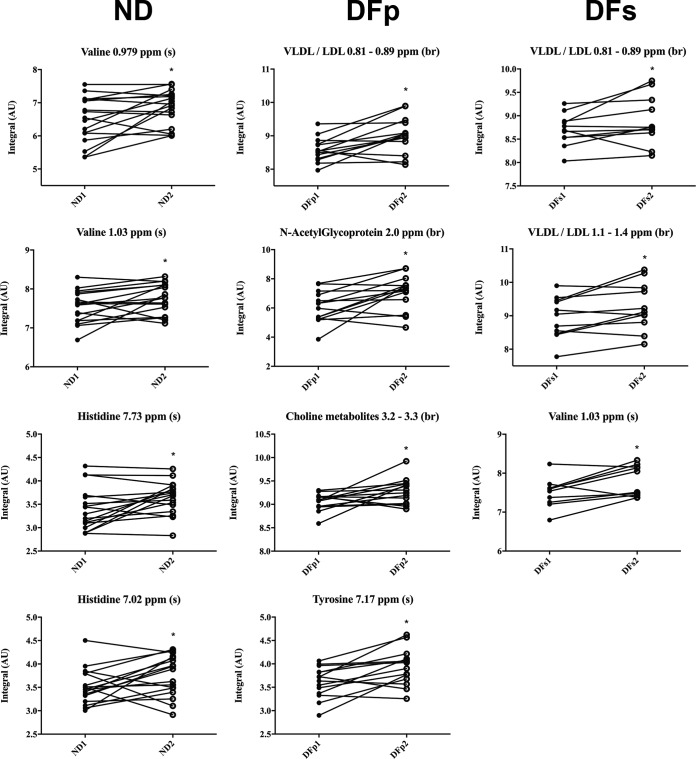
The potential differences in plasma metabolite and lipid concentrations within individuals observed by the multivariate analysis were further investigated at the univariate level (Fig. 7). In the ND group, there was a significant increase in the valine and histidine concentrations in the defervescence phase of the disease compared to those at the onset of symptoms. In DFp subjects, an increase in N-acetyl glycoprotein, choline metabolite, and tyrosine concentrations was observed as a function of time. In DFs subjects, there was also an increase in the valine concentration between the onset of symptoms and the defervescence phase. In both DF groups, an increase in VLDL/LDL content was observed from the first day of symptoms to the defervescence phase. In the ND group, alterations in lipoprotein concentrations were not observed as a function of time (data not shown).
FIG 7.
Longitudinal variations of plasma metabolite concentrations observed in ND and DF subjects at the onset of symptoms and at the defervescence phase. The variations in the CPMG spectra for all compounds were measured. Numbers correspond to the onset of symptoms (indicated by the suffix 1) and the defervescence phase (indicated by the suffix 2). br, broad resonance; s, singlet; m, multiplet. *, P < 0.05, as assessed by the Kruskal-Wallis test. Significance was evaluated by Dunn's multiple-comparison test.
Even though sPLS-DA showed patterns that could be used to discriminate the DHFp and DHFs groups as a function of disease progression (Fig. 5D and E), at the univariate level there were no significant differences in metabolite concentrations between the defervescence phase and the time of onset of symptoms (data not shown).
DISCUSSION
The search for biological markers of prognostic and diagnostic value is a central theme in research in frontier areas, such as medical virology and cancer. In the current study, we were able to demonstrate that DENV infection promotes specific alterations in plasma metabolite concentrations, and we worked with the hypothesis that those changes are related to liver dysfunction. One important strength of the current study is the fact that control subjects represented a group of nonhealthy individuals presenting with a nonspecific infection. Therefore, all results can be discussed in the context of characteristics discriminating DENV-infected subjects.
The VLDL/LDL concentration strongly contributed to the plasma metabolic signatures of the subject groups, and our results indicated that the lipid profile is indeed altered in DENV-infected subjects (Fig. 6 and Table 2). We did not specifically assess the differences in high-molecular-weight plasma components, as CPMG analysis provides broad residual signals of high-molecular-weight lipoproteins. On the other hand, VLDL and LDL broad signals truly reflect mobile lipoprotein signals, as it was already confirmed by using diffusion-edited spectra (32, 33). One interesting observation was that while a decrease in plasma lipoprotein concentrations discriminated DHF (both primary and secondary infections) from ND-related illness, it also discriminated primary and secondary infections with DF from ND-related illness. In addition, DHF subjects presented an even greater decrease in lipoprotein concentrations than subjects with DF primary infection (Fig. 6). Consistent with the findings of the current study, several years ago it was observed that children with severe DHF had a more pronounced decrease in plasma lipoprotein content than the controls and less severe cases of dengue disease (34). Moreover, recent work has shown that a reduction in plasma cholesterol content, especially the content of LDL particles, correlated with dengue pathophysiology and an increased risk of developing DHF (35). Therefore, dengue disease severity is indeed associated with a decrease in the plasma lipoprotein concentration, and the alterations in lipid metabolism observed in self-limiting infections, which is the case in DF subjects, are feeble.
As already shown by our group and other groups, the decrease in plasma LDL/VLDL concentration as a function of dengue severity might be a result of (i) an increase in LDL uptake and in hydroxymethylglutaryl coenzyme A (CoA) activity in liver cells (36), (ii) an increase in the level of lipid droplet synthesis (37), and/or (iii) an increase in β-oxidation as a function of autophagy induction (38). Taken together, these events indicate that DENV entry and replication depend upon the interaction of DENV with cellular lipid components, which alters cellular lipid metabolism. Since the liver is a central organ involved in lipid metabolism, it is plausible to suggest that the alterations in lipoprotein concentrations in DENV-infected subjects indicate liver dysfunction.
Another interesting aspect to be discussed is the time course of the variation in plasma VLDL/LDL content. It has been shown that a longitudinal increase in the levels of different lipid mediators is associated with an anti-inflammatory response in primary, self-limiting infection in patients with DF (22). Therefore, an eventual rise in lipoprotein levels in DENV-infected patients might indicate recovery from disease and improved liver function. Indeed, this was observed in the current study for patients with DF, primary and secondary infection, since the VLDL/LDL concentration presented a longitudinal rise from the first day of symptoms to the defervescence phase (Fig. 7; Table 3). On the other hand, in DHF subjects who presented with the more severe forms of infection, an increase in lipoprotein levels over time was not observed, confirming that plasma VLDL and LDL concentrations are potential markers of prognostic importance (data not shown).
In addition to changes in lipoprotein levels, residual signals of N-acetyl glycoproteins were found to be decreased in DFp subjects compared to ND subjects. N-Acetyl glycoproteins might include signals from acute-phase proteins, such as α1-acid glycoprotein, α1-antitrypsin, and haptoglobin (39). The physiological/metabolic significance of this decrease might involve the active participation of the liver in infection. N-Acetyl glycoprotein signals also presented a longitudinal increase from the onset of symptoms to the defervescence phase in DFp subjects, whereas a longitudinal decrease was found in DHFp subjects (Fig. 7 and Table 3). This result might suggest an effect of DENV replication and the host response in self-limiting infection distinct from those in severe disease.
Regarding low-molecular-weight compounds, glutamine was the metabolite that contributed to discriminating ND and DENV-infected subjects at the multivariate and univariate levels. It was observed that a decrease in plasma glutamine concentration was a characteristic of DHFp and DHFs subjects compared to ND subjects (Table 2 and Fig. 6). Additionally, DHFs subjects also presented a decrease in plasma glutamine concentration compared to DFp subjects, suggesting that the glutamine concentration might be a marker of disease progression and severity (Table 2 and Fig. 6). The fact that these differences among DENV-infected subjects were seen in DFp versus DHFs subjects might indicate a better prognosis for patients with DFp than patients with DFs. Indeed, the metabolic profile of DFs subjects was very different from that of the subjects in the other groups during overall multivariate analyses (Fig. 2, dark blue). In agreement with that finding, a longitudinal decrease in the plasma glutamine concentration from the onset of symptoms to the defervescence phase of the disease was seen in DFs and DHFs subjects (Table 3). Since longitudinal variations in plasma glutamine concentration were not seen in all groups, we reckon that changes in plasma glutamine concentrations within the same subject have smaller effects on disease recovery. On the other hand, on the basis of reports in the literature linking secondary DENV infection to disease severity, these results might indicate that the plasma glutamine concentration is indeed a candidate biomarker for both DENV infection and disease (40).
A decrease in the plasma glutamine concentration as a function of DENV infection is in line with several observations showing that in hypermetabolic situations, the demand for glutamine exceeds the body's ability to provide this amino acid (41). Therefore, in the exacerbated immune response scenario during DENV infection, a decrease in the plasma glutamine concentration might be explained by an increase in its utilization by the liver to synthesize proteins during the acute phase of infection. This would also be the case for immune cells, since it has already been shown that glutamine is a crucial substrate for the provision of energy and, most importantly, biosynthetic precursors (purine and pyrimidines) for lymphocyte proliferation and for the phagocytic activity of neutrophils and macrophages (42). Additionally, both energy and carbon sources are important for cytokine and chemokine synthesis and secretion, which favor increased glutamine utilization as dengue disease progresses. An increase in glutamine utilization for leukocyte proliferation would power both viral replication and cytokine and chemokine production by these cells.
Since it has already been suggested that the level of cortisol, a proteolytic hormone, is increased in DENV infection (22), glutamine release after proteolysis in the peripheral tissues might be potential sources to supply liver and immune cells. Adding to the importance of glutamine as a source of carbon and energy for host cells, DENV replication can possibly rely upon glutamine, which might explain the decrease in its concentration in plasma. It has been shown that this is the case for vaccinia virus (43), human cytomegalovirus (44), and human herpes simplex virus 1 (45) replication in cell culture models. On the other hand, it was recently shown that glucose and not glutamine is essential for DENV replication in vitro in human foreskin fibroblasts (46). However, DENV targets different types of cells, such as hepatic, endothelial, and several immune cells, which possess distinct metabolisms and requirements for ATP and, therefore, carbon sources. Thus, it is possible that glutamine is important for DENV replication in vivo.
Acetate was another metabolite with the strong potential to discriminate among groups (Fig. 6 and Table 2). Plasma acetate concentrations were increased in samples from both DHFp and DHFs subjects compared to samples from ND subjects. An increase in the plasma acetate concentration, along with a decrease in glutamine and lipoprotein concentrations, in samples from DHF patients can also be discussed in the context of liver dysfunction. The major source of plasma acetate is the intestinal flora, and it has been described that the acetate concentration in portal venous blood is considerably greater than its concentration in the systemic circulation. In this context, it has been shown that an increase in the plasma acetate concentration is found in several liver disease conditions due to a decrease in the activity of acetyl-CoA synthetase, the enzyme that converts acetate to acetyl-CoA (47). It remains to be determined if this is the case for DENV patients. In agreement with this, it has been demonstrated that the replication of picornavirus depends upon long-chain acyl-CoA synthetase activity (48). At first, a decrease in acetyl-CoA synthesis may be seem to be a mechanism to tamper viral spread, since the deviation of lipid metabolism is a necessary mechanism for DENV replication and infection. On the other hand, other sites of replication (e.g., immune cells) might be actively producing viral particles, irrespective of liver dysfunction.
Changes in other metabolites possibly related to DENV-mediated changes in lipid metabolism were increases in the concentrations of citrate and choline metabolites in DHFs subjects compared to those in ND subjects (Fig. 6 and Table 2). These changes add to the discussion that, as the infection worsens, an increase in lipid synthesis is important to drive viral replication and that in self-limiting DF changes in lipid metabolism-related compounds are tampered (22).
Valine was another amino acid which presented a longitudinal increase in ND and DFs subjects (Fig. 7). An overall increase in plasma BCAA concentrations might suggest improved liver function, as reported for patients with cirrhosis (49). The fact that this increase was not observed in DHF patients (data not shown) is in line with our discussion where metabolic changes are associated with liver function, especially in the more severe cases of disease caused by DENV infection.
Tyrosine content was significantly increased in DHFs subjects compared to that in DFp subjects. Alterations in the metabolism of phenylalanine, a precursor of tyrosine, have been reported in infectious diseases, including disease caused by DENV infection (22), which could cause an increase in the plasma tyrosine concentration as infection worsens. Corroborating this result, the sPLS-DA statistics showed a significant increase in plasma tyrosine concentrations in DHFp and DHFs patients from the onset of symptoms to the defervescence phase of the disease (Table 3). Although our analysis did not indicate that phenylalanine is a potential metabolite marker, an increase in the plasma phenylalanine concentrations (22) and in intracellular tyrosine concentrations (18) have been reported in DENV infection.
In the context of the inflammatory character of dengue disease, the decrease in plasma histidine concentrations in DHFp and DHFs subjects compared to those in ND subjects suggests an increase in its conversion to histamine (Fig. 6). An interesting observation was the fact that ND subjects presented a longitudinal increase in the histidine concentration, indicating that the decrease in the plasma histidine concentration is specific not only to inflammatory DENV infection but also to disease severity (Fig. 7).
The results presented in this work add to the ongoing discussion that viruses are metabolic engineers. In this regard, we have already demonstrated that the replication of DENV in human hepatic cells institutes the virus's own metabolic program, which is related to mitochondrial dysfunction (50) and alterations in the secretion pattern of specific molecules (12).
One of the strengths of this work, as already mentioned, is the fact that the results are specific to DENV infection and dengue disease, since control subjects represented a group of nonhealthy individuals presenting with a nonspecific infection. We believe that it is possible to distinguish nonhealthy individuals who seek health care and DENV-infected subjects by the use of markers of DENV disease. Our results therefore could be of great value for both the screening of patients and the diagnosis of disease. Additionally, we were able to stratify samples not only according to disease severity but also according to primary and secondary infections, suggesting that these results could be used for prognostic purposes. Multivariate statistics robustly demonstrated that the plasma metabolome of DENV-infected subjects could be discriminated from the plasma metabolome of ND subjects. Likewise, DF and DHF in patients with primary and secondary infections cause particular metabolic changes in plasma. Since the multivariate statistical analysis considers repetitive measures, all the observed changes took into account individual responses, which shows the consistency of the results. Additionally, we also observed that longitudinal variations in plasma metabolite concentrations were involved in the discrimination of all groups, irrespective of the infection type. Our results are in agreement with those recently presented in the literature that point to the fact that metabolomics is a powerful tool to identify biomarkers of DENV infection and dengue disease outcomes (51). The results of an exploratory metabolomics analysis, as already pointed out (22), reflect the combined result of DENV replication in different cells and tissues and the responses to the intricate mechanisms underlying DENV-host interactions. Even though the multilevel multivariate analysis revealed that plasma metabolome changes are, indeed, affected by the severity of disease caused by DENV infection, other factors would be involved in the distinct metabolic patterns observed. The reason for that is the fact that differences in metabolite concentrations were not always confirmed by univariate analysis. Nevertheless, taken together, these results suggest that monitoring of the plasma metabolome during the course of DENV infection could help discriminate individuals at risk of development of severe disease and predict the outcome of disease. Moreover, the specific changes observed in the current study related to a decrease in LDL/VLDL and glutamine concentrations are highly suggestive of liver dysfunction, setting this organ as a central target for monitoring for prognostic purposes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christian Ludwig from the Institute of Metabolism and Systems Research, University of Birmingham, for the assistance with the use of the software NMRLab to process the NMR spectra.
T.E.-B. was responsible for the conception of the study, performed the majority of the experiments, analyzed the data, and prepared the manuscript; C.J.S. designed the statistical models and analyzed the data; F.C.L.A. assisted with the study design and with the NMR and data analyses; M.T.C. and E.T.D.A.M. were responsible for the cohort, provided the samples, and assisted with data interpretation; and A.T.D.P. was responsible for the conception of the study and formulation of research questions and assisted with data interpretation.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00187-16.
REFERENCES
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2015. Dengue and severe dengue. Fact sheet no. 117. Updated May 2015 World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs117/en/. [Google Scholar]
- 3.Brazil Ministry of Health. 2015. Boletim epidemiológico, vol 48 http://portalsaude.saude.gov.br/index.php/situacao-epidemiologica-dados-dengue. [Google Scholar]
- 4.World Health Organization. 2009. Dengue guidelines for diagnosis, treatment, prevention and control, 3rd ed World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 5.World Health Organization. 2011. Comprehensive guidelines for prevention and control of dengue and dengue hemorrhagic fever. Revised and expanded edition. World Health Organization, Regional Office for South-East Asia, New Delhi, India. [Google Scholar]
- 6.Nascimento EJ, Hottz ED, Garcia-Bates TM, Bozza F, Marques ET Jr, Barratt-Boyes SM. 2014. Emerging concepts in dengue pathogenesis: interplay between plasmablasts, platelets, and complement in triggering vasculopathy. Crit Rev Immunol 34:227–240. doi: 10.1615/CritRevImmunol.2014010212. [DOI] [PubMed] [Google Scholar]
- 7.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, Rey LC, Dietze R, Luz K, Rivas E, Miranda Montoya MC, Cortés Supelano M, Zambrano B, Langevin E, Boaz M, Tornieporth N, Saville M, Noriega F, CYD15 Study Group. 2015. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 8.Brasier AR, Zhao Y, Wiktorowicz JE, Spratt HM, Nascimento EJ, Cordeiro MT, Soman KV, Ju H, Recinos A III, Stafford S, Wu Z, Marques ET Jr, Vasilakis N. 2015. Molecular classification of outcomes from dengue virus-3 infections. J Clin Virol 64:97–106. doi: 10.1016/j.jcv.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nascimento EJ, Braga-Neto U, Calzavara-Silva CE, Gomes AL, Abath FG, Brito CA, Cordeiro MT, Silva AM, Magalhaes C, Andrade R, Gil LH, Marques ET Jr. 2009. Gene expression profiling during early acute febrile stage of dengue infection can predict the disease outcome. PLoS One 4:e7892. doi: 10.1371/journal.pone.0007892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pando-Robles V, Oses-Prieto JA, Rodríguez-Gandarilla M, Meneses-Romero E, Burlingame AL, Batista CV. 2014. Quantitative proteomic analysis of Huh-7 cells infected with dengue virus by label-free LC-MS. J Proteomics 5:16–29. doi: 10.1016/j.jprot.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Albuquerque LM, Trugilho MR, Chapeaurouge A, Jurgilas PB, Bozza PT, Bozza FA, Perales J, Neves-Ferreira AG. 2009. Two-dimensional difference gel electrophoresis (DiGE) analysis of plasmas from dengue fever patients. J Proteome Res 8:5431–5441. doi: 10.1021/pr900236f. [DOI] [PubMed] [Google Scholar]
- 12.Higa LM, Caruso MB, Canellas F, Soares MR, Oliveira-Carvalho AL, Chapeaurouge DA, Almeida PM, Perales J, Zingali RB, Da Poian AT. 2008. Secretome of HepG2 cells infected with dengue virus: implications for pathogenesis. Biochim Biophys Acta 1784:1607–1616. doi: 10.1016/j.bbapap.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Nie CY, Han T, Zhang L, Li Y, Liu H, Xiao SX, Li Y, Kang H, Liu SY. 2014. Cross-sectional and dynamic change of serum metabolite profiling for hepatitis B-related acute-on-chronic liver failure by UPLC/MS. J Viral Hepat 21:53–63. doi: 10.1111/jvh.12122. [DOI] [PubMed] [Google Scholar]
- 14.Saito T, Sugimoto M, Igarashi K, Saito K, Shao L, Katsumi T, Tomita K, Sato C, Okumoto K, Nishise Y, Watanabe H, Tomita M, Ueno Y, Soga T. 2013. Dynamics of serum metabolites in patients with chronic hepatitis C receiving pegylated interferon plus ribavirin: a metabolomics analysis. Metabolism 62:1577–1586. doi: 10.1016/j.metabol.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Cassol E, Misra V, Holman A, Kamat A, Morgello S, Gabuzda D. 2013. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis 13:203. doi: 10.1186/1471-2334-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu C, Jiang Z, Fan X, Liao G, Li S, He C, Han L, Luo S, Liu Y, Lin H, Li L, Li X, Liang Q, Wang Y, Luo G. 2012. A metabonomic approach to the effect evaluation of treatment in patients infected with influenza A (H1N1). Talanta 100:51–56. doi: 10.1016/j.talanta.2012.07.076. [DOI] [PubMed] [Google Scholar]
- 17.Munshi SU, Taneja S, Bhavesh NS, Shastri J, Aggarwal R, Jameel S. 2011. Metabonomic analysis of hepatitis E patients shows deregulated metabolic cycles and abnormalities in amino acid metabolism. J Viral Hepat 18:e591–e602. doi: 10.1111/j.1365-2893.2011.01488.x. [DOI] [PubMed] [Google Scholar]
- 18.Birungi G, Chen SM, Loy BP, Ng ML, Li SF. 2010. Metabolomics approach for investigation of effects of dengue virus infection using the EA.hy926 cell line. J Proteome Res 9:6523–6534. doi: 10.1021/pr100727m. [DOI] [PubMed] [Google Scholar]
- 19.Shahfiza N, Osman H, Hock TT, Shaari K, Abdel-Hamid AH. 2015. Metabolomics for characterization of gender differences in patients infected with dengue virus. Asian Pac J Trop Med 8:451–456. doi: 10.1016/j.apjtm.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Tiziani S, Lodi A, Khanim FL, Viant MR, Bunce CM, Günther UL. 2009. Metabolomic profiling of drug responses in acute myeloid leukaemia cell lines. PLoS One 4:e4251. doi: 10.1371/journal.pone.0004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westerhuis JA, van Velzen EJ, Hoefsloot HC, Smilde AK. 2010. Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metabolomics 6:119–128. doi: 10.1007/s11306-009-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui L, Lee YH, Kumar Y, Xu F, Lu K, Ooi EE, Tannenbaum SR, Ong CN. 2013. Serum metabolome and lipidome changes in adult patients with primary dengue infection. PLoS Negl Trop Dis 7:e2373. doi: 10.1371/journal.pntd.0002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordeiro MT, Silva AM, Brito CA, Nascimento EJ, Magalhães MC, Guimarães GF, Lucena-Silva N, de Carvalho EM, Marques ET Jr. 2007. Characterization of a dengue patient cohort in Recife, Brazil. Am J Trop Med Hyg 77:1128–1134. [PubMed] [Google Scholar]
- 24.Dona AC, Jiménez B, Schäfer H, Humpfer E, Spraul M, Lewis MR, Pearce JT, Holmes E, Lindon JC, Nicholson JK. 2014. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal Chem 86:9887–9894. doi: 10.1021/ac5025039. [DOI] [PubMed] [Google Scholar]
- 25.Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, Nicholson JK. 2007. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc 2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 26.Dieterle F, Ross A, Schlotterbeck G, Senn H. 2006. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem 78:4281–4290. [DOI] [PubMed] [Google Scholar]
- 27.Parsons HM, Ludwig C, Günther UL, Viant MR. 2007. Improved classification accuracy in 1- and 2-dimensional NMR metabolomics data using the variance stabilizing generalised logarithm transformation. BMC Bioinformatics 8:234. doi: 10.1186/1471-2105-8-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludwig C, Günther UL. 2011. MetaboLab—advanced NMR data processing and analysis for metabolomics. BMC Bioinformatics 12:366. doi: 10.1186/1471-2105-12-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lê Cao K-A, González I, Déjean S. 2009. intergrOmics: an R package to unravel relationships between two omics data sets. Bioinformatics 25:2855–2856. doi: 10.1093/bioinformatics/btp515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González I, Lê Cao K-A, Déjean S. 2011. mixOmics: Omics Data Integration Project 2011. mixOmics, Toulouse, France: http://www.mixomics.org. [Google Scholar]
- 31.R Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- 32.Pinto J, Barros AS, Domingues MR, Goodfellow BJ, Galhano E, Pita C, Almeida MDC, Carreira IM, Gil AM. 2015. Following healthy pregnancy by NMR metabolomics of plasma and correlation to urine. J Proteome Res 14:1263–1274. doi: 10.1021/pr5011982. [DOI] [PubMed] [Google Scholar]
- 33.Wijeyesekera A, Selman C, Barton RH, Holmes E, Nicholson JK, Withers DJ. 2012. Metabotyping of long-lived mice using 1H NMR spectroscopy. J Proteome Res 11:2224–2235. doi: 10.1021/pr2010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Gorp EC, Suharti C, Mairuhu AT, Dolmans WM, van der Ven J, Demacker PN, van der Meer JW. 2002. Changes in the plasma lipid profile as a potential predictor of clinical outcome in dengue hemorrhagic fever. Clin Infect Dis 34:1150–1153. doi: 10.1086/339539. [DOI] [PubMed] [Google Scholar]
- 35.Biswas HH, Gordon A, Nuñez A, Perez MA, Balmaseda A, Harris E. 2015. Lower low-density lipoprotein cholesterol levels are associated with severe dengue outcome. PLoS Negl Trop Dis 9:e0003904. doi: 10.1371/journal.pntd.0003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soto-Acosta R, Mosso C, Cervantes-Salazar M, Puerta-Guardo H, Medina F, Favari L, Ludert JE, del Angel RM. 2013. The increase in cholesterol levels at early stages after dengue virus infection correlates with an augment in LDL particle uptake and HMG-CoA reductase activity. Virology 442:132–147. doi: 10.1016/j.virol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Samsa MM, Mondotte JA, Iglesias NG, Assunção-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. 2009. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog 5:e1000632. doi: 10.1371/journal.ppat.1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heaton NS, Randall G. 2010. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8:422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell JD, Brown JC, Nicholson JK, Sadler PJ. 1987. Assignment of resonances for ‘acute-phase’ glycoproteins in high resolution proton NMR spectra of human blood plasma. FEBS Lett 215:311–315. doi: 10.1016/0014-5793(87)80168-0. [DOI] [PubMed] [Google Scholar]
- 40.Halstead SB. 2014. Dengue antibody-dependent enhancement: knowns and unknowns. Microbiol Spectr 2:6. doi: 10.1128/microbiolspec.AID-0022-2014. [DOI] [PubMed] [Google Scholar]
- 41.Dunham CM, Fabian M, Siegel JH, Gettings L. 1991. Hepatic insufficiency and increased proteolysis, cardiac output, and oxygen consumption following hemorrhage. Circ Shock 35:78–86. [PubMed] [Google Scholar]
- 42.Newsholme P, Curi R, Pithon Curi TC, Murphy CJ, Garcia C, Pires de Melo M. 1999. Glutamine metabolism by lymphocytes, macrophages, and neutrophils: its importance in health and disease. J Nutr Biochem 10:316–324. doi: 10.1016/S0955-2863(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 43.Fontaine KA, Camarda R, Lagunoff M. 2014. Vaccinia virus requires glutamine but not glucose for efficient replication. J Virol 88:4366–4374. doi: 10.1128/JVI.03134-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers JW, Maguire TG, Alwine JC. 2010. Glutamine metabolism is essential for human cytomegalovirus infection. J Virol 84:1867–1873. doi: 10.1128/JVI.02123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vastag L, Koyuncu E, Grady SL, Shenk TE, Rabinowitz JD. 2011. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog 7:e1002124. doi: 10.1371/journal.ppat.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fontaine KA, Sanchez EL, Camarda R, Lagunoff M. 2015. Dengue virus induces and requires glycolysis for optimal replication. J Virol 89:2358–2366. doi: 10.1128/JVI.02309-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tollinger CD, Vreman HJ, Weiner MW. 1979. Measurement of acetate in human blood by gas chromatography: effects of sample preparation, feeding, and various diseases. Clin Chem 25:1787–1790. [PubMed] [Google Scholar]
- 48.Nchoutmboube JA, Viktorova EG, Scott AJ, Ford LA, Pei Z, Watkins PA, Ernst RK, Belov GA. 2013. Increased long chain acyl-CoA synthetase activity and fatty acid import is linked to membrane synthesis for development of picornavirus replication organelles. PLoS Pathog 9:e1003401. doi: 10.1371/journal.ppat.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishida H, Kato T, Takehana K, Tatsumi T, Hosui A, Nawa T, Kodama T, Shimizu S, Hikita H, Hiramatsu N, Kanto T, Hayashi N, Takehara T. 2013. Valine, the branched-chain amino acid, suppresses hepatitis C virus RNA replication but promotes infectious particle formation. Biochem Biophys Res Commun 437:127–133. doi: 10.1016/j.bbrc.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 50.El-Bacha T, Midlej V, Pereira da Silva AP, Silva da Costa L, Benchimol M, Galina A, Da Poian AT. 2007. Mitochondrial and bioenergetic dysfunction in human hepatic cells infected with dengue 2 virus. Biochim Biophys Acta 1772:1158–1166. doi: 10.1016/j.bbadis.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Voge NV, Perera R, Mahapatra S, Gresh L, Balmaseda A, Loroño-Pino MA, Hopf-Jannasch AS, Belisle JT, Harris E, Blair CD, Beaty BJ. 2016. Metabolomics-based discovery of small molecule biomarkers in serum associated with dengue virus infections and disease outcomes. PLoS Negl Trop Dis 10:e0004449. doi: 10.1371/journal.pntd.0004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.