Abstract
The aim of this study was the evaluation of fermentation by lactic acid bacteria (LAB) contains lactobacillus (L.) casei- casei and L. reuteri on acrylamide formation and physicochemical properties of the Iranian flat bread named, Sangak, and Bread roll. Sangak and Bread roll were made with whole and white wheat flour, respectively. Whole-wheat flour had upper content of protein, sugar, ash, fiber, damaged starch and the activity of amylase than the white wheat flour. After 24 h of fermentation, the pH values of the sourdoughs made from whole-wheat flour (3.00, 2.90) were lower, in compared to sourdoughs prepared from white wheat flour (3.60, 3.58). In addition, in Sangak bread, glucose, and fructose were completely utilized after fermentation, but in bread roll, the reduced sugar levels increased after fermentation and baking that represent microorganisms cannot be activated and utilized sugars. Acrylamide formation was impacted by pH of sourdough and total reducing sugar (r = 0.915, r = 0.885 respectively). Bread roll and Sangak bread were fermented by L. casei- casei contained lowest acrylamide content, in two bread types (219.1, 104.3 μg/kg respectively). As an important result, the acrylamide content of Sangak bread in all cases was lower than in the Bread roll.
Keywords: Fermentation, Sourdough, Fiber, Bread roll, Sangak bread
Introduction
Acrylamide is produced through the Maillard reaction in many foods that have been heated to high temperatures during preparation at home or factory. Acrylamide comes primarily from the reaction between reducing sugars (carbonyl groups) and the amino group of free L-asparagine. Both the substrates are required for the reaction. The minimum temperature for production of acrylamide is 120 °C and long heating procedure significantly increases acrylamide levels (Bemiller and Huber 2008; Capuano et al. 2008; Claus et al. 2008). Acrylamide is also influenced by the pH of surrounding ingredients. Reduced acrylamide production in the acidic pH is thought to be the result, in part to protonation of the amino group of asparagine, decreasing its nucleophilic potential (Bemiller and Huber 2008). French-fried potatoes, potato chips, bread, biscuits, and coffee are the major sources of dietary exposure of acrylamide to humans (EPA 1994).
The European Chemicals Agency (ECHA) added acrylamide to the list of substances of terribly high concern. As acrylamide has been classified as a probable carcinogenic substance to humans (IARC 1994; EPA 1994) and is thought as a neurotoxin, national and international regulatory agencies have focused their attention on the detection of Acrylamide in food items (WHO 2002). Daily intake of acrylamide has been depending on local cooking and eating styles. No direct legislative regulation for the maximum level of acrylamide in food products has been developed until now. However, in some countries, such as Germany the so-called signal value and minimization model has been implemented. In this plan, signal values are determined for each group of food products. If acrylamide levels of food specified above the signal value are found, food control authorities contact the food producer and start a negotiation on minimization (Kornbrust et al. 2010). Attempts to minimize formation of acrylamide in food commonly consist of one or more of three approaches: 1- Elimination of either one or both of the substrates, 2- Modification of processing conditions, and 3- Acrylamide elimination or reduction of food following its formation. It is likely that a combination of mitigation methods will be required to limit effectively the acrylamide formation in food products, with the engaged methods likely variable according to the kinds of a specific food (Bemiller and Huber 2008). Among the food mentioned above, bread is one of the most consumed product worldwide. It is a good basis of energy, protein, dietary fiber (DF), minerals, vitamins, and many alternative bioactive compounds (Bartkiene et al. 2013). Fermentation processes by the lactic acid bacteria (LAB) and yeast could reduce the acrylamide content of bread. One of the main reasons is the precursors consumption by microorganisms exists in sourdough (Fredriksson et al. 2004; Baardseth et al. 2004; Fink et al. 2006; Claus et al. 2008; Bartkiene et al. 2013).
Sourdough is an ancient method to improve texture and flavor of bread, also increase the shelf life of bakery products. Sourdough uses in bread production are growing in recent years because of the consumers switching to products that are more natural and containing less chemical preservers. The most important bacteria isolated from sourdough are owned by the “genus” Lactobacillus. Isolated lactobacilli strains can be obligate homofermentative, obligate heterofermentative or facultative heterofermentative strains (Katina 2005).
In Iran, a traditional formulation and method are applied for the production of a type of bread, which contributes to the excellent quality and long shelf life of the bread. Its name consists of 2 parts: ‘Sang’ in Persian means that the stone and ‘Sangak’ means very little stone. The dough is baked on a bed of tiny stream stones in an exceedingly furnace. Sangak bread is created by using whole-grain wheat flour. Because of the big amount of fiber in Sangak bread, it is simply digestible. Increased intake of DF has been joined with a reduced risk of overweight, cardiovascular disease, diabetes, certain cancers, and constipation. Bran in bread is in a position to slow sugar absorption (Didar et al. 2010; Katina 2005). The utilization of refined flour in bread decreases its content of the DF and associates bioactive compounds. Thus, in many sorts of bread in the world, whole grain flour, compound flour, bran, or fiber concentrates are added to bread as supplementing it with the DF (Gamel et al. 2015).
Therefore, the aim of this study was to evaluate the impact of L. casei- casei as an obligate homofermentative strains and L. reuteri as an obligate heterofermentative strains, yeast, and the kind of bread in the fermentation process, on reducing acrylamide of breads. For this purpose, totally, different formulations of Sangak and Bread roll were made and acrylamide, sugar, and some physicochemical specifications were determined after baking.
Material and methods
Chemicals
All chemical substances and solvents utilized in the tests were of analytical and HPLC grade were purchased from Merck Chemical Company (Darmstadt, Germany) and Sigma-Aldrich Company (St. Louis, MO, USA).
Ingredients
Two types of wheat flour obtained from an area mill (Karaj, Iran), were used for bread preparation. Dried instant yeast (Iran Maye Co., Iran), salt and different ingredients were purchased domestically.
Lactic acid bacteria strains
The LAB strains were L. casei- casei (DSM 20011) and L. reuteri (DSM 20016), that obtained from the culture collection of the food microbiology laboratory of faculty of Agricultural Engineering and Technology, Tehran University. The LAB strains were previously selected based on their ability for improvement dough rheological properties, flavor, crumb firmness, shelf life and overall acceptableness (Corsetti and Settanni 2007; Didar et al. 2010; Gamel et al. 2015; Katina 2005).
Working cultures were prepared from glycerol stock stored at −70 °C. Strains were streaked on MRS (de Man, Rogosa and Sharpe) agar plates. Then the single colonies were subcultured and propagated doubly in MRS broth medium for 24 h at 37 °C until 109 CFU/g, before the experiment.
Sourdough preparation
Stationary phase cells of the LAB strains, as described above, were harvested by centrifugation 8000 × g, for 10 min (min), at 4 °C. Then they washed twice with sterile 0.85 % saline solution, suspended in the milk, and incubated in 37 °C for 24 h. The cell suspension was added to the substrates contains: “whole-wheat flour and water” and “white wheat flour and water” to obtain 109 CFU/g in the dough.
Bread making
Bread roll
A commercial local bakery (Karaj, Iran) made the bread in their laboratory. The dough of Bread roll was prepared according to the approved American Association of Cereal Chemists methodology (AACC 10.10B). The Bread roll formula consisted of 100 g white wheat flour, 1.7 g instant dry yeast, 13 g sourdough, 16 g bread improver, and 5 g vegetable oil. It should be mentioned that, Bread roll dough was chemically acidified by bread improver.
After combining the dry ingredients in a mixer, 50 g tap water was slowly added, whereas mixing continues at low speed for 3 min, followed by 5 min at medium speed till dough shaped.
The dough was separated into 50 g portions and formed as the round roll. After fermentation and proofing at 29 ± 0.5 °C for 90 min, dough baked at 190 °C for 20 min in a convection oven. Bread was cooled at room temperature until packaging to transfer the laboratory. Control bread was prepared exactly as described above, without sourdough.
Sangak bread
Experimental Sangak bread was prepared according to the traditional procedure used for this bread making in Iran, and a bakery was selected locally in Karaj, Iran.
The Sangak bread formula contained 100 g whole-wheat flour, 90 g tap water, 1.2 g salt, 1 g instant dry yeast, 13 g sourdough. The ingredients were blended and the dough mixed at a low speed in a mixer for three min followed by seven min at medium speed until dough shaped. This dough fermented at 29 ± 0.5 °C for 90 min, and then was flattened on the little stones in the traditional furnace. Baking was performed at the beginning at 330 °C for 2 min, followed by 300 °C for 1 min. Control bread was prepared precisely as defined above, without sourdough.
Analytical techniques
Preparation of test samples
Sangak bread and Bread roll were sliced, and then dried in an oven at 30–40 °C. The drying temperature was set relatively low to avoid acrylamide formation throughout the drying process. Then bread samples were ground and homogenized by using a mixer (National-Model Mj-176NR-Japan).
Acrylamide analysis
Acrylamide determination was performed according to a sensitive technique of Lim and Shin (2013) and Ghasemzadeh-Mohammadi et al. (2012) with minor modifications. Gas chromatography with mass spectrometry (GC-MS) (A 7890A GC system from Agilent Technologies, Palo Alto, CA, USA, with a triple-axis detector coupled with a 5975C inert MSD network mass selective detector) was applied to determine acrylamide in bread. For the first time, the planned technique terribly sensitively determined ultra-trace levels of acrylamide concentration in bread after derivatization with xanthydrol and Dispersive liquid–liquid microextraction (DLLME).
In order to acrylamide primary extraction from the matrix, 12 mL of mixing solution containing potassium hydroxide, and ethyl alcohol (75:25) were added to 1 g of the sample. Microwaving (Delonghi type MW 602) at 500 MHz for 2 min was employed to hydrolyze the sample. After cooling, the compounds were transferred into the centrifuge tube and centrifuged at 4000 × g for 5 min (Heltich Rotorfix 32A). Then the aqueous phase was transferred to another vessel, and pH decreased to 6.5 by adding hydrochloric acid. Finally, to precipitate the proteins and carbohydrates, 2 mL of Carrez solutions I and II (50:50) were added to the vessel, which was centrifuged again at 4000 × g for 5 min. 10 mL of filtered sample was shaken for 30 min at 300 rpm using a mechanical shaker after adding 30 μL of 0.5 M xanthydrol solution, 80 μL of 6.0 M HCl, and 2 μL of acrylamide-d3 (1.0 mg/L in methanol). The derivatization reaction was conducted at ambient temperature for 30 min in the dark, and then the solution was neutralized with 6.0 M KOH. The pH of the solution was adjusted to 9.0 by 0.2 g of NaHCO3/K2CO3 (3:1, w/w). Then DLLME procedure was applied to extract of xanthyl-acrylamide.
Briefly, a solution consisting of 100 μL tetrachloroethylene as the extracting solvent, 600 μL of acetone as the disperser solvent was injected rapidly into the xanthyl-acrylamide solution. The mixture was gently shaken and centrifuged at 4000 × g for 10 min. The dispersed fine particles of the extraction phase were sedimented at the bottom of the vessel. The upper aqueous phase was separated with a syringe and about 1.5 μL of the sediment phase was injected directly into the GC–MS using a microsyringe.
Determination of water activity
Water activity (Aw) was determined using a Thermo constanter (Novasina, RS 232, Swiss) at 25 °C.
Determination of moisture, ash, wet gluten content and gluten index
Moisture (ISO 712), ash (ISO 2171), wet gluten and gluten index (ISO 21415-2) and damaged starch (ISO 17715) of flours and bread samples were determined according to the ISO methods (ISO 2006, 2007, 2009, 2013).
Determination of pH, acidity, protein, fiber, and falling number
The hydrogen ion activity (pH) of flour, sourdough, and bread was measured by preparing a 10 % solution in water. The measurements were done by employing a pH meter (Metrohm Model 632), based on Association of Official Analytical Chemists (AOAC) method 943.02 (AOAC 1995).
The acid content was measured by the amount of 0.1 N NaOH required to neutralize solution 947.05 (AOAC 1995).
Protein, fiber, and falling number of flours were determined based on AACC methods 46–12; 32–05; 56–81 (AACC 2000).
Sugars analysis
Fructose, glucose and maltose were separated and quantitated by ultra-high pressure liquid chromatography (UHPLC) (Knauer, platin blue, Germany) equipped by Refractive Index (RI) Detector (Knauer, smart line, 2300) after aqueous extraction from the sample matrix based on AACC method 80–04 (AACC 2000).
Sensory evaluation
A panel of 10-trained specialists was used to evaluate the sensory characteristics of the bread. They were asked to evaluate the overall quality (color, odor, taste, and chewiness) of each sample concerning general properties. The ranking scale ranged from zero (unacceptable) to 5 (ideal). The average of the panelists scores were calculated (AACC 2000).
Firmness measurements
The objective of this method is to quantitatively determine the force required to compress loaf bread by a preset distance or to penetrate flat bread.
In Bread roll, Crumb firmness was measured on days 1, 2, and 3 to evaluate the shelf life of the bread. Bread firmness during storage was determined based on the modified AACC method (AACC 74-09), as a maximum compression force (40 % compression or 10 mm compression depth), with the texture profile analysis test. The plate probe 40 mm diameter was used. The thickness of each bread slice was 2.5 cm, and edges of the slice were cut off before measurement (AACC 2000).
In Sangak bread, also, firmness was measured on days 1, 2, and 3 and bread firmness was determined by a penetration into the bread by probe 12.7 mm diameters.
Statistical analysis
A two-way ANOVA was applied to bread samples with the SPSS software package (IBM, SPSS, Statics, and Version 22.0). When the difference between the samples in ANOVA was statistically significant, pairwise comparisons of those samples were analyzed with Duncan’s test (Significance of variations at P < 0.05). All analysis was carried out on dry weight basis (d.w.b.) in triplicate.
Results
Tables 1 and 2 show the flour and bread characteristics tested in this study. Whole-wheat flour had upper content of protein, ash, fiber and damaged starch than the white wheat flour. In addition, the activity of alpha-amylase in whole-wheat flour showed more than the white flour based on falling number (FN) determination. These specifications can effect on acrylamide contents of samples.
Table 1.
Flours specificationsa
| Specifications | Whole wheat flour | White wheat flour |
|---|---|---|
| Moisture (%) | 10.4 ± 0.01 | 11.2 ± 0.01 |
| Ash(%) | 1.63 ± 0.01 | 0.70 ± 0.01 |
| Protein (%) | 15.5 ± 0.05 | 11.3 ± 0.03 |
| Falling number(s) | 479 ± 1.1 | 574 ± 1.4 |
| Damaged starch (UCD) | 21.5 ± 0.3 | 18.1 ± 0.1 |
| Gluten (%) | 27 ± 0.1 | 29.6 ± 0.1 |
| Gluten Index | 92 ± 0.5 | 72 ± 0.3 |
| pH | 6.2 ± 0.03 | 5.6 ± 0.02 |
| Fiber (%) | 8.2 ± 0.1 | 1.4 ± 0.1 |
aResults are mean ± SD of three determinations on d.w.b
Table 2.
Specifications of breada,b
| Type of bread | Acrylamide (μg/kg) | Aw (%) | Moisture (%) | Acidity (%) | pH of bread | pH of sourdough | Scores of sensory evaluation |
|---|---|---|---|---|---|---|---|
| Bread roll + sourdough (L. Reuteri) | 289.75 ± 8.7 a | 0.76 ± 0.001 e | 22.80 ± 0.05 d | 2.01 ± 0.03 a | 5.00 ± 0.02 d | 3.60 ± 0.10 a | 4.80 ± 0.0 a |
| Bread roll + sourdough (L. Casei-Casei) | 219.1 ± 5.3 c | 0.79 ± 0.001 d | 21.90 ± 0.05 e | 2.01 ± 0.03 a | 5.02 ± 0.01 d | 3.58 ± 0.12 a | 4.70 ± 0.5 a |
| Control Bread roll | 239.1 ± 1.9 b | 0.79 ± 0.001 d | 21.81 ± 0.05 f | 2.00 ± 0.02 a | 5.19 ± 0.01 c | - | 4.00 ± 0.0 c |
| Sangak bread + sourdough (L. Reuteri) | 127.36 ± 2.8 e | 0.84 ± 0.002 c | 32.29 ± 0.01 c | 1.75 ± 0.01 b | 5.67 ± 0.02 b | 3.00 ± 0.10 b | 4.50 ± 0.3 b |
| Sangak bread + sourdough (L. Casei-Casei) | 104.33 ± 2.9 f | 0.92 ± 0.003 a | 35.71 ± 0.01 a | 1.50 ± 0.02 c | 5.68 ± 0.02 b | 2.90 ± 0.10 b | 4.50 ± 0.0 b |
| Control Sangak bread | 135.06 ± 3.3 d | 0.86 ± 0.006 b | 33.51 ± 0.01 b | 1.25 ± 0.02 d | 5.91 ± 0.01 a | - | 4.10 ± 0.5 c |
aDifferent letters in the same column indicate significant differences between the values (p < 0.05) by Duncan’s multiple-comparison test
bResults are mean ± SD of three determinations on d.w.b
Glucose, fructose, maltose, and total reducing sugar in two kinds of flours and bread are shown in Table 3. Total reducing sugar was considered as the sum of glucose, fructose, and maltose content. In whole-wheat flour, initially higher amounts of sugars tested, were available compared to white wheat flour.
Table 3.
Sugar content (mg/g d.w.b.) in flour and bread a,b
| Sample | Added strain in sourdough | Maltose | Glucose | Fructose | Total reducing sugar |
|---|---|---|---|---|---|
| White wheat flour c | - | 2.36 ± 0.11 f | 2.77 ± 0.09 d | 8.39 ± 0.05 f | 13.52 ± 0.07 g |
| Bread roll | L. Reuteri | 10.17 ± 0.16 a | 6.33 ± 0.18 b | 36.42 ± 0.18 b | 52.92 ± 0.20 b |
| Bread roll | L. Csaei-Casei | 9.48 ± 0.12 b | 5.64 ± 0.11 c | 35.91 ± 0.08 c | 51.06 ± 0.78 c |
| Control Bread roll | - | 9.50 ± 0.10 b | 12.14 ± 0.11 a | 45.70 ± 0.10 a | 67.35 ± 0.10 a |
| Whole wheat flour d | - | 3.42 ± 0.12 e | 6.86 ± 0.14 b | 15.58 ± 0.05 d | 25.86 ± 0.10 d |
| Sangak bread | L. Reuteri | 7.22 ± 0.11c | 1.41 ± 0.17 d | 8.52 ± 0.14 f | 15.14 ± 0.10 f |
| Sangak bread | L. Csaei-Casei | 7.33 ± 0.18 c | 1.64 ± 0.12 d | 10.32 ± 0.13 e | 19.29 ± 0.11 e |
| Control Sangak bread | - | 5.70 ± 0.10 d | 1.14 ± 0.035 e | 5.52 ± 0.03 g | 12.35 ± 0.05 h |
aDifferent letters in the same column indicate significant differences between the values(p < 0.05) by Duncan’s multiple-comparison test
bResults are mean ± SD of three determinations on d.w.b
cWhite wheat flour is an ingredient of bread roll
dWhole wheat flour is an ingredient of sangak bread
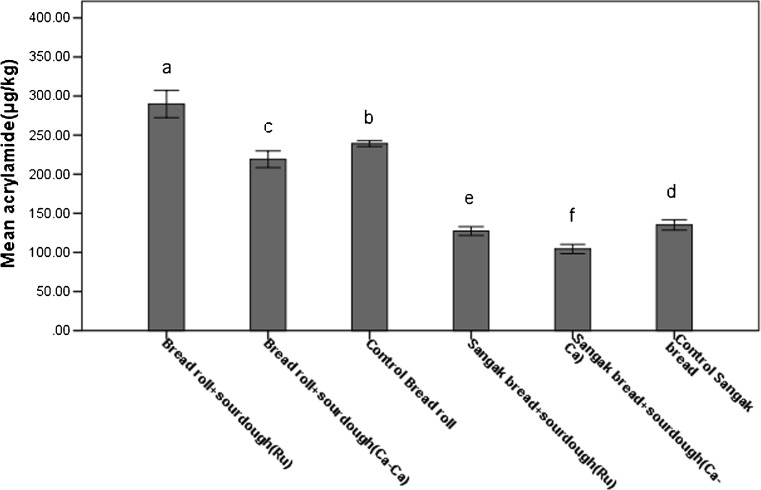
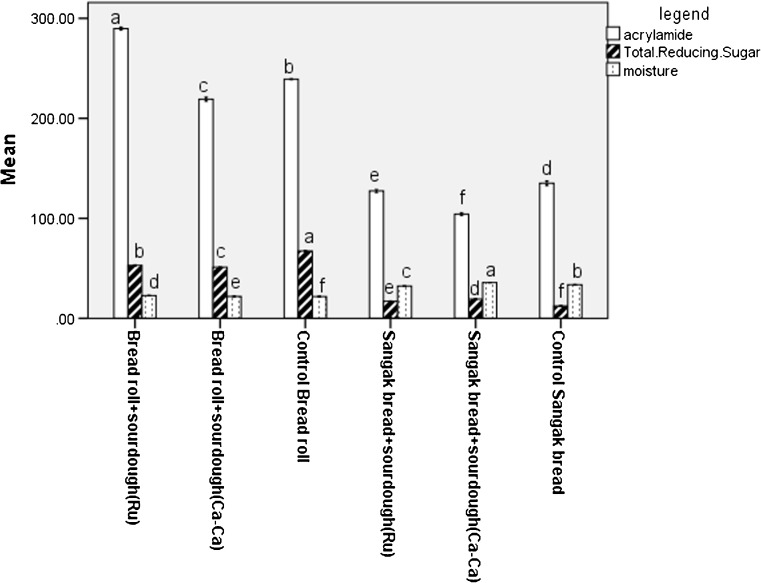
Acrylamide concentrations in bread samples ranged from 104.3 until 289.7 (μg/Kg) that shown in Table 2 and Fig. 1 in details. The acrylamide content of Sangak bread in all cases was lower than in the Bread roll. The correlation between acrylamide, sugars, and moisture of samples is significant at the 0.01 level (Fig. 3).
Fig. 1.
Compare means of acrylamide in Sangak bread and Bread roll. Different letters indicate significant differences between the values (p < 0.05) by Duncan’s multiple-comparison test. Results are mean ± 2 SD (error bars) of three determinations on d.w.b
Fig. 3.
Compare means of acrylamide, sugars and moisture of bread. Different letters in each legend, indicate significant differences between the values (p < 0.05) by Duncan’s multiple-comparison test
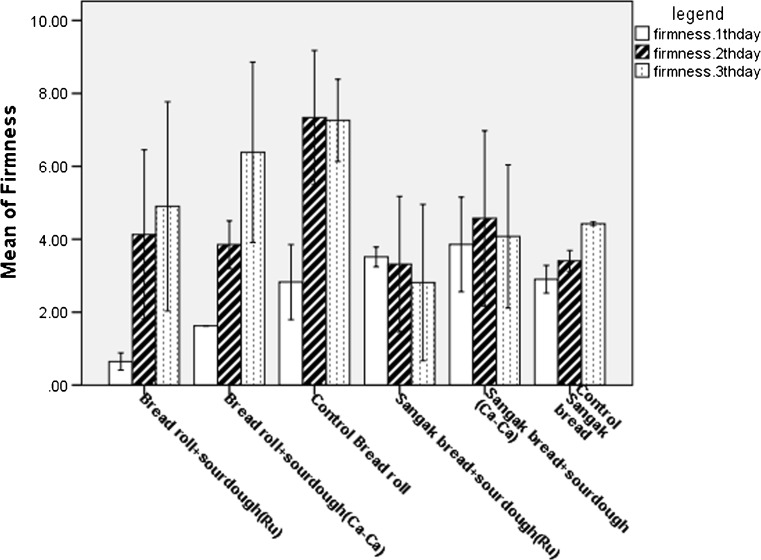
Table 2 and Fig. 2 show scores of sensory evaluation and bread firmness. Overall quality of fermented bread by sourdough is higher than to control, in two types of bread.
Fig. 2.
Compare means of bread firmness on first, 2th and 3th day after baking. Results are mean ± 2 SD (error bars) of three determinations
Discussion
The influence of pH, acidity, and lactic acid bacteria on the acrylamide formation in bread
Bread containing whole grain such as Sangak bread contains high level of enzymes as amylases, decarboxylases, proteases, and transaminases due to outer layers of wheat. During this study, the activity of alpha-amylase enzyme in whole-wheat flour almost 20 % greater than to white wheat flour based on falling number analysis (Table 2). As well, damaged starch is sensitive to the action of amylolytic enzymes and will simply denaturation by enzymes. In the present study, damaged starch in whole-wheat flour almost 16 % more than the other (Table 2).
When the enzyme activity is high in the flour, the breakdown of proteins and starch to amino acids and simple sugars easily happen and nutrients available for microorganisms increase in the dough. This is often caused by an excessive amount of acid and reduction of pH that was evaluated in the present study. Therefore, the variations in terms of FN will affect the final acidity of the sourdough (Katina et al. 2005).
The extraction level of the flour is one of the most important elements influencing the acidity of Sourdough (Banu et al. 2011). Our results showed that pH values of sourdoughs (with whole-wheat flour and white wheat flour, respectively), fermented with L. reuteri, L. casei-casei at the end of the fermentation, for making Sangak bread was 3.00 and 2.90, and for preparing Bread roll was 3.60 and 3.58, respectively (Table 2.). A significant reduction of acrylamide in Sangak bread was obtained by the pH of sourdough less or equal 3. Bread improver chemically acidified the bread roll dough, which did not effect on acrylamide reduction.
These results are in agreement with other authors that low pH values by microorganism sources will be one of the solutions to prevent the Maillard reaction, so decreasing the acrylamide content in bread. The primary step in acrylamide formation in Maillard reaction is that the formation of the Schiff base which will change to form 3-aminopropionamide, a potent precursor of acrylamide (Bemiller and Huber 2008; Granvogl et al. 2004).
The results showed that the amount of acrylamide in bread made with LAB starters in two kinds of bread was less than in the control sample except Bread roll fermented by L. Reuteri with a 21 % increase. Moreover, the acrylamide content of Sangak bread in all cases was lower than in the Bread roll that are presented in Fig.1 and Table 2.
A reduction effect of lactofermentation on the acrylamide formation in bread samples depended on the specifications of flour, the activity of the LAB strain used in sourdough and adaptability or competition with yeast in the utilization of nutrients and production process. The fermentation with commercial strain L. casei-casei was found to have a higher effect on acrylamide reduction in bread samples.
Bartkiene et al. (2013) showed that the fermentation with a commercial strain L. casei had a higher impact on acrylamide reduction in bread samples compared to L. sakei, Pediococcus acidilactici and Pediococcus pentosaceus strains that confirmed by our results. Some lactic acid bacteria seemed to have a strongly negative effect on yeast fermentation, which might elevated acrylamide levels in bread produced with sourdough as compared with samples exclusively fermented with yeast (Claus et al. 2008).
Low pH in the dough inhibits the formation of the Schiff base by protonation of the amine group of amino acid. Furthermore, associate acidic flavor of bakery products is just accepted in the case of sourdough, which, might attribute to improvement of bread quality and prevention of acrylamide formation that to be confirmed by the other researchers (Bartkiene et al. 2013; Fredriksson et al. 2004).
The Bread roll produced from sourdough had lower firmness compared with the control bread. As well, in the second and third days after baking, the firmness of Sangak bread fermented by sourdough (L. reuteri) was lower than the first day. The firmness of fermented Sangak bread by L. casei-casei on the third day had not significantly increased compared to the first day. Overall, the Sangak bread had the longer shelf life until three days (Fig. 2). In addition, fermented bread by sourdough had higher scores of sensory analysis than to control, in two types of bread. Organic acids, alcohols, esters, carbonyls, carbon dioxide, diacetyl, hydrogen peroxide and exopolysaccharides produced by LAB during sourdough fermentation can improve the flavor, aroma, volume, texture, nutritional quality, microbial safety and shelf life of bread (Katina 2005; Corsetti and Settanni 2007; Gamel et al. 2015). Control of staling and keeping the quality of bread for longer periods can lead to important economic benefits.
Acrylamide content in bread and relation to sugars
Homofermentative strains ferment hexoses to produce mostly lactic acid. Numerous heterofermentative strains can also ferment pentosans to produce lactic acid, acetic acid, and ethanol. The activity of heterofermentative strains is dependent on the treating situations of sourdough and the type of heterofermentative strain. Sugars used by lactic acid bacteria as an energy source varies by species and even by strain. The most common lactic acid bacteria identified in sourdoughs are capable of fermenting pentose, hexose, sucrose, and maltose (Katina 2005).
In this study, before the fermentation, maltose, glucose, and fructose levels (3.42, 6.86, 15.58 mg/g, respectively) were higher in whole-wheat flour than in white wheat flour (2.36, 2.77, 8.39 mg/g respectively) (Table 3).
Sugars increased over 24 h fermentation and baking in the bread roll. Glucose and fructose were higher in control Bread roll than in bread fermented with L. casei and L. reuteri.
In contrast, in Sangak bread maltose levels increased after fermentation and baking, but glucose and fructose decreased in all cases.
Fructose was existing either as a free monosaccharide or/and released through bread making from fructooligosaccharides and fructan naturally present in wheat flour. In Sangak bread, glucose and fructose were completely utilized after fermentation by LAB and yeast. In comparison, in Bread roll the reduced sugar levels increased after fermentation and baking that represent microorganisms cannot activate and utilization of sugars. The extraction rate of the flour is one of the most important elements influencing the level of enzymes, consequently alteration on microbial activity (Katina 2005). Recent studies have established the relationship between the strain and species-specific genetic characteristics of LAB, their metabolic activities, and their act in sourdough fermentation (Ganzle et al. 2007). As well, endogenous and added (exogenous) enzymes may cooperate with the sourdough LAB thus promoting special effects on the microbial metabolism. In a research, eleven LAB species were used alone or in association with added microbial enzymes to produce sourdough. Only three species were positively influenced by added enzymes and Lactic acid fermentation was markedly enhanced (Gobbetti et al. 2005).
The activity of the glucoamylases during fermentation is released glucose that favored the growth of L. casei, since glucose is the preferable carbohydrate of homofermentative LAB (Ganzle et al. 2007). The preferred use of fructose as an electron acceptor is observed in most heterofermentative lactobacilli. The utilization of electron acceptors permits the production of acetate from acetyl-phosphate and the synthesis of an extra ATP.
The obligate heterofermentative Lactobacilli as Lactobacillus reuteri also show maltose phosphorylase activity. Glucose-1-P is metabolized via the pentose-phosphate shunt; glucose is generated until the external glucose concentration is high enough to persuade the expression of hexokinase activity (Ganzle et al. 2007).
The results showed that maltose, glucose, fructose and total reducing sugars of bread influenced (r = 0.885, r = 0.769, r = 0.895 and r = 0.885 respectively) the formation of acrylamide in the bread that this correlation was significant at the 0.01 level (Fig. 3). This is a consequence of a consumption of the precursors by microorganisms in the fermentation process. Sugar seems to be the most important ingredient in the dough formula for acrylamide formation in bakery products because the free asparagine is relatively low in wheat flour. This result was confirmed by other scientists (Keramat et al. 2011).
The impact of moisture and aw on the acrylamide formation in bread
The less content of acrylamide in Sangak bread versus Bread roll could also be achieved due to short heat treatment and higher moisture contents (Table 2, Fig. 3) that was approved by the other scientist (Claus et al. 2008). Acar and Gökmen (2009) showed that the acrylamide concentration can be influenced by the product depth and alter of temperature in different locations of the bread.
Since low moisture contents enhance acrylamide formation throughout the Maillard reaction, these effects might, at least partially, be avoided by use of a higher relative humidity throughout baking (FEDIL 2011). During baking of dough, the water content on the surface of the loaf quickly decreases providing optimum conditions for the formation of Maillard reaction product and intense brown color. Inside the dough, the temperature is lower and the Aw remains relatively high. Therefore, the crumb is just weakly colored with a low concentration of Maillard reaction products (Capuano et al. 2008).
Flat bread such as Naan had amazingly low levels of acrylamide, despite their larger proportion of crust to the crumb. This could be because of only a minor proportion of the crust surface was powerfully colored during baking (Sadd and Hamlet 2005).
Low moisture content could be a more important promoter of acrylamide than temperature, thus crust moisture could be a key factor controlling acrylamide levels, and this accounts for a lot of the variation seen in different kinds of bakery products (Sadd and Hamlet 2005).
The obtained results showed that moisture and Aw of bread reversely influenced the formation of acrylamide in bread (r = −0.929, r = −0.926 respectively). The bread made with high moisture and Aw had lower acrylamide concentrations.
Conclusion
The important results obtained during this study clearly showed the following items:
Low pH through increased microbial and bran endogenous enzymatic activities, as well, formulation and baking technology powerfully have an effect on acrylamide content in bakery products.
Lactofermentation increased of bread shelf life and improved of sensory characteristics via proteolysis and lipolysis activity, which cause to produce aromatic compounds.
Overall, bread containing whole grain as well as the addition of sourdough starters, have become more important in the bakery industry due to improving health.
However, additional study based on this research should be applied to achieve the least amount of acrylamide and appropriate organoleptic characteristics for each specific type of bread according to bakery conditions and formulations.
Acknowledgments
The authors gratefully acknowledge the financial support from the University of Tehran, Standard Research Institute, Iranian Center of Excellence for Application of Modern Technologies for producing functional foods and drink and Parsian Enzyme Iranian Co.
Contributor Information
Farnaz Dastmalchi, Email: farnazdastmalchi@yahoo.com.
Seyed Hadi Razavi, Email: srazavi@ut.ac.ir.
Mohammad Faraji, Email: mohammadfaraji2010@gmail.com.
References
- Acar ÖÇ, Gökmen V. Investigation of acrylamide formation on bakery products using a crust-like model. Mol Nutr Food Res. 2009;53(12):1521–1525. doi: 10.1002/mnfr.200800585. [DOI] [PubMed] [Google Scholar]
- American Association of Cereal Chemists (AACC) (2000) Approved methods of analysis. 10th ed. St. Paul, MN, U.S.A: AACC International
- Association of Official Analytical Chemists (AOAC) Official methods of analysis of AOAC international. 16th. VA: Arlington; 1995. [Google Scholar]
- Baardseth P, Blom H, Enersen G, Skrede G, Slinde E, Sundt T, Thomassen T (2004) Reduction of acrylamide formation in cereal based food processing. Patent WO2004028276
- Banu I, Vasilean I, Barbu V, Iancu C. The effect of some technological factors on the rye sourdough bread. Chem Chemical Eng Biotec Food Indu. 2011;12(2):197–202. [Google Scholar]
- Bartkiene E, Jakobsone I, Juodeikiene G, Vidmantiene D, Pugajeva I, Bartkevics V. Study on the reduction of acrylamide in mixed rye bread by fermentation with bacteriocin-like inhibitory substances producing lactic acid bacteria in combination with Aspergillus niger glucoamylase. Food Control. 2013;30:35–40. doi: 10.1016/j.foodcont.2012.07.012. [DOI] [Google Scholar]
- Bemiller JN, Huber KC. Carbohydrates. In: Damodaran S, Parkin KL, Fennema OR, editors. Fennemas Food chemistry. 4th. Boca Raton: CRC Press, Taylor& Francis Group; 2008. pp. 101–105. [Google Scholar]
- Capuano E, Ferrigno A, Acampa I, Ait-Ameur L, Fogliano V. Characterization of the maillard reaction in bread crisps. Eur Food Res Technol. 2008;228:311–319. doi: 10.1007/s00217-008-0936-5. [DOI] [Google Scholar]
- Claus A, Carle R, Schieber A. Acrylamide in cereal products: a review. J Cereal Sci. 2008;47:118–133. doi: 10.1016/j.jcs.2007.06.016. [DOI] [Google Scholar]
- Corsetti A, Settanni L. Lactobacilli in sourdough fermentation. Food Res Int. 2007;40:539–558. doi: 10.1016/j.foodres.2006.11.001. [DOI] [Google Scholar]
- Didar Z, Pourfarzad A, Haddad Khodaparast MH. Effect of different lactic acid bacteria on phytic acid content and quality of whole wheat toast bread. World Acad Sci Eng Technol. 2010;68:1443–1448. [Google Scholar]
- FEDIL. Food drink Europe acrylamide toolbox (2011) Available at: ec.europa.eu/food/./ciaa_acrylamide_toolbox09
- Fink M, Andersson R, Rosen J, Aman P. Effect of added asparagine and glycine on acrylamide content in yeast-leavened bread. Cereal Chem. 2006;83:218–222. doi: 10.1094/CC-83-0218. [DOI] [Google Scholar]
- Fredriksson H, Tallving J, Rosen J, Aman P. Fermentation reduces free asparagine in dough and acrylamide content in bread. Cereal Chem. 2004;81:650–653. doi: 10.1094/CCHEM.2004.81.5.650. [DOI] [Google Scholar]
- Gamel TH, Abdel-Aal ESM, Tosh SM. Effect of yeast-fermented and sourdough making processes on physicochemical characteristics of b-glucan in whole wheat/oat bread. LWT Food Sci Technol. 2015;60:78–85. doi: 10.1016/j.lwt.2014.07.030. [DOI] [Google Scholar]
- Ganzle MG, Vermeulen N, Vogel RF. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 2007;24:128–138. doi: 10.1016/j.fm.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh-Mohammadi V, Mohammadi A, Hashemi M, Khaksar R, Haratian P. Microwave-assisted extraction and dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry for isolation and determination of polycyclic aromatic hydrocarbons in smoked fish. J Chromatogr A. 2012;1237:30–36. doi: 10.1016/j.chroma.2012.02.078. [DOI] [PubMed] [Google Scholar]
- Gobbetti M, De Angelis M, Corsetti A, Di Cagno R. Biochemistry and physiology of sourdough lactic acid bacteria. Trends Food Sci Technol. 2005;16:57–69. doi: 10.1016/j.tifs.2004.02.013. [DOI] [Google Scholar]
- Granvogl M, Jezussek M, Koehler P, Schieberle P. Quantitation of 3- amino -propionamide in potatoes-a minor but potent precursor in acrylamide formation. J Agric Food Chem. 2004;52:4751–4757. doi: 10.1021/jf049581s. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) (1994) Acrylamide. In: IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans Vol. 60, pp 389–433
- International Organization for Standardization (ISO) (2006) Approved Method of the ISO, No. 21415–2. Wheat and wheat Flour, Gluten Content, Part 2:Determination Of wet gluten by mechanical means
- International Organization for Standardization (ISO) (2007) Approved Method of the ISO, No. 2171. Cereals, Pulses and by Products, Determination of ash yield by incineration
- International Organization for Standardization (ISO) (2009) Approved Method of the ISO, No. 712. Cereal and cereal products, Determination of moisture content, Reference Method
- International Organization for Standardization (ISO) (2013) Approved Method of the ISO, No. 17715. Flour from wheat (Triticum aestivum L.) - Amperometric method for starch damage measurement
- Katina K (2005) Sourdough: a tool for the improved flavour, texture and shelf-life of wheat bread. Academic dissertation. Espoo. VTT Publications 569, Finland, pp 17–25
- Katina K, Arendt E, Liukkonen KH, Autio K, Flander L, Poutanen K. Potential of sourdough for healthier cereal products. Trends Food Sci Technol. 2005;16:104–112. doi: 10.1016/j.tifs.2004.03.008. [DOI] [Google Scholar]
- Keramat J, LeBail A, Prost C, Jafari M. Acrylamide in baking products: a review article. Food Bioprocess Tech. 2011;4:530–543. doi: 10.1007/s11947-010-0495-1. [DOI] [Google Scholar]
- Kornbrust BA, Stringer MA, Krebs NE, Hendriksen LV, Hendriksen HV (2010) Asparaginase – an enzyme for acrylamide reduction in food products. In: Whitehurst RJ, Oort MV (eds.) Enzymes in food technology, Second edition, Blackwell Publishing, pp 59–70
- Lim H, Shin H. Ultra trace level determinations of acrylamide in surface and drinking water by GC–MS after derivatization with xanthydrol. J Sep Sci. 2013;36(18):3059–3066. doi: 10.1002/jssc.201300209. [DOI] [PubMed] [Google Scholar]
- Sadd P, Hamlet C. The formation of acrylamide in UK cereal products. In: Friedman M, Mottram D, editors. Chemistry and safety of acrylamid in food. UK: Springer Science Business Media, Inc,; 2005. pp. 415–418. [Google Scholar]
- US Environmental Protection Agency (EPA) Chemical, summary for acrylamide. Washington DC: Office of Toxic Substances; 1994. [Google Scholar]
- World Health Organisation (WHO) (2002) Health implications of acrylamide in food. Report of a Joint FAO/WHO Consultation, WHO Headquarters. Geneva, Switzerland