Abstract
Two fresh red vegetables smoothies based on tomato, carrots, pepper and broccoli and rich in health-promoting compounds were developed. The smoothies showed a viscoelastic behaviour. According to sensory analyses, a shelf life of 28 days at 5 °C for fresh blended smoothies was established while thermally-treated ones (3 min, 80 °C) reached up to 40 days at 20 °C and 58 days at 5 °C. For those mild heat treated smoothies, total vitamin C degradation was 2-fold reduced during storage at 5 °C compared to samples stored at 20 °C while the initial total carotenoids, lycopene and total chlorophylls contents were not greatly affected. A 250-g portion of such smoothies covers in a great extend the established recommended daily nutrient intakes for dietary fibre, minerals and vitamin C of different population groups. As main conclusion, a mild thermal treatment and low temperature storage greatly increased the shelf life of red fresh vegetables smoothies and reduced total vitamin C degradation.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-015-2143-2) contains supplementary material, which is available to authorized users.
Keywords: Vitamin C, Lycopene, Chlorophylls, Bioactive compounds, Fibre, Beverages
Introduction
The Mediterranean diet has been particularly studied for its positive effects on the prevention of heart diseases and its potential to reduce the incidence of chronic degenerative diseases such as diabetes, high blood pressure and to avoid the low-density lipoprotein oxidation (Mitjavila et al. 2013). Epidemiological studies conducted by the PREDIMED (2015) suggest that most of those beneficial effects are derived from the phytochemical constituents of fruit, vegetables and olive oil, which are the main components of that diet. Tomato, red pepper, carrot and broccoli have high contents of those health-promoting phytochemicals such as carotenoids, phenolic compounds, vitamins C and E, folates, glucosinolates and minerals, among others (Serrano et al. 2010; Martínez-Hernández et al. 2015a; Fernández-León et al. 2013; Sánchez-Rangel et al. 2014). Dietary fibre activates intestinal peristalsis, binds bile acids and water, and reduces blood cholesterol level and, consequently, the risk of incidence of ischemic heart disease and postprandial glycaemia (Chen et al. 2011).
The current lifestyle does not allow the time needed for the preparation of these vegetables. Thus, their consumption should be promoted through the development of ready-to-eat products that should be processed with minimal non-aggressive treatments to preserve as much as possible their quality (Artés-Hernández et al. 2009). Smoothies are non-alcoholic beverages prepared from fresh or frozen fruit and/or vegetables, which are blended without filtering and usually mixed with crushed ice to be immediately consumed. Often, some smoothies may include other components like yogurt, milk, ice-cream, lemonade or tea. They have a milk shake-like consistency that is thicker than slush drinks. Accordingly, smoothies represent an excellent and convenient alternative to promote the daily consumption of fruit and vegetables. The smoothie processing involves a breakdown of plant parenchyma, which leads to a dispersed solution consisting in a liquid phase (pectin and other soluble solids) and a solid phase composed of insoluble solids (cell wall). The main challenge of the smoothie preparation is the limited shelf-life of these products since they are susceptible to spoilage and quality degradation. For that reason, in order to increase the shelf-life while keeping quality, mild thermal treatments must be used during processing (Di Cagno et al. 2011) and storage at low temperature is recommended. However, the thermal treatment should be as mild as possible in order to preserve the nutritional and sensory quality of the smoothie. Thermal treatments (generally in the range of 80 °C to 95 °C) are commercially applied for the inactivation of spoilage enzymes in fruit purees and juices (Di Cagno et al. 2011). However, these treatments may reduce phytochemical contents of smoothies thus affecting related antioxidant properties. To the best of our knowledge, there is no information available about the effects of mild thermal processing and subsequent storage on quality changes of fresh vegetable smoothies. For that reason, the aim of this work was to study the effect of a mild conventional pasteurization, compared to a non-thermal treatment, on sensory, microbial and physicochemical quality changes, as well as on selected bioactive compounds of two red fresh vegetable smoothies throughout storage at 5 and 20 °C.
Materials and methods
Plant material and smoothie preparation
Fresh vegetables (tomato, red pepper, broccoli and carrot) were purchased at a local supermarket from Cartagena (Spain) in September. All produce was firstly sanitized with 75 mg L−1 NaOCl during 2 min and then rinsed with tap water during 1 min. Tomatoes and carrots were peeled and all vegetables were then cut and blended (MX2050 blender, Braun, Germany). R1 (tomato, red pepper, broccoli and spices) and R2 (tomato, red pepper, broccoli carrot and spices) smoothies formulations (Supplementary material 1) were developed based on previous sensory studies being well accepted by a trained panel.
Thermal treatment and storage conditions
After preparation, smoothies were immediately placed in 15 mL Falcon tubes and heat treated in an agitated water bath at 83 °C (J.P. Selecta, Barcelona, Spain). The temperature of the samples increased during 3 min until the core reached 80 °C. At that moment temperature was kept for 3 min and then immediately cooled up to 5 or 20 °C in iced water. Samples were then stored in darkness at 5 and 20 °C. Fresh blended unheated samples were used as control (CTRL) being stored at 5 °C. Five replicates per treatment and sampling day, for each storage temperature, were prepared. Samples from each treatment were taken on each sampling day and stored at −80 °C until further analysis.
Rheological properties of smoothies
Rheological measurements were executed using an ARG2 stress-controlled rheometer (TA Instruments, New Castle, DE, USA) equipped with serrated (to prevent wall depletion phenomena) plate-plate geometry (20 mm, gap 2 mm). A solvent trap saturated with water was used to avoid evaporation. For every measurement the smoothie sample was transferred to the rheometer geometry and then the sample was allowed to equilibrate between the plates at 25 °C for 1 min. Oscillatory tests were performed within the linear viscoelastic region. Storage modulus (G’) and loss modulus (G”) were determined in a frequency range of 100 to 0.2 Hz. The strain value was obtained by preliminary strain sweep oscillatory trials to determine the linear viscoelastic region. The strain sweep oscillatory tests were carried out at a frequency of 1 Hz and in a range of shear strain of 0.01 to 10 %. Flow tests were also used to cover shear rate range between 10 −2 /s and 10 2 /s. All experiments were carried out at 25 °C. Rheological data is presented as Supplementary material 2. Three repetitions of the dynamic-mechanical experiments were performed for each smoothie sample.
Total dietary fibre and mineral content
The contents of pectin, hemicellulose, cellulose, lignin and ash in the smoothies were studied by thermogravimetric analysis (TGA), conducted on a TGA/DSC HT thermogravimetric analyser (Mettler-Toledo GmbH, Schwerzenbach, Switzerland) by the method described by Boluda-Aguilar et al. (2010) slightly modified. Fine powder from dried samples (105 °C for 24 h) was obtained by a mincer (IKA, A 11basic, Berlin, Germany). Approximately 10 mg of sample powder was used. Derivative thermogravimetric (DTG) curves were analysed by derivative weight loss (see Supplementary material 3). The TG-DTG curves are also presented in Supplementary material 3. The temperature for the maximal weight loss (Tmax) at 90 °C is attributed to the free water loss. The decomposition peaks at the Tmax of 190, 270 and 321 °C are assigned to pectin, hemicelluloses and cellulose, respectively (Boluda-Aguilar et al. 2010). The weight percentage of each component in the analysed samples was obtained as the mass loss produced during volatilization.
The mineral content of the samples was analysed by X-ray fluorescence (XRF) according to Martínez-Hernández et al. (2015a). For the XRF analyses a spectrometer S4 Pioneer (Bruker Corporation, Billerica, MA, USA) was used, equipped with a Rh anticathode X-ray tube (20–60 kV, 5-150 mA and 4 kW maximum), five analyser crystals (LiF200, LiF220, Ge, PET, and XS-55), sealed proportional counter for light elements detection and a scintillation counter for heavy elements with slight modifications. The recorded spectrum was evaluated by the fundamental parameters method using the Spectra plus software EVA 1.7. Mineral content was expressed as g kg−1 dry weight (dw) and mg kg−1dw for major minerals and trace elements, respectively. All samples were analysed in triplicate.
Sensory evaluation
Sensory analyses were performed according to international standards (ASTM 1986). Tests were conducted in a standard room (UNE-EN ISO 8589 2007) equipped with ten individual taste boxes. Samples (about 30 mL) were served at room temperature in transparent plastic glasses coded with three random digit numbers. Still mineral water was used as palate cleanser. The trained sensory panel consisted of twelve assessors (six women/six men, aged 22–68 years) screened for sensory ability (colour, flavour, visual appearance and texture). A 5-point scale of damage incidence and severity was scored for off-colours, off-odours, lumpiness, turbidity and precipitation/phase separation (5: none; 4: slight; 3: moderate, limit of usability; 2: severe; 1: extreme). Visual appearance, flavour, texture and overall quality (5: excellent, 4: good, 3: fair, limit of usability, 2: poor; 1: extremely bad).
Colour
Colour was determined using a colorimeter (Minolta CR-300 Series, Japan) calibrated with a white reference plate (light source C), 2° observer and 8-mm viewing aperture. Samples were introduced in a special glass tube mounted on a device connected to the colorimeter. Measurements were recorded using the standard tristimulus parameters (L*, a*, b*) of the CIE Lab system on three equidistant points of each replicate. Three colour readings were taken turning the tube at every read and all three measurements were automatically averaged by the device and recorded. Total colour differences (ΔE) throughout storage compared to their respective initial values were calculated according to equations previously described (Walkling-Ribeiro et al. 2010).
Microbial analysis
To determine the mesophilic, psychrophilic, Enterobacteria, and yeast and mould growth, standard enumeration methods were used. Samples of 5 g were homogenised in 45 mL of sterile peptone saline solution (pH 7; Scharlau Chemie SA, Barcelona, Spain) for 10 s in a sterile stomacher bag (model 400 Bags 6141, London, UK) using a masticator (Colwort Stomacher 400 Lab, Seward Medical, London, UK). For the enumeration of each microbial group, 10-fold dilution series were prepared in 9 mL of sterile peptone saline solution. Mesophilic, Enterobacteria and psychrotrophic were pour plated, and yeast and mould were spread plated. The following media and incubation conditions were used: plate count modified agar (PCA) (Scharlau Chemie, Barcelona, Spain) for mesophilic and psychrotrophic aerobic bacteria, incubated at 30 °C for 48 h and at 5 °C for 7 days, respectively; violet red bile dextrose agar (Scharlau Chemie, Barcelona, Spain) for Enterobacteria, incubated at 37 °C for 48 h; and rose Bengal agar (Scharlau Chemie, Barcelona, Spain) for yeasts and moulds, incubated for 3–5 days at 22 °C. All microbial counts were reported as log colony forming units per gram of product (log CFU g−1). Each of the three replicates was analysed by duplicate. The presence of Salmonella spp., Listeria monocytogenes and generic Escherichia coli was monitored according to the European legislation (Regulation EC 1441/2007 2007).
Physiochemical analyses
The pH, titratable acidity (TA) and total soluble solids content (SSC) of red vegetables smoothies were studied. A pH-meter was used to analyse the pH. The SSC of the smoothies was determined by a digital hand-held refractometer (Atago N1, Tokyo, Japan) at 25 °C and expressed as °Brix. TA was determined by the titration of 5 mL of juice plus 45 mL of distilled water with 0.1 mol L−1 NaOH to pH 8.1 (T50, Metter Toledo, Milan, Italy) and expressed as % (g citric acid 100 mL−1). Three replicates per treatment were analysed.
Bioactive compounds
Vitamin C
The ascorbic (AA) and dehydroascorbic (DHA) acids were measured according to Martínez-Hernández et al. (2013). Derivatised samples (20 μL) were injected onto a Gemini NX (250 mm × 4.6 mm, 5 μm) C18 column (Phenomenex, Torrance CA, USA), using an HPLC (Series 1100 Agilent Technologies, Waldbronn, Germany) equipped with a G1322A degasser, G1311A quaternary pump, G1313A autosampler, G1316A column heater and G1315B photodiode array detector. The HPLC system was controlled by the software ChemStation Agilent, v. 08.03. AA and DHA were quantified using commercial standards (Sigma, St Louis, MO, USA). Calibration curves were made with at least six data points for each standard. Total vitamin C was calculated as the sum of AA and DHA and expressed as mg kg−1 fw. Each of the three replicates was analysed by triplicate.
Total carotenoids and chlorophylls content
Sample preparation for total carotenoids and chlorophylls determinations was conducted according to Martínez-Hernández et al. (2011). An UV-visible spectrophotometer (8453, Hewlet Packard, Columbia, USA) was used to registered absorbances at 662, 644 and 470 nm. The equations developed by Wellburn (1994) were used to determine the individual levels of chlorophyll a (Cha = 10.05 × A662 – 0.766 × A644), chlorophyll b (Ch b = 16.37 × A644 – 3.14 × A662), total chlorophyll amount (Ca + Cb) and total carotenoids [Total carotenoids = (1000 × A470– 1.28 × Ca– 56.7 × Cb)/205]. Total chlorophyll and total carotenoids contents were expressed as mg kg−1 fw. Each of the three replicates was analysed by triplicate.
Lycopene
Lycopene content was determined according to Davis et al. (2003). Briefly, 1 g of ground frozen sample was mixed with 5 mL of acetone containing 0.05 % (w/v) butylhydroxytoluene, 5 mL 95 % ethanol and 10 mL hexane. The extraction was carried out for 15 min in darkness inside a polystyrene box with ice and shaken continuously at 200 × g with the orbital shaker. After extraction, 3 mL of distilled water was added, samples were shaken again for 5 min in the orbital shaker and the upper of the three layers formed was used as lycopene extract. Absorbances of the extracts were measured at 503 nm in the UV-visible spectrophotometer. The lycopene content was calculated according to Fish et al. (2002) as: lycopene = (A503 × MW × DF)/(Ɛ); where MW is the lycopene molecular weight, DF the dilution factor and ε is the lycopene molar extinction coefficient (172,000 L mol cm−1 in hexane). Lycopene contents were expressed as mg kg−1 fw. Each of the three replicates was analysed by triplicate.
Statistical analysis
The experiment was a one-factor (treatment) design subjected to analysis of variance (ANOVA) using Statgraphics Plus software (vs. 5.1, Statpoint Technologies Inc, Warrenton, USA). Statistical significance was assessed at the level P = 0.05, and Tukey’s multiple range test was used to separate means.
Results and discussion
Rheological properties of smoothies
The texture of a smoothie has to provide a balance between desired mechanical stability (for storage and handling) and desired instability (to elicit a specific texture attribute during mastication). Rheological properties are useful in determining the proportions of most ingredients for the product development, its quality, and correlation of food texture to sensory attributes. Smoothies are viscoelastic food materials that exhibit both solid-like and fluid-like behaviour. The storage modulus (G’) of smoothies was greater than the loss modulus (G”) at any given point in the frequency sweep tests (see Supplementary material 2). This fact indicates a dominant contribution of the elastic component to the viscoelasticity of the investigated smoothies, typical behaviour of a viscoelastic solid. This means that the attractive forces become dominant due to the strong hydrogen bond and hydrophobic association (Basu et al. 2011). Apparent viscosity of CTRL-R1 was higher than CTRL-R2, probably owed to the higher pectin content of R1 smoothie (see Supplementary material 2 and Table 1). The tanδ value (ratio between loss and storage modulus, also known as loss tangent) is a direct measure of the relative importance of viscous and elastic effects in the sample. For all the considered samples, tanδ was lower than 1 thus indicating a gel-like behaviour. While apparent viscosity of R1 smoothie was reduced after thermal treatment, R2 smoothie showed the opposite behaviour (see Supplementary material 2) which may be explained by the different composition of smoothies. The effective shear rate range in the mouth is 40–50 s−1, which would have implied actual sensory consistency (Wood and Goff 1973). The viscosity of CTRL-R1 was higher than CTRL-R2 within the shear rate range 40–50 s−1. Accordingly, panellists scored better texture of R1 smoothies than R2 (as described latter), which is related to a greater smoothie viscosity of R1 smoothie.
Table 1.
Total dietary fibre and moisture contents of red fresh vegetables smoothies (R1 and R2)
| R1 | R2 | |
|---|---|---|
| Chemical composition (% wet basis) | ||
| Moisture content | 88.9 | 88.9 |
| Insoluble total dietary fiber a | 4.8 | 4.7 |
| Pectin | 1.5 | 1.4 |
| Hemicellulose | 1.2 | 1.2 |
| Cellulose | 2.1 | 2.1 |
a expressed as % on wet basis. Calculated as the sum of pectin, hemicellulose and cellulose
Total dietary fibre and mineral content
The total dietary fibre content (DF), as well as their main components as pectin, hemicellulose and cellulose are depicted in Table 1. The total DF content of R1 and R2 smoothies were 4.7 and 4.8 % wet basis (wb), respectively. The slightly higher total DF of R2 smoothie compared to R1 may be explained by the presence of carrots and higher pepper and broccoli contents in the smoothie formulation, having all those vegetables higher fibre contents. Pectin and hemicellulose contents of smoothies accounted 1.4-1.5 and 1.2 % wb, respectively. Cellulose accounted 2.1 % wb for both smoothies. According to The Code of Federal Regulations (FDA et al. 2015), food products which contain 20 % or more of the recommended daily nutrient intakes (RNIs) for fibre (25 g day−1) are considered as an ‘excellent source of fibre’. Accordingly, the fresh red smoothies developed in this study can be considered as an ‘excellent source of fibre’ since a portion of 250 g provides 50 % of the RNIs for fibre.
The minerals content of both red smoothies are presented in Table 2. R1 smoothie presented 1.1-1.5-fold higher P, Na, Al and Mn contents than R2 smoothie. On the other side, R2 smoothie presented 1.1-1.4-fold higher Fe, K, Ca, Zn and Sr contents than R1. A smoothie portion of 250 g provides 8–11, 2–3, 2–4 and 3–4 % of the RNIs for Mg, Ca, Fe and Zn, respectively, covering population groups with special nutritional requirements such as elders, pregnant women or adolescents (FAO/WHO 2004).
Table 2.
Mineral content of red fresh vegetables smoothies (R1 and R2) (n = 5 ± SD)
| Mineral nutrients a | R1 | R2 |
|---|---|---|
| P | 2.55 ± 0.02 | 2.31 ± 0.02 |
| S | 1.74 ± 0.01 | 1.77 ± 0.02 |
| Na | 13.70 ± 0.14 | 10.60 ± 0.12 |
| K | 14.51 ± 0.04 | 16.84 ± 0.04 |
| Ca | 0.85 ± 0.01 | 1.02 ± 0.01 |
| Mg | 0.75 ± 0.01 | 0.74 ± 0.01 |
| Cl | 18.22 ± 0.06 | 15.14 ± 0.05 |
| Al | 0.15 ± 0.01 | 0.10 ± 0.01 |
| Mineral traces found b | ||
| Fe | 2.00 ± 0.08 | 11.00 ± 0.94 |
| Mn | 8.10 ± 0.83 | 7.60 ± 0.88 |
| Zn | 9.40 ± 0.42 | 10.50 ± 0.46 |
| Si | 38.00 ± 5.70 | 8.00 ± 0.49 |
| Br | 10.80 ± 0.35 | 10.80 ± 0.38 |
| Sr | 7.21 ± 0.27 | 8.56 ± 0.30 |
a (g kg−1 dw)
b (mg kg−1 dw)
Sensory analysis
Visual appearance, flavour, texture, off-colours, off-odours, lumpiness, turbidity, precipitation/phase separation and overall quality of CTRL smoothies were reported to be over the limit of acceptability up to 28 days at 5 °C. Thermally-treated smoothies maintained their sensory acceptation up to 40 days at 20 °C and 58 days at 5 °C (data not shown). Accordingly, the shelf-life of the smoothies was established based on the sensory analyses.
Soluble solids content, pH and titratable acidity
The initial SSC of CTRL-R1 and CTRL-R2 smoothies were 8.37 and 7.07 °Brix, respectively (Table 3). The higher SSC of R1 smoothie compared to R2 may be explained by the higher tomato content of R1 (75 %) when compared to R2 (56 %). Di Cagno et al. (2011) reported a SSC of 13.1 °Brix in red fruit smoothies. The higher tomato content (56 and 75 %) of our smoothies compared with the low tomato (8 %) and high fruit contents (31 % prunes and 26 % cherries) of those fruit smoothies may explain the observed SSC differences. The thermal treatment did not induce significant SSC changes in R1 smoothie but SSC of R2 lightly increased in 1.4 °Brix after treatment. Accordingly, the SSC increase of R2 smoothie may be explained by its carrot content. The hard texture of carrot tissue may lead to the presence of carrot particles after blending. Accordingly, the soluble solids extraction can be enhanced after thermal treatment as observed in R2 samples. SSC of both untreated and thermally-treated smoothies did not significantly change during storage either at 5 or 20 °C.
Table 3.
pH, soluble solids content, titratable acidity, and total colour differences of untreated (CTRL) and heat-treated (HT) red fresh vegetables smoothies (R1 and R2) stored at 5 and 20 °C (n = 5 ± SD)
| Days of storage | R1 | R2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | SSC (° Brix) | TA ( g citric acid 100 mL−1) | ΔE | pH | SSC (° Brix) | TA ( g citric acid 100 mL−1) | ΔE | ||
| 0 | CTRL5C | – | – | ||||||
| HT5C | – | – | |||||||
| HT20C | – | – | |||||||
| 7 | CTRL5C | ||||||||
| HT5C | |||||||||
| HT20C | |||||||||
| 14 | CTRL5C | ||||||||
| HT5C | |||||||||
| HT20C | |||||||||
| 21 | CTRL5C | ||||||||
| HT5C | |||||||||
| HT20C | |||||||||
| 28 | CTRL5C | ||||||||
| HT5C | |||||||||
| HT20C | |||||||||
| 35 | HT5C | ||||||||
| HT20C | |||||||||
| 40 | HT5C | ||||||||
| HT20C | |||||||||
| 49 | HT5C | ||||||||
| 58 | HT5C | ||||||||
Different capital letters denote significant differences (P ≤ 0.05) among treatments for the same sampling day and smoothie. Different lowercase letters denote significant differences (P ≤ 0.05) among sampling days for the same treatment and smoothie
The initial pH of untreated R1 and R2 smoothies were 4.36 and 4.31, respectively (Table 3). Di Cagno et al. (2011) reported lower pH levels (3.5) in a red fruit smoothie due to its high content of fruits, which have lower pH than vegetables. The pH of both smoothies did not significantly change after the thermal treatment. Similarly, the pH of treated and untreated smoothies did not greatly change (<0.2 pH units) during storage either at 5 or 20 °C.
The initial TA of untreated R1 and R2 smoothies were 0.25 and 0.22 mg citric acid 100−1 g fw, respectively (Table 3). Keenan et al. (2010) reported higher TA values of 0.56 mg citric acid 100−1 g fw in a fruit smoothie owed to the higher TA of fruit compared to vegetables. Throughout conservation, TA of CTRL smoothies registered increases up to 34 and 54 % after 21 and 28 days at 5 °C, respectively. Thermal treatment and storage at 5 °C may reduce metabolic reactions since no great TA changes (<0.07 mg citric acid 100−1 g fw) were observed in those smoothies. Similarly, Di Cagno et al. (2011) did not observe significant TA differences in heat-treated (80 °C for 10 min) fruit/vegetable smoothies throughout storage at 4 °C. However, storage at 20 °C of thermally-treated smoothies induced a gradual TA reduction with values approximately 30 % lower at the end of storage compared to their respective initial levels. The latter behaviour may be explained by the higher storage temperature, which enhances metabolic reactions that produce acidic compounds. In general, the TA behaviour of samples during storage was inversely correlated to pH behaviour.
Colour
The L*, a* and b* values of R1/R2 smoothies were 92.1/91.5, 16.1/13.4 and 37.4/38.7, respectively (data not shown). Thermal treatment induced slight colour changes with ΔE values for R1 and R2 smoothies of 5.6 and 9.6, respectively. Walkling-Ribeiro et al. (2010) reported lower ΔE value (1.2) after a short thermal treatment (72 °C for 15 s) of a fruit smoothie. A great ΔE, of approximately 20 units, was observed after 3 days of storage of untreated smoothies, while treated smoothies only achieved ΔE of approximately 2–11 units after 7 days of storage at both temperatures. As observed, colour changes of smoothies during storage were greatly reduced in those treated samples, which are mostly due to the thermal inactivation of colour degradative enzymes such as polyphenoloxidase (PPO) and peroxidase (POD). Accordingly, great to nearly complete PPO and POD inactivations have been reported in broccoli and spinach purees after similar thermal treatments (Morales-Blancas et al. 2002; Wang et al. 2012, 2013). As expected, ΔE levels gradually increased throughout storage. However, storage at low temperature reduced the colour changes since ΔE of 20–21 and 24–26 were registered in those smoothies after 40 days at 5 and 20 °C, respectively.
Microbial analysis
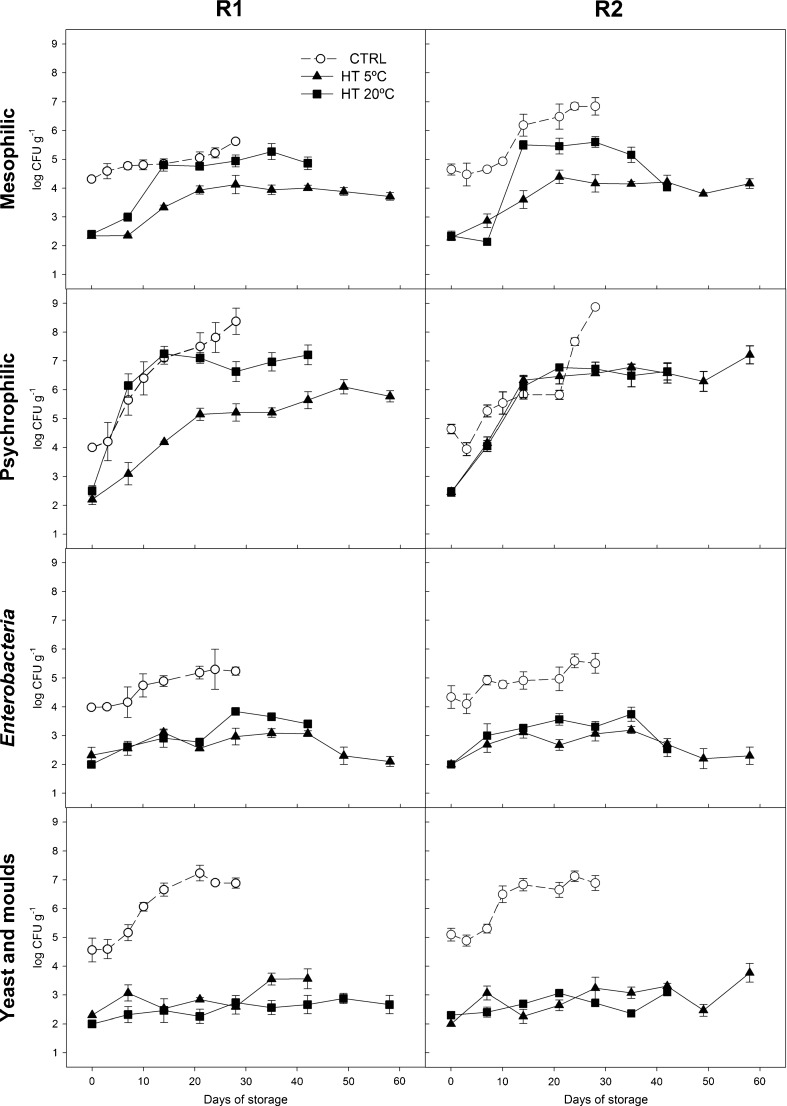
The initial microbial counts of CTRL-R1/CTRL-R2 smoothies were 4.3/4.6, 4.0/4.6, 3.9/4.3 and 4.6/5.9 log CFU g−1 for mesophiles, psychrophiles, Enterobacteria and yeast and moulds, respectively (Fig. 1). Thermal treatment of R1/R2 smoothies achieved mesophilic, psychrophilic, Enterobacteria and yeast and moulds reductions of approximately 2.0/2.4, 1.7/2.2, 1.8/2.3 and 2.3/2.8 log units, respectively. Walkling-Ribeiro et al. (2010) reported mesophilic and yeast and moulds reductions of 3.5 and 3.7 log CFU g−1, respectively, in a fruit smoothie after a thermal treatment of 70 °C for 15 s. The dynamic system used by Walkling-Ribeiro et al. (2010) during heat treatment compared to our static system may explain the better microbial reductions achieved by those authors.
Fig. 1.
Mesophilic, psychrophilic, Enterobacteria and yeast and moulds counts (log CFU g−1) of untreated (CTRL) and heat-treated (HT) red fresh vegetables smoothies (R1 and R2) stored at 5 and 20 °C (n = 5 ± SD). Different capital letters denote significant differences (P ≤ 0.05) among treatments for the same sampling day and smoothie. Different lowercase letters denote significant differences (P ≤ 0.05) among sampling days for the same treatment and smoothie
During the first 10 days of storage, mesophilic counts of CTRL-R1 and CTRL-R2 smoothies increased by 0.5 and 0.3 log CFU g−1, respectively. However, thermally-treated R1/R2 smoothies stored at 5 and 20 °C showed mesophilic increases of 0.6/1.0 and 1.7/1.9 log units, respectively, after 10 days. As expected, the microbial growth rates were higher at high storage temperatures. Similarly, Walkling-Ribeiro et al. (2010) reported a mesophilic increment of 0.1-0.7 log CFU g−1 in a fruit smoothie after 7–14 days at 4 °C. The higher mesophilic growth observed for treated samples could be explained by any of the following hypotheses: 1) the presence of vegetative or spore cells, which resisted to the thermal treatment, due to their higher thermal resistance and/or the protecting effects of the smoothie matrix, could grow better due to the lower microbial competence for the nutrients. 2) The heat treatment could completely inactivated the myrosinase (163.0 nmoles sinigrin transformed per g fw of sample; data not shown), being this enzyme responsible for the glucosinolates conversion to isothiocyanates. Isothiocyanates from broccoli have shown high antimicrobial activities contrary to glucosinolates (Vig et al. 2009). Accordingly, the glucosinolate-isothiocyanate conversion was possible in untreated unheated samples, contrary to heat-treated samples, with the observed preserving benefits from the isothiocyanates throughout storage of smoothies. Therefore, our previous preliminary data showed that mesophilic increase of 2 log units in untreated R1 smoothie after 28 days at 5 °C was doubled when that untreated R1 smoothie was prepared without broccoli (data not shown).
Attending to mesophilic counts of treated smoothies stored at 20 °C, a typical microbial growth curve was observed. Accordingly, lag (0-3rd day), exponential (3rd-14th day; increases of 2–3 log units regarding initial levels), stationary (14-28th day) and decline phases were observed. The absence of lag phase in the R1 smoothie could be an artefact since this phase can be shorter than 3 days at this high storage temperature but could be extended due to the initial antimicrobial effect achieved by using oregano in the formulation of R2 smoothie. As expected, the reduction of storage temperature to 5 °C extended the exponential phase until approximately day 21th, with lower counts increments (approximately 1 log unit) compared to those treated samples stored at 20 °C.
Psychrotrophes showed a similar behaviour to mesophiles. However, increments of psychrophiles were higher regarding mesophiles increases with approximately 3–4 log unit increases for CTRL and treated samples stored either at 5 or 20 °C for 28 days. Psychotropic count changes of treated smoothies from day 28 to the end of their shelf-life were below 1 log unit.
Enterobacteria counts of heat treated and CTRL samples increased progressively during storage achieving approximately 1 log unit increases after 28 days at 5 °C. However, treated smoothies stored at 20 °C registered Enterobacteria increments 2-fold higher than those samples stored at 5 °C after 28–35 days. After that maximum Enterobacteria counts, those levels started to decrease until the end of the smoothies shelf-life reaching, in general, similar levels to their respective initial counts.
Conclusively, thermal treatments of smoothies reduced 2–3 log units their initial microbial loads being microbial growth rates of such treated samples better controlled during storage at 5 °C up to 58 days compared to samples stored at 20 °C. Microbial loads of treated smoothies were below 7 log CFU g−1 at the end of their shelf-lives.
Vitamin C
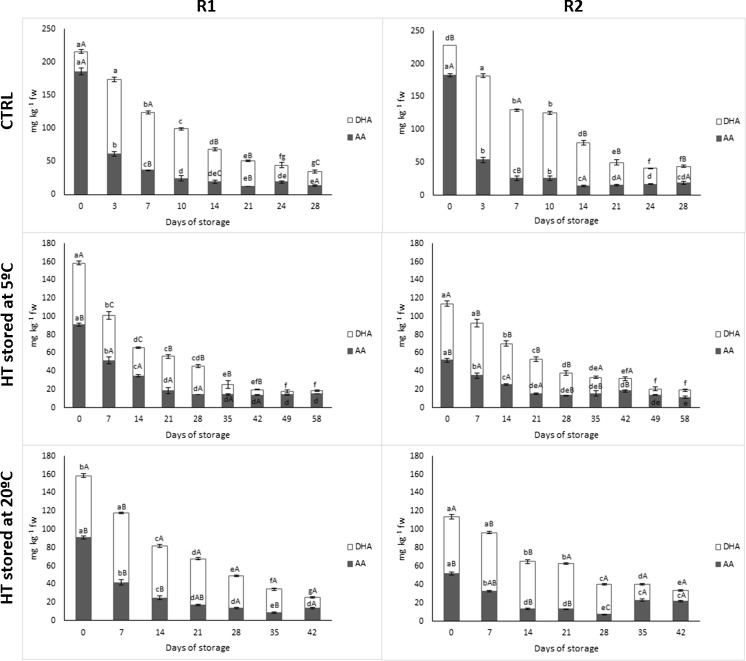
Total vitamin C contents, expressed as the sum of AA and DHA, of CTRL-R1 and CTRL-R2 smoothies were 216 and 229 mg kg−1 fw, respectively (Fig. 2). A smoothie portion of 250 g would provide approximately 130 % of the RNIs for vitamin C for adults and 80 % for lactating women, which is the population group with the highest vitamin C RNIs (FAO/WHO 2004). Vitamin C content of red pepper is approximately 11-fold higher than that of tomato (Vanderslice et al. 1990). Accordingly, the higher red pepper content (21 %) of R2 smoothie compared to R1 (12 %) was more relevant than the tomato concentrations of 56 and 75 %, respectively. DHA contents of untreated R1 and R2 smoothies accounted the 14 and 20 % of the total vitamin C content, respectively. Similarly, it has been reported that DHA of fresh tomatoes and red peppers accounted the 3 and 22 % of total vitamin C, respectively, although these proportions may differ depending of the variety (Lee and Kader 2000). Thermal treatment significantly degraded vitamin C of R1/R2 smoothies by 27/50 %. However, a 250 g portion of thermally-treated R1/R2 smoothie still provides approximately 100/71 % of the RNIs for vitamin C for adults and 56/41 % for lactating women (FAO/WHO 2004). Similarly, Benlloch-Tinoco et al. (2014) reported 27 % vitamin C degradation in kiwifruit puree after thermal processing at 84 °C for 5 min. AA content is easily oxidized during thermal treatments to DHA (Lee and Kader 2000). Accordingly, AA contents of R1/R2 smoothies decreased by 51/72 % after thermal treatment with DHA increments of 70/40 %.
Fig. 2.
Total vitamin C (ascorbic acid and dehydroascorbic acid) content of untreated (CTRL) and heat-treated (HT) red fresh vegetables smoothies (R1 and R2) stored at 5 and 20 °C (n = 5 ± SD). Different capital letters denote significant differences (P ≤ 0.05) among treatments for the same sampling day and smoothie. Different lowercase letters denote significant differences (P ≤ 0.05) among sampling days for the same treatment and smoothie
Storage of fresh fruit and vegetables implies AA oxidation to DHA being considered ascorbic acid oxidase (AAO) as the major enzyme responsible of this oxidation process (Lee and Kader 2000). AAO of crushed broccoli florets was almost inactivated after thermal treatment at 65 °C for 8 min (Munyaka et al. 2010). Accordingly, great AA decreases/DHA increments of approximately 67/275 and 71/180 % were observed in CTRL-R1 and CTRL-R2 smoothies, respectively, after 3 days at 5 °C. That behaviour was not observed in treated smoothies. Total vitamin C degradation rates were greatly reduced after 14–21 days. As expected, AA and DHA degradations were better controlled at lower storage temperature. Accordingly, while AA/DHA degradation of 75/42 % was observed in treated samples stored at 5 °C after 21 days, treated samples stored at 20 °C showed similar reductions earlier (14 days). At the end of shelf-life, total vitamin C contents of R1/R2 smoothies accounted approximately 14/17 % of their respective initial levels.
Total carotenoids and lycopene contents
The initial total carotenoids contents of CTRL-R1 and CTRL-R2 smoothies were 52.5 and 65.2 mg kg−1 fw, respectively (Table 4). Lycopene accounted 53 and 74 % of the total carotenoids contents of R1 and R2 smoothies, respectively (Table 4). Since lycopene is the main carotenoid of tomatoes (Martínez-Hernández et al. 2015b), the high tomato content of smoothies may explain the high lycopene proportion. Carotenes are sensitive to heat, among other factors such as light, oxygen, and pH, and might be lost during thermal processing due to isomerization and oxidative degradation. However, lycopene is likely to remain in a crystalline form during thermal processing of tomato and it is therefore relatively stable (Martínez-Hernández et al. 2015b). Accordingly, thermal treatment of smoothies did not significantly affect their total carotenoids or lycopene contents. Similarly, lycopene content of tomato flesh was not changed after blanching at 85 °C for 4 min (Urbonaviciene et al. 2012).
Table 4.
Total carotenoids, lycopene and total chlorophylls contents of untreated (CTRL) and heat-treated (HT) red fresh vegetables smoothies (R1 and R2) stored at 5 and 20 °C (n = 5 ± SD)
| Days of storage | R1 | R2 | |||||
|---|---|---|---|---|---|---|---|
| Total carotenoids (mg kg−1 fw) | Lycopene (mg kg−1 fw) | Total chlorophylls (mg kg−1 fw) | Total carotenoids (mg kg−1 fw) | Lycopene (mg kg−1 fw) | Total chlorophylls (mg kg−1 fw) | ||
| 0 | CTRL5C | ||||||
| HT5C | |||||||
| HT20C | |||||||
| 7 | CTRL5C | ||||||
| HT5C | |||||||
| HT20C | |||||||
| 14 | CTRL5C | ||||||
| HT5C | |||||||
| HT20C | |||||||
| 21 | CTRL5C | ||||||
| HT5C | |||||||
| HT20C | |||||||
| 28 | CTRL5C | ||||||
| HT5C | |||||||
| HT20C | |||||||
| 35 | HT5C | ||||||
| HT20C | |||||||
| 40 | HT5C | ||||||
| HT20C | |||||||
| 49 | HT5C | ||||||
| 58 | HT5C | ||||||
Different capital letters denote significant differences (P ≤ 0.05) among treatments for the same sampling day and smoothie. Different lowercase letters denote significant differences (P ≤ 0.05) among sampling days for the same treatment and smoothie
The total carotenoids contents of CTRL smoothies were quite stable during storage registering maximum reductions of up to 13–16 % after 21 days keeping these levels until the end of its shelf-life. A great total carotenoids decrease of 30–40 % was registered in treated smoothies after 14–21 days at both storage temperatures. However, total carotenoids contents of treated smoothies were well maintained from days 14–21 registering even a slight and progressive total carotenoids increment until the end of storage. Hence, treated smoothies registered 10–20 % lower total carotenoids contents after 58 days at 5 °C and 40 days at 20 °C, respectively. Since lycopene mainly contributed to total carotenoids content, the lycopene behaviour during storage of smoothies was similar to that of total carotenoids. Consequently, a heat treatment of the smoothies just after blended greatly extended their shelf-lives registering final total carotenoids levels similar to those of CTRL samples independently (p < 0.05) of the storage temperature.
Total chlorophylls
The initial total chlorophylls contents of CTRL-R1 and CTRL-R2 smoothies were 26.8 and 27.4 mg kg−1 fw, respectively (Table 4). Since smoothies contained approximately 12 % of broccoli, being this vegetable the main source of chlorophylls, its contents are in accordance to those previously reported by Fernández-León et al. (2013) in fresh-cut broccoli (Cv. Parthenon). Chlorophyll a and b equally (50 %) accounted to the total chlorophylls content. The thermal treatment did not significantly affect the chlorophylls contents of smoothies.
No great chlorophylls changes were observed throughout the storage. Chlorophylls are highly susceptible to enzymatic or non-enzymatic degradation during processing and storage. Pheideaoxygenase (PaO) pathway is the chlorophyll degradation pathway, which involves the following enzymes: chlorophyllase, Mg-dechelatase and peroxidase. According to data from Holden (1961), the low pH of smoothies presented here (4.35-4.40) inactivated chlorophyllase, which is responsible of the first step in PaO pathway. However, spinach puree with pH of 5.89 registered chlorophyll degradation up to approximately 25 % after 43 days at 4 °C (Wang et al. 2013).
Conclusions
Two red fresh vegetables smoothies rich in health-promoting compounds were developed. The shelf-life for freshly blended (CTRL) smoothies, according to sensory and microbiological quality, was established in 28 days at 5 °C. A mild thermal treatment of 3 min at 80 °C extended their shelf-life up to 40 days at 20 ° C maintaining their health-promoting properties related to lycopene, total carotenoids and chlorophylls and with no great changes in other quality parameters (total soluble solids content and pH). Additionally, when the storage temperature of those thermally-treated smoothies was reduced to 5 °C an increase in shelf-life up to 58 days with better colour and vitamin C content retention was achieved. A 250-g portion of these smoothies can highly cover the established recommended daily nutrient intakes for dietary fibre, minerals and vitamin C of different population groups.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 14 kb)
(DOCX 1937 kb)
(DOCX 159 kb)
Acknowledgments
The authors are grateful to SAKATA SEEDS IBÉRICA, Spanish Ministry of Economy and Competitiveness (MINECO) Project AGL2013-48830-C2-1-R and FEDER UPCA13-2E-1653 for financial support. We are also grateful to A. Margola and M. Otón Alcaraz for their skilful technical assistance.
References
- Artés-Hernández F, Escalona VH, Robles PA, Martínez-Hernández GB, Artés F. Effect of UV-C radiation on quality of minimally processed spinach leaves. J Sci Food Agric. 2009;89:414–421. doi: 10.1002/jsfa.3460. [DOI] [Google Scholar]
- ASTM (1986) Physical requirements guidelines for sensory evaluation. American society for testing and materials publications. Publication 913. Philadelphia, USA
- Basu S, Shivhare US, Singh TV, Beniwal VS. Rheological, textural and spectral characteristics of sorbitol substituted mango jam. J Food Eng. 2011;105:503–512. doi: 10.1016/j.jfoodeng.2011.03.014. [DOI] [Google Scholar]
- Benlloch-Tinoco M, Igual M, Salvador A, Rodrigo D, Martínez-Navarrete N. Quality and acceptability of microwave and conventionally pasteurised kiwifruit puree. Food Bioprocess Technol. 2014;7:3282–3292. doi: 10.1007/s11947-014-1315-9. [DOI] [Google Scholar]
- Boluda-Aguilar M, García-Vidal L, González-Castañeda F, López-Gómez A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour Technol. 2010;101:3506–3513. doi: 10.1016/j.biortech.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ma KY, Liang Y, Peng C, Zuo Y. Role and classification of cholesterol-lowering functional foods. J Funct Foods. 2011;3:61–69. doi: 10.1016/j.jff.2011.02.003. [DOI] [Google Scholar]
- Davis AR, Fish WW, Perkins-Veazie P. A rapid spectrophotometric method for analyzing lycopene content in tomato and tomato products. Postharvest Biol Technol. 2003;28:425–430. doi: 10.1016/S0925-5214(02)00203-X. [DOI] [Google Scholar]
- Di Cagno R, Minervini G, Rizzello CG, Angelis D, Gobbetti M. Effect of lactic acid fermentation on antioxidant, texture, colour and sensory properties of red and green smoothies. Food Microbiol. 2011;28:1062–1071. doi: 10.1016/j.fm.2011.02.011. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2004) Vitamin and mineral requirements in human nutrition, 2nd edn, ed. by WHO, Bangkok, Thailand
- FDA, CFR-code of federal regulations, title 21, volume 2. [Online]. Available: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.54. 12 May 2015
- Fernández-León MF, Fernández-León AM, Lozano M, Ayuso MC, Amodio ML, Colelli G, González-Gómez D. Retention of quality and functional values of broccoli ‘Parthenon’ stored in modified atmosphere packaging. Food Control. 2013;31:302–313. doi: 10.1016/j.foodcont.2012.10.012. [DOI] [Google Scholar]
- Fish WW, Perkins-Veazie P, Collins JK. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J Food Compos Anal. 2002;15:309–317. doi: 10.1006/jfca.2002.1069. [DOI] [Google Scholar]
- Holden M. The breakdown of chlorophyll by chlorophyllase. Biochem J. 1961;78:359–364. doi: 10.1042/bj0780359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DF, Brunton NP, Gormley TR, Butler F, Tiwari BK, Patras A. Effect of thermal and high hydrostatic pressure processing on antioxidant activity and colour of fruit smoothies. Innov Food Sci Emerg Technol. 2010;11:551–556. doi: 10.1016/j.ifset.2010.07.003. [DOI] [Google Scholar]
- Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20:207–220. doi: 10.1016/S0925-5214(00)00133-2. [DOI] [Google Scholar]
- Martínez-Hernández GB, Gómez PA, Pradas I, Artés F, Artés-Hernández F. Moderate UV-C pretreatment as a quality enhancement tool in fresh-cut Bimi® broccoli. Postharvest Biol Technol. 2011;62:327–337. doi: 10.1016/j.postharvbio.2011.06.015. [DOI] [Google Scholar]
- Martínez-Hernández GB, Artés-Hernández F, Gómez PA, Artés F. Induced changes in bioactive compounds of kailan-hybrid broccoli after innovative processing and storage. J Funct Foods. 2013;5:133–143. doi: 10.1016/j.jff.2012.09.004. [DOI] [Google Scholar]
- Martínez-Hernández GB, Gómez PA, Artés F, Artés-Hernández F. Nutritional quality changes throughout shelf-life of fresh-cut kailan-hybrid and ‘Parthenon’ broccoli as affected by temperature and atmosphere composition. Food Sci Technol Int. 2015;21:14–23. doi: 10.1177/1082013213502352. [DOI] [PubMed] [Google Scholar]
- Martínez-Hernández GB, Boluda-Aguilar M, Taboada-Rodríguez A, Soto S, Marín-Iniesta F, López-Gómez A (2015b) Review: processing, packaging, and storage of tomato products. Influence on the lycopene content. Food Eng Rev. doi:10.1007/s12393-015-9113-3
- Mitjavila MT, Fandos M, Salas-Salvadó J, Covas MI, Borrego S, Estruch R, Sáez GT. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized controlled trial. Clin Nutr. 2013;32:172–178. doi: 10.1016/j.clnu.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Morales-Blancas EF, Chandia VE, Cisneros-Zevallos L. Thermal inactivation kinetics of peroxidase and lipoxygenase from broccoli, green asparagus and carrots. J Food Sci. 2002;67:146–154. doi: 10.1111/j.1365-2621.2002.tb11375.x. [DOI] [Google Scholar]
- Munyaka AW, Oey I, Van Loey A, Hendrickx M. Application of thermal inactivation of enzymes during vitamin C analysis to study the influence of acidification, crushing and blanching on vitamin C stability in broccoli (Brassica oleracea L var. Italica) Food Chem. 2010;120:591–598. doi: 10.1016/j.foodchem.2009.10.029. [DOI] [Google Scholar]
- PREDIMED [Online]. Available: http://www.predimed.es/. 06 May 2015
- Regulation EC 1441/2007 Commission regulation on microbiological criteria for foodstuffs. Off J Eur Union. 2007;32:12–29. [Google Scholar]
- Sánchez-Rangel JC, Jacobo-Velázquez DA, Cisneros-Zevallos L, Benavides J (2014) Primary recovery of bioactive compounds from stressed carrot tissue using aqueous two-phase systems strategies. J Chem Technol Biotechnol. doi:10.1002/jctb.4553
- Serrano M, Zapata PJ, Castillo S, Guillén F, Martínez-Romero D, Valero D. Antioxidant and nutritive constituents during sweet pepper development and ripening are enhanced by nitrophenolate treatments. Food Chem. 2010;118:497–503. doi: 10.1016/j.foodchem.2009.05.006. [DOI] [Google Scholar]
- UNE-EN ISO 8589 (2007) Sensory analysis- general guidance for the design of test rooms. Reviewed on 2011
- Urbonaviciene D, Viskelis P, Viskelis J, Jankauskiene J, Bobinas C. Lycopene and b-carotene in non-blanched and blanched tomatoes. J Food Agri Environ. 2012;10:142–146. [Google Scholar]
- Vanderslice JT, Higgs DJ, Hayes JM, Block G. Ascorbic acid and dehydroascorbic acid content of foods-as-eaten. J Food Compos Anal. 1990;3:105–118. doi: 10.1016/0889-1575(90)90018-H. [DOI] [Google Scholar]
- Vig AP, Rampal G, Thind TS, Arora S. Bio-protective effects of glucosinolates. A review. LWT Food Sci Technol. 2009;42:1561–1572. doi: 10.1016/j.lwt.2009.05.023. [DOI] [Google Scholar]
- Walkling-Ribeiro M, Noci F, Cronin DA, Lyng JG, Morgan DJ. Shelf life and sensory attributes of a fruit smoothie-type beverage processed with moderate heat and pulsed electric fields. LWT Food Sci Technol. 2010;43:1067–1073. doi: 10.1016/j.lwt.2010.02.010. [DOI] [Google Scholar]
- Wang R, Wang T, Zheng Q, Hu X, Zhang Y, Liao X. Effects of high hydrostatic pressure on colour of spinach puree and related properties. J Sci Food Agric. 2012;92:1417–1423. doi: 10.1002/jsfa.4719. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu Q, Yao J, Zhang Y, Liao X, Hu X, Wu J. Post-effects of high hydrostatic pressure on green colour retention and related properties of spinach puree during storage. Innov Food Sci Emerg Technol. 2013;17:63–71. doi: 10.1016/j.ifset.2012.11.007. [DOI] [Google Scholar]
- Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- Wood FW, Goff TC. The determination of the effective shear rate in the brabenderviscograph and in other systems of complex geometry. Starch. 1973;25:89–91. doi: 10.1002/star.19730250305. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)
(DOCX 1937 kb)
(DOCX 159 kb)