Abstract
In 2014, NAFLD was confirmed as the fastest growing aetiology for hepatocellular cancer in the USA. However, 2014 also saw progress in our understanding of the heritability and pathogenesis of NAFLD, and an important clinical trial targeting the farnesoid X receptor pathway has illustrated advances in developing a pharmacological therapy.
NAFLD has emerged as the most important liver disease of this decade, which is in part related to the global epidemic of obesity and type 2 diabetes mellitus—two of the most common risk factors for NAFLD.1 However, in many parts of the world, NAFLD is increasing in prevalence even in those without traditional risk factors.2 The key histological phenotypes, a fatty liver or NASH, can be produced via a multitude of genetic, molecular and metabolic perturbations that converge on common cellular pathways driving the histological phenotype of the disease. 2014 has seen advances in many aspects of the disease, including mechanisms of cardiovascular risk, identification of novel gene associations, noninvasive assessment and therapeutic agents. Here, we highlight some of these findings that are likely to have a major influence on the field.
Cardiovascular disease remains the leading cause of death in patients with NAFLD, followed by malignancy and liver disease.3 Importantly, the increased morbidity and mortality owing to cardiovascular disease, liver disease and cancer is attributable to NASH—the more‐aggressive phenotype of NAFLD. Siddiqui et al.4 systematically evaluated the atherogenic risk profile in patients with NAFLD compared with lean or obese individuals without NAFLD as controls. The researchers confirmed an increase in various factors—including insulin, triglycerides, apolipoprotein B, VLDL particle size and concentration, LDL particle concentration, small‐dense LDL‐cholesterol, and percentage of small‐dense LDL‐cholesterol—in patients with NAFLD compared with both lean and obese controls. These data extended our prior understanding that NAFLD is associated with increased hepatic triglyceride and cholesterol synthesis to show an association with larger triglyceride and cholesterol‐rich VLDL particles. Large VLDL particles are incompletely hydrolyzed by peripheral lipoprotein lipase, yielding intermediate density lipoproteins and LDLs that contain excess triglyceride. Excess triglyceride renders LDL‐cholesterol more susceptible to hepatic lipases, which breaks it into multiple small‐dense LDL particles. Small‐dense LDL‐cholesterol is more atherogenic than larger particle LDL.5 Together, these data further our understanding of how NAFLD can increase atherogenic risk. Long‐term studies are now needed to determine if these changes translate into worse patient outcomes or if therapies directed at reducing VLDL triglyceride are needed in addition to statins in patients with NAFLD.
An area of considerable interest is the influence of genetics on NAFLD. In a paper published by Kozlitina et al.6 in 2014, a single nucleotide polymorphism in TM6SF2, a gene whose function is currently unknown, has been associated with the presence of liver fat in an exome‐wide association study of patients from the Dallas Heart Study. TM6SF2 is more common in individuals of European ancestry than PNPLA3, a gene that has been shown to be associated with NAFLD. In two large European cohorts, patients homozygous for the target allele had more hepatic fat, higher alanine aminotranferase levels and lower plasma triglyceride levels compared with those without it.6 Additional in vivo studies indicated that loss‐of‐function of the protein encoded by TM6SF2, as seen in mice with the variant allele, led to hepatic triglyceride accumulation, recapitulating the human disease phenotype.6 These findings provide additional clues about the underlying complexity of genetic predisposition and potential pathways responsible for NAFLD development.
Of increasing concern is the association between NAFLD and hepatocellular carcinoma (HCC). Although traditional dogma states that nearly all HCC occurs in the setting of cirrhosis, it is now widely appreciated that HCC can develop in the absence of cirrhosis in up to 50% of cases.7 Wong et al.8 analysed the US national transplant database from 2002–2012 to ascertain the prevalence of HCC in liver transplant recipients. Of 10,061 patients with HCC who underwent liver transplantation, NASH was the second most common underlying risk factor for HCC after hepatitis C; however, the prevalence of NASH‐related HCC has increased nearly fourfold compared with approximately 2.5‐fold for hepatitis.3 The increase in NASH‐related HCC persisted after controlling for diabetes and obesity, two independent risk factors for HCC commonly associated with NAFLD. This association provides important evidence that NASH is a driver of HCC leading to transplantation. These data should inform public health and policy decisions to support population‐based approaches for the prevention, early detection and treatment of NASH.
Currently, the only accurate method to diagnose NASH is liver biopsy. Biopsy has numerous limitations such as patient discomfort, risk and sampling variability. Such limitations have provided impetus to a search for noninvasive methods to accurately diagnose NASH. Bastati and colleagues9 published a proof‐of‐concept study in which patients with NAFLD underwent a gadoxetic‐acid‐enhanced MRI. The ability to distinguish NASH from isolated hepatic steatosis was achieved with a probability, sensitivity and specificity of 85%, 97% and 63%, respectively.9 One important limitation of this technique is that similar changes can be seen in the setting of major fibrosis and other causes of liver injury. Therefore, the reported poor specificity might have been related to substantial fibrosis in the NASH cohort. Despite these drawbacks, this study suggests that it might eventually be feasible to establish a noninvasive profile of NAFLD that identifies the population at risk of worse outcomes and disease progression, and to assess response to therapy.
The role of bile acids in metabolic disease and NASH is receiving increased focus. An important trial performed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) NASH Clinical Research Network demonstrated the efficacy and safety of obeticholic acid for the treatment of NASH.10 Obeticholic acid is an agonist of the farnesoid X receptor, which has been shown to increase insulin sensitivity and glucose disposal, and reduce hyper‐triglyceridaemia and liver enzyme levels in patients with suspected NAFLD (Figure 1). In the FLINT trial, 283 patients with NASH were randomly allocated to receive obeticholic acid (25 mg daily) or placebo for 72 weeks.10 The primary end point was an improvement in the NAFLD activity score by ≥2; in 46% of those on obeticholic acid an improvement in NAFLD activity score was seen, compared with 21% of those on placebo (P <0.001). Importantly, all of the histological features of NASH such as steatosis, lobular inflammation, ballooning and fibrosis improved substantially in the obeticholic acid group. These exciting data are, however, tempered by the observation of a mean 6 mg/dl (equivalent to 0.16 mmol/l) increase in LDL‐cholesterol and a 1 mg/dl (0.3 mmol/l) drop in HDL‐cholesterol in the experimental group. The clinical significance of these lipid changes is unclear and needs further clarification. Also, whether a decrease in all the features of NASH in the short term (72 weeks) translates into a decrease in progression to cirrhosis will require long‐term trials. Nonetheless, the FLINT trial does provide proof that all of the features of NASH can be improved with pharmacological therapy and sets the stage for larger long‐term trials both with farnesoid X receptor agonists and other compounds that will hopefully provide the evidence‐base needed to have effective therapies available for patients with NASH.
Figure 1.
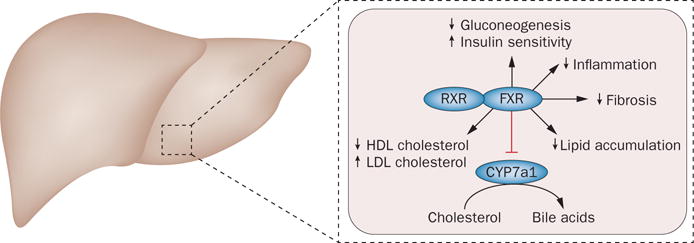
Potential effects of obeticholic acid on NASH. Bile acid ligands bind to FXR, which forms a heterodimer with RXR and reduces bile acid synthesis via CYP7a1 inhibition. FXR is involved in many metabolic processes that regulate lipid and glucose metabolism. Obeticholic acid, a semi-synthetic bile acid ligand for FXR, 100× more potent than its natural ligand, might be able to ameliorate several metabolic derangements seen in NASH such as hepatic steatosis, glucose tolerance, inflammation and even hepatic fibrosis by inhibiting hepatic stellate cell activity. Abbreviations: CYP7a1, cytochrome P450 7A1; FXR, farnesoid X receptor; RXR, retinoid X receptor.
In summary, the pace of progress in NAFLD is accelerating with rapid translation of basic discoveries towards diagnostics and therapeutics that will help alleviate the burden of disease. Particularly, the possibility of diagnosing the condition noninvasively and effectively reversing the condition with pharmacological therapy seems well within reach in the next few years.
Key advances.
-
■
TM6SF2 links genetics to NASH6
-
■
NAFLD is the most rapidly increasing aetiology of hepatocellular cancer requiring liver transplantation8
-
■
NAFLD extends the atherogenic risk profile beyond increased triglycerides and low HDL-cholesterol to include increased small-dense LDL-cholesterol5
-
■
MRI-based techniques might enable distinction of steatohepatitis from a fatty liver9
-
■
Farnesoid X receptor agonists can reverse all of the individual histological components of NASH but is associated with a modest increase in LDL-cholesterol10
Acknowledgments
This work of the authors is supported in part by a grant from the NIH T32 DK 07150 and RO1 DK 81410 to A.J.S.
Footnotes
Competing interests
M.E.R. has served as a consultant to AbbVie, Fibrogen, Genentech, NGM Biopharmaceuticals, Takeda and W.L. Gore and Associates. A.J.S. has stock options in Genfit. He has served as a consultant to AbbVie, Astra Zeneca, Exhalenz, Fibrogen, Genfit, Immuron, Nimbus, Nitto Denko, Salix, Takeda and Tobira. He has been an unpaid consultant to Echosens and Intercept. His institution has received grant support from Gilead, Novartis, Salix and Tobira.
References
- 1.Neuschwander-Tetri BA, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 3.Dunn W, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqui MS, et al. Severity of nonalcoholic fatty liver disease and progression to cirrhosis associate with atherogenic lipoprotein profile. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2014.10.008. http://dx.doi.org/10.1016/j.cgh.2014.10.008. [DOI] [PMC free article] [PubMed]
- 5.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002;90:89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 6.Kozlitina J, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 9.Bastati N, et al. Noninvasive differentiation of simple steatosis and steatohepatitis by using gadoxetic acid-enhanced MR imaging in patients with nonalcoholic fatty liver disease: a proof-of-concept study. Radiology. 2014;271:739–747. doi: 10.1148/radiol.14131890. [DOI] [PubMed] [Google Scholar]
- 10.Neuschwander-Tetri BA, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. doi: 10.1016/S0140-6736(14)61933-4. http://dx.doi.org/10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed]


