Abstract
A comparative study was done on the health promoting and functional properties of the fibers obtained as by-products from six fruits viz., pomace of carambola (Averrhoa carambola L.) and pineapple (Ananas comosus L. Merr), peels of watermelon (Citrullus lanatus), Burmese grape (Baccurea sapida Muell. Arg) and Khasi mandarin orange (Citrus reticulata Blanco), and blossom of seeded banana (Musa balbisiana, ABB). Highest yield of fiber was obtained from Burmese grape peel (BGPL, 79.94 ± 0.41 g/100 g) and seeded banana blossom (BB 77.18 ± 0.20 g/100 g). The total dietary fiber content (TDF) was highest in fiber fraction derived from pineapple pomace (PNPM, 79.76 ± 0.42 g/100 g) and BGPL (67.27 ± 0.39 g/100 g). All the samples contained insoluble dietary fiber as the major fiber fraction. The fiber samples showed good water holding, oil holding and swelling capacities. The fiber samples exhibited antioxidant activity. All the samples showed good results for glucose adsorption, amylase activity inhibition, glucose diffusion rate and glucose diffusion reduction rate index.
Keywords: Dietary fiber, Total phenolic content, Oil holding capacity, Glucose adsorption, Glucose lowering
Introduction
Plants are naturally very rich in dietary fiber, which has health promoting properties. Dietary fiber binds with bile and water, helps in bowel movement, increases the fecal mass, and controls irritated bowel syndrome conditions as well as lowers blood glucose and cholesterol levels (Kahlon and Chow 2000). The glucose and cholesterol lowering properties in turn helps in prevention and control of obesity, diabetes and coronary heart diseases. Dietary fiber enriched diet prevents colon cancer. This could be due to the fermentation and production of short chain fatty acids in the large intestine by the gut micro flora residing there. A healthy adult person is usually recommended to consume between 25 and 30 g of fiber daily (Buttriss and Stokes 2008). Fruits and vegetables are naturally rich in dietary fiber. Fruit and vegetable byproducts like peel, pomace, seed and seed coats are the major sources of dietary fiber. In most cases, the dietary fibers derived from fruits and vegetable byproducts contain bioactive compounds embedded in their matrix (Evans and Halliwell 2001) and therefore these could impart health benefits both as fiber and antioxidant source. The bioactive compounds, mainly phenolic acids and flavonoids are the secondary plant metabolites that have health promoting properties like antioxidant and radical quenching properties. The awareness of the health benefits of dietary fiber and other bioactive compounds in the diet has led to a rapid development of fiber and antioxidant enriched food products (Dhingra et al. 2012). Fernandez-Gines et al. (2003) reported that addition of citrus fiber with associated antioxidant bioactive compounds to meat products inhibited lipid oxidation and decreased residual nitrite levels. Similarly, when mango peel powder was fortified into macaroni, an increase in the dietary fiber, polyphenols and carotenoid content was reported (Ajila et al. 2010).
Although a number of studies have been reported regarding the dietary fiber rich food sources and their health promoting role, still there are many fruit waste residues which could be potential sources of dietary fiber that could cater to the rising need of developing newer and novel functional food products. Therefore, the present work intended to do a preliminary study of the health promoting ability and functional properties of the fibers obtained as by-products from six sources viz., pomace of carambola (Averrhoa carambola L.) and pineapple (Ananas comosus L. Merr), peels of watermelon (Citrullus lanatus), Burmese grape (Baccurea sapida Muell. Arg) and Khasi mandarin orange (Citrus reticulata Blanco), and blossom of seeded banana (Musa balbisiana Colla., ABB).
Materials and methods
Chemicals and reagents
All the chemicals and reagents used were of analytical grade and supplied by Merck (India). The dietary fiber enzyme kit was purchased from Sigma and the glucose assay kit was supplied by Tulip group, India.
The six fruits taken for the study were procured from the local market, Tezpur. The peels or outer rind from Khasi mandarin (KMPL), Burmese grape (BGPL) and watermelon (WMPL) were removed and chopped into 1cm2 size and dried. Pomace from pineapple (PNPM) and carambola (CMPM) was collected after the extraction of their juice using a household juicer (Philips). In case of banana blossom (BB), the outer hard layer of the flower was discarded and the whole blossom was shredded and dried. All the samples were dried at 50 °C in a tray drier for 12 h. The dried samples were ground in a laboratory grain miller (Fritsch Pulverisette) to pass through 0.5 mm screen and stored at 4 °C in airtight containers for further analysis.
Proximate composition
The dried samples were analyzed for content of moisture, crude lipid, crude protein and ash following AOAC (1995) methods. The results were expressed on dry basis.
Preparation of the fiber rich fraction
The dried samples were first subjected to hexane treatment for removal of crude lipid and pigments. The samples were kept overnight in hexane in ratio of 1:10 (sample: hexane) at room temperature and then filtered through a muslin cloth. The residue was dried and again treated twice with 80 % hot (80 °C) ethanol for 30 min and filtered. The residue was rinsed with 95 % ethanol and then dried at 50 °C in a tray drier. The final dried solids were then ground to 0.5 mm particle size in a grain mill. The yield of the obtained fiber powder was calculated on dry basis using the equation below.
Dietary fiber content
The total dietary fiber (TDF), insoluble dietary fiber (IDF) and soluble dietary fiber (SDF) content of the prepared fiber samples were determined by enzymatic gravimetric method (AOAC 1995).
Physicochemical properties
The moisture content and bulk density of the prepared fiber samples were determined by AOAC methods (1995). Water holding capacity and swelling capacity were determined by the method of Robertson et al. (2000). while oil holding capacity was determined according to Lin et al. (1974) using cellulose as a standard control.
Phytochemical content and antioxidant activity
Sample extraction
The fiber samples were extracted in 80 % acetone for 90 min at 20 °C in a ratio of 1:10 (sample: solvent) in a shaking incubator (Sartorius). After the completion of the incubation period the extraction mixture was then centrifuged (Hettich-Zentrifugen, Germany) at 3000 rpm for 15 min and the supernatant was collected and stored at −20 °C until further analysis of phytochemical content and antioxidant activity.
Determination of total phenolic content (TPC)
TPC in the sample extracts was assessed using the Folin-Ciocalteau assay (Slinkard and Singleton 1977) with slight modification. For the analysis, 20 μL each of extract, gallic acid standard and blank were taken in separate test tubes and to each 1.58 mL of distilled water was added, followed by 100 μL of Folin-Ciocalteau reagent, mixed well and within 8 min, 300 μL of sodium carbonate was added. The samples were vortexed immediately and the tubes were incubated in the dark for 30 min at 40 °C. The absorbance was then measured at 765 nm in a UV-Vis spectrophotometer (Cecil, Aquarius7400). The results were expressed in mgGAE/100 g.
Determination of total flavonoid content (TFC)
TFC was determined by aluminium trichloride method (Chang et al. 2002). Briefly, 0.5 mL of the extract was mixed with 1.5 mL of 95 % ethanol, 0.1 mL of 10 % aluminum trichloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of deionised water. After incubation at room temperature for 40 min, the absorbance of reaction mixture was measured at 415 nm against deionised water taken as blank in a UV-Vis spectrophotometer (Cecil, Aquarius 7400). Results were expressed as quercetin equivalent (mgQE/100 g) of sample.
Determination of ferric reducing antioxidant property (FRAP)
FRAP activity of the samples was measured by the method of Benzie and Strain (1996). Briefly, a 40 μL an aliquot of sample extract was mixed with 3.0 mL of FRAP solution. The reaction mixture was incubated at 37 °C for 4 min and the absorbance was determined at 593 nm in a UV-Vis spectrophotometer (Cecil, Aquarius 7400) against a blank that was prepared using distilled water. FRAP solution was pre warmed at 37 °C and prepared freshly by mixing 2.5 mL of a 10 mM 2,4,6-TPTZ [2,4,6-tri(2-pyridyl)-1,3,5-triazine] solution in 40 mM hydrochloric acid with 2.5 mL of 20 mM ferric chloride and 25 mL of 0.3 M acetate buffer (pH 3.6). A calibration curve was prepared, using an aqueous solution of ferrous sulfate (1–10 mM). FRAP values were expressed as μmol of ferrous equivalent Fe (II)/100 g of sample.
Determination of DPPH activity
Radical scavenging activity of the sample extracts was measured by determining the inhibition rate of DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical (Brand-Williams et al. 1995). For the experiment, 100 μL of extracts were added to 1.4 mL of 10–4 M DPPH radical methanolic solution. The absorbance at 517 nm was measured at 30 min against blank (100 μL methanol in 1.4 mL of DPPH radical solution) using a UV-Vis Spectrophotometer (Cecil Aquarius 7400). The results were expressed in terms of radical scavenging activity.
Where, Ao is absorbance of control blank, and As is absorbance of sample extract.
Determination of metal chelation capacity (MCC)
MCC was determined based on the method of Dinis et al. (1994). For the estimation, 1.0 mL of 0.125 mM ferrous sulphate, and 1.0 mL of 0.3125 mM ferrozine were mixed with 0.2 mL sample extract. The mixture was allowed to equilibrate for 10 min at room temperature and the absorbance at 562 nm in a UV-Vis spectrophotometer (Cecil, Aquarius 7400) was recorded. The control contained all the reaction reagents except the extract. Decreased absorbance of the reaction mixture indicated increased activity.
Where, Ao is absorbance of control blank, and As is absorbance of sample extract.
HPLC study of the polyphenols
RP-HPLC (Waters system) gradient elution method was used to identify the major phenolic acids in the fiber samples. Symmetry 300 C18 (4.6 × 250 mm) column with a binary pump and a UV-VIS detector was used. The ethanolic extract was concentrated under vacuum and then redissolved in 1 mL methanol. Mobile phases used were acidified ultrapure water (0.1 % acetic acid, pH 3.2, mobile phase A) and methanol (mobile phase B). The gradient method of Saikia et al. (2015) was followed. The method consisted of 80 % A (0–8 min), 65 % A (9–12 min), 45 % A (13–16 min), 30 % A (17–20 min), 20 % A (21–30 min), 10 % of A (31–34 min) and then washing of the column with 65 % A (35–39 min) and finally with 80 % A (40–45 min). Sample volume of 20 μL was used. The flow rate was maintained at 0.8 mL/min and wavelengths used for UV-Vis detector were 254 and 325 nm. The standards used for comparison and identification were (±) catechin, caffeic acid, coumaric acid, gallic acid, syringic acid, chlorogenic acid, rutin hydrate, quercetin and ascorbic acid.
Functional properties
Glucose adsorption capacity and amylase activity inhibition rate
Glucose adsorption capacity and amylase activity inhibition rate were determined by the methods given by Ou et al. (2001). The glucose content of the supernatant was determined using glucose assay kit (Coral, Tulip group, India). For the above study, cellulose was taken as control for comparison.
Glucose diffusion rate and glucose diffusion reduction index (GDRI)
Glucose diffusion and GDRI were determined according to the method of Ou et al. (2001) with slight modifications. The fiber sample weighing 0.5 g was mixed with 25 mL of 50 mmol/L glucose solution. The mixture was then dialyzed in 80 mL of deionised water at 37 °C using a dialysis membrane with a cut off molecular weight of 12,000 kDa. After 20, 30, 60 and 120 min, the glucose content in the dialysate was determined using a glucose assay kit (Coral, Tulip group, India). A control was also taken without the fiber sample and cellulose as a standard for comparison purpose.
Where, G1 is the glucose content with fiber and G2 is the glucose content of the control.
Statistical analysis
All experiments were carried out at least in triplicate and reported as mean ± standard deviation of mean (S.E.M) using SPSS version 11.5. The data were statistically analyzed by Duncan’s multiple range tests at ≤0.05 significance level.
Results and discussion
Proximate composition
The proximate values of the fiber rich fractions of fruit samples are given in Table 1. The crude protein in WMPL and CMPM was high with 16.45 ± 0.18 g/100 g and 13.65 ± 0.10 g/100 g, respectively. Crude lipid for the fiber samples ranged from 1.43–3.73 g/100 g with highest content in KMPL and WMPL samples. The BB fiber sample showed highest ash content. Overall, these fiber samples were found to be good sources of protein and minerals.
Table 1.
Moisture content and proximate composition of the dried samples
| Sample | Moisture content (g/100 g, as is) | Crude protein (g/100 g, db) | Crude lipid (g/100 g, db) | Ash content (g/100 g, db) |
|---|---|---|---|---|
| PNPM | 4.45 ± 0.21b | 5.95 ± 0.16a | 1.43 ± 0.10a | 1.93 ± 0.08a |
| BGPL | 3.59 ± 0.19a | 9.10 ± 0.11b | 1.53 ± 0.15b | 6.86 ± 0.12b |
| KMPL | 6.81 ± 0.11c | 8.05 ± 0.18b | 3.73 ± 0.11e | 2.31 ± 0.09a |
| CMPM | 9.75 ± 0.12e | 13.65 ± 0.10d | 1.94 ± 0.08c | 2.84 ± 0.15a |
| WMPL | 7.65 ± 0.17d | 16.45 ± 0.18e | 3.32 ± 0.20d | 7.86 ± 0.17c |
| BB | 7.83 ± 0.23d | 10.50 ± 0.11c | 1.58 ± 0.11b | 11.67 ± 0.11d |
Mean ± S.D. with the same letter between the rows are not significantly different at p ≤ 0.05 by DMRT
PNPM pineapple pomace, BGPL Burmese grape peel, KMPL Khasi mandarin peel, CMPM carambola pomace, WMPL watermelon, BB banana blossom
Yield and total dietary fiber
The fiber samples after defatting with hexane and treating with 80 % hot alcohol were calculated for their yield (Table 2). Yield was higher for BGPL and BB than the rest. PNPM contained highest total dietary fiber (79.76 ± 0.42 g/100 g). In all samples, insoluble fiber content (28.57–62.21 g/100 g) was more than soluble fiber (9.23–17.55 g/100 g). Davidson and McDonald (1998) reported that cellulose, hemicelluloses and lignin are the major polysaccharide constituents in insoluble fiber fraction.
Table 2.
Yield and dietary fiber of the obtained fiber samples
| Sample | Yield (g/100 g) | TDF (g/100 g) | IDF (g/100 g) | SDF (g/100 g) |
|---|---|---|---|---|
| PNPM | 66.07 ± 0.48c | 79.76 ± 0.42d | 62.21 ± 0.33e | 17.55 ± 0.11c |
| BGPL | 79.94 ± 0.41d | 67.27 ± 0.39e | 56.41 ± 0.38d | 10.86 ± 0.17a |
| KMPL | 52.83 ± 0.14a | 37.82 ± .33a | 28.57 ± 0.41a | 9.23 ± 0.23a |
| CMPM | 62.08 ± 0.23c | 60.17 ± 0.29d | 46.32 ± 0.22c | 13.84 ± 0.27b |
| WMPL | 58,07 ± 0.19b | 47.48 ± 0.47c | 32.45 ± 0.25b | 15.03 ± 0.14c |
| BB | 77.18 ± 0.20d | 42.12 ± 0.35b | 29.21 ± 0.19a | 12.91 ± 0.25b |
Mean ± S.D. with the same letter between the rows are not significantly different at p ≤ 0.05 by DMRT
PNPM pineapple pomace, BGPL Burmese grape peel, KMPL Khasi mandarin peel, CMPM carambola pomace, WMPL watermelon, BB banana blossom
Physicochemical properties
The physicochemical properties of the fiber samples are mentioned in Table 3. The bulk density of the fibers was quite comparable with that of cellulose taken as control (0.48 ± 0.03 g/mL). The water holding capacity (WHC) was higher in KMPL, WMPL and BB while oil holding capacity (OHC) was more in BGPL and KMPL. Both WHC and OHC were significantly greater in the studied samples as compared to cellulose (4.47 ± 0.21 g/g and 3.05 ± 0.08 g/g, respectively).
Table 3.
Physicochemical properties of the fiber samples
| Sample | Bulk density (g/mL) | Water holding capacity (g/g) | Oil holding capacity (g/g) | Swelling capacity (mL/g) |
|---|---|---|---|---|
| PNPM | 0.29 ± 0.09a | 9.49 ± 0.08d | 7.85 ± 0.11c | 11.93 ± 0.11c |
| BGPL | 0.30 ± 0.06a | 7.56 ± 0.10c | 10.64 ± 0.16f | 9.40 ± 0.13a |
| KMPL | 0.46 ± 0.05c | 13.96 ± 0.12e | 12.00 ± 0.12g | 10.30 ± 0.07b |
| CMPM | 0.46 ± 0.03c | 6.83 ± 0.17b | 8.60 ± 0.09d | 9.39 ± 0.20a |
| WMPL | 0.39 ± 0.10b | 15.29 ± 0.11g | 6.06 ± 0.19b | 16.97 ± 0.25e |
| BB | 0.50 ± 0.05d | 14.17 ± 0.19f | 9.33 ± 0.14e | 13.97 ± 0.18d |
| CEL | 0.48 ± 0.03e | 4.47 ± 0.21a | 3.05 ± 0.08a | 10.40 ± 0.39b |
Mean ± S.D. with the same letter between the rows are not significantly different at p ≤ 0.05 by DMRT
PNPM pineapple pomace, BGPL Burmese grape peel, KMPL Khasi mandarin peel, CMPM carambola pomace, WMPL watermelon, BB banana blossom
The swelling capacity of WMPL and BB was high at 16.97 ± 0.25 mL/g and 13.97 ± 0.18 mL/g, respectively. The swelling capacity of all the samples was comparable with that of cellulose (10.40 ± 0.39 mL/g). WHC and swelling capacity indicates the hydration properties of the fiber and gives an insight into the behavior of the fiber in a particular food system which is a vital parameter for consideration while developing a new food product. WHC and swelling capacity also affects the transit of food through the gastrointestinal tract. Fiber with good hydration property will hold more water and thus contribute to the fecal bulking process (Thibault et al. 1992). Similarly, good oil holding property indicates reduced absorption of fat in intestine and increased excretion. This may play a positive role in lowering serum cholesterol as well as reduce the deposition of cholesterol in the aorta and liver.
Phytochemical content and antioxidant activity
Dietary fiber derived from byproducts of fruits and vegetables contains significant amount of phytochemicals in bound form within the fiber matrix and are released inside the human gut on action of the digestive enzymes and fermentation by colonic bacteria (Evans and Halliwell 2001; Saura-Calixto and Goñi 2006). In the present study all the fiber samples showed good phytochemical content and antioxidant activity (Table 4). Relatively, the highest total phenolic content (TPC) was observed in BGPL followed by CMPM, KMPL and BB. PNPM and WMPL had relatively low TPC values compared to the rest of the samples. Highest total flavonoid content (TFC) was observed in KMPL followed by CMPM and WMPL. Perez-Jimenez and Saura-Calixto (2008) reported that polyphenols concentration in grape antioxidant dietary fiber was 19,740 mg/100 g dry weight. Rubilar et al. (2007) found glycosylated flavonols (quercetin, kaempferol) in extracts of white and red grape pomace. Likewise, Schieber et al. (2001) mentioned the presence of hesperidin and eriocitrin in peel and solid residue of lemon waste.
Table 4.
Phytochemical content and antioxidant activities of the fiber samples
| Samples | TPC (mgGAE/100 g) | TFC (mgQE/100 g) | FRAP (μm/100 g) | DPPH (%) | MCC (%) |
|---|---|---|---|---|---|
| PNPM | 368.50 ± 0.45b | 60.38 ± 0.23b | 2868.06 ± 0.27b | 68.56 ± 0.11b | 2.21 ± 0.21b |
| BGPL | 1029.00 ± 0.39f | 69.88 ± 0.11c | 6006.94 ± 0.33d | 91.98 ± 0.17d | 1.28 ± 0.17a |
| KMPL | 867.50 ± 0.21d | 251.75 ± 0.17f | 5833.33 ± 0.41c | 93.72 ± 0.20e | 3.49 ± 0.11c |
| CMPM | 989.00 ± 0.27e | 91.85 ± 0.25e | 12,250.00 ± 0.47f | 94.30 ± 0.14e | 8.83 ± 0.14f |
| WMPL | 182.00 ± 0.18a | 73.88 ± 0.15d | 685.76 ± 0.42a | 25.58 ± 0.19a | 5.27 ± 0.09e |
| BB | 433.00 ± 0.14c | 33.75 ± 0.19a | 6791.66 ± 0.39e | 87.50 ± 0.21c | 4.49 ± 0.08d |
Mean ± S.D. with the same letter between the rows are not significantly different at p ≤ 0.05 by DMRT
PNPM pineapple pomace, BGPL Burmese grape peel, KMPL Khasi mandarin peel, CMPM carambola pomace, WMPL watermelon, BB banana blossom
The antioxidant activity of the fiber samples were determined by FRAP and DPPH methods. FRAP values in the fiber samples ranged from 685.76–12,250.00 μmol/100 g. The DPPH radical scavenging activity values ranged from 25.58–94.30 %. The metal chelating activity ranged from 1.28–8.83 %. Overall, KMPL, BGPL, CMPM and BB showed good residual phytochemical content as well as antioxidant activity.
Phenolic compounds in the selected fibers determined by RP-HPLC
The phenolic composition varied depending on the fiber source and only the results obtained at 254 nm are given and discussed as the peak intensity of the phenolic compounds identified were very low at 325 nm (Table 5 and Fig. 1). Gallic acid (RT = 3.23 min), catechin (RT = 11.89 min), chlorogenic acid (RT = 13.54 min), caffeic acid (RT = 14.49 min), syringic acid (RT = 14.73 min), ferulic acid (RT = 16.55), coumaric acid (RT = 16.72 min), rutin (RT = 17.31 min) and quercetin (RT = 19.89 min) were identified. Gallic acid was predominantly present in all the six samples; the highest content was reported in BB sample (27.00 ± 0.10 mg/100 g). Catechin was present in CMPM, BGPL and WMPL. Chlorogenic acid was present only in WMPL. Similarly, KMPL showed highest caffeic acid content (53.09 ± 0.12 mg/100 g) followed by PNPM (11.09 ± 0.09 mg/100 g). In rest of the samples, caffeic acid was not detected. Likewise, syringic acid was highest in KMPL (22.94 ± 0.09 mg/100 g) but was not detected in CMPM and BGPL. Ferulic acid was present in all the samples except in WMPL and KMPL. Similarly, coumaric acid was present only in BGPL and WMPL. The rutin hydrate content was highest in KMPL sample. BB and CMPM samples also showed the presence of rutin hydrate. Lastly, CMPM, BGPL and BB samples exhibited the presence of low amount of quercetin in them. Differences in phenolic compounds present in fruits are dependent on type of samples, environmental conditions, locations and agronomic factors, maturity stages and type of processing techniques (Naczk and Shahidi 2006). Therefore, it can be inferred that, even after processing treatments given during the fiber extraction, the samples retained reasonable amount of phenolic compounds consisting of hydroxybenzoic and hydroxycinnamic acid derivatives and flavonoids. Comparatively good retention was observed in KMPL, BB, CMPM followed by PNPM and thus these fiber samples could be referred to as antioxidant dietary fiber.
Table 5.
Phenolic acids (mg/100 g) compositions of the selected fibers determined by RP-HPLC
| Sample | GA* | CTH* | CGA* | CFA* | SA* | FA* | CMA* | RH* | QTH* |
|---|---|---|---|---|---|---|---|---|---|
| PNPM | 7.61 ± 0.09 | ND | ND | 11.09 ± 0.09 | 0.63 ± 0.04 | 0.12 ± 0.01 | ND | ND | ND |
| BGPL | 0.46 ± 0.11 | 2.33 ± 0.05 | ND | ND | ND | 1.14 ± 0.03 | 1.28 ± 0.07 | ND | 0.43 ± 0.02 |
| KMPL | 7.07 ± 0.02 | ND | ND | 53.09 ± 0.12 | 22.94 ± 0.09 | ND | ND | 38.62 ± 0.08 | ND |
| CMPM | 6.50 ± 0.09 | 10.72 ± 0.05 | ND | ND | ND | 13.02 ± 0.06 | ND | 1.47 | 1.22 ± 0.05 |
| WMPL | 6.39 ± 0.14 | 2.46 ± 0.02 | 4.53 ± 0.05 | ND | 2.01 ± 0.02 | ND | 4.53 ± 0.05 | ND | ND |
| BB | 27.00 ± 0.10 | ND | ND | ND | 4.68 ± 0.05 | 4.83 ± 0.02 | ND | 7.86 ± 0.03 | 1.43 ± 0.07 |
Results are mean ± S.D. of triplicates values
GA gallic acid, CTH catechin, CGA chlorogenic acid, CFA caffeic acid, SA syringic acid, FA ferulic acid, CMA coumaric acid, RH rutin hydrate, QTH quercetin, PNPM pineapple pomace, BGPL Burmese grape peel, KMPL Khasi mandarin peel, CMPM carambola pomace, WMPL watermelon, BB banana blossom
Fig. 1.
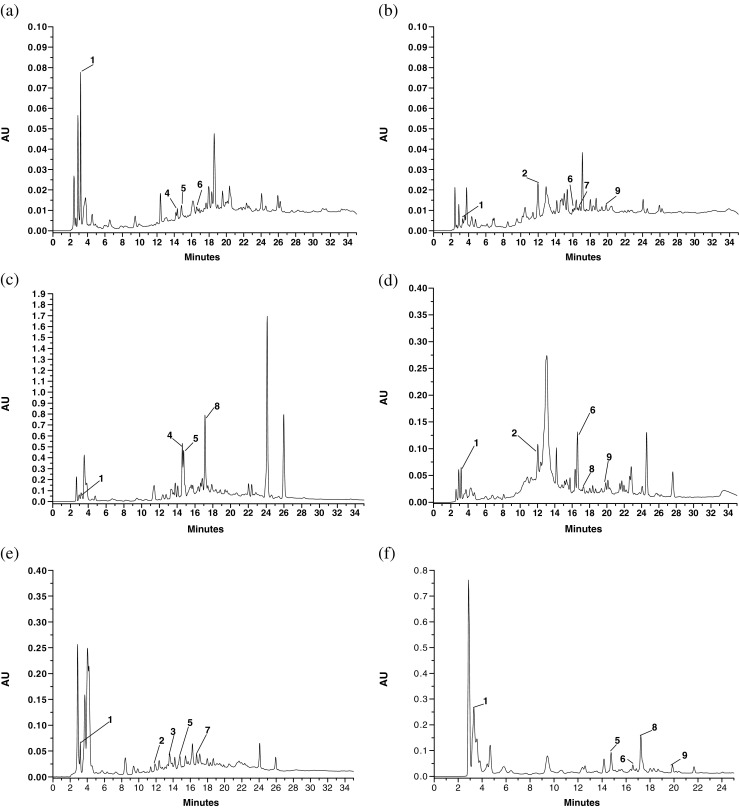
Phenolic acid compositions determined by RP-HPLC of a PNPM b BGPL c KMPL d CMPM e WMPL and f BB fibre samples at 254 nm. PNPM pineapple pomace, BGPL Burmese grape peel, KMPL Khasi mandarin peel, CMPM carambola pomace, WMPL watermelon, BB banana blossom. Footnote: 1- gallic acid; 2- catechin; 3- chlorogenic acid; 4- caffeic acid; 5- syringic acid; 6- ferulic acid; 7- coumaric acid; 8- rutin hydrate and 9- quercetin
Functional properties
Glucose adsorption and amylase activity inhibition rate
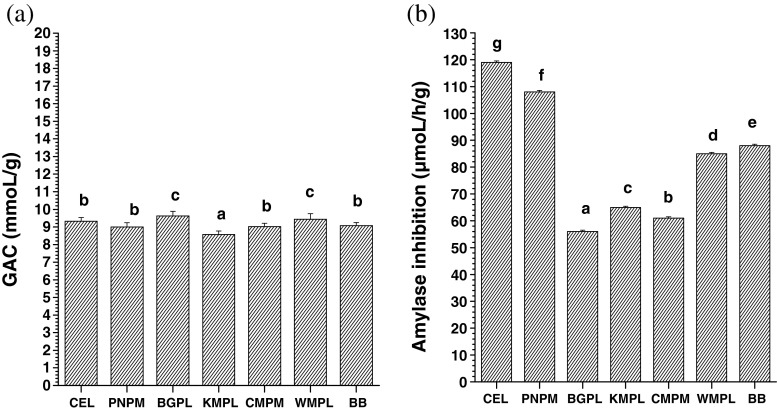
The adsorption of glucose by the different fibers ranged from 8.57–9.62 mmoL/g (Fig. 2a). All the fiber samples showed adsorption capacity comparable to that of cellulose (9.33 ± 0.11 mmoL/g). It can be seen from Fig. 2b that all the fiber samples showed significant variation in amylase inhibition rate with values ranging from 56 to 108 μmoL/h/g. Only PNPM showed inhibition of 108 ± 0.14 μmoL/h/g which was comparable with that of cellulose (119 ± 0.17 μmoL/h/g). Lowest inhibition was observed in BGPL. The inhibition of amylase is usually dependent on the insoluble fiber content. However, in the present study, BGPL and CMPM with high insoluble dietary fiber showed low inhibition of amylase whereas in comparison WMPL and BB with low insoluble dietary fiber exhibited higher amylase inhibition rate. This exception could be due to variation in the non sweet sugar components constituting the fiber samples. Another possible reason for amylase inhibition not being in accordance with the insoluble dietary fiber content might be due to the reduced accessibility of the enzyme to their substrate because of the encapsulation of starch and enzyme by the fiber (Ou et al. 2001; Gourgue et al. 1992).
Fig. 2.
a Glucose adsorption capacity and b amylase inhibition of the fibre sample. PNPM pineapple pomace, BGPL Burmese grape peel, KMPL Khasi mandarin peel, CMPM carambola pomace, WMPL watermelon, BB banana blossom. Same letter between the bars means no significant difference at p ≤ 0.05 by DMRT
Amylase inhibition property of the fiber samples indicates their positive role in delaying the rate of glucose absorption in the intestine due to delayed release of glucose from starch digestion which ultimately lowers postprandial serum glucose (Chau et al. 2003).
Glucose diffusion rate (GDR) and glucose diffusion reduction index (GDRI)
The glucose diffusion rate indicates the release of glucose during the metabolic processes in the human gut and its subsequent absorption by the body. The fiber in diet plays a crucial role in controlling the rate of glucose diffusion. The glucose diffusion rate thus determines the GDRI. The GDRI measures or predicts the role of fiber in reducing the glucose absorption in the GI tract (Lopez et al. 1996).
Table 6 shows the GDR and GDRI values for the studied samples. GDR was slower in BGPL, CMPM and BB compared to cellulose, PNPM, WMPL and KMPL. GDR was inversely proportional to GDRI and also, GDRI values decreased with increase in time. The decrease in GDRI was highest in PNPM, but CMPM showed relatively less decrease in GDRI value after 30 min incubation time.
Table 6.
Glucose diffusion rate and GDRI of the selected fibers
| Sample | 20 min | 30 min | 60 min | 120 min | ||||
|---|---|---|---|---|---|---|---|---|
| GDR (μmoL) | GDRI | GDR (μmoL) | GDRI | GDR (μmoL) | GDRI | GDR (μmoL) | GDRI | |
| Control | 138.00 ± 0.18d | 0 | 195.00 ± 0.42e | 0 | 280.00 ± 0.45g | 0 | 371.00 ± 0.33g | 0 |
| CEL | 119.00 ± 0.20c | 13.77b | 157.00 ± 0.33b | 19.44d | 258.00 ± 0.52e | 7.86a | 332.00 ± 0.45d | 10.51c |
| PNPM | 125.00 ± 0.24c | 9.42b | 189.00 ± 0.31e | 3.08a | 260.00 ± 0.13f | 7.14a | 357.00 ± 0.23f | 3.71a |
| BGPL | 100.00 ± 0.09b | 27.54c | 155.00 ± 0.24b | 20.51d | 220.00 ± 0.11b | 21.43d | 306.00 ± 0.27b | 17.52e |
| KMPL | 134.00 ± 0.12d | 2.90a | 184.00 ± 0.27d | 5.64 b | 246.00 ± 0.25d | 12.14b | 359.00 ± 0.49f | 3.23a |
| CMPM | 104.00 ± 0.11b | 24.64c | 163.00 ± 0.11c | 16.41c | 235.00 ± 0.34c | 16.07c | 317.00 ± 0.37c | 14.56d |
| WMPL | 122.00 ± 0.10c | 11.59b | 183.00 ± 0.16d | 6.15b | 255.00 ± 0.38e | 8.93a | 343.00 ± 0.26e | 7.55b |
| BB | 78.00 ± 0.23a | 43.48d | 132.00 ± 0.23a | 32.31e | 210.00 ± 0.21a | 25.00e | 295.00 ± 0.31a | 20.48f |
Mean ± S.D. with the same letter between the rows are not significantly different at P ≤ 0.05 by DMRT
PNPM pineapple pomace, BGPL Burmese grape peel, KMPL Khasi mandarin peel, CMPM carambola pomace, WMPL watermelon, BB banana blossom
The individual glucose adsorption capacity of the fiber may contribute to the final glucose absorption rate in the GI tract. Another reason could be the entrapment of glucose molecule between the networks formed by fibers (Nishimune et al. 1991) which in turn acted as a physical barrier towards the glucose absorption process.
Lastly, good glucose adsorption, amylase inhibition and high GDRI properties in the selected fibers would allow them to be incorporated as a functional fiber source in the development of low calorie, glucose lowering food products.
Conclusion
The present study of the six fiber samples showed varied proximate, physicochemical, phytochemical and functional properties. WMPL and BB were rich in crude protein and ash content. Even though PNPM had highest amount of dietary fiber, other samples showed comparatively better results for all the parameters tested. All the samples showed good water holding, oil holding and swelling capacity capacities. The fiber samples contained good phytochemical content and antioxidant activity. CMPM contained highest TPC and FRAP values. The samples showed considerably good values for glucose adsorption capacity, amylase inhibition, glucose diffusion rate and GDRI. Among the six samples, BGPL, BB, CMPM showed high GDRI values. All the selected fibers can be suitably used as sources of dietary fiber and antioxidants for development of low calorie, high fiber functional food products in the food industries. In vivo studies could be carried out to determine the health promoting properties of these fibers.
Acknowledgments
One of us (SS) is very grateful to the Council of Scientific & Industrial Research (CSIR), New Delhi, India for providing the Senior Research Fellowship.
References
- Ajila CM, Aalami M, Leelavathi K, Rao UJSP. Mango peel powder: a potential source of antioxidant and dietary fiber in macaroni preparations. Innov Food Sci Emerg Technol. 2010;11:219–224. doi: 10.1016/j.ifset.2009.10.004. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 16th. Washington, DC: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power” the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Buttriss JL, Stokes CS. Dietary fiber and health: an overview. Nutr Bull. 2008;33:186–200. doi: 10.1111/j.1467-3010.2008.00705.x. [DOI] [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colourimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chau CF, Huang YL, Lee MH (2003) In vitro hypoglycemic effects of different insoluble fiber-rich fractions prepared from the peel of Citrus Sinensis L. cv. Liucheng. J Agric Food Chem 51:6623–6626 [DOI] [PubMed]
- Davidson MH, McDonald A. Fiber: forms and functions. Nutr Res. 1998;18:617–624. doi: 10.1016/S0271-5317(98)00048-7. [DOI] [Google Scholar]
- Dhingra D, Mona M, Rajput H, Patil RT. Dietary fiber in foods: a review. J Food Sci Technol. 2012;49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Evans P, Halliwell B. Micronutrients: oxidant/antioxidant status. Br J Nutr. 2001;85:67–74. doi: 10.1079/BJN2000296. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gines JM, Fernandez-Lopez J, Sayas-Barbera E, Sendra E, Pérez-Alvarez JA. Effect of storage conditions on quality characteristics of bologna sausages made with citrus fiber. J Food Sci. 2003;68:710–714. doi: 10.1111/j.1365-2621.2003.tb05737.x. [DOI] [Google Scholar]
- Gourgue CMP, Champ MMJ, Lozano Y, Delort-Laval J. Dietary fiber from mango byproducts: characterization and hypoglycemic effects determined by in vitro methods. J Agric Food Chem. 1992;40:1864–1868. doi: 10.1021/jf00022a027. [DOI] [Google Scholar]
- Kahlon TS, Chow FI. In vitro binding of bile acids by rice bran, oat bran, wheat bran and corn bran. Cereal Chem. 2000;77:518–521. doi: 10.1094/CCHEM.2000.77.4.518. [DOI] [Google Scholar]
- Lin MJY, Humbert E S, Sosulki FW. Certain functional properties of sunflower meal products. J Food Sci. 1974;39:368–370. doi: 10.1111/j.1365-2621.1974.tb02896.x. [DOI] [Google Scholar]
- Lopez G, Ros G, Rincon F, Periago MJ, Martınez MC, Ortuno J. Relationship between physical and hydration properties of soluble and insoluble fiber of artichoke. J Agric Food Chem. 1996;44:2773–2778. doi: 10.1021/jf9507699. [DOI] [Google Scholar]
- Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Nishimune T, Yakushiji T, Sumimoto T, Taguchi S, Konishi Y, Nakahara S, Ichikawa T, Kunita N. Glycemic response and fiber content of some foods. Am J Clin Nutr. 1991;54:414–419. doi: 10.1093/ajcn/54.2.414. [DOI] [PubMed] [Google Scholar]
- Ou S, Kwok KC, Li Y, Fu L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J Agric Food Chem. 2001;49:1026–1029. doi: 10.1021/jf000574n. [DOI] [PubMed] [Google Scholar]
- Perez-Jimenez J, Saura-Calixto F. Grape products and cardiovascular disease risk factors. Nutr Res Rev. 2008;21:158–173. doi: 10.1017/S0954422408125124. [DOI] [PubMed] [Google Scholar]
- Robertson JA, de Monredon FD, Dysseler P, Guillon F, Amado R, Thibault JF. Hydration properties of dietary fiber and resistant starch: a European collaborative study. LWT Food Sci Technol. 2000;33:72–79. doi: 10.1006/fstl.1999.0595. [DOI] [Google Scholar]
- Rubilar M, Pinelo M, Shene C, Sineiro J, Nunez MJ. Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: almond hulls and grape pomace. J Agric Food Chem. 2007;55:10101–10109. doi: 10.1021/jf0721996. [DOI] [PubMed] [Google Scholar]
- Saikia S, Mahnot NK, Mahanta CL. Optimization of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015;171:144–152. doi: 10.1016/j.foodchem.2014.08.064. [DOI] [PubMed] [Google Scholar]
- Saura-Calixto F, Goñi I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2006;94:442–447. doi: 10.1016/j.foodchem.2004.11.033. [DOI] [Google Scholar]
- Schieber A, Stintzing FC, Carle R. Byproducts of plant food processing as a source of functional compounds – recent developments. Trends Food Sci Technol. 2001;12:401–413. doi: 10.1016/S0924-2244(02)00012-2. [DOI] [Google Scholar]
- Slinkard S, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- Thibault JF, Lahaye M, Guillon F. Physiochemical properties of food plant cell walls. In: Schweizer E, Edwards C, editors. Dietary fiber, a component of food. Nutritional function in health and disease, ILSI Europe. Berlin: Springer-verlag; 1992. pp. 21–39. [Google Scholar]