Abstract
Extensive traditional use of medical plants leads to research dealing with chemical composition of essential oils. The aim of this work was evaluation of quality of the essential oil and extending of the knowledge about chemical composition of essential oil from ribwort (Plantago lanceolata L.) and proportional representation of compounds. Extractions of essential oils from samples of ribwort were performed by hydrodistillation. GC-MS and GC-FID techniques were used for investigation of the qualitative and semi-quantitative content of aromatic compounds in the essential oils, respectively. Major aroma constituents of ribwort leaves were groups of fatty acids 28.0–52.1 % (the most abundant palmitic acid 15.3–32.0 %), oxidated monoterpenes 4.3–13.2 % (linalool 2.7–3.5 %), aldehydes and ketones 6.9–10.0 % (pentyl vinyl ketone 2.0–3.4 %) and alcohols 3.8–9.2 % (1-octen-3-ol 2.4–8.2 %). In relative high amount were identified apocarotenoids (1.5–2.3 %) which are important constituents because of their intense fragrant. The importance is in potential manufacture control of feedstocks before producing of food supplements.
Keywords: Plantago lanceolata L., Essential oil, Hydrodistillation, GC-MS, GC-FID
Introduction
Plantago lanceolata L., a perennial herb belonging to the family Plantaginaceae, usually grows to a high about 30–60 cm and produces a rosette of leaves 10–25 cm, sometimes up to 40 cm, long (Kubát 2002). The size of the plant depends on the growth habitats. It occurs as a wild plant in field crops, but due to the large consumption in the pharmaceutical industry is also cultivated.
The plant belongs historically to Eurasia continent, but gradually expanded around the world together with the colonizers from Europe. Plantain has been proclaimed for medicinal usage dating back to the ancient Greeks and Romans, who used it frequently on herpes, skin infections but also as an antidote for rabies. Notable pharmacological properties of Plantago lanceolata L. have been reported, such as anti-inflammatory (Beara et al. 2010), antimicrobial activity (Nostro et al. 2000), antioxidant and cytotoxic activity (Beara et al. 2012), anti-tumoural activity (Herbert et al. 1991), and antispasmodic effect (Fleer and Verspohl 2007). Furthermore, Plantago lanceolata L. is also included in the diet as herbal tea, which is used in folk medicine for treatment of disorders of the respiratory tract (Fons et al. 2008).
Many constituents of ribwort have been reported, such as phenolic compounds (phenolic acids, flavonoids and coumarins) (Beara et al. 2012; Fons et al. 1999), iridoid glycosides (e.g. catalpol, aucubin) and phenylethanoid glycosides (e.g. verbascoside, plantamajoside) (Adler et al. 1995), volatile components (Fons et al. 1998), etc. But no published study has previously dealed with the chemical composition of the essential oil extracted by hydrodistillation from leaves of Plantago lanceolata L.
Our interest was not in the extraction process, but in the quality of the essential oil and extending of our knowledge about chemical composition of essential oil from Plantago lanceolata L. and proportional representation of compounds. Individual components of the essential oils are often used as food flavourings extracted from plant material. Potential use of essential oils are application as additives in many types of foods (beverages odourisers) and food supplements (like cough drops), with different organoleptic effects. The importance is in potential manufacture control of feedstocks before producing of food supplements. Separation of volatile compounds of essential oils and subsequent calculation of proportional representation has been carried out by gas chromatography with flame ionization detector (GC-FID). Identification of compounds has been carried out by gas chromatography/mass spectrometry system (GC-MS) and by comparing to linear retention indices.
Materials and methods
Plant material
Three samples of Plantago lanceolata L. (Table 1) were purchased from local companies in Czech Republic.
Table 1.
List of plant material
| Sample | Company/producer |
|---|---|
|
Plantago lanceolata L. – sample 1 (crushed leaves) |
Dr. Müller Pharma s.r.o. (Hradec Králové, Czech Republic) |
|
Plantago lanceolata L. – sample 2 (crushed leaves) |
Valdemar Grešík – Natura s.r.o. (Děčín, Czech Republic) |
|
Plantago lanceolata L. – sample 3 (crushed leaves packed in tea bags) |
Megafyt Pharma s.r.o. (Vrané n. Vltavou, Czech Republic) |
Chemicals
n-Hexane, and n-alkane mixture standard solutions C8-C20 and C21-C40 in concentrations of 40 mg·l−1 dissolved in n-hexane and in toluene, respectively, were purchased from Sigma-Aldrich (Prague, Czech Republic). Distilled water was purified using a Milli-Q® Water Purification System (Millipore SAS, Molsheim, France).
Sample preparation
Sample was not treated before extraction, treatment (drying and crushing) was done by producers. A 40 g of crushed dry sample were extracted with 600 mL of water in an apparatus (Kavalierglass a.s., Prague, Czech Republic) of Clevenger type for 5 h, until no more essential oil was obtained. The essential oil was collected into 1 ml of n-hexane and analysed. Each extraction was performed at least three times.
GC-MS analysis
A gas chromatograph, model GC-2010 Plus, coupled with mass spectrometry detector TQ-8030 and auto-sampler AOC-5000 Plus (all from Shimadzu, Kyoto, Japan) was used for analysis. Injections have been performed in split mode 1:5. The GC-MS system has been equipped with a capillary column SLB-5 ms with length 30 m, 0.25 mm inner diameter and 0.25 μm film thickness (Supelco, Bellefonte, PA, USA). Helium 5.0 (Linde Gas a.s., Prague, Czech Republic) was used as a carrier gas at a constant linear velocity of 30 cm/s. The injector and the interface temperature were maintained at 250 °C. The column temperature has been programmed as follows: the initial temperature was 40 °C (1 min) then increased at a rate of 2 °C/min up to 200 °C (10 min). The mass spectrometer was operated in the full scan mode over a mass range of m/z 45–500 and in the electron ionization (EI) mode (70 eV). The mixtures of n-alkanes (C8-C20, C21-C40) were injected using the above temperature program in order to calculate the retention index (RI) for each peak. Identification of the components was done by comparison of mass spectral fragmentation patterns stored in MS data libraries (NIST 11, FFNSC 2), and verified by comparison of retention indices (RIs) of identified compounds with published index data (Adams 2007; Goodner 2008; Nijssen et al. 1963–2015; NIST 2011) and RIs from MS data library FFNSC 2.
GC-FID analysis
GC-FID analysis was accomplished on a Shimadzu GC2010 equipped with a flame ionization detector (FID). The detector temperature was 220 °C. The other analytic conditions including the column type and column temperature, the injector temperature, carrier gas and the linear velocity were the same as those of GC–MS analysis.
Results and discussion
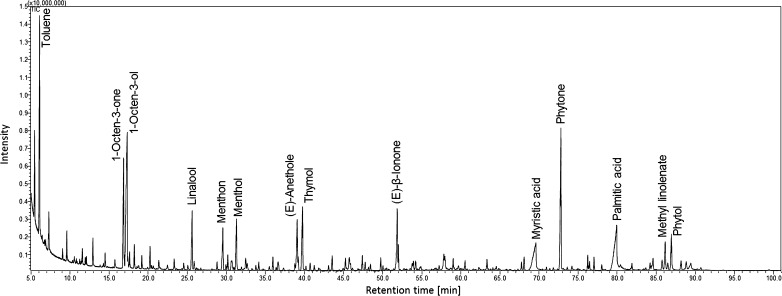
Totally, up to 236 peaks were observed in chromatograms of the extracted compounds from ribwort. However, relatively large number of the peaks was not identified, often due to absence of appropriate mass spectrum in libraries or absence of retention indexes calculated for given column. Figure 1 depicts representative chromatogram. The identified peaks are listed in Table 2. Semi-quantitative data were obtained from the integration of the FID peak areas.
Fig. 1.
GC-MS chromatogram of the essential oil (sample 3) with labeling of the most intensive peaks
Table 2.
Comparison of volatile compounds presented in extracts obtained by hydrodistillation of three samples of Plantago lanceolata L., contents of individual compounds are expressed as average relative percent peak area of GC-FID after three replicates (n = 3), n.i. = not identified
| Compound | CAS number | RIa | Sample 1 [% rel.] |
Sample 2 [% rel.] |
Sample 3 [% rel.] |
|---|---|---|---|---|---|
| Monoterpenes | |||||
| α-Pinene | 80–56-8 | 934 | n.i. | n.i. | 0.05 |
| δ-3-Carene | 13,466–78-9 | 1010 | n.i. | n.i. | <0.01 |
| p-Cymene | 99–87-6 | 1025 | n.i. | 0.05 | 0.07 |
| Limonene | 138–86-3 | 1029 | 0.09 | 0.14 | 0.76 |
| β-cis-Ocimene | 3338–55-4 | 1037 | 0.02 | 0.10 | 0.02 |
| α-Ocimene | 502–99-8 | 1047 | 0.47 | 0.70 | 0.27 |
| γ-Terpinene | 99–85-4 | 1058 | 0.03 | 0.08 | 0.02 |
| α-Terpinolene | 586–62-9 | 1086 | 0.04 | 0.04 | 0.02 |
| Σ Identified monoterpenes | 0.65 | 1.11 | 1.21 | ||
| Oxidated monoterpenes | |||||
| 1,8-Cineole | 470–82-6 | 1033 | 0.01 | 0.04 | 0.07 |
| Fenchone | 1195–79-5 | 1089 | n.i. | 0.10 | 0.30 |
| Linalool | 78–70-6 | 1104 | 2.86 | 3.54 | 2.66 |
| β-Thujone | 33,766–30-2 | 1119 | n.i. | 0.05 | 0.07 |
| Camphor | 76–22-2 | 1147 | n.i. | 0.18 | 0.37 |
| Menthone | 10,458–14-7 | 1157 | 0.05 | 0.65 | 1.80 |
| Isomenthon | 491–07-6 | 1166 | 0.11 | 0.06 | 0.15 |
| Neomenthol | 3623–51-6 | 1172 | n.i. | n.i. | 0.95 |
| Menthol | 1490–04-6 | 1180 | 0.43 | 1.03 | 2.12 |
| α-Terpineol | 98–55-5 | 1197 | 0.34 | 0.58 | 0.36 |
| Nerol | 106–25-2 | 1228 | 0.28 | 0.17 | 0.35 |
| Pulegone | 89–82-7 | 1239 | n.i. | <0.01 | 0.05 |
| Carvone | 99–49-0 | 1246 | 0.02 | 0.06 | 0.16 |
| Geraniol | 106–24-1 | 1255 | 0.04 | 0.03 | 0.02 |
| Thymol | 89–83-8 | 1298 | 0.15 | 0.02 | 3.75 |
| Carvacrol | 499–75-2 | 1306 | n.i. | <0.01 | <0.01 |
| Σ Identified ox. monoterpenes | 4.29 | 6.51 | 13.18 | ||
| Sesquiterpenes | |||||
| β-Bourbonene | 5208–59-3 | 1382 | n.i. | n.i. | 0.40 |
| α-Gurjunene | 489–40-7 | 1406 | n.i. | n.i. | 0.04 |
| β-Caryophyllene | 87–44-5 | 1418 | 0.14 | 0.07 | 0.05 |
| (E)-α-bergamotene | 13,474–59-4 | 1433 | n.i. | 0.14 | 0.26 |
| (E)-β-Farnesene | 18,794–84-8 | 1454 | 0.01 | 0.02 | 0.01 |
| (E,E)-α-Farnesene | 502–61-4 | 1505 | n.i. | 0.21 | n.i. |
| β-Bisabolene | 4891–79-6 | 1508 | n.i. | 0.03 | 0.08 |
| β-Sesquiphellandrene | 20,307–83-9 | 1524 | n.i. | n.i. | 0.28 |
| Σ Identified sesquiterpenes | 0.15 | 0.47 | 1.11 | ||
| Oxidated sesquiterpenes | |||||
| Cabreuva oxide A | 107,602–54-0 | 1440 | n.i. | 0.13 | n.i. |
| Cabreuva oxide B | 107,602–53-9 | 1458 | 0.30 | 0.47 | 0.44 |
| Cabreuva oxide D | 107,602–52-8 | 1475 | n.i. | <0.01 | 0.02 |
| (E)-Nerolidol | 40,716–66-3 | 1563 | 0.03 | 0.01 | 0.03 |
| Spathulenol | 6750–60-3 | 1579 | 1.21 | n.i. | n.i. |
| Viridiflorol | 552–02-3 | 1595 | n.i. | 0.22 | 0.33 |
| Fokienol | 33,440–00-5 | 1599 | 1.29 | 0.66 | 0.32 |
| Humulene epoxide II | 19,888–34-7 | 1611 | n.i. | 0.01 | 0.05 |
| Isospathulenol | 88,395–46-4 | 1631 | 0.67 | n.i. | n.i. |
| α-Bisabolol oxide B | 26,184–88-3 | 1656 | n.i. | 0.01 | 0.02 |
| β-eudesmol | 473–15-4 | 1657 | 0.19 | n.i. | n.i. |
| Valeranone | 1803–39-0 | 1675 | 0.18 | n.i. | n.i. |
| α-Bisabolone oxide A | 58,985–73-2 | 1682 | n.i. | 0.03 | 0.06 |
| Valerenal | 4176–16-3 | 1717 | 0.11 | n.i. | n.i. |
| α-Bisabolol oxide A | 22,567–36-8 | 1750 | n.i. | 0.09 | n.i. |
| Xanthorhizol | 30,199–26-9 | 1752 | n.i. | n.i. | 0.08 |
| Σ Identified ox. sesquiterpenes | 3.98 | 1.64 | 1.35 | ||
| Oxidated diterpenes | |||||
| Phytone | 502–69-2 | 1846 | 1.81 | 2.16 | 2.99 |
| Isophytol | 505–32-8 | 1947 | n.i. | 0.04 | 0.10 |
| Geranyl linalool | 1113–21-9 | 2022 | 0.08 | <0.01 | 0.06 |
| 13-Epi-manool | 1438–62-6 | 2050 | n.i. | <0.01 | <0.01 |
| Phytol | 150–86-7 | 2111 | 3.60 | 1.01 | 0.88 |
| Σ Identified ox. diterpenes | 5.49 | 3.21 | 4.03 | ||
| Apocarotenoids | |||||
| β-Isophorone | 471–01-2 | 1043 | n.i. | n.i. | <0.01 |
| α-Isophorone | 78–59-1 | 1124 | n.i. | <0.01 | <0.01 |
| Safranal | 116–26-7 | 1200 | 0.34 | 0.58 | n.i. |
| β-cyclocitral | 432–25-7 | 1221 | 0.13 | 0.20 | 0.37 |
| β-Damascenone | 23,726–93-4 | 1380 | 0.79 | 0.40 | 0.39 |
| Tetrahydrogeranylacetone | 1604–34-8 | 1404 | n.i. | n.i. | <0.01 |
| Geranylacetone | 3796–70-1 | 1450 | 0.01 | 0.02 | 0.02 |
| (E)-β-Ionone | 79–77-6 | 1482 | 0.11 | 0.12 | 0.05 |
| 5,6-Epoxy-β-ionone | 23,267–57-4 | 1484 | 0.03 | 0.05 | 0.41 |
| (E,E)-Pseudoionone | 3548–78-5 | 1585 | n.i. | 0.77 | 0.77 |
| Megastigmatrienon | 38,818–55-2 | 1626 | 0.06 | 0.10 | n.i. |
| (5E,9E)-Farnesyl acetone | 1117–52-8 | 1912 | 0.07 | 0.01 | 0.03 |
| Σ Identified apocarotenoids | 1.54 | 2.25 | 2.04 | ||
| Aldehydes and ketones | |||||
| Propyl vinyl ketone | 1629–60-3 | 778 | 0.03 | 0.06 | 0.07 |
| (3E,5E)-1,3,5-Heptatriene | 17,679–93-5 | 785 | 0.16 | n.i. | n.i. |
| Ethyl propyl ketone | 589–38-8 | 789 | 0.06 | 0.05 | 0.05 |
| Methyl butyl ketone | 591–78-6 | 794 | 0.03 | 0.03 | 0.04 |
| Capronaldehyde | 66–25-1 | 805 | 1.00 | 0.44 | 0.69 |
| (E)-2-hexenal | 6728–26-3 | 854 | 1.27 | 0.70 | 0.89 |
| Heptan-2-one | 110–43-0 | 893 | 0.03 | 0.02 | 0.16 |
| (Z)-4-Heptenal | 6728–31-0 | 903 | 0.05 | 0.03 | 0.02 |
| Heptanal | 111–71-7 | 905 | 0.49 | 0.28 | 0.34 |
| (E)-3-Hepten-2-one | 5609–09-6 | 937 | n.i. | 0.01 | n.i. |
| α-Ethylcaproaldehyde | 123–05-7 | 949 | n.i. | n.i. | 0.05 |
| (E)-2-Heptenal | 18,829–55-5 | 958 | 0.37 | 0.01 | 0.02 |
| Benzaldehyd | 100–52-7 | 962 | 0.19 | 0.23 | 0.20 |
| Pentyl vinyl ketone | 4312–99-6 | 980 | 2.01 | 2.55 | 3.40 |
| 2,3-Octanedione | 585–25-1 | 988 | 0.27 | 0.09 | 0.09 |
| Caprylaldehyde | 124–13-0 | 1006 | 0.12 | 0.06 | 0.06 |
| (E,E)-2,4-Heptadienal | 4313–03-5 | 1015 | 1.28 | 0.69 | 0.39 |
| 2,2,6-Trimethylcyclohexanone | 2408–37-9 | 1035 | 0.10 | 0.04 | 0.08 |
| 3-Octen-2-one | 1669–44-9 | 1041 | <0.01 | <0.01 | <0.01 |
| Phenylacetaldehyde | 122–78-1 | 1045 | 0.02 | 0.02 | 0.03 |
| 2-Octenal | 2363–89-5 | 1060 | 0.29 | 0.12 | 0.14 |
| Acetophenone | 98–86-2 | 1067 | n.i. | <0.01 | <0.01 |
| (E,E)-3,5-Octadien-2-one | 30,086–02-3 | 1072 | <0.01 | <0.01 | <0.01 |
| (E,Z)-3,5-octadien-2-one | 4173–41-5 | 1096 | <0.01 | <0.01 | <0.01 |
| Nonanal | 124–19-6 | 1107 | 0.53 | 0.23 | 0.15 |
| (E,E)-2,4-octadienal | 30,361–28-5 | 1114 | 0.02 | 0.02 | 0.04 |
| 3-Nonen-2-one | 14,309–57-0 | 1142 | n.i. | n.i. | <0.01 |
| (E,Z)-2,6-nonadienal | 557–48-2 | 1156 | 0.59 | 0.21 | 0.35 |
| (E)-2-nonenal | 18,829–56-6 | 1163 | 0.30 | 0.06 | 0.05 |
| p-Ethylbenzaldehyde | 4748–78-1 | 1177 | n.i. | n.i. | <0.01 |
| p-Methylacetophenone | 122–00-9 | 1187 | 0.08 | 0.13 | 0.24 |
| Capraldehyde | 112–31-2 | 1208 | 0.11 | 0.12 | 0.04 |
| Undecanal | 112–44-7 | 1310 | n.i. | <0.01 | <0.01 |
| (E,E)-2,4-decadienal | 25,152–84-5 | 1321 | 0.63 | 0.67 | 0.35 |
| Benzophenone | 119–61-9 | 1629 | n.i. | n.i. | <0.01 |
| Pentadecanal | 2765–11-9 | 1717 | n.i. | 0.03 | 0.02 |
| p-Methylbenzophenone | 134–84-9 | 1758 | n.i. | 0.01 | 0.24 |
| Palmitaldehyde | 629–80-1 | 1819 | n.i. | n.i. | 0.03 |
| Σ Identified aldehydes and ketones | 10.03 | 6.91 | 8.23 | ||
| Alcohols | |||||
| 2-Hexanol | 626–93-7 | 808 | <0.01 | <0.01 | <0.01 |
| (Z)-3-hexen-1-ol | 928–96-1 | 859 | n.i. | n.i. | <0.01 |
| 1-Octen-3-ol | 3391–86-4 | 984 | 2.39 | 6.94 | 8.24 |
| 3-Octanol | 589–98-0 | 1001 | 0.97 | 0.53 | 0.39 |
| Octanol | 111–87-5 | 1076 | 0.46 | 0.22 | 0.35 |
| Tetradecanol | 112–72-1 | 1674 | n.i. | 0.19 | 0.21 |
| Σ Identified alcohols | 3.82 | 7.88 | 9.19 | ||
| Phenols and phenolic ethers | |||||
| p-Allylanisole | 140–67-0 | 1200 | n.i. | n.i. | 0.36 |
| (E)-anethole | 4180–23-8 | 1289 | <0.01 | 0.78 | 3.21 |
| p-Vinylguaiacol | 7786–61-0 | 1313 | 0.01 | 0.12 | 0.22 |
| Eugenol | 97–53-0 | 1355 | 0.06 | 0.10 | 0.22 |
| 2,5-Di-tert-butylphenol | 5875–45-6 | 1513 | n.i. | n.i. | 0.05 |
| Σ Identified phenols and phenolic ethers | 0.07 | 1.00 | 4.06 | ||
| Fatty acids | |||||
| Capric acid | 334–48-5 | 1390 | 0.69 | 0.61 | 0.47 |
| Myristic acid | 544–63-8 | 1785 | 4.63 | 5.99 | 4.48 |
| Pentadecanoic acid | 1002–84-2 | 1875 | n.i. | 1.11 | 0.53 |
| Palmitic acid | 57–10-3 | 1990 | 27.86 | 31.97 | 15.26 |
| Margaric acid | 506–12-7 | 2071 | 0.15 | 0.16 | 0.11 |
| Linoleic acid | 60–33-3 | 2149 | 4.68 | 6.85 | 2.89 |
| Linolenic acid | 463–40-1 | 2160 | 6.29 | 5.36 | 4.23 |
| Σ Identified fatty acids | 44.30 | 52.05 | 27.97 | ||
| Esters | |||||
| Methyl salicylate | 119–36-8 | 1193 | n.i. | 0.10 | n.i. |
| Linalyl acetate | 115–95-7 | 1252 | n.i. | 0.13 | 0.51 |
| Bornyl acetate | 92,618–89-8 | 1285 | n.i. | n.i. | 0.13 |
| Menthyl acetate | 16,409–45-3 | 1291 | 0.05 | 0.19 | 0.27 |
| α-Terpinyl acetate | 80–26-2 | 1348 | n.i. | 0.03 | 0.02 |
| Neryl acetate | 141–12-8 | 1361 | n.i. | n.i. | 0.03 |
| 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | 6846–50-0 | 1587 | n.i. | 0.64 | n.i. |
| Isopropyl laurate | 10,233–13-3 | 1628 | n.i. | 0.04 | n.i. |
| Methyl myristate | 124–10-7 | 1727 | n.i. | n.i. | 0.06 |
| Methyl palmitate | 112–39-0 | 1928 | 0.05 | 0.05 | 0.21 |
| Methyl linoleate | 112–63-0 | 2092 | 0.32 | 0.38 | 0.25 |
| Methyl linolenate | 301–00-8 | 2099 | 0.06 | 0.07 | 0.26 |
| Ethyl linoleate | 544–35-4 | 2161 | n.i. | n.i. | 0.08 |
| Σ Identified esters | 0.48 | 1.63 | 1.82 | ||
| Aliphatic hydrocarbons | |||||
| Dodecane | 112–43-3 | 1201 | n.i. | 0.12 | n.i. |
| Tridecane | 629–50-5 | 1301 | n.i. | <0.01 | n.i. |
| Tetradecane | 629–59-4 | 1401 | n.i. | 0.03 | 0.02 |
| Pentadecane | 629–62-9 | 1501 | n.i. | 0.04 | 0.05 |
| Cetane | 544–76-3 | 1601 | n.i. | n.i. | 0.32 |
| Heptadecane | 629–78-7 | 1701 | n.i. | 0.08 | 0.05 |
| Octadecane | 593–45-3 | 1801 | n.i. | <0.01 | <0.01 |
| Nonadecane | 629–92-5 | 1893 | n.i. | <0.01 | 0.02 |
| Σ Identified aliphatic hydrocarbons | n.d. | 0.27 | 0.46 | ||
| Aromatic hydrocarbons | |||||
| Toluene | 108–88-3 | 765 | 0.21 | 0.31 | 6.92 |
| 1,3-Diisopropylnaphthalene | 57,122–16-4 | 1667 | n.i. | 0.15 | n.i. |
| 1,4-Diisopropylnaphthalene | 24,157–79-7 | 1714 | n.i. | 0.01 | n.i. |
| 2,7-Diisopropylnaphthalene | 40,458–98-8 | ||||
| 1,6-Diisopropylnaphthalene | 51,113–41-8 | 1719 | n.i. | 0.02 | n.i. |
| 2,6-Diisopropylnaphthalene | 24,157–81-1 | 1724 | n.i. | <0.01 | n.i. |
| Diisobutyl phthalate | 84–69-5 | 1862 | 0.07 | 0.05 | 0.01 |
| Dibutyl phthalate | 84–74-2 | 1957 | 0.08 | 0.04 | 0.13 |
| Σ Identified aromatic hydrocarbons | 0.36 | 0.58 | 7.06 | ||
| Others | |||||
| Dimethylfulvene | 2175–91-9 | 870 | n.i. | 0.03 | 0.04 |
| Cyclooctatetraene | 629–20-9 | 896 | n.i. | 0.06 | 0.20 |
| Dimethyl trisulfide | 3658–80-8 | 967 | n.i. | 0.23 | n.i. |
| 2-Amylfuran | 3777–69-3 | 992 | 0.77 | 0.44 | 0.54 |
| Σ Identified other compounds | 0.77 | 0.76 | 0.78 | ||
| Number of identified compounds | 85 | 125 | 131 | ||
aRI, retention index on capillary column SLB-5 ms, RI = 100·n + 100·(tx-tn)/(tn+1-tn); n, the number of carbon atoms in the alkane; tx, retention time of peak of unknown compound; tn+1 and tn, the retention time of alkane with n + 1 and n carbon atoms
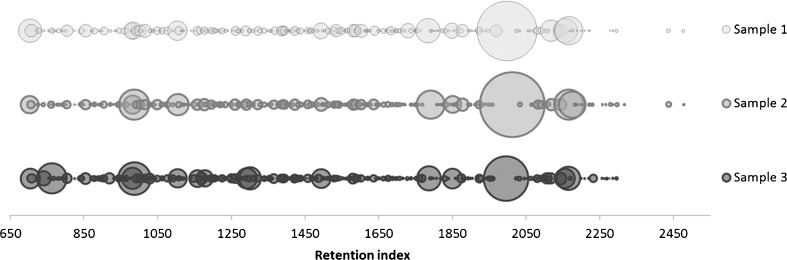
As is seen in Fig. 2 showing aroma profiles of ribwort using bubbles whose sizes correspond to the representation of the individual compounds, a composition of all three samples is very similar. The biggest portion of the essential oils is generated by fatty acids (28.0–52.0 %), particularly myristic acid (RI 1785), palmitic acid (RI 1990) linoleic acid (RI 2145) and linolenic acid (RI 2163). Fatty acids are common components of the essential oils (Clarke 2008), for instance palmitic acid was found in ribwort leaves by Fons (Fons et al. 1998). Furthermore, in the present work, methyl esters of some fatty acids were also identified.
Fig. 2.
Comparison of three hydrodistillation extracts of ribwort according to RI; area of the bubbles represents the relative peak area of GC-FID
Oxidated monoterpenes, which commonly generate a large part of the essential oils contented in aromatic herbs, made up 4.3–13.2 %. From non-oxidated monoterpenes only 8 compounds were identified, the biggest part was created by limonene (0.8 % in sample 3) and α-ocimene (0.7 % and 0.5 % in sample 2 and 3, respectively). Similar representation of non-oxidated compounds was observed also in sesquiterpenes. Sixteen oxidated sesquiterpenes were identified in all three samples. It is the same as in the case of oxidated monoterpenes, but amount in percents was lower, 1.4–4.0 %.
One group of the main identified components of the essential oils was oxidated diterpenes (particularly phytol (RI 2111) and phytone (RI 1846)), which generated 3.2–5.5 %. Free phytol is created by chlorophyll hydrolysis by an enzyme chlorophyllase as an integral part of plants catabolism in fruits ripening or leave yellowing, and it does not have any influence to plant aroma (Velíšek et al. 2009). Phytone is created by its oxidation which was most likely invoked during distillation.
Unlike Fons (Fons et al. 1998) who identified great amount of apocarotenoids in ribwort, namely (E)-9-hydroxymegastigma-4,7-dien-3-on a 3-hydroxy-5,6-epoxy-β-ionol, in the present work these compounds were not found. Nevertheless, some other apocarotenoids were observed in the aroma profile of ribwort and generated 1.5–2.3 % of aroma. Moreover in this work, (E)-β-damascenone (RI 1380) was identified. It is a compound belonging among the most aromatic ones (Velíšek and Hajšlová 2009), which is ordinarily used in perfumes production (Pybus and Sell 1999).
Regarding the content of phenolic compounds, quite big differences among individual samples appeared. They were most abundantly contained in sample 3 (4.1 %). In samples 1 and 2 only 0.1 % and 1.0 %, respectively, were identified. These differences are obvious in the Fig. 2, showing comparison of aroma profiles using bubbles corresponding to (E)-anethol (RI 1289).
Oxidated compounds as alcohols, aldehydes, and ketones (except oxidated terpenes) were the second most abundant family of compounds generating together 14.1–17.4 % (in that the carbonyl compounds 6.9–10.0 %). High content of 1-octen-3-ol (up to 8.2 %) is in the compliance with the work of Fons (Fons et al. 1998).
Also some aromatic compounds were identified, particularly toluene, diisobutyl phthalate or dibutyl phthalate. However, those were probably contaminants. Equally, Fons (Fons et al. 1998) identified in the sample of ribwort toluene and o-, m- and p-xylene, which were also stated as contaminants. The highest content of toluene (6.9 %) was presented in sample 3. This sample was finely ground and packed in teabags individually closed by plastic packaging. The other two samples were packed as whole leaves (sample 1) and coarsely ground leaves (sample 2) into a paper back. Therefore, it is expected toluene most likely appeared from the plastic packaging. Moreover, isomers of diisopropylnaphthalene (DIPN) were identified in essential oil obtained from sample 2 in amount less than 0.2 %. DIPNs are used as a solvent in the manufacture of certain printing materials and as plant growth regulator in agriculture (Brzozowski et al. 2002). DIPNs probably originated from recycled paper packaging, which were dried plants packed. A carton was evaluated as a source of DIPN contamination also by study examining contaminants in dried foods (Großmann-Kühnau 2011).
Different composition of individual samples could be affected by growing environment, time and conditions of collection, method of drying or storage conditions. These effects were described for various plants (Figueiredo et al. 2008; Gil et al. 2002; Mahmoodi Sourestani et al. 2014; Orav et al. 2004; Rowshan et al. 2013). Moreover, it is expected presence of different chemotypes of ribwort which differ in quantitative as well as in qualitative content of aromatic compounds.
Conclusion
In this work, the essential oil’s analysis of three ribwort samples (Plantago lanceolata L.) were conducted in order to observe qualitative and semi-quantitative composition. Hydrodistillation enabled to gain great amount of volatile compounds, which is obvious from a number of peaks (up to 236) occurred in chromatograms. The most abundant family of compounds were fatty acids. There were also identified methyl esters of fatty acids and great amount of aliphatic alcohols, aldehydes and ketones. Significant constituents of essential oils contented in ribwort showed to be apocarotenoids that belong among the most intense fragrant compounds. On the other hand, non-oxidated terpenes were found in relatively small amount and most of terpenes were oxidated as monoterpenes, sesquiterpenes and diterpenes. Novelty of the study up to now, there is no published study deals with the chemical composition of the essential oil extracted by hydrodistillation from leaves of Plantago lanceolata L. The importance is in potential manufacture control of feedstocks before producing of food supplements, too.
Footnotes
Research highlights
•Hydrodistillation was used for extraction of essential oil from ribwort
•Separation of volatiles was performed by gas chromatography
•Totally, 153 compounds were identified using mass spectra and retention indices
•Semi-quantitative determination was performed by GC-FID
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, IL, USA: Allured Publishing Corporation; 2007. [Google Scholar]
- Adler LS, Schmitt J, Bowers MD. GENETIC-VARIATION IN DEFENSIVE CHEMISTRY IN PLANTAGO-LANCEOLATA (PLANTAGINACEAE) AND ITS EFFECT ON THE SPECIALIST HERBIVORE JUNONIA-COENIA (NYMPHALIDAE) Oecologia. 1995;101:75–85. doi: 10.1007/BF00328903. [DOI] [PubMed] [Google Scholar]
- Beara IN, Orcic DZ, Lesjak MM, Mimica-Dukic NM, Pekovic BA, Popovic MR. Liquid chromatography/tandem mass spectrometry study of anti-inflammatory activity of plantain (plantago L.) species. J Pharm Biomed Anal. 2010;52:701–706. doi: 10.1016/j.jpba.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Beara IN, Lesjak MM, Orcic DZ, Simin ND, Cetojevic-Simin DD, Bozin BN, Mimica-Dukic NM. Comparative analysis of phenolic profile, antioxidant, anti-inflammatory and cytotoxic activity of two closely-related plantain species: plantago altissima L. and plantago lanceolata L. LWT-Food Sci Technol. 2012;47:64–70. doi: 10.1016/j.lwt.2012.01.001. [DOI] [Google Scholar]
- Brzozowski R, Skupiński W, Jamróz MH, Skarżyński M, Otwinowska H. Isolation and identification of diisopropylnaphthalene isomers in the alkylation products of naphthalene. J Chromatogr A. 2002;946:221–227. doi: 10.1016/S0021-9673(01)01571-0. [DOI] [PubMed] [Google Scholar]
- Clarke S. Essential chemistry for aromatherapy. London: Churchill Livingstone; 2008. [Google Scholar]
- Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Frag J. 2008;23:213–226. doi: 10.1002/ffj.1875. [DOI] [Google Scholar]
- Fleer H, Verspohl EJ. Antispasmodic activity of an extract from plantago lanceolata L. and some isolated compounds. Phytomedicine. 2007;14:409–415. doi: 10.1016/j.phymed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Fons F, Rapior S, Gargadennec A, Andary C, Bessiere JM. Volatile components of plantago lanceolata (plantaginaceae) Acta Bot Gall. 1998;145:265–269. doi: 10.1080/12538078.1998.10516306. [DOI] [Google Scholar]
- Fons F, Tousch D, Rapior S, Gueiffier A, Roussel JL, Gargadennec A, Andary C. Phenolic profiles of untransformed and hairy root cultures of plantago lanceolata. Plant Physiol Biochem. 1999;37:291–296. doi: 10.1016/S0981-9428(99)80027-8. [DOI] [Google Scholar]
- Fons F, Gargadennec A, Rapior S. Culture of plantago species as bioactive components resources: a 20-year review and recent applications. Acta Bot Gall. 2008;155:277–300. doi: 10.1080/12538078.2008.10516109. [DOI] [Google Scholar]
- Gil A, de la Fuente EB, Lenardis AE, López Pereira M, Suárez SA, Bandoni A, van Baren C, Di Leo Lira P, Ghersa CM. Coriander essential oil composition from two genotypes grown in different environmental conditions. J Agric Food Chem. 2002;50:2870–2877. doi: 10.1021/jf011128i. [DOI] [PubMed] [Google Scholar]
- Goodner KL. Practical retention index models of OV-101, DB-1, DB-5, and DB-wax for flavor and fragrance compounds. LWT-Food Sci Technol. 2008;41:951–958. doi: 10.1016/j.lwt.2007.07.007. [DOI] [Google Scholar]
- Großmann-Kühnau Für Sie gelesen! Mineralöl-Übergänge aus Kartonverpackungen auf Lebensmittel — Ist eine Dekontamination im Haushalt möglich? Dtsch Lebensm-Rundsch. 2011;107:362–365. [Google Scholar]
- Herbert JM, Maffrand JP, Taoubi K, Augereau JM, Fouraste I, Gleye J. VERBASCOSIDE ISOLATED FROM LANTANA-CAMARA, AN INHIBITOR OF PROTEIN-KINASE-C. J Nat Prod. 1991;54:1595–1600. doi: 10.1021/np50078a016. [DOI] [PubMed] [Google Scholar]
- Kubát K. Klíč ke květeně české republiky. Prague: Academia; 2002. [Google Scholar]
- Mahmoodi Sourestani M, Malekzadeh M, Tava A. Influence of drying, storage and distillation times on essential oil yield and composition of anise hyssop [agastache foeniculum (pursh.) kuntze] J Essent Oil Res. 2014;26:177–184. doi: 10.1080/10412905.2014.882274. [DOI] [Google Scholar]
- Nijssen LM, Ingen-Visscher CAv, Donders JJH (1963–2015) VCF Volatile Compounds in Food : database/Nijssen, L.M.; Ingen-Visscher, C.A. van; Donders, J.J.H. [eds]. – Version 16.1 – Zeist (The Netherlands). http://www.vcf-online.nl/VcfHome.cfm. Accessed 25 April 2015
- NIST (2011) National institute of standard and technology (2011) NIST standard reference database number 69. http://webbook.nist.gov/. Accessed 25 April 2015
- Nostro A, Germano MP, D'Angelo V, Marino A, Cannatelli MA. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol. 2000;30:379–384. doi: 10.1046/j.1472-765x.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- Orav A, Stulova I, Kailas T, Muurisepp M. Effect of storage on the essential oil composition of piper nigrum L. fruits of different ripening states. J Agric Food Chem. 2004;52:2582–2586. doi: 10.1021/jf030635s. [DOI] [PubMed] [Google Scholar]
- Pybus DH, Sell CS. The chemistry of fragrances. Cambridge: The Royal Society of Chemistry; 1999. [Google Scholar]
- Rowshan V, Bahmanzadegan A, Saharkhiz MJ. Influence of storage conditions on the essential oil composition of thymus daenensis celak. Ind Crop Prod. 2013;49:97–101. doi: 10.1016/j.indcrop.2013.04.029. [DOI] [Google Scholar]
- Velíšek J, Hajšlová J. Chemie potravin 2. Tábor: OSSIS; 2009. [Google Scholar]