Abstract
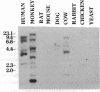
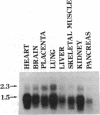
Two functional human dUTP pyrophosphatase (dUTPase; EC 3.6.1.23) cDNAs were isolated from a cDNA expression library by genetic complementation in Escherichia coli. These cDNAs differed in size but exhibited a common overlapping DNA sequence. Contained within this sequence was a single long open reading frame sufficient to encode a polypeptide of 141 amino acids with a calculated molecular mass of 16.6 kDa. The amino acid sequence of this protein exhibits 35% identity with the E. coli dUTPase and 53% identity with the Saccharomyces cerevisiae enzyme. The human dUTPase was found to contain five characteristics amino acid sequence motifs that are common to the dUTPases of E. coli, yeast, and herpesviruses and to dUTPase-like sequences encoded by some retrovirus gag and pol genes. A high degree of amino acid sequence identity (greater than 60%) was also observed between the human dUTPase and the putative pseudoproteases of two poxviruses, indicating that these virus proteins are dUTPases. Northern hybridization analysis reveals that dUTPase is encoded by at least two species of poly(A)+ mRNA and possibly a third, smaller species. All of these mRNAs are present in a variety of human tissues but their relative levels vary between tissues. Southern analysis indicates that the dUTPase gene has been conserved to some extent throughout vertebrate evolution; however, the gene may be very large, or its organization somewhat complex in some systems. We suggest that dUTPase may generally perform an essential role in DNA replication and therefore could serve as a target enzyme for the development of chemotherapeutic compounds.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay B. J., Kunz B. A., Little J. G., Haynes R. H. Genetic and biochemical consequences of thymidylate stress. Can J Biochem. 1982 Mar;60(3):172–184. doi: 10.1139/o82-023. [DOI] [PubMed] [Google Scholar]
- Caradonna S. J., Adamkiewicz D. M. Purification and properties of the deoxyuridine triphosphate nucleotidohydrolase enzyme derived from HeLa S3 cells. Comparison to a distinct dUTP nucleotidohydrolase induced in herpes simplex virus-infected HeLa S3 cells. J Biol Chem. 1984 May 10;259(9):5459–5464. [PubMed] [Google Scholar]
- Elder J. H., Lerner D. L., Hasselkus-Light C. S., Fontenot D. J., Hunter E., Luciw P. A., Montelaro R. C., Phillips T. R. Distinct subsets of retroviruses encode dUTPase. J Virol. 1992 Mar;66(3):1791–1794. doi: 10.1128/jvi.66.3.1791-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Mulligan J. T., Ramer S. W., Spottswood M., Davis R. W. Lambda YES: a multifunctional cDNA expression vector for the isolation of genes by complementation of yeast and Escherichia coli mutations. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1731–1735. doi: 10.1073/pnas.88.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Spottswood M. R. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991 Sep;10(9):2653–2659. doi: 10.1002/j.1460-2075.1991.tb07808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goulian M., Bleile B., Tseng B. Y. The effect of methotrexate on levels of dUTP in animal cells. J Biol Chem. 1980 Nov 25;255(22):10630–10637. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hughes S. J., Johnston L. H., de Carlos A., Smith G. L. Vaccinia virus encodes an active thymidylate kinase that complements a cdc8 mutant of Saccharomyces cerevisiae. J Biol Chem. 1991 Oct 25;266(30):20103–20109. [PubMed] [Google Scholar]
- Impellizzeri K. J., Anderson B., Burgers P. M. The spectrum of spontaneous mutations in a Saccharomyces cerevisiae uracil-DNA-glycosylase mutant limits the function of this enzyme to cytosine deamination repair. J Bacteriol. 1991 Nov;173(21):6807–6810. doi: 10.1128/jb.173.21.6807-6810.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham H. A., Dickey L., Goulian M. DNA fragmentation and cytotoxicity from increased cellular deoxyuridylate. Biochemistry. 1986 Jun 3;25(11):3225–3230. doi: 10.1021/bi00359a022. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Goulian M. Deoxyuridine triphosphatase: a potential site of interaction with pyrimidine nucleotide analogues. Biochem Biophys Res Commun. 1982 Dec 15;109(3):746–752. doi: 10.1016/0006-291x(82)92003-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg L. G., Karlström O. H., Nyman P. O., Neuhard J. Isolation and characterization of the dut gene of Escherichia coli. I. Cloning in thermoinducible plasmids. Gene. 1983 Apr;22(1):115–126. doi: 10.1016/0378-1119(83)90070-7. [DOI] [PubMed] [Google Scholar]
- Lundberg L. G., Thoresson H. O., Karlström O. H., Nyman P. O. Nucleotide sequence of the structural gene for dUTPase of Escherichia coli K-12. EMBO J. 1983;2(6):967–971. doi: 10.1002/j.1460-2075.1983.tb01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahagaokar S., Rao P. N., Orengo A. Deoxyuridine triphosphate nucleotidohydrolase of HeLa cells. Int J Biochem. 1980;11(5):415–421. doi: 10.1016/0020-711x(80)90312-2. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. Protein sequence comparisons show that the 'pseudoproteases' encoded by poxviruses and certain retroviruses belong to the deoxyuridine triphosphatase family. Nucleic Acids Res. 1990 Jul 25;18(14):4105–4110. doi: 10.1093/nar/18.14.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer A. A., Fraser K. M., Stockwell P. A., Robinson A. J. A homologue of retroviral pseudoproteases in the parapoxvirus, orf virus. Virology. 1989 Oct;172(2):665–668. doi: 10.1016/0042-6822(89)90212-2. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nation M. D., Guzder S. N., Giroir L. E., Deutsch W. A. Control of Drosophila deoxyuridine triphosphatase. Existence of a developmentally expressed protein inhibitor. Biochem J. 1989 Apr 15;259(2):593–596. doi: 10.1042/bj2590593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Yasunaga T., Miyata T., Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 1986 Nov;60(2):589–598. doi: 10.1128/jvi.60.2.589-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. G., Sowers L. C., Laszlo J., Sedwick W. D. The occurrence and consequences of deoxyuridine in DNA. Adv Enzyme Regul. 1984;22:157–185. doi: 10.1016/0065-2571(84)90013-x. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Kutler M., Brown O. E. Antifolate-induced misincorporation of deoxyuridine monophosphate into DNA: inhibition of high molecular weight DNA synthesis in human lymphoblastoid cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):917–921. doi: 10.1073/pnas.78.2.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. Deoxyuridine triphosphatase of Escherichia coli. Purification, properties, and use as a reagent to reduce uracil incorporation into DNA. J Biol Chem. 1978 May 10;253(9):3305–3312. [PubMed] [Google Scholar]
- Slabaugh M. B., Roseman N. A. Retroviral protease-like gene in the vaccinia virus genome. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4152–4155. doi: 10.1073/pnas.86.11.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. F., Weiss B. Role of exonuclease III in the base excision repair of uracil-containing DNA. J Bacteriol. 1982 Jul;151(1):351–357. doi: 10.1128/jb.151.1.351-357.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Lehman I. R. Excision repair of uracil incorporated in DNA as a result of a defect in dUTPase. J Mol Biol. 1977 Dec 5;117(2):293–306. doi: 10.1016/0022-2836(77)90128-0. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Duncan B. K. In vivo synthesis and properties of uracil-containing DNA. Nature. 1978 Mar 2;272(5648):32–34. doi: 10.1038/272032a0. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A., Pai E. F. The structure of Ras protein: a model for a universal molecular switch. Trends Biochem Sci. 1991 Oct;16(10):382–387. doi: 10.1016/0968-0004(91)90156-p. [DOI] [PubMed] [Google Scholar]
- el-Hajj H. H., Zhang H., Weiss B. Lethality of a dut (deoxyuridine triphosphatase) mutation in Escherichia coli. J Bacteriol. 1988 Mar;170(3):1069–1075. doi: 10.1128/jb.170.3.1069-1075.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]