Abstract
The effects of technical cashew nut shell liquid (TCNSL) on the trans isomerization of edible oils during heating are investigated. Edible oils were subjected to thermal treatment at various heating times and temperatures. Our results show that the addition of TCNSL to edible oils at the appropriate concentration during heating suppresses trans fatty acid formation and induces formation of conjugated linoleic acid (CLA) isomers. A concentration of 0.2 % TCNSL demonstrates the best ability to inhibit formation of trans-oleic acid, trans-linoleic acid, and trans-linolenic acid isomers as well as increase the formation of 9 t,11 t-CLA and 10 t,12 t-CLA isomers. Our analysis indicates that the presence of 0.2 % TCNSL in corn oil does not significantly reduce the acid value, but may significantly lower the peroxide value. TCNSL is also observed to have better function compared to Vitamin E (VE) and tertiary butylhydroquinone (TBHQ), indicating that it may be considered an effective additive in edible oils.
Keywords: Technical cashew nut shell liquid, Heat treatment, trans isomers, Edible oils, Peroxide number
Introduction
Edible oils are a major component of the human diet due to their high-energy value, large quantities of easily solubilized vitamins, and essential fatty acid content. Unsaturated fatty acids within edible oils, such as oleic acids (OAs), linoleic acids (LAs), and linolenic acids (LNAs), can undergo cis,trans (c,t) isomerization during the refining and/or deep-fat frying processes, leading to the formation of trans fatty acids (TFAs). Consumption of TFAs has been linked to an increased risk of cardiovascular disease (Ganguly and Pierce 2012), as well as other symptoms, such as inflammation and endothelial dysfunction (Mozaffarian 2006). Due to the substantial health benefits associated with reducing intake of TFAs, governments around the world are actively passing legislation to limit, and in some cases, ban the use of TFAs (FAO/WHO 2009; Li 2013).
The TFAs found in most plant oils are composed mainly of trans-LAs and trans-LNAs, which are formed from the isomerization of 9c,12c-LA and 9c,12c,15c-LNA triacylglycerol, respectively. The chemistry of TFA isomers in edible oils, including triolein, trilinolein, and trilinolenin, has been previously investigated. 9 t-OA, 9 t,12 t-LA, 9c,12 t-LA, and 9 t,12c-LA are the trans isomers formed from processing OA and LA above 180 °C (Li et al. 2013; Tsuzuki 2011). Trans isomerization of LNA can be achieved at high temperatures, producing 9 t,12c,15 t-LNA, 9 t,12 t,15c-LNA, 9c,12c,15c-LNA, 9c,12 t,15c-LNA, and 9 t,12c,15c-LNA as the predominant trans products (Tsuzuki et al. 2008). Previous studies have also reported that the formation of these trans isomers at longer heating times and higher temperatures was also associated with a decrease in unsaturated fatty acid content (Li et al. 2012).
In addition, other studies have highlighted the formation of conjugated linoleic acids (CLAs) during the heating of edible oils, showing that trans,trans CLA isomers are the predominant products, followed by cis,trans and trans,cis CLA isomers (Christy 2009; Guo et al. 2015). Recently, interest in CLAs has increased due to the biological activities associated with their numerous isomers. These CLA isomers have been found to be beneficial for body composition, and inhibit atherosclerosis, carcinogenesis, and diabetogenesis (Park 2014; Yang et al. 2015). Beppu et al. (2006) reported that 9 t,11 t-CLA has a stronger inhibitory effect on human colon cancer cell lines compared to 10 t,12c-CLA and 9c,11 t-CLA. These studies highlight the potential benefits of increasing formation of CLA isomers formed during the heating of oils.
The edible oils are not only as utritional purpose, but even as ingredients in many recipe and food processing. The stability of edible oil goes bad when it subjects to higher temperature. In order to increasing the amounts of high quality oils with beneficial properties, many studies try to explore the optimum stabilization conditions using a suitable additive. Butyl-hydroxyanisol (BHA), butylated hydroxytoluene (BHT), and tertiary butylhydroquinone (TBHQ), are synthetic antioxidants commonly added to edible oils to inhibit oxidative changes that are usually introduced during storage and/or heating. In previous study, the same authors reported that these antioxidants were shown to reduce TFA formation and induce CLA formation in heated edible oils, with TBHQ exhibiting the greatest activity (Guo et al. 2015). However, the safety and toxicity of these synthetic antioxidants draw great attention recently, and some countries limit the use of them (Fan and Eskin 2015). Therefore, it is necessary to explore a natural additive that can be used to decrease TFA formation, increase CLA formation, and improve resistance to oxidation in heated oils.
Cashew nut shell liquid (CNSL), a byproduct of cashew nut production, is a brownish viscous oil composed of anacardic acids, cardanol, and cardol. The low thermal stability of the carboxyl group in anacardic acid gives it a tendency to convert to cardanol. Therefore, the major components of technical CNSL (TCNSL) obtained after thermal processing are monophenol, cardanol, and the resorcinol derivative cardol (Attanasi et al. 2012). These compounds possess antioxidant, antigenotoxic, anti-tumour, and antimicrobial activities (dos Santos Andrade et al. 2011; Kubo et al. 1993), as well as anti-inflammatory action (Schmourlo et al. 2005) and anti-lipoxigenase activity (Ha and Kubo 2005).
With the goal of finding new applications for TCNSL, we hypothesized that the presence of TCNSL in commercially available edible oils may be responsible for the noted reduction in TFA formation during heating. Therefore, the objective of the current study is to use GC-FID to investigate the effects of TCNSL on the formation of trans isomers during heating of edible oils. Results from this study may provide insight into the influence of TCNSL on edible oils during the deep-fat frying process and may also suggest new methods for reducing the formation of TFAs and inducing the formation of CLAs.
Materials and methods
Materials
Refined edible corn oil, soybean oil, and sunflower oil were purchased from a local market (Beijing, China). Technical cashew nut shell liquid (TCNSL, cardol to cardanol ratio = 7:3) was purchased from Shenyang Shizhongyetai New Materials Co., Ltd (Beijing, China). Standard mixtures of the methyl esters, including oleic acid isomers, linoleic acid isomers, and linolenic acid isomers, were purchased from Sigma-Aldrich (St. Louis, MO, USA). 9 t,11 t linoleic acid methyl ester was purchased from Matreya (State College, PA, USA). Vitamin E (VE) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Tertiary butylhydroquinone (TBHQ) was obtained from Sinopharm Chemical Reagent Beijing Co., Ltd (Beijing, China). Triundecanoin was obtained from NU-CHEK Prep and used as an internal standard for GC-FID analysis. Isooctane was purchased from Fisher Scientific (Fair Lawn, NJ, USA) and used as a chromatographic organic solvent. Silicone oil (viscosity 500 cSt) was used as a heating material and obtained from Dingye Industrial Co., Ltd. (Beijing, China).
Thermal processing
2.0 g of corn oil, soybean oil, and sunflower sample with 0.03, 0.2, 0.8 and 2.0 % TCNSL were transferred into a 4 mL micro-glass ampoule with a length of 4 cm, an internal diameter of 1.5 mm, and a wall thickness of 1 mm. The ampoules, in the absence (control) and presence of TCNSL, were then sealed using a propane-oxygen flame and heated at 230 °C for 8 h in a silicone oil bath. In order to investigate the effects of heating time and temperature on thermally-induced trans isomers after adding TCNSL, corn oil was chosen as a model system for edible oils. Corn oil sample with TCNSL was heated at 230 °C for 2, 4, 8, 12, 16, 24, 32, and 48 h, and heated at 180, 200, 220, 230, and 240 °C for 8 h, respectively. For further comparing the effects of TCNSL with other additives in edible oils, corn oil samples with 0.2 % VE and 0.2 % TBHQ were heated at 180 °C for 8 h. Temperature control during heating was accurate to ± 2 °C. After incubation, the glass tubes were removed from the silicone oil bath and cooled prior to analysis.
Preparation of fatty acid methyl esters
After heating, the samples were subjected to a derivatization procedure according to the method described in the AOCS Official Method, chapters 1–91 (AOCS 1997). Briefly, the heated sample (200 mg) was weighed in a stoppered centrifuge vial and mixed with 2 mL of the internal standard, triundecanoin (C11:0, dissolved in isooctane), and 0.1 mL of a 2 mol/L methanolic KOH. The mixture was then shaked well for 30 s, and placed in a centrifuge for 10 min at 4000 rpm. The supernatant layers were then removed and dried using anhydrous magnesium sulphate.
GC-FID analysis
The dried supernatant (20 μL) was diluted with isooctane to a final volume of 1 mL and analyzed using a GC-2010 chromatograph (Shimadzu, Kyoto, Japan) equipped with a CP-SIL 88 fused silica capillary column (100 m × 0.25 μm × 0.2 mm) and a flame ionization detector. An initial temperature of 60 °C was maintained for 5 min and then increased to 160 °C at a rate of 25 °C/min. After a 5 min incubation period at 160 °C, the temperature was raised again at a rate of 2 °C/min until a final temperature of 225 °C was achieved. The sample was then held at this final temperature for 15 min. The injection volume was 1 μL with a split ratio of 1:10, and nitrogen (99.999 %) was used as the carrier gas at a flow rate of 6.3 mL/min. The injector and interface temperatures were both set to 230 °C. Specific fatty acids were identified by comparison of their retention times to the retention times of known standards, according to a calculation method from Li et al. (2013). The amount of 9 t,11 t-CLA and 10 t,12 t-CLA mixture present was calculated by comparing samples to a 9 t,11 t-CLA standard. Accurate quantification of the fatty acid concentrations was dependent on the absence of any chemical interactions between the molecules.
Statistical analysis
Experiments were performed in triplicate following a completely randomized design. Sigmaplot 12.0 software (Systat Software Inc., San Jose, CA, USA) was used for data plotting. One-way analysis of variance (ANOVA) was applied to all samples in order to verify significant differences at the 5 % level using SPSS (Version 16.0, SPSS Inc., Chicago, IL, USA).
Results and discussion
Effect of TCNSL on trans isomers in edible oils after processing at 230 °C for 8 h
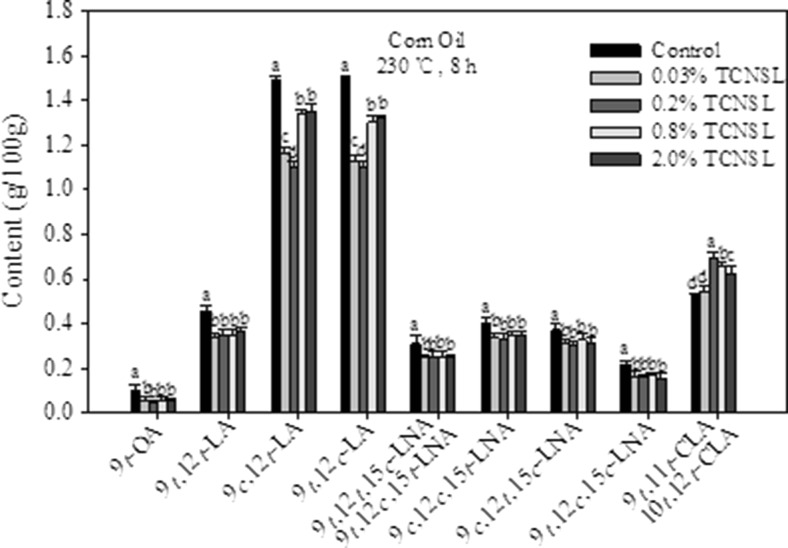
In order to elucidate the effect of TCNSL on heat-induced formation of trans isomers in edible oils, corn oil was incubated at 230 °C for 8 h. As shown in Fig. 1, eleven different trans isomers were identified, including one OA isomer (9 t-OA), five LA isomers (9 t,12 t-LA, 9 t,12 t-LA, 9c,12 t-LA, 9 t,12c-LA, and 9 t,11 t-LA) and five LNA isomers (9 t,12 t,15c-LNA, 9 t,12c,15 t-LNA, 9c,12c,15 t-LNA, 9c,12 t,15c-LNA, and 9 t,12c,15c-LNA). The total content of trans-OA, trans-LA, and trans-LNA isomers in control was 0.08 g/100 g, 4.03 g/100 g, and 1.25 g/100 g, respectively. These results indicate that the trans-LA isomers are the major products formed among the trans isomers in heated corn oil. Interestingly, the primary trans isomers (9c,12 t-LA and 9 t,12c-LA) were formed in nearly equal quantities in heated corn oil, while the 9 t-OA isomer was the minor product. These findings are in agreement with previous reports (Chen et al. 2014; Liu et al. 2007). The amounts of 9c,12c,15 t-LNA and 9c,12 t,15c-LNA in heated corn oil were almost equal and were higher than the other three trans-LNA isomers. Our results showed that the content of 9 t,11 t-CLA and 10 t,12 t-CLA in corn oil was 0.52 g/100 g.
Fig. 1.
Effect of technical cashew nut shell liquid (TCNSL) on trans isomers in corn oil heated at 230 °C for 8 h. Vertical error bars represent the standard deviation of the mean
In this study, the addition of 0.03%TCNSL to corn oil resulted in a significant reduction in the formation of 9 t-OA (44.9 %), and total trans-LA (23.3 %) and LNA (19.2 %) isomers during heating (Fig. 1). The inhibitory effect of TCNSL showed no significant improvement at higher concentrations for 9 t-OA, 9 t,12 t-LA, 9 t,12 t,15c-LNA, 9 t,12c,15 t-LNA, 9c,12c,15 t-LNA, 9c,12 t, 15c-LNA, and 9 t,12c15c-LNA. Furthermore, the amount of 9 t,11 t-CLA and 10 t,12 t-CLA formed in heated corn oil initially increased with the addition of TCNSL, but subsequently decreased at higher concentrations. 0.2 % TCNSL significantly increased the amount of 9 t,11 t-CLA and 10 t,12 t-CLA (33.6 %) compared to that of the control.
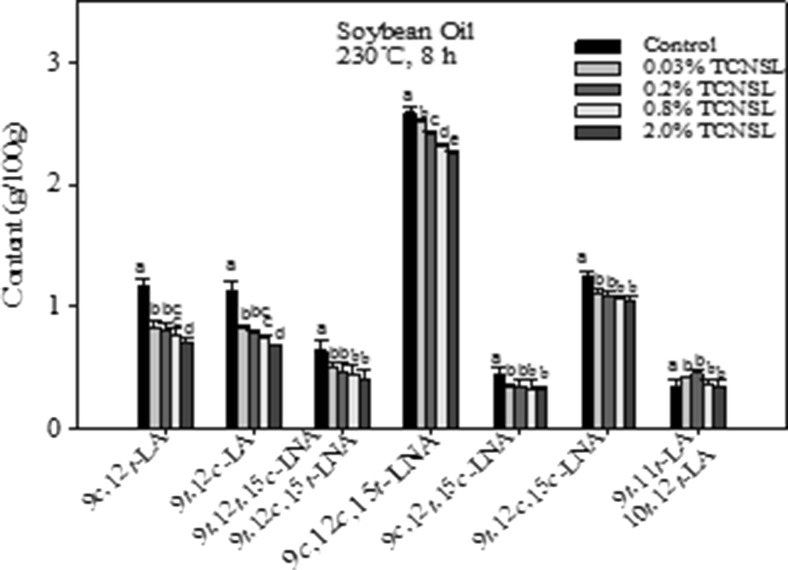
As reported in Fig. 2, soybean oil was found to contain four different LA and five LNA isomers after heating at 230 °C for 8 h. Out of these products, the trans-LNA isomers were the major products formed (4.57 g/100 g), followed by the trans-LA isomers (2.10 g/100 g). The amount of 9c,12 t-LA was nearly equal to that of 9 t,12c-LA in the heated soybean oil, which was consistent with the measurements for heated corn oil (Fig. 1). The amount of 9c,12c,15 t-LNA was the most abundant among trans-LNA isomers in the heated soybean oil. The content of 9 t,11 t-CLA and 10 t,12 t-CLA in heated soybean oil was measured to be 0.39 g/100 g.
Fig. 2.
Effect of technical cashew nut shell liquid (TCNSL) on trans isomers in soybean oil heated at 230 °C for 8 h. Vertical error bars represent the standard deviation of the mean
In soybean oil, the formation of trans-LA and trans-LNA isomers was significantly reduced with increasing concentrations of TCNSL, suggesting that the addition of TCNSL can inhibit the formation of TFA isomers at higher temperatures. The 9 t,11 t-CLA and 10 t,12 t-CLA content increased 21.1 and 32.8 % compared to that of control by addition of 0.03 % TCNSL and 0.2 % TCNSL, respectively.
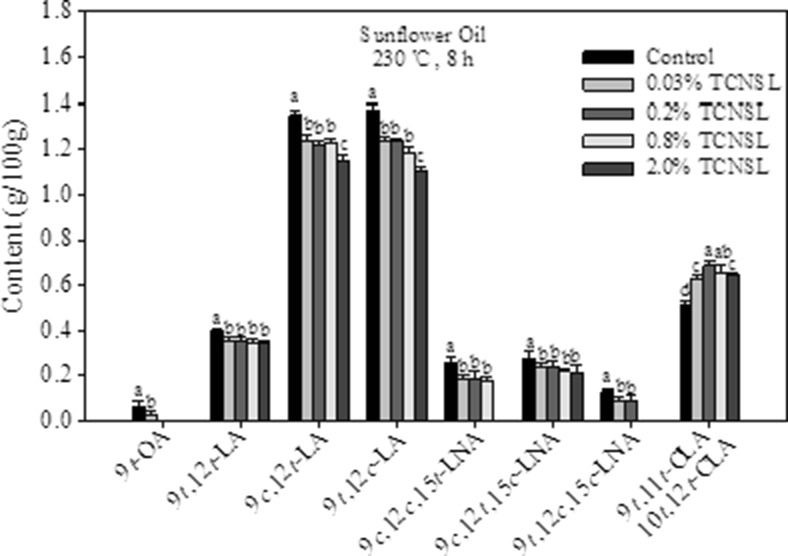
Figure 3 are reported the results for thermal treatment of sunflower oil. There were nine different trans-isomers identified in heated sunflower oil, including a single OA isomer, five LA isomers, and three LNA isomers, with a total content in control of 0.03/100 g, 3.00/100 g, and 1.51/100 g, respectively. The trans-LA isomers were the major products in heated sunflower oil, while the amount of 9c,12 t-LA was nearly equal to that of 9 t,12c-LA, in agreement with results measured for heated corn oil and soybean oil. However, the amount of 9 t,12 t-LA was lower compared to that of 9c,12 t-LA and 9 t,12c-LA in the three different oils. This observation is in agreement with previous reports showing that 9c,12 t-LA and 9 t,12c-LA are present at similar levels that are often higher than the levels of 9 t,12 t-LA in many non-hydrogenated dietary fats (Glew et al. 2006; Kramer et al. 2008). These results suggest that LA molecules in oils readily isomerize to form 9c,12 t-LA and 9 t,12c-LA, but isomerization to form 9 t,12 t-LA is unfavorable. The amount of trans-LNA in heated sunflower oil also varies among the different isomers, with levels of 9 t,12 t,15c-LNA and 9 t,12c,15 t-LNA below the detection limit compared to corn oil and soybean oil under the same conditions. In addition, 9c,12c,15 t-LNA and 9 t,12c,15c-LNA were not detected at concentrations higher than 2.0 and 0.8 %, respectively. The amount of 9 t,11 t-CLA and 10 t,12 t-CLA in heated sunflower oil was observed to be 0.62 g/100 g.
Fig. 3.
Effect of technical cashew nut shell liquid (TCNSL) on trans isomers in sunflower oil heated at 230 °C for 8 h. Vertical error bars represent the standard deviation of the mean
The formation of trans-LA and trans-LNA isomers was significantly reduced by the addition of TCNSL in sunflower oil that had been processed at 230 °C for 8 h. Similar to heated corn and soybean oil, the formation of 9 t,11 t-CLA and 10 t,12 t-CLA in heated sunflower oil was initially enhanced, but decreased with increasing concentrations of TCNSL. However, the amount of 9 t,11 t-CLA and 10 t,12 t-CLA in sunflower oil increased 22.2 % compared to that of control after adding 0.03 % TCNSL.
In summary, the addition of TCNSL was observed to lower the amounts of trans-OA, trans-LA, and trans-LNA in heated corn, soybean, and sunflower oil, indicating that TCNSL suppresses the cis-to-trans isomerization of the double bonds in edible oils to varying degrees. These inhibitory effects can be attributed to the antioxidant activity of TCNSL, during which a hydroxyl group donates a hydrogen atom to a lipid radical, forming a more stable product and mitigating the propagation phase of lipid oxidation during heat-induced trans isomerization of edible oils.
The increased amounts of CLAs (9 t,11 t-CLA and 10 t,12 t-CLA) observed upon addition of TCNSL in heated corn, soybean, and sunflower oil, suggests that TCNSL is able to promote migration of the double bonds in the fatty acids of edible oils to form CLAs (Guo et al. 2015). After donating a hydrogen atom, TCNSL can form radicals that may not be stabilized by neighboring molecules or radicals (Elisia et al. 2013). This behavior may result in pro-oxidant activity, in which the radicals participate in the propagation of lipid radicals and thus promote the formation of conjugated structures. Therefore, in the range of 0.03 to 0.2 %, addition of TCNSL can be effective in decreasing TFA content while increasing CLA formation in edible oils, with 0.2 % TCNSL exhibiting the best inhibition and promotion of TFA and CLA formation, respectively.
Effect of TCNSL on trans isomers in corn oil at 230 °C for various heating times
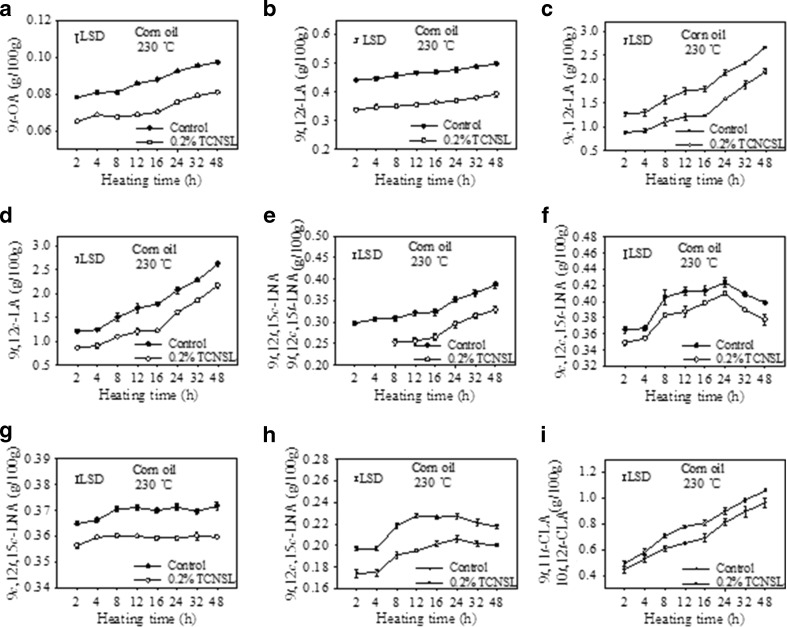
In order to investigate the effect of heating time on the formation of trans isomers after adding TCNSL, 0.2 % TCNSL was added to the corn oil samples and heated at 230 °C (Fig. 4). The 9 t-OA content in the corn oil increased with increasing heating time. The presence of 0.2 % TCNSL reduced 9 t-OA formation significantly in heated corn oil over a 48 h heating period. The formation of trans-LAs, including 9 t,12 t-LA, 9c,12 t-LA and 9 t,12c-LA, also increased with increasing heating time and was significantly inhibited by the addition of 0.2 % TCNSL during the 48 h heating period (Fig. 4b, c and d).
Fig. 4.
Effect of technical cashew nut shell liquid (TCNSL) on trans isomers in corn oils heated at 230 °C for various amounts of time. Vertical error bars represent the standard deviation of the mean
The formation of 9 t,12 t,15c-LNA and 9 t,12c,15 t-LNA in control corn oil increased slowly at 230 °C over 48 h, while 9 t,12 t,15c-LNA and 9 t,12c,15 t-LNA were not detected after the first 4 h of heating in samples containing 0.2 % TCNSL. After 8 h of heating, the levels of these LNAs were significantly decreased by the addition of 0.2 % TCNSL (Fig. 4e). In comparison, there was no remarkable change observed in the amount of 9c,12c,15 t-LNA formed in the control corn oil sample after the first 4 h of heating, the content of 9c,12c,15 t-LNA eventually increased and then finally decreased after heating for 24 h (Fig. 4f). A similar trend was also observed for 9 t,12c,15c-LNA (Fig. 4 h). However, the presence of 0.2 % TCNSL was able to reduce the amounts of both 9c,12c,15 t-LNA and 9 t,12c,15c-LNA in corn oil heated at 230 °C for 48 h. The formation of 9c,12 t,15c-LNA increased gradually with longer heating times, and was significantly reduced by the addition of 0.2 % TCNSL (Fig. 4 g). These results suggest that the presence of 0.2 % TCNSL is able to inhibit the formation of trans-OA, trans LA, and trans-LNA isomers over longer heating times at a constant temperature.
The formation of 9 t,11 t-CLA and 10 t,12 t-CLA in corn oil was observed to increase gradually with increased heating time at 230 °C (Fig. 4i). Furthermore, the 9 t,11 t-CLA and 10 t,12 t-CLA content in corn oil was higher upon addition of 0.2 % TCNSL compared to the control, suggesting that the presence of 0.2 % TCNSL is able to promote the formation of 9 t,11 t-CLA and 10 t,12 t-CLA. These results indicate that the observed activity of TCNSL in corn oil is not influenced by heating time.
Effect of TCNSL on trans isomers in corn oils heated at various temperatures for 8 h
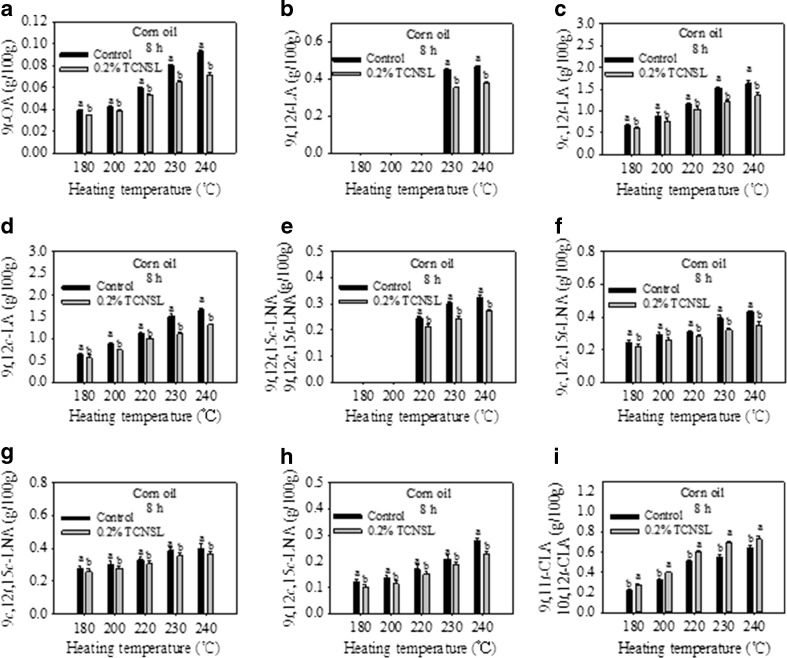
The effect of heating temperature on trans isomer formation after adding 0.2 % TCNSL to corn oil were investigated (Fig. 5). The amount of 9 t-OA in the control increased with heating temperature over the course of 8 h. The addition of 0.2 % TCNSL was able to remarkably reduce the level of 9 t-OA produced in the corn oil at 180, 200, 220, 230, and 240 °C (Fig. 5a). However, 9 t,12 t-LA was undetectable at 180, 200, and 220 °C, and decreased in concentration upon the addition of 0.2 % TCNSL when heated at 230 and 240 °C (Fig. 5b). The amount of 9c,12 t-LA and 9 t,12c-LA increased with heating temperatures, but was inhibited by the addition of 0.2 % TCNSL at all temperatures investigated (Fig. 5c and d). The formation of 9 t,12 t,15c-LNA and 9 t,12c,15 t-LNA was undetectable at 180 and 200 °C, and was significantly reduced by the addition of 0.2 % TCNSL at 220 °C (Fig. 5e). The formation of 9c,12c,15 t-LNA, 9c,12 t,15c-LNA, and 9 t,12c,15c-LNA increased with increasing heating temperature, but the addition of 0.2 % TCNSL was also able to significantly reduce the formation of these isomers (Fig. 5f, g and h). These results demonstrate that 0.2 % TCNSL is able to generally inhibit the formation of trans-OA, trans-LA, and trans-LNA isomers. In contrast, while the formation of 9 t,11 t-CLA and 10 t,12 t-CLA in corn oil increased with heating temperature (Fig. 5i), the amounts of 9 t,11 t-CLA and 10 t,12 t-CLA increased by the presence of 0.2 % TCNSL. This suggests that 0.2 % TCNSL induces the formation of 9 t,11 t-CLA and 10 t,12 t-CLA. The results also indicate that the inhibitory and induction abilities of TCNSL in corn oil increase with increased heating temperature.
Fig. 5.
Effect of technical cashew nut shell liquid (TCNSL) on trans isomers in corn oils heated at various temperatures for 8 h. Vertical error bars represent the standard deviation of the mean
Effect of TCNSL on the acid value and peroxide value of corn oil
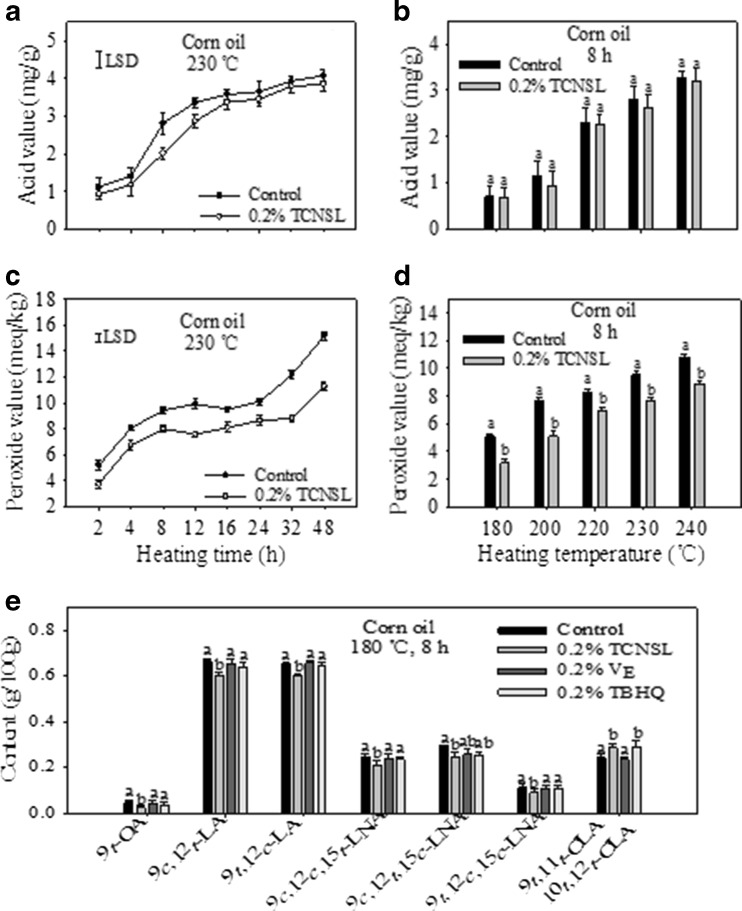
Acidity value, an indicator of free fatty acid content in oil, is an important parameter for the evaluation of oil quality and an indicator of the degree of refining (Cho et al. 2013; Bastos et al. 2015). The lower the acidity value of an oil, the better the quality, freshness, and degree of refining (Grossi et al. 2014; Zhang et al. 2015). In the present study, the acidity value of corn oil was shown to increase gradually with the increasing of heating time (Fig. 6a) and higher temperature (Fig. 6b). According to the hygienic standard for edible vegetable oil in the People’s Republic of China (Ministry of Health of the People’s Republic of China and Standardization Administration of the People’s Republic of China 2005), vegetable oil with an acidity value higher than 3 mg/g is considered unqualified and cannot be consumed directly. The results shown in Fig. 6a and b demonstrated that the acidity value of corn oil became higher than 3 mg/g after heating at 230 °C for 12 h, and after heating at 240 °C for 8 h. Consumption of edible oils with high acidity values may lead to human gastrointestinal discomfort, diarrhea, and liver damage (Thomas et al. 2000; Hammond et al. 2005. Thus, it is not recommended to heat corn oil for an extended period of time at high temperatures. The addition of 0.2 % TCNSL was able to reduce the acidity value of corn oil heated at 230 °C and at other heating temperatures, but the observed reduction was not significant. Overall, results indicate that the addition of 0.2 % TCNSL has no practical influence on the acidity value of corn oil processed at different heating times and temperatures.
Fig. 6.
Effect of technical cashew nut shell liquid (TCNSL) on the acid value (a) and peroxide value (b) of corn oil heated at 230 °C for various amounts of time, and the acid value (c) and peroxide value (d) of corn oil heated at various temperatures for 8 h; Effects of TCNSL, VE, and TBHQ on the formation of heat-induced trans isomers in corn oils heated at 180 °C for 8 h (e). Vertical error bars represent the standard deviation of the mean
The peroxide value is a common parameter to describe the primary oxidation of oils and fats. This value shows the degree of oxidation and measures the amount of total peroxides in a substance, and is expressed as milliequivalents of oxygen per kilogram of fat (Grossi et al. 2015). The peroxide value of corn oil increased with longer heating times (Fig. 6c) and higher temperatures (Fig. 6d). From the perspective of food quality and safety, the evaluation of the peroxide value is among the most important quality control measures for edible oils as it is an indicator of primary oxidation status (Akinoso et al. 2010; Pizarro et al. 2013). Our results showed that the addition of 0.2 % TCNSL significantly reduced the peroxide value of corn oil at different heating times and temperatures, with similar effects to those measured for additives such as vitamin E (VE), BHA, BHT, and TBHQ. The results of the present study indicate that TCNSL maintains the quality of corn oil processed at high temperatures, suggesting it may be an effective additive in edible oils.
In order to compare the effects of TCNSL with other additives in edible oils, the effects of VE and TBHQ on the formation of trans isomers in oil processed at 180 °C for 8 h were investigated. VE, a natural antioxidant, is commonly present in plant-derived edible oils. TBHQ, a synthetic antioxidant that is used to extend the shelf life of oils, has a greater ability to reduce trans-LAs and induce the formation of CLAs in heated oils when compared to BHA and BHT (Guo et al. 2015). In the present study, 0.2 % VE and 0.2 % TBHQ had no significant influence on the formation of trans-OA, trans-LA, or trans-LNA isomers when corn oil was heated at 180 °C for 8 h, consistent with the results of Guo et al. (2015), who also reported that trans-LA isomers were significantly reduced by the addition of 0.2 % VE and 0.2 % TBHQ when corn oil was heated at 240 °C for 8 h. These results indicate that the inhibitory ability of VE and TBHQ is related to heating temperature. The amount of 9 t,11 t-CLA and 10 t,12 t-CLA formed during heating (180 °C for 8 h) also increased due to the addition of 0.2 % TCNSL and 0.2 % TBHQ (Fig. 6e). However, 0.2 % VE had no significant effect on the formation of 9 t,11 t-CLA and 10 t,12 t-CLA isomers under these conditions. However, 0.2 % TCNSL exhibited better inhibition of trans-OA, trans-LA, and trans-LNA isomers, and better induction of the formation of CLA isomers when compared to 0.2 % VE and 0.2 % TBHQ. These results confirm that TCNSL has the potential to be a useful additive in edible oils due to its ability to reduce the formation of TFAs, reduce the peroxide value, and induce the formation of CLAs.
Conclusion
The addition of an appropriate concentration of TCNSL to heated edible oils can inhibit TFA formation and induce CLA formation. A concentration of 0.2 % TCNSL proved to be optimal for reducing formation of trans-OAs, trans-LAs, and trans-LNAs formation, as well as inducing formation of 9 t,11 t-CLA and 10 t,12 t-CLA. While the presence of 0.2 % TCNSL did not significantly reduce the acid value of corn oil, it was able to significantly reduce the peroxide value. In addition, TCNSL had better abilities as an additive than either VE or TBHQ, and therefore should be considered as a promising additive for the improvement of edible oils.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (31271851), Beijing Natural Science Foundation (6154033), and China Postdoctoral Science Foundation Funded Project (2014 M561105, 2015 T80158).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
References
- Akinoso R, Aboaba S, Olayanju T. Effects of moisture content and heat treatment on peroxide value and oxidative stability of un-refined sesame oil. Afr J Food Agric Nutr Dev. 2010;10:4268–4285. [Google Scholar]
- AOCS (1997) Preparation of methyl esters of long-chain fatty acids. AOCS Official Method Ch 1–91, IL
- Attanasi OA, Behalo MS, Gianfranco F, Lomonaco D, Mazzetto SE, Mele G, Piod I, Vasapollo G. Solvent free synthesis of novel mono- and bis-benzoxazines from cashew nut shell liquid components. Curr Org Chem. 2012;16:2613–2621. doi: 10.2174/138527212804004616. [DOI] [Google Scholar]
- Bastos RK, Frigo EP, Alves HJ, Dieter J, de Souza SNM, da Silva AAF, Kothe V (2015) Effect of swine wastewater on Jatropha curcas L. oil acidity. Ind Crop Prod 74:642–647
- Beppu F, Hosokawa M, Tanaka L, Kohno H, Tanaka T, Miyashita K. Potent inhibitory effect of trans9, trans11 isomer of conjugated linoleic acid on the growth of human colon cancer cells. J Nutr Biochem. 2006;17(12):830–836. doi: 10.1016/j.jnutbio.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Y, Nie S, Yang X, Wang Y, Yang M, Li C, Xie M. The analysis of trans fatty acid profiles in deep frying palm oil and chicken fillets with an improved gas chromatography method. Food Control. 2014;44:191–197. doi: 10.1016/j.foodcont.2014.04.010. [DOI] [Google Scholar]
- Cho YJ, Kim TE, Gil B. Correlation between refractive index of vegetable oils measured with surface plasmon resonance and acid values determined with the AOCS official method. LWT- Food Sci Technol. 2013;53(2):517–521. doi: 10.1016/j.lwt.2013.03.016. [DOI] [Google Scholar]
- Christy AA. Evidence in the formation of conjugated linoleic acids from thermally induced 9t12t linoleic acid: a study by gas chromatography and infrared spectroscopy. Chem Phys Lipids. 2009;161(2):86–94. doi: 10.1016/j.chemphyslip.2009.07.002. [DOI] [PubMed] [Google Scholar]
- dos Santos Andrade TJA, Araújo BQ, das Graças Lopes Citó AM, da Silva J, Saffi J, Richter MF, de Barros Falcão Ferraz A. Antioxidant properties and chemical composition of technical cashew nut shell liquid (tCNSL) Food Chem. 2011;126(3):1044–1048. doi: 10.1016/j.foodchem.2010.11.122. [DOI] [Google Scholar]
- Elisia I, Young JW, Yuan YV, Kitts DD. Association between tocopherol isoform composition and lipid oxidation in selected multiple edible oils. Food Res Int. 2013;52(2):508–514. doi: 10.1016/j.foodres.2013.02.013. [DOI] [Google Scholar]
- Fan L, Eskin NAM (2015) 15 - The use of antioxidants in the preservation of edible oils. In: Shahidi F (ed) Handbook of antioxidants for food preservation. Woodhead Publishing, pp 373–388
- FAO/WHO (2009) Fat and fatty acids in human nutrition. Proceedings of the Joint FAO/WHO Expert Consultation. November 10–14, 2008 Geneva, Switzerland: Annals of Nutrition & Metabolism 55(1–3): 5–300 [DOI] [PubMed]
- Ganguly R, Pierce GN. Trans fat involvement in cardiovascular disease. Mol Nutr Food Res. 2012;56(7):1090–1096. doi: 10.1002/mnfr.201100700. [DOI] [PubMed] [Google Scholar]
- Glew RH, Herbein JH, Ma I, Obadofin M, Wark WA, VanderJagt DJ. The trans fatty acid and conjugated linoleic acid content of fulani butter oil in Nigeria. J Food Compos Anal. 2006;19(6–7):704–710. doi: 10.1016/j.jfca.2005.06.004. [DOI] [Google Scholar]
- Grossi M, Di Lecce G, Gallina Toschi T, Riccò B. A novel electrochemical method for olive oil acidity determination. Microelectron J. 2014;45(12):1701–1707. doi: 10.1016/j.mejo.2014.07.006. [DOI] [Google Scholar]
- Grossi M, Di Lecce G, Arru M, Gallina Toschi T, Riccò B. An opto-electronic system for in-situ determination of peroxide value and total phenol content in olive oil. J Food Eng. 2015;146:1–7. doi: 10.1016/j.jfoodeng.2014.08.015. [DOI] [Google Scholar]
- Guo Q, Ha Y, Li Q, Jin J, Deng Z, Li Y, Zhang S. Impact of additives on thermally-induced trans isomers in 9c,12c linoleic acid triacylglycerol. Food Chem. 2015;174:299–305. doi: 10.1016/j.foodchem.2014.11.063. [DOI] [PubMed] [Google Scholar]
- Ha TJ, Kubo I. Lipoxygenase inhibitory activity of anacardic acids. J Agric Food Chem. 2005;53:4350–4354. doi: 10.1021/jf048184e. [DOI] [PubMed] [Google Scholar]
- Hammond EG, Johnson LA, Su C, Wang T, White PJ (2005) Soybean oil. In: Bailey’s industrial oil and fat products. Wiley
- Kramer JK, Hernandez M, Cruz-Hernandez C, Kraft J, Dugan ME. Combining results of two GC separations partly achieves determination of all cis and trans 16:1, 18:1, 18:2 and 18:3 except CLA isomers of milk fat as demonstrated using Ag-ion SPE fractionation. Lipids. 2008;43(3):259–273. doi: 10.1007/s11745-007-3143-4. [DOI] [PubMed] [Google Scholar]
- Kubo I, Muroi H, Himejima M, Yamagiwa Y, Mera H, Tokushima K. Structure-antibacterial activity relationships of anacardic acids. J Agric Food Chem. 1993;41:1016–1019. doi: 10.1021/jf00030a036. [DOI] [Google Scholar]
- Li A (2013) Mechanism of thermally induced isomerization of unsaturated fatty acids in soybean oil and safety analysis of the products. Chinese Academy of Agricultural Sciences, pp 6–7
- Li A, Ha Y, Wang F, Li W, Li Q. Determination of thermally induced trans-fatty acids in soybean oil by attenuated total reflectance fourier transform infrared spectroscopy and gas chromatography analysis. J Agric Food Chem. 2012;60(42):10709–10713. doi: 10.1021/jf3033599. [DOI] [PubMed] [Google Scholar]
- Li A, Yuan B, Li W, Wang F, Ha Y. Thermally induced isomerization of linoleic acid in soybean oil. Chem Phys Lipids. 2013;166:55–60. doi: 10.1016/j.chemphyslip.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Liu W, Stepheninbaraj B, Chen B. Analysis and formation of trans fatty acids in hydrogenated soybean oil during heating. Food Chem. 2007;104(4):1740–1749. doi: 10.1016/j.foodchem.2006.10.069. [DOI] [Google Scholar]
- Ministry of Health of the People’s Republic of China and Standardization Administration of the People’s Republic of China (2005) Hygienic standard for edible vegetable oil. GB 2716–2005
- Mozaffarian D. Trans fatty acids - effects on systemic inflammation and endothelial function. Atheroscler Suppl. 2006;7(2):29–32. doi: 10.1016/j.atherosclerosissup.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Park Y. Chapter 37 - Conjugated linoleic acid in human health effects on weight control. In: Watson RR, editor. Nutrition in the prevention and treatment of abdominal obesity. San Diego: Academic; 2014. pp. 429–446. [Google Scholar]
- Pizarro C, Esteban-Díez I, Rodríguez-Tecedor S, González-Sáiz JM. Determination of the peroxide value in extra virgin olive oils through the application of the stepwise orthogonalisation of predictors to mid-infrared spectra. Food Control. 2013;34:158–167. doi: 10.1016/j.foodcont.2013.03.025. [DOI] [Google Scholar]
- Schmourlo G, Mendonca-Filho RR, Alviano CS, Costa SS. Screening of antifungal agents using ethanol and bioautography of medicinal and precipitation food plants. J Ethnopharmacol. 2005;15:563–568. doi: 10.1016/j.jep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Thomas A, Matthäus B, Fiebig HJ (2000) Fats and fatty oils. In: Ullmann’s Encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA
- Tsuzuki W. Effects of antioxidants on heat-induced trans fatty acid formation in triolein and trilinolein. Food Chem. 2011;129(1):104–109. doi: 10.1016/j.foodchem.2011.04.036. [DOI] [Google Scholar]
- Tsuzuki W, Nagata R, Yunoki R, Nakajima M, Nagata T. Cis/trans-isomerisation of triolein, trilinolein and trilinolenin induced by heat treatment. Food Chem. 2008;108(1):75–80. doi: 10.1016/j.foodchem.2007.10.047. [DOI] [Google Scholar]
- Yang B, Chen H, Stanton C, Ross RP, Zhang H, Chen YQ, Chen W. Review of the roles of conjugated linoleic acid in health and disease. J Funct Foods. 2015;15:314–325. doi: 10.1016/j.jff.2015.03.050. [DOI] [Google Scholar]
- Zhang W, Li N, Feng Y, Su S, Li T, Liang B. A unique quantitative method of acid value of edible oils and studying the impact of heating on edible oils by UV–vis spectrometry. Food Chem. 2015;185:326–332. doi: 10.1016/j.foodchem.2015.04.005. [DOI] [PubMed] [Google Scholar]