Abstract
Blackberry aqueous extract acidified with 2 % citric acid was spray-dried using gum Arabic (GA) and polydextrose (PD) as encapsulating agents at concentrations of 10 and 15 % and temperatures of 140 to 160 °C. All powders presented high solubility, ranging from 88.2 to 97.4 %, and the encapsulation conditions did not significantly affect the hygroscopicity. The powders produced with gum Arabic showed higher brightness than those with polydextrose. The anthocyanins retention in the microcapsules was 878.32 to 1300.83 mg/100 g, and the phenolics was 2106.56 to 2429.22 mg (GAE)/100 g. The antioxidant activity was quantified according to DDPH and ABTS methods, with values ranging from 31.28 to 40.26 % and 27 to 45.15 %, respectively. The microscopy showed spherical particles for both encapsulating agents, and smooth surface with some concavities with the gum Arabic, and smooth or slightly rough surface when using polydextrose. The Pearson correlation coefficient showed a high correlation between the color parameters, L*, a*, b*, Hue, Chroma and browning index (BI), which were also strongly correlated with anthocyanins. Phenolic presented correlation with DPPH and ABTS values. The results showed that the best encapsulation condition was atomization at 140 °C and 15 % gum Arabic.
Keywords: Blackberry, Spray drying, Anthocyanins, Antioxidant activity
Introduction
Besides being a source of fiber, minerals, and vitamins C and A (Turemis et al. 2003), blackberry is rich in phenolic compounds such as quercetin, gallic acid (Elisia et al. 2007), catechins (Tsanova-Savova et al. 2005), epicatechins (Jakobek et al. 2009), epigallocatechin (Pascual-Teresa et al. 2000), and anthocyanins (Wang and Lin 2000). These compounds, especially the anthocyanins, give the blackberry high antioxidant activity (Elisia et al. 2007; Jakobek et al. 2007).
The basic chemical structure of anthocyanins is the flavylium cation, which consists of two aromatic rings connected by the chain of three carbon atoms and an oxygen bridge. The molecule may be glycosylated linked to sugar molecules (Francis 1989), but in some cases it can be acylated with organic aromatic or aliphatic acids (cinnamic acid, malonic acid, and acetic acid, to name a few) through ester bonds (He and Giusti 2010). Anthocyanins are responsible for the colors violet, red and blue in flowers and fruits. The antioxidant activity of anthocyanins plays important health benefits such as anti-inflammatory effect, prevention of neuronal and cardiovascular diseases, diabetes (Tulio et al. 2008), and cancer treatments. The predominant anthocyanin in blackberry is the cyanidin-3-glucoside (Wang et al. 1997).
The efficiency and stability of anthocyanins and other phenolic compounds in blackberry can be affected by many factors, including the presence of light, enzymes such as polyphenol oxidase and peroxidase, ascorbic acid (Francis 1989), pH (>3), moisture and oxygen (Fang and Bhandari 2011). In this context, microencapsulation of anthocyanins may be a good alternative for maintaining the stability of these bioactive compounds when extracted from foods.
Spray drying is one of the most widely used microencapsulation techniques, since it provides rapid evaporation of water and maintains the low temperature in the particles. Prior to spray drying, the wall material is mixed with the suspension containing encapsulated components through intensive homogenization. According to Fang and Bhandari (2012), spray drying is the most suitable method for the microencapsulation of polyphenols.
Gum Arabic is the most commonly used encapsulating agents in spray drying, due to its high soluble fiber content, prebiotic effect, high digestive tolerance, and low caloric value (Badreldin et al. 2009). In addition, it is suitable for various formulations of functional foods once it is non-cariogenic.
Polydextrose is a non-digestible polysaccharide, which was used as encapsulating agent in the present study as a new alternative for microencapsulation. It is soluble, has low caloric value (approximately 1 kcal/g), and is used as sugar substitute. Polydextrose is randomly bounded into the water molecule, has an average degree of polymerization about 10 glucose residues, and is obtained by thermal polymerization of D-glucose in the presence of sorbitol and phosphoric acid (Ribeiro et al. 2003).
The objective of the present study was to evaluate the encapsulation of bioactive compounds from blackberry aqueous extract by spray drying technique, using gum Arabic and polydextrose as wall materials.
Material and methods
Material
The blackberry cultivar Tupy, from the city of Vacaria in the state of Rio Grande do Sul, Brazil was acquired in frozen form and remained stored in a freezer at −18 ± 2 °C until used.
Experimental procedure
The fruits were thawed and cut into slices with 3 mm thickness. Then, in order to inactivate both peroxidase and polyphenol oxidase enzymes, the fruit was subjected to blanching in which the slices were placed in an autoclave, generating steam at 100 °C at atmospheric pressure for 2 min. After blanching, the sample was rapidly cooled in an ice bath for 3 min. Water acidified with citric acid 2 % (w/v) at a ratio of 1:3 (fruit: water) was added to the blanched sample. After 4 h kept in the dark, the suspension was filtered through Whatman filter paper No.1 for the separation of insoluble solids, obtaining the blackberry aqueous extract (pH 2.5).
The blackberry extract was mixed to the encapsulating agent under stirring using Ultra Turrax (IKA/T25) for 4 min at 6000 rpm until complete dissolution. Gum Arabic Instantgum BA (Nexira Brasil Comercial Ltda, São Paulo, Brasil) and Polydextrose (MasterSense Ing Alim Ltda Sao Paulo, Brasil) at concentrations of 10 and 15 % were used as encapsulating agents. The spray drying process was performed in a two-fluid pneumatic Mini Spray Dryer-LM 1.0 MSDI, tire type (LABMAQ, Brasil), with 1.0 mm diameter nozzle. The mixture was fed into the chamber through a peristaltic pump at a flow rate of 0.60 L/h; temperature of drying air 140 and 160 °C; compressed air pressure of 3.5 kgf/cm2 and airflow of 40.5 L/h.
The powders were characterized for solubility, hygroscopicity, anthocyanins content, phenolic compounds, antioxidant activity, and microstructure. All analyses were performed in triplicate and in the absence of light.
Determination of anthocyanins
The total anthocyanin content was determined by the pH differential (Giusti and Wrolsted 2001) method. The powder was dissolved in KCl buffer (0.025 M, pH 1.0) and CH3COONa (0.4 M, pH 4.5) at a ratio of 1:90. To quantify anthocyanin content in the blackberry extract, the same buffer was used in the ratio of 1:20.
The absorbance (A) was measured in UV-visible (Shimadzu-1800) spectrophotometer at 520 and 700 nm and the results were calculated according to the equation:
| 1 |
where A520 is the sample absorbance at 520 nm, and A700 is the sample absorbance at 700 nm.
The concentration of monomeric anthocyanins (MA) was calculated according to Eq. 2:
| 2 |
where M is the molar mass ratio of cyanidin-3-glucoside (449.2 g.mol−1), DF is the dilution factor, ε is the molar extinction coefficient (26,900 L.mol−1.cm−1), l is the optical path for the cuvette (1 cm).
Determination of total phenolics
The phenolic content in the blackberry powder was determined by the Folin-Ciocalteu method, according to the protocol described by Singleton and Rossi (1965) with modifications. For this, 0.5 g blackberry powder was resuspended in 20 mL distilled water. For the extract, an aliquot of 1 mL was placed in 25 mL volumetric flask and the volume was completed with methanol. Then, 1 mL extract solution or powder was mixed with 10 mL distilled water and 500 μL Folin-Ciocalteu reagent, and allowed to react for 3 min. Then, 1.5 mL sodium carbonate (20 %, w/v) were added, and allowed to stand for 2 h. The reading was performed in UV-visible spectrophotometer (Shimadzu-1800) at 765 nm, using distilled water as blank. The results were expressed in mg gallic acid per 100 g sample.
Determination of antioxidant activity by DPPH and ABTS+
For sample preparation, 1 g blackberry powder was resuspended in 50 mL methanol (50 %, v/v) for DPPH assay, and 20 mL for ABTS. The mixture was homogenized and kept for 60 min at room temperature in the dark. For the blackberry extract, a 1 mL aliquot was added to 3 mL methanol (50 % v/v) for DPPH assay, and 2 mL for ABTS, homogenized and kept for 60 min at room temperature in the dark.
DPPH values were determined according to the method described by Brand-Williams et al. (1995) based on the capture of DPPH radicals by antioxidants. To prepare 60 mM DPPH solution, 2.4 mg DPPH was resuspended in 100 mL methanol. The solution was homogenized and transferred to dark bottles, and used only on the day of analysis. Aliquots of 0.1 mL blackberry powder solution or extract were transferred to test tubes containing 3.9 mL DPPH solution, and homogenized. An aliquot of 0.1 mL methanol (50 %, v/v) and 3.9 mL DPPH solution was used as control. After 45 min, the scavenging activity was monitored by spectrophotometer at 515 nm by the decrease in absorbance, using methanol as a blank. The results were expressed as a percentage of radical sequestered:
| 3 |
The antioxidant activity by ABTS was determined by the method described by Re et al. (1999). The radical cation ABTS was generated through a reaction between 7 mM stock solution of ABTS (prepared by dissolving 192 mg ABTS in 50 mL distilled water) and 140 mM potassium persulfate solution (378.4 mg potassium persulfate in 10 mL distilled water). This solution was kept in the dark for 12 h at room temperature before use. The ABTS solution was diluted with phosphate buffer (5 mM, pH 7.0) to obtain an absorbance of 0.7 (± 0.02) at 734 nm. Aliquots of 10 μL blackberry powder solution or extract were diluted with 1 mL ABTS solution and the absorbance (734 nm) was monitored after 6 min. The results were expressed as percent of sequestered radicals (%) according to Eq. (3).
Determination of powder solubility and hygroscopicity
The solubility was determined according to the method proposed by Cano-Chauca et al (2005), with some modifications. For that, 1 g powder was resuspended in 100 mL distilled water under magnetic stirring for 5 min. Then, the solution was centrifuged at 3000 × g for 15 min and a 25 ml supernatant was transferred to a 50 mL beaker and dried at 105 °C for 24 h. Solubility (%) was calculated by weight difference using the following equation.
| 4 |
Where: Pa (g) is the mass of the beaker plus sample after dried, and Pb (g) is the initial mass of the weighed beaker.
The hygroscopicity was determined in accordance with the method proposed by Tonon et al. (2009). Approximately 1 g powder was weighed into aluminum caps and placed in a container with saturated (75 %) sodium chloride (NaCl). The samples were placed in an incubator (model 411/FDP, Ethik Techology, Brazil) at 25 °C, and weighed at 48 h intervals until equilibrium was reached. The hygroscopicity was expressed in percentage or 1 g of adsorbed moisture per 100 g dry solids (g/100 g) using the following equation (Caparino et al. 2012):
| 5 |
where Δm (g) is the weight increase of the powder after equilibrium was reached, M is the initial mass of the powder and Mi is the free water content in the powder before exposure to moist air environment.
Color measurements
Color measurements were performed by direct readings on a Minolta colorimeter (CR400/410) using CIE Lab system, where L* indicates the lightness (0 – black, 100 - white), a* coordinate varying from green (−60) to red (60), and b* from blue (−60) to yellow (60). The instrument was standardized with a white ceramic plate (L* = 97.47; a* = 0.08; b* = 1.76). The parameters L*, a* and b* were used to calculate Chroma, Hue angle and browning index (BI) according to the following equations:
| 6 |
| 7 |
| 8 |
where,
| 9 |
Scanning electron microscopy
The microstructures of spray-dried samples were observed by scanning electron microscopy according to the method reported by Toneli et al. (2008), which consisted of weighing approximately 1 g sample in double-faced adhesive tape, mounted on stubs and sputter coated with a thin layer of gold in a sputter coater (Bal-Tec, SCD050, New York, USA). The samples were viewed in a scanning electron microscope (JSM 6060, Tokyo, Japan) operating at 10 kV and 1 × 10−6 mbar, using a 3000 × magnification.
Statistical analysis
Three factors were studied: drying air temperature, at levels of 140 and 160 °C; concentration of encapsulating agent in the dispersion, at levels of 10 and 15 %; and a single qualitative variable (encapsulating material); with two repetitions for each treatment. The statistical design used was full factorial experiment. ANOVA was used and the differences between means were compared by Tukey’s test, using SAS 9.3 software (SAS Institute Inc.). Pearson correlation analysis between color parameters (L*, a*, b*, Hue, Chroma and BI), phenolics, anthocyanins, and antioxidant activity of blackberry powder were realized, and correlation coefficients (r) were also calculated with SAS 9.3.
Results and discussion
Centesimal composition
The centesimal composition of blackberry was: 87.45 % moisture, 1.30 % protein, 0.15 % lipids, 0.21 % ash, and 10.89 % carbohydrate content.
Characterization of blackberry extract
The anthocyanin content was 2765.14 ± 5.4 mg/100 g, which was higher than that found by Ivanovic et al. (2014), who found from 1150 to 1380 mg/100 g, and by Elisia et al. (2007) who reported 1710 mg/100 g in blackberry ethanolic extract.
The phenolics content was 10,039 ± 162.5 mg (GAE)/100 g, which was higher than the values found by Wang and Lin (2000) that ranged from 7400 to 9160 mg (GAE)/100 g in the blackberry extract obtained using phosphate buffer (75 mM, pH 7.0). The antioxidant activity of the extract determined by DPPH and ABTS was 55 ± 0.7 % and 67 ± 0.2 %, respectively.
Blackberry extract microcapsules
Solubility and hygroscopicity
Figure 1 shows the solubility (A) and hygroscopicity (B) of the blackberry microparticles. All encapsulated samples were very soluble, with values ranging from 88.2 to 97.4 %. For both wall materials, no significant difference (p > 0.05) was observed in solubility values with increasing temperature and concentration.
Fig. 1.
Solubility (%) (a) and hygroscopicity (%) (b) of spray-dried blackberry powder, at 140 and 160 °C, with 10 and 15 % gum Arabic (GA) and polydextrose (PD). Values expressed as mean ± standard deviation. Same letters indicate no significant difference (p > 0.05)
The hygroscopicity ranged from 14.49 to 19.42 %. Tonon et al. (2009) reported hygroscopicity of 19.74 % for açai powder encapsulated with gum Arabic. Kuck and Noreña (2016) found hygroscopicity values ranged from 11.67 % to 16.61 % for grape skin powder produced with gum Arabic, partially hydrolyzed guar gum and polydextrose. The hygroscopicity of the spray-dried powders did not change significantly (p > 0.05) with the drying temperature and the concentration of the encapsulating agent. Concerning the nature of the encapsulating agent, although the particles spray-dried with gum Arabic presented lower hygroscopicity when compared with polydextrose, these values were not significant (p > 0.05). Gum Arabic is a low hygroscopic material, thus when it is used as encapsulating agent it tends to give less hygroscopic and more stable particles (Fazaeli et al. 2012). According to Tonon et al. (2010), the low hygroscopicity of these particles with respect to the others, results in lower water adsorption and thus, lower molecular mobility.
Anthocyanins and phenolics content
The contents of anthocyanins and phenolic compounds in the powders are shown in Fig. 2a and b, respectively, which decreased when compared to the levels found in the extract. Adams (1973) reported that the exposure of anthocyanins to high temperatures causes the opening of the flavylium ring, resulting in the formation of a chalcone, which is the first step of thermal degradation of anthocyanins.
Fig. 2.
Anthocyanins content (mg/100 g) (a) and phenolics content (mg (GAE)/100 g) (b) of spray-dried blackberry powder, at 140 and 160 °C, with 10 and 15 % gum Arabic (GA) and polydextrose (PD). Values expressed as mean ± standard deviation. Same letters indicate no significant difference (p > 0.05)
The anthocyanins content ranged from 878.32 to 1300.83 mg/100 g on dry basis. The results showed that the anthocyanins content decreased significantly (p < 0.05) with increasing temperature, and the anthocyanin retention was higher in the treatment with gum Arabic than in the treatment with polydextrose (p < 0.05). Adams (1973) reported that high temperatures and pH values in the range from 2 to 4 induce the hydrolysis of the glycosyl groups of the anthocyanin molecule. This hydrolysis leads to a loss of color of anthocyanins, since aglucones are less stable than the glycosylated molecules. Tonon et al. (2008) found that the anthocyanin content decreased from 86.01 to 79.09 % when increasing temperature from 150 to 190 °C in spray-dried açai using maltodextrin as encapsulating agent. According to Quek et al. (2007) powders produced at lower drying temperatures are more prone to adhesion between the particles, thus reducing the exposure to oxygen, and protecting anthocyanins from oxidation. Regarding the type of encapsulating agent, Dangles and Brouillard (1992) stated that gum Arabic is an excellent encapsulating agent that adequately involves the anthocyanin molecule, so that the flavylium cation is less vulnerable to the degradation factors, increasing anthocyanins stability.
Regarding the effect of concentration of the encapsulating agent, the highest concentration caused no significant changes in the anthocyanins, except with polydextrose at 140 °C, which decreased anthocyanins levels (p < 0.05).
With respect to the phenolics content, the values ranged from 2106.56 to 2429.22 mg (GAE)/100 g, and were higher in the samples containing gum Arabic, at 140 °C, since the levels decreased significantly (p < 0.05) at lower temperatures. The losses of phenolic compounds during drying process may be due to the oxidative reactions and elevated temperatures used in spray drying, since some phenolic compounds are thermolabile (Moure et al. 2001).
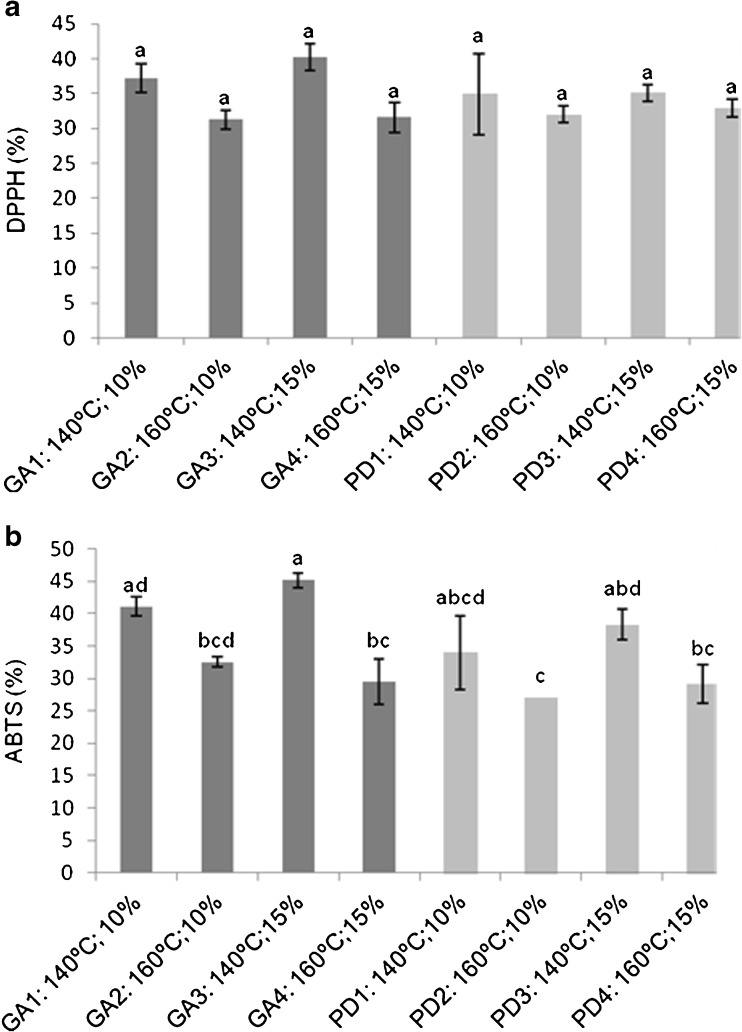
Antioxidant activity
The antioxidant activity of the encapsulated powders was determined by both DPPH and ABTS methods, and the results are shown in Fig. 3a and b, with values ranging from 31.28 to 40.26 %, and 29.15 to 45.15 %, for DPPH and ABTS, respectively. These values were lower than those values found in the blackberry extract, which were 55 and 67 % by DPPH and ABTS, respectively. High temperatures adversely affect the structure of phenolic compounds, causing structural breakage, resulting in the formation of different compounds, thereby reducing the antioxidant activity (Mishra et al. 2013).
Fig. 3.
Antioxidant activity (%) by DPPH (a) and by ABTS (b) of spray-dried blackberry powder, at 140 and 160 °C, with 10 and 15 % gum Arabic (GA) and polydextrose (PD). Values expressed as mean ± standard deviation. Same letters indicate no significant difference (p > 0.05)
No significant difference (p > 0.05) was observed for the antioxidant activity measured by DPPH, for all treatments. For the ABTS, the highest activities were obtained at 140 °C (p < 0.05).
Both methods have the principle of stabilization of radicals by hydrogen atom donation (Sudha et al. 2012). The percent differences in DPPH and ABTS assays may be due to the different stereo selectivity of radicals reaction, and the different compounds in the powder capable to react and eliminate different radicals (Zhu et al. 2008).
Color measurements
The color parameters are presented in Table 1. The powders encapsulated with gum Arabic had higher luminosity than the powders with polydextrose. Increasing the drying temperature and the concentration of encapsulating agents did not significantly (p > 0.05) affect L* parameters of the powder encapsulated with gum Arabic. In contrast, significant (p < 0.05) differences were observed for the powders with polydextrose as encapsulating agent.
Table 1.
Color parameters of blackberry powder obtained by spray drying at 140 to 160 °C, using 10 and 15 % gum Arabic and polydextrose
| Treatment | L* | a* | b* | Hue | Chroma | BI |
|---|---|---|---|---|---|---|
| GA1 | 67.91 ± 0.82ab | 35.42 ± 0.40ª | 4.87 ± 0.11ª | 7.82 ± 0.08ª | 35.75 ± 0.41ª | 41.72 ± 0.43ª |
| GA2 | 68.72 ± 0.26b | 33.73 ± 0.45b | 4.88 ± 0.18ª | 8.23 ± 0.20ª | 34.08 ± 0.47b | 39.78 ± 0.84a |
| GA3 | 68.78 ± 1.63b | 30.78 ± 0.28c | 3.22 ± 0.05b | 5.98 ± 0.04a | 30.95 ± 0.29c | 33.76 ± 0.44b |
| GA4 | 69.63 ± 0.16b | 30.53 ± 0.02c | 3.54 ± 0.01b | 6.62 ± 0.02a | 30.74 ± 0.02c | 34.34 ± 0.07b |
| PD1 | 63.96 ± 0.23c | 40.94 ± 0.06d | 8.26 ± 0.01c | 11.41 ± 0.00ab | 41.76 ± 0.07d | 55.48 ± 0.28c |
| PD2 | 52.40 ± 0.57d | 41.03 ± 0.30d | 9.00 ± 0.67c | 16.76 ± 0.81b | 42.00 ± 0.44d | 60.29 ± 1.13d |
| PD3 | 65.59 ± 1.10ae | 36.81 ± 0.47e | 6.35 ± 0.24d | 9.79 ± 0.24ab | 37.35 ± 0.51e | 46.99 ± 1.61e |
| PD4 | 62.50 ± 0.33c | 38.44 ± 0.04f | 8.46 ± 0.10d | 12.41 ± 0.13ab | 39.36 ± 0.06f | 54.78 ± 0.50c |
Values expressed as mean ± standard deviation
Same lowercase letters in the same column indicate no significant difference (p > 0.05)
10 % gum Arabic: 140 °C (GA1), 160 °C (GA2); 15 % gum Arabic: 140 °C (GA3), 160 °C (GA4). 10 % polydextrose at 140 °C (PD1), 160 °C (PD2); 15 % polydextrose at 140 °C (PD3), 160 °C (PD4)
The a* and b* values reduced significantly (p < 0.05) with increasing concentration of gum Arabic and polydextrose. Jiménez-Aguilar et al. (2011) reported that the coordinate a* is attributed to the anthocyanins content of the fruit, which are responsible for the red color of the spray-dried blueberry microcapsules. According to Ahmed et al. (2010), color changes in products microencapsulated with bioactive compounds can occur due to the formation of polymeric anthocyanins. Furthermore, the relative amounts of flavylium cation (red or orange), quinoidal forms (blue), carbinol pseudobase (colorless) and chalcone (colorless or slightly yellowish) in equilibrium conditions may vary depending on the pH and the anthocyanin structure (Giusti and Wrolstad 2001).
The parameters concentration of encapsulating agent and temperature did not affect the Hue angle, once no significant differences (p > 0.05) were observed for all treatments. Furthermore, the lower values evidenced predominantly red-blue color in the samples. With respect to Chroma, there was a significant reduction (p < 0.05) with increasing the concentration of gum Arabic and polydextrose for all treatments. Concerning the browning index (BI), it did not increase significantly with temperature (p > 0.05) for the powders encapsulated with gum Arabic. In contrast, the powders with polydextrose presented higher BI with increasing temperature (p < 0.05), indicating a higher degree of browning. Browning may occur due to thermal degradation of anthocyanins, especially in the presence of oxygen and enzymes such as polyphenol oxidase, since it catalyzes the oxidation of chlorogenic acid to the corresponding o-quinone, which reacts with anthocyanins, leading to the formation of brown products (Kader et al. 1999).
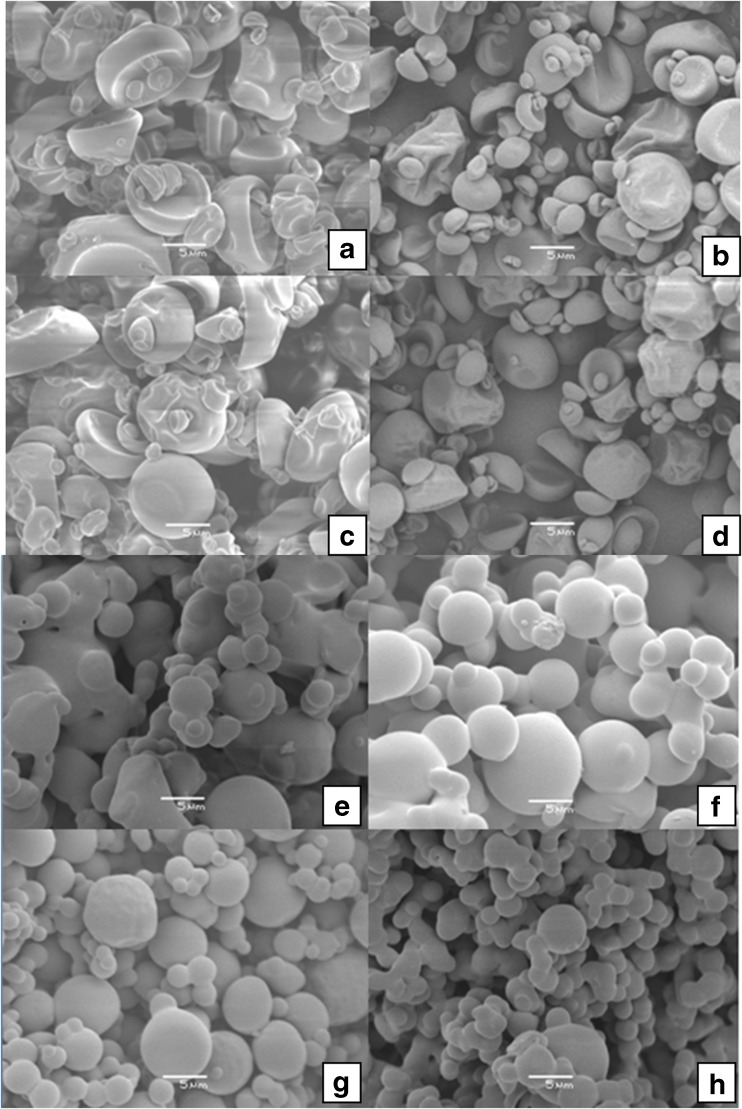
Structure microscopic analysis
The microstructure of the blackberry powder produced by spray drying at 140 and 160 °C was evaluated by scanning electron microscopy. Figure 4 shows the images of the microparticles encapsulated with gum Arabic (Fig. 4a, b, c and d). The microparticles exhibited spherical shape, smooth surface and variable sizes with some concavities. Rosenberg et al. (1985) reported that the concavities are typical of spray-dried samples due to the rapid water evaporation. Aguilera and Stanley (1999) reported that spray-dried products generally result in hollow spherical particles, and the formation of concavities occurs due to shrinkage after hardening of the outer surface of the sphere followed by expansion of air bubbles trapped inside the particle.
Fig. 4.
Microscopic images of the spray-dried blackberry powder with 10 % gum Arabic at 140 °C (a), 160 °C (b); 15 % gum Arabic at 140 °C (c), 160 °C (d); 10 % polydextrose at 140 °C (e), 160 °C (f); 15 % polydextrose at 140 °C (g), 160 °C (h); at 3000× magnification
When polydextrose was used (Figs. 4e, f, g and h), the particles exhibited spherical shape, mostly with smooth surface, but with some slightly rough surface (Fig. 4g and h). The formation of microstructures with rough surfaces is undesirable, since it affects the flow properties of the wall material (Rosenberg et al. 1985). It was also observed that smaller particles were arranged around the larger particles. According to Cano-Chauca et al. (2005), the presence of few cracks and surface pores, and the strong adhesion of smaller particles around the larger ones can be due to the absence of crystalline surfaces, which is characteristic of amorphous products.
The analysis of correlation of Pearson
Pearson analysis was used to assess the correlations between color measurements (L*, a*, b*, Hue, Chroma and BI), phenolics, anthocyanins, and antioxidant activity for the different drying conditions. The Pearson correlation analysis shows the interdependence between the variables and can be seen in Table 2.
Table 2.
Pearson correlation coefficients (r) between color parameters (L*, a*, b*, Hue, Chroma and BI), phenolics, anthocyanins, and antioxidant activity of blackberry powder
| L* | a* | b* | Hue | Chroma | BI | Phenolics | Anthocyanins | DPPH | ABTS | |
|---|---|---|---|---|---|---|---|---|---|---|
| L* | 1.000 | |||||||||
| a* | −0.792 | 1.000 | ||||||||
| b* | −0.833 | 0.966 | 1.000 | |||||||
| Hue | −0.967 | 0.888 | 0.936 | 1.000 | ||||||
| Chroma | −0.799 | 0.999 | 0.973 | 0.896 | 1.000 | |||||
| BI | −0.867 | 0.978 | 0.995 | 0.951 | 0.983 | 1.000 | ||||
| Phenolics | 0.454 | −0.449 | −0.582 | −0.573 | −0.463 | −0.541 | 1.000 | |||
| Anthocyanins | 0.736 | −0.831 | −0.906 | −0.832 | −0.840 | −0.887 | 0.554 | 1.000 | ||
| DPPH | 0.317 | −0.245 | −0.369 | −0.433 | −0.256 | −0.330 | 0.863 | 0.344 | 1.000 | |
| ABTS | 0.552 | −0.422 | −0.569 | −0.639 | −0.437 | −0.538 | 0.950 | 0.516 | 0.925 | 1.000 |
The color parameters, L*, a*, b*, Hue angle, Chroma and BI were highly correlated with each other (r > 0.792), and with anthocyanins (r > 0.736). Phenolic compounds were strongly correlated with the antioxidant activity (DPPH: r = 0.86; ABTS: r = 0.95). Jakobek et al. (2009) reported that the phenolic compounds in blackberry have high antioxidant activity, and Huang et al. (2012) found a positive correlation between the phenolic compounds and antioxidant activity in blueberry.
The results presented above evidenced that the encapsulation with 15 % gum Arabic at 140 °C was the best encapsulation condition, resulting in a powder with good retention of bioactive compounds, with anthocyanins levels of 1279.11 mg/100 g cyanidin-3-glucoside, 2429.22 mg (GAE)/100 g phenolic compounds, 40.26 % antioxidant activity by DPPH, and 45.15 % by ABTS, in addition to the positive results for solubility, hygroscopicity, and color.
Conclusion
The samples encapsulated with gum Arabic and polydextrose under different concentration and temperature conditions showed that the solubility was higher than 88.2 %, and the hygroscopicity was not affected by the conditions used in the encapsulation process. With respect to the color parameters, samples were reddish and the browning index increased significantly with temperature in the samples with polydextrose.
The higher drying temperature caused a decrease in anthocyanins and phenolic compounds, while the antioxidant activity by DPPH assay was not significantly different. The antioxidant activity measured by ABTS method was not significantly affected by the higher concentrations of encapsulating agent.
The microstructure exhibited spheric shape and smooth surface, and presented concavities when gum Arabic was used as encapsulating agent. According to the results, it was found that the spray-dried samples containing 15 % gum Arabic at 140 °C had higher retention of bioactive compounds, with anthocyanins contents of 1279.11 mg/100 g cyanidin-3-glucoside, 2429.22 mg (GAE)/100 g phenolic compounds, 40.26 % antioxidant activity by DPPH method, and 45.15 % by ABTS.
Acknowledgments
This work was supported by FAPERGS, CAPES and CNPq, Brazil.
References
- Adams JB. Thermal degradation of anthocyanins with particular reference to the 3-glycosides of cyanidin. I. In acidified aqueous solution at 100 °C. J Sci Food Agric. 1973;24:747–762. doi: 10.1002/jsfa.2740240702. [DOI] [Google Scholar]
- Aguilera JM, Stanley DW (1999) Microstructural principles of food processing and engineering. Aspen, Gaithersburg, 432 p
- Ahmed M, Akter MS, Lee JC, Eun JB (2010) Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT Food Sci Technol 43:1307–1312
- Badreldin HA, Ziada A, Blunden G. Biological effects of gum Arabic: a review of some recent research. Food Chem Toxicol. 2009;47:1–8. doi: 10.1016/j.fct.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cano-Chauca M, Stringheta PC, Ramos AM, Cal-Vidal J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov Food Sci Emerg. 2005;6:420–428. doi: 10.1016/j.ifset.2005.05.003. [DOI] [Google Scholar]
- Caparino OA, Tang J, Nindo CI, Sablani SS, Powers JR, Fellman JK. Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘carabao’ var.) powder. J Food Eng. 2012;111:135–148. doi: 10.1016/j.jfoodeng.2012.01.010. [DOI] [Google Scholar]
- Dangles O, Brouillard R. A spectroscopic method based on the anthocyanin copigmentation interaction and applied to the quantitative study of molecular complexes. J Chem Soc Perkin Trans. 1992;2:247–257. doi: 10.1039/p29920000247. [DOI] [Google Scholar]
- Elisia I, Hu C, Popovich DG, Kitts DD. Antioxidant assessment of an anthocyanin-enriched blackberry extract. Food Chem. 2007;101:1052–1058. doi: 10.1016/j.foodchem.2006.02.060. [DOI] [Google Scholar]
- Fang Z, Bhandari B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011;129:1139–1147. doi: 10.1016/j.foodchem.2011.05.093. [DOI] [PubMed] [Google Scholar]
- Fang Z, Bhandari B. Comparing the efficiency of protein and maltodextrin on spray drying of bayberry juice. Food Res Int. 2012;48:478–483. doi: 10.1016/j.foodres.2012.05.025. [DOI] [Google Scholar]
- Fazaeli M, Emam-Djomeh Z, Ashtari AK, Omid M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod Process. 2012;90:667–675. doi: 10.1016/j.fbp.2012.04.006. [DOI] [Google Scholar]
- Francis FJ. Food colorants: anthocyanins. Crit Rev Food Sci Nutr. 1989;28:273–314. doi: 10.1080/10408398909527503. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Wrolsted RE. Anthocyanins: characterization and measurement with UV–visible spectroscopy. In: Wrolstad RE, Schwartz SJ, editors. Current protocols in food analytical chemistry. New York: Wiley; 2001. pp. 1–13. [Google Scholar]
- He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang H, Liu W, Li C. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J Zhejiang University. 2012;13:94–102. doi: 10.1631/jzus.B1100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovic J, Tadic V, Dimitrijevic S, Stamenic M, Petrovic S, Zizovic I. Antioxidant properties of the anthocyanin-containing ultrasonicextract from blackberry cultivar “cacanska bestrna”. Ind Crop Prod. 2014;53:274–281. doi: 10.1016/j.indcrop.2013.12.048. [DOI] [Google Scholar]
- Jakobek L, Seruga M, Seruga B, Novak I, Medvidovic-Kosanovic M. Phenolic compound composition and antioxidant activity of fruits of rubus and prunus species From Croatia. Int J Food Sci Technol. 2009;44:860–868. doi: 10.1111/j.1365-2621.2009.01920.x. [DOI] [Google Scholar]
- Jiménez-Aguilar DM, Ortega-Regules AE, Lozada-Ramírez JD, Pérez-Pérez MCI, Vernon-Carter EJ, Welti-Chanes J (2011) Color and chemical stability of spray-dried blueberry extract using mesquite gum as wall material. J Food Comp Anal 24:889–894
- Kader F, Rovel B, Girardin M, Metche M. Mechanism of browning in fresh highbush blueberry fruit (vaccinium corymbosum L.). role of blueberry polyphenol oxidase, chlorogenic acid and anthocyanins. J Sci Food Agric. 1999;74:31–34. doi: 10.1002/(SICI)1097-0010(199705)74:1<31::AID-JSFA764>3.0.CO;2-9. [DOI] [Google Scholar]
- Kuck LS, Noreña CPZ. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016;194:569–576. doi: 10.1016/j.foodchem.2015.08.066. [DOI] [PubMed] [Google Scholar]
- Mishra A, Sharma AM, Kumar S, Saxena AK, Pandey AK (2013) Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Res Int. doi:10.1155/2013/915436 [DOI] [PMC free article] [PubMed]
- Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H, Núñes MJ, Parajó JC. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J Agric Food Chem. 2000;48:5331–5337. doi: 10.1021/jf000549h. [DOI] [PubMed] [Google Scholar]
- Quek SY, Chok NK, Swedlund P. The physicochemical properties of spray-dried watermelon powder. Chem Eng Process. 2007;46:386–392. doi: 10.1016/j.cep.2006.06.020. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Panala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro C, Zimeri JE, Yildiz E, Kokini JL. Estimation of effective diffusivities and glass transition temperature of polydextrose as a function of moisture content. Carbohydr Polym. 2003;51:273–280. doi: 10.1016/S0144-8617(02)00182-0. [DOI] [Google Scholar]
- Rosenberg M, Kopelman I J, Talmon Y. A scanning electron microscopy study of microencapsulation. J. Food Sci. 1985;50:139–144. doi: 10.1111/j.1365-2621.1985.tb13295.x. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic– phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Sudha G, Vadivukkarasi S, Sheree R B I, Lakshmanan P. Antioxidant activity of various extracts from an edible mushroom pleurotus eous. Food Sci Biotechnol. 2012;21:661–668. doi: 10.1007/s10068-012-0086-1. [DOI] [Google Scholar]
- Toneli JCL, Park KJ, Murr FEX, Negreiros AA. Effect of moisture on the microstructure of inulin powder. Food Sci Technol. 2008;28:122–131. doi: 10.1590/S0101-20612008000100018. [DOI] [Google Scholar]
- Tonon RV, Brabet C, Hubinger MD. Influence of process conditions on the physicochemical properties of açai (euterpe oleraceae mart.) powder produced by spray drying. J Food Eng. 2008;88:411–418. doi: 10.1016/j.jfoodeng.2008.02.029. [DOI] [Google Scholar]
- Tonon RV, Brabet C, Hubinger MD. Anthocyanin stability and antioxidant activity of spray-dried açaí (Euterpe oleracea mart.) juice produced with different carrier agents. Food Res Int. 2010;43:907–914. doi: 10.1016/j.foodres.2009.12.013. [DOI] [Google Scholar]
- Tonon RV, Brabet C, Pallet D, Brat P, Hubinger DM. Physicochemical and morphological characterisation of açai (euterpe oleraceae mart.) powder produced with different carrier agents. Int J Food Sci Technol. 2009;44:1950–1958. doi: 10.1111/j.1365-2621.2009.02012.x. [DOI] [Google Scholar]
- Tsanova-Savova S, Ribarova F, Gerova M. (+)-Catechin and (−)-epicatechin in Bulgarian fruits. J Food Compos Anal. 2005;18:691–698. doi: 10.1016/j.jfca.2004.06.008. [DOI] [Google Scholar]
- Tulio JrAZ, Reese RN, Wyzgoski FJ, Rinaldi PL, Fu R, Scheerens JC, Miller AR. Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry. J Agric Food Chem. 2008;56:1880–1888. doi: 10.1021/jf072313k. [DOI] [PubMed] [Google Scholar]
- Turemis N, Kafkas E, Kafkas S, Kurkcuoglu M, Baser KHC. Determination of aroma compounds in blackberry by GC/MS analysis. Chem Nat Compd. 2003;39:174–176. doi: 10.1023/A:1024809813305. [DOI] [Google Scholar]
- Wang H, Cao G, Prior RL. Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem. 1997;45:304–309. doi: 10.1021/jf960421t. [DOI] [Google Scholar]
- Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem. 2000;48:140–146. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]