Abstract
Although many fruit by-products are good sources of nutrients, little is known about their prebiotic potential. This research was aimed at establishing the prebiotic effect of pineapple wastes on probiotics including Lactobacillus (L.) acidophilus (ATCC® 4356™), L. casei (ATCC® 393™) and L. paracasei spp. paracasei (ATCC® BAA52™) and the subsequent release of antioxidant and antimutagenic peptides in yogurt during their growth. Oven- and freeze- dried peel and pomace were milled separately into powders and tested for prebiotic activities. The net probiotic growth (1.28–2.14 log cfu/g) in customized MRS broth containing the pineapple powders as a direct carbohydrate source was comparable to MRS broth containing glucose. The powders were also separately added to milk during the manufacturing of yogurt with or without probiotics. An increase (by 0.3–1.4 log cycle) in probiotic populations was observed in the yogurts as a consequence of pineapple powder supplementation. Crude water-soluble peptide extracts, prepared by high-speed centrifugation of the yogurts, displayed remarkable antioxidant activities assessed through in vitro assays, namely scavenging activity of 1,1-diphenyl-2-picrylhydrazyl radicals (IC50 = 0.37–0.19 mg/ml) and hydroxyl radicals (58.52–73.55 %). The peptide extracts also exhibited antimutagenic activities (18.60–32.72 %) as sodium azide inhibitor in the Salmonella mutagenicity test. Together, these results suggest that pineapple by-products exhibited prebiotic properties and could possibly be commercially applied in new functional food formulations.
Keywords: Pineapple by-products, Probiotics, Peptide, Antioxidant activity, Antimutagenic activity
Introduction
Yogurt is a fermented dairy product, which has long been considered a prolific source for nutritious and therapeutic constituents. Some of these are attributable to metabolic activity of starter cultures and can be further enhanced by addition of probiotic organisms. According to FAO/WHO (2002), probiotics are “live microorganisms which when administered in adequate amounts confer a health benefit on the host”. Despite the importance of minimum level of these bacteria to achieve probiotic effects, many studies showed poor viability of these bacteria in yogurt (Lourens-Hattingh and Viljoen 2001). Several strategies have been employed to enhance probiotic growth in milk medium. Fortunately, some legislations authorize addition of total solids (thickeners, stabilizers, emulsifiers, or gelling agents) up to 2 % (Canadian Legal Legislation Institute 2014). This opens an opportunity of supplementation to yogurt for the enhancement of probiotic growth and prebiotic supplementation could be an approach. A prebiotic is a “selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host wellbeing and health” (Gibson et al. 2004). Non-digestible oligosaccharides and polysaccharides usually exhibited prebiotic properties, such as fructooligosaccharides, and galactooligosaccharides, inulin, resistant starch, and lactulose derived from various sources including fruits and vegetables (Thammarutwasik et al. 2009). Some of them including fructooligosaccharides, galactooligosaccharides, and inulin are established prebiotics and are available commercially. Combining probiotics and prebiotics into a single product creates a synbiotic (Donkor et al. 2007a; Sah et al. 2016). Industry and scientific community are very keen to explore and introduce new prebiotic ingredients with added functionalities such as fibre-rich fraction from fruits, vegetables, and cereals due to several reasons including commercial importance, sustainability, and health benefits. Several studies have sought to enhance the growth of probiotic organisms by fortifying the fibre-rich fractions from herbs (Chowdhury et al. 2008), cereals (Vasiljevic et al. 2007), and banana, passion, or apple processing by-products (Espírito Santo et al. 2012).
Pineapple waste contains mainly peel and pomace, presenting more than one third of the whole fruit mass (Huang et al. 2011) and is only partially utilized as feed or composting for fertilization but mainly discarded, creating environmental issues such as pollution and climate change and consequently economic loss. However, these by-products have been reported to contain dietary fibres, sugars, proteins, and minerals (Huang et al. 2011), and availability of these nutrients greatly depends on the recovery processes such as drying techniques employed in the preparation of powder. The drying techniques, namely oven drying and freeze drying have significantly different effects on the functional properties like, solubility, flow behaviours, water- and oil-holding capacity, and foaming capacity of the dried powder (Mirhosseini and Amid 2013). Sogi et al. (2013) also suggested that freeze-drying has advantages over conventional drying techniques in solubility, preservation of nutrients etc. These nutrients could serve as a prominent source of growth factors such as prebiotic factors for lactic acid bacteria (LAB) and may enhance protease production and activity leading to liberation of a range of protein hydrolysates, when incorporated in yogurt.
There is a growing interest in protein hydrolysates as they may provide enhanced nutritional value (Pownall et al. 2010) in addition to chemotherapeutic potentialities in the treatment and management of many diseases and disorders such as cancer (Sah et al. 2015a). Mota et al. (2006); Sah et al. (2015b) reported that bioactivity of protein hydrolysates depends on the protein source, enzyme profile and activity, and hydrolysis time.
In this context, the aim of the present research was to establish a prebiotic potential of a fibre-rich fraction from pineapple wastes to enhance the metabolic performance of the selected probiotic organisms– Lactobacillus (L.) acidophilus (ATCC® 4356™), L. casei (ATCC® 393™) and L. paracasei spp. paracasei (ATCC® BAA52™). This would consequently lead to augmented liberation of antioxidant and antimutagenic peptides encrypted into milk protein primary structures during manufacture of yogurt.
Materials and methods
Substrates and chemicals
Trifluoroacetic acid, vancomycin, clindamycin, 1,1-diphenyl-2-picrylhydrazyl, o-phthaldialdehyde (OPA), serine, salicylic acid and sodium azide were purchased from Sigma-Aldrich Corporation (St Louis, Missouri, USA). De Man Rogosa and Sharpe (MRS) and M17 media were supplied by Oxoid Australia (West Heidelberg, Victoria, Australia) while Becton Dickinson Pty Ltd. (BD) (Sydney, NSW, Australia) supplied Davis minimal agar (DMA). Skim milk powder was procured from a local supermarket (Woolworths Limited, Australia). All aqueous solutions were prepared using Milli-Q water (18.2 MΩcm).
Propagation of cultures
Streptococcus thermophilus ASCC 1275 (S. thermophilus) and L. delbrueckii spp. bulgaricus Lb1466 (L. bulgaricus) were collected from the Victoria University Culture Collection (Werribee, Australia). Cell Biosciences Pty Ltd. (Heidelberg, Victoria, Australia) supplied L. acidophilus ATCC 4356 (L. acidophilus), L. casei ATCC 393 (L. casei) and L. paracasei spp. paracasei ATCC BAA52 (L. paracasei). All strains were preserved at −80 °C in MRS broth containing 40 % (v/v) glycerol. The strains were resuscitated and starter cultures were prepared as described by Sah et al. (2014).
Preparation and analysis of pineapple waste powder (PWP)
Whole pineapples (Ananas comosus [L.] Merrill) without crown were purchased from a local supermarket (Woolworths Limited, Australia). Peel and pomace powder were prepared as described by Espírito Santo et al. (2012) with some modifications. Briefly, crushed peel and pomace were dipped in hot water (90 °C) for 30 min to inactivate potential pathogens and enzymes, dried separately using an oven dryer (Memmert GmbH+ Co. KG., Schwabach, Germany) at 60 °C for 24 h and a Dynavac FD 300 freeze dryer (Airvac Engineering Pty. Ltd., Rowville, Australia), and milled to fine powders. Particle size of the powders was standardized to less than 180 μm using sieves (Mesh Series S410/1986; Endecotts Ltd., London, UK) and then sterilized in UV irradiation for 30 min.
Proximate analysis of PWPs was performed by measuring moisture (AOAC official method 934.06), crude protein (AOAC official method 920.152), crude fat (AOAC official method 963.15), total ash (AOAC official method 940.26), and total dietary fibre (AOAC official method 985.29) (Horwitz and Latimer 2006). Total available carbohydrates were calculated by subtracting % moisture, % crude protein, % crude fat, % total ash, and % total dietary fibre from 100. Trace metals were also estimated by using an ICPE-9000 Multitype Inductively Coupled Plasma Atomic Emission Spectrometer (Shimadzu Corporation, Kyoto, Japan) according to AOAC official method 985.01 (Horwitz and Latimer 2006).
Assessment of prebiotic activity of PWPs
The prebiotic activity of the PWPs was assessed through the growth of L. acidophilus, L. casei and L. paracasei according to Moreno-Vilet et al. (2014) with some modifications. The customized culture media were prepared by substituting glucose with the pineapple powders in the same formulations of MRS broth (CM0359, Oxoid). Customized MRS medium without glucose, hereafter referred to as cMRS broth, was prepared by mixing different ingredients in Milli-Q water, i.e. 10 g/l of bacteriological peptone, 8 g/l Lab Lemco powder, 4 g/l yeast extract, 1 g/l of Tween 80, 2 g/l of di-potassium hydrogen phosphate, 5 g/l of sodium acetate trihydrate, 2 g/l of ammonium citrate tribasic, 0.2 g/l of magnesium sulphate heptahydrate, and 0.05 g/l of manganese sulphate tetrahydrate. The pH was adjusted to 6.2 ± 0.2 at 25 °C. The PWPs, whose amounts were quantified on the basis of total soluble carbohydrates content determined by phenol-sulphuric acid method (Dubois et al. 1956), were mixed separately at the rate of 20 g/l to the cMRS and autoclaved at 121 °C for 15 mins. The broths (at room temperature) were aseptically inoculated with 1 % (v/v) of each probiotic cultures (1–2 × 109 cfu/ml) separately and incubated at 37 °C for 24 h with agitation (120 rpm). The bacterial colonies were enumerated on MRS agar after anaerobic incubation at 37 °C for 48 h. The net growth of probiotic organisms was determined by subtracting count as log cfu/g at 0 h to that at 24 h. The experiment was repeated using cMRS supplemented with glucose as the positive control and cMRS only as the negative control.
Lactic and acetic acids of the samples collected at 24 h were also determined as described by Donkor et al. (2007a) employing a Varian HPLC (Varian Analytical Instruments, Walnut Creek, CA, USA) fitted with an Aminex HPX-87 H (300 × 7.8 mm) ion-exchange column (Biorad Life Science Group, Hercules, CA, USA) maintained at 65 °C. Flow rate of the mobile phase (4.5 mM H2SO4) was 0.6 ml/min and peaks were detected at 210 nm.
Preparation of yogurt supplemented with PWPs
Three replicate experiments were performed by making set-type yogurt as described by Sah et al. (2014) with some modifications. Briefly, the quantity of PWP corresponding to 1 % (w/v) of the yogurt mix was dissolved separately in a small quantity of Milli-Q water, pH adjusted to 6.7, blanched to inactivate bromelain and other proteolytic enzymes, and supplemented separately to four lots of milk bases prepared by reconstituting skim milk powder in Milli-Q water at 14 % (w/v). The fortified milk bases were homogenized, and heated to 85 °C for 30 min, cooled to 45 °C and inoculated aseptically with 1 % (v/v) of each S. thermophilus and L. bulgaricus monocultures. They were divided into two equal portions; one portion was further inoculated with each of L. acidophilus, L. casei and L. paracasei monocultures at 1 % (v/v) (Table 1). The final mixes were poured into polystyrene cups, incubated at 42 °C until pH of 4.5 ± 0.05 was achieved.
Table 1.
Experimental design and the coding used in the study to evaluate the effect of pineapple waste powder on probiotic growth, antioxidant and antimutagenic activities of yogurt
| Pineapple waste powder (1 % w/v) | Code | Combination of cultures (1 % v/v each) |
|---|---|---|
| Oven dried peel powder | 1 | S. thermophilus + L. bulgaricus |
| 2 | S. thermophilus + L. bulgaricus + L. acidophilus + L. casei + L. paracasei | |
| Freeze dried peel powder | 3 | S. thermophilus + L. bulgaricus |
| 4 | S. thermophilus + L. bulgaricus + L. acidophilus + L. casei + L. paracasei | |
| Oven dried pomace powder | 5 | S. thermophilus + L. bulgaricus |
| 6 | S. thermophilus + L. bulgaricus + L. acidophilus + L. casei + L. paracasei | |
| Freeze dried pomace powder | 7 | S. thermophilus + L. bulgaricus |
| 8 | S. thermophilus + L. bulgaricus + L. acidophilus + L. casei + L. paracasei |
Yogurt culture = S. thermophilus + L. bulgaricus; Probiotic cultures = L. acidophilus + L. casei + L. paracasei
Enumeration of yogurt and probiotic cultures
S. thermophilus, L. bulgaricus, L. acidophilus, L. casei and L. paracasei in yogurt samples were enumerated by spreading 0.1 ml of the appropriate dilutions of the sample in sterile peptone salt solution (bacteriological peptone 0.1 % w/v; sodium chloride 0.85 % w/v, pH 7.0 ± 0.2) onto selective agar plates and expressed as log cfu/g. The selective agar plates and incubation conditions were M17 medium supplemented with lactose and 45 °C for 24 h aerobically for S. thermophilus, acidified MRS agar (pH 5.2) and 45 °C for 72 h anaerobically for L. bulgaricus, MRS-clindamycin agar (pH 6.2; 0.5 ppm clindamycin) and 37 °C for 72 h anaerobically for L. acidophilus, and MRS-vancomycin agar (pH 6.2; 1 ppm vancomycin) and 37 °C for 72 h anaerobically for both L. casei/L. paracasei together (Sah et al. 2014).
Determination of pH and titratable acidity of yogurt samples
The pH of yogurt samples was measured using pH 720 precision pH meter (WTW inoLab®, Weilheim, Germany) and titratable acidity was estimated according to the AOAC official method 947.05 and expressed as % lactic acid (Horwitz and Latimer 2006).
Determination of proteolysis in yogurt samples
Proteolysis was assessed using the OPA method as described by Sah et al. (2014) and expressed as percentage degree of hydrolysis (DH) with some modifications in the sample preparation. Briefly, 1 ml Milli-Q water was added to a 5 g aliquot of yogurt sample and the final volume adjusted to 10 ml with TCA solution (0.75 M). The mix was filtered using a 0.45 μm Phenex syringe filter (Phenomenex Inc., Lane Cove, Australia) after centrifugation at 2684 × g for 30 mins at 4 °C and analyzed using a Biochrom Libra S12 UV/Vis spectrophotometer (Biochrom Ltd., Cambridge, UK).
Preparation and profiling of water-soluble peptide extract (WSPE)
The WSPEs from yogurts and heat treated reconstituted skim milk (RSM; pH adjusted to 4.5 ± 0.05) were prepared and profiled using a HPLC system consisting of a Varian 9012 solvent delivery system, a Varian 9100 auto-sampler, and a 9065 Polychrom UV/Vis detector (Varian Inc., Palo Alto, California, USA) as described by Sah et al. (2014).
Determination of antioxidant activities
DPPH radical scavenging activity
The radical scavenging activity (RSA) of WSPEs was measured against DPPH• radical as described by Siow and Gan (2013) with some modifications. 1.0 ml of DPPH reagent (0.075 mM in ethanol) was added to 0.1 ml of aqueous WSPE of different protein concentrations and left to stand in dark at room temperature for 30 mins. The mix was clarified by centrifuging at 16,000× g (5415C microcentrifuge, Eppendorf, Hamburg, Germany) for 5 mins at room temperature and subjected for absorbance measurement at 517 nm. Milli-Q water was used for blank and % RSA was calculated using Eq. (1).
| 1 |
IC50 value, which is the concentration that scavenges 50 % of DPPH radicals, was calculated from a linear regression curve of protein content of the WSPE (mg/ml) versus the % RSA.
Hydroxyl radical (•OH) scavenging activity (HRSA)
The HRSA of WSPEs was assayed as described by Zheng et al. (2015) with some modifications. Briefly, 500 μl aqueous FeSO4·7H2O (2 mM) and 100 μl aqueous H2O2 (2 mM) were added to 20 μl of sample (0.1 mg protein/ml), allowed to stand for 10 min at room temperature and 500 μl of aqueous salicylic acid (2.5 mM) was then added. Absorbance of the reaction mixture was measured at 510 nm after incubation at 37 °C for 30 min. Milli-Q water was used for the blank (instead of salicylic acid solution) and the control (instead of sample) in the reaction mixture. The HRSA was calculated using Eq. (2), where As, Ab, and Ac represented absorbance for sample, blank, and control respectively.
| 2 |
Determination of antimutagenic activity by the Ames test
The antimutagenicity of WSPEs was conducted using Salmonella enterica spp. enterica serovar typhimurium (ATCC® 29629™) (genotype: his, rfa, uvrB-bio) through the preincubation protocol of the Ames test as described by Sah et al. (2014). Briefly, 0.1 ml of test sample (50 ± 1 μg peptide), 0.1 ml sodium azide solution (1 μg) were mixed to 0.5 ml of sodium phosphate buffer (0.1 M; pH 7.4). After inoculating 0.1 ml culture, the mix was preincubated for 20 min at 37 °C. The mix was then poured over a Davis minimal agar plate with 2 ml molten top agar. The plate was incubated aerobically for 48 h at 37 °C and revertant colonies were enumerated. The antimutagenic activity as percentage of inhibition was calculated using Eq. (3).
| 3 |
Where, S1 = revertant counts/plate induced by mutagen in the presence of peptide extract; M = revertant counts/plate induced by mutagen alone; S0 = spontaneous revertant counts/plate.
Statistical analysis
Experiments were conducted in triplicate and results were averaged and expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) was conducted to explore the influence of pineapple fibres as the main effect, followed by Tukey Honestly Significant Difference post hoc test to evaluate significant differences between the means at P < 0.05. Two-way ANOVA was carried out to examine the interaction effect of yogurt types and different pineapple fibres on the measured variables. A two-tailed Pearson’s correlation test was carried out to measure the correlations between proteolysis and antioxidant activity or antimutagenic activity. Hierarchical cluster analysis (HCA) and principal component analysis (PCA) were conducted to show correlation among the measured variables and their relationship with different yogurt samples. Statistical analyses were performed using the SPSS 22.0 for windows (SPSS Inc., Chicago, Illinois, USA).
Results and discussion
Chemical composition and prebiotic effect of PWPs
The powders were prepared from pineapple peel and pomace and subjected to proximate and elemental analyses; the results are presented in Table 2. Although significant (P < 0.05) differences in the composition between peel and pomace were noted, oven and freeze-drying methods employed in the preparation of powders had no pronounced (P > 0.05) effect. The high dietary fibre content (44.90–58.48 g/100 g of dry matter), comparable in the pineapple peel and pomace powders, reflected those fibre-rich fractions in wine grape pomace (61.32 g/100 g of dry matter) (Tseng and Zhao 2013) and passion fruit seed fibres (64.8 g/100 g of dry matter) (Chau and Huang 2004). Crude protein, crude fat, and total ash were found in low concentrations: 3.82–6.89 g/100 g of dry matter, 0.34–1.35 g/100 g of dry matter, and 2.52–4.76 g/100 g of dry matter, respectively. Of the minerals tested, potassium was found at the highest level (5967.28–10,144.32 mg/kg of dry matter), followed by calcium (893.03–2206.75 mg/kg of dry matter) and sodium (610.69–1033.57 mg/kg of dry matter). Other trace elements such as copper, zinc, and cobalt were available at very low concentrations. Chromium and iron were only detected in peel powders and manganese was only detected in oven- dried peel. Overall, the pineapple powders, due to their high dietary fibre and essential minerals contents, can be considered an excellent source of growth factors including prebiotic components for probiotic bacteria.
Table 2.
Proximate and elemental analysis of the powders prepared by milling oven- and - freeze dried pineapple peel and pomace
| Parameters | Pineapple waste powder | |||
|---|---|---|---|---|
| Oven-dried peel | Freeze-dried peel | Oven-dried pomace | Freeze-dried pomace | |
| Moisture† | 4.32 ± 0.09c | 5.25 ± 0.14a | 4.77 ± 0.11b | 4.55 ± 0.06bc |
| Crude protein§ | 6.89 ± 0.13a | 6.89 ± 0.04a | 3.91 ± 0.09b | 3.82 ± 0.13b |
| Crude fat§ | 1.19 ± 0.06b | 1.35 ± 0.06a | 0.47 ± 0.02c | 0.34 ± 0.03d |
| Total ash§ | 4.56 ± 0.16a | 4.76 ± 0.05a | 2.69 ± 0.15b | 2.52 ± 0.03b |
| Total dietary fiber§ | 57.76 ± 1.33a | 58.48 ± 0.49a | 46.19 ± 1.41b | 44.90 ± 0.71b |
| Total available carbohydrates§ | 29.60 ± 1.18b | 28.52 ± 0.53b | 46.73 ± 1.42a | 48.43 ± 0.59a |
| Total soluble carbohydrates§ | 26.27 ± 0.87b | 27.12 ± 0.82b | 37.88 ± 1.64a | 38.29 ± 1.13a |
| Calcium* | 1997.97 ± 10.13b | 2206.75 ± 20.41a | 893.03 ± 3.06c | 911.34 ± 7.66c |
| Magnesium* | 883.36 ± 3.92b | 1031.70 ± 7.44a | 713.47 ± 2.75d | 846.16 ± 3.14c |
| Sodium* | 967.97 ± 4.89b | 1033.57 ± 6.34a | 610.69 ± 4.57d | 915.73 ± 18.87c |
| Potassium* | 8574.78 ± 8.31b | 10,144.32 ± 51.62a | 5967.28 ± 11.28d | 6395.15 ± 58.55c |
| Cobalt* | 5.21 ± 0.16a | 3.25 ± 0.12b | 1.21 ± 0.12c | 1.16 ± 0.03c |
| Manganese* | 1.22 ± 0.07a | ND | ND | ND |
| Iron* | 9.90 ± 0.98b | 14.82 ± 0.62a | ND | ND |
| Zinc* | 62.66 ± 0.08b | 85.46 ± 0.69a | 38.04 ± 0.08d | 40.00 ± 0.37c |
| Copper* | 285.47 ± 6.83b | 400.44 ± 2.69a | 17.83 ± 0.02c | 20.47 ± 0.13c |
| Chromium* | 36.96 ± 0.08b | 57.68 ± 0.68a | ND | ND |
†Results were expressed as g/100 g of pineapple peel/pomace powder; §Results were expressed as g/100 g of dry matter; *Results were expressed as mg/kg of dry matter; ND Not Detected, abcdResults were expressed as mean ± standard deviation (n = 3) and values with different superscripts within a row were significantly different (P < 0.05)
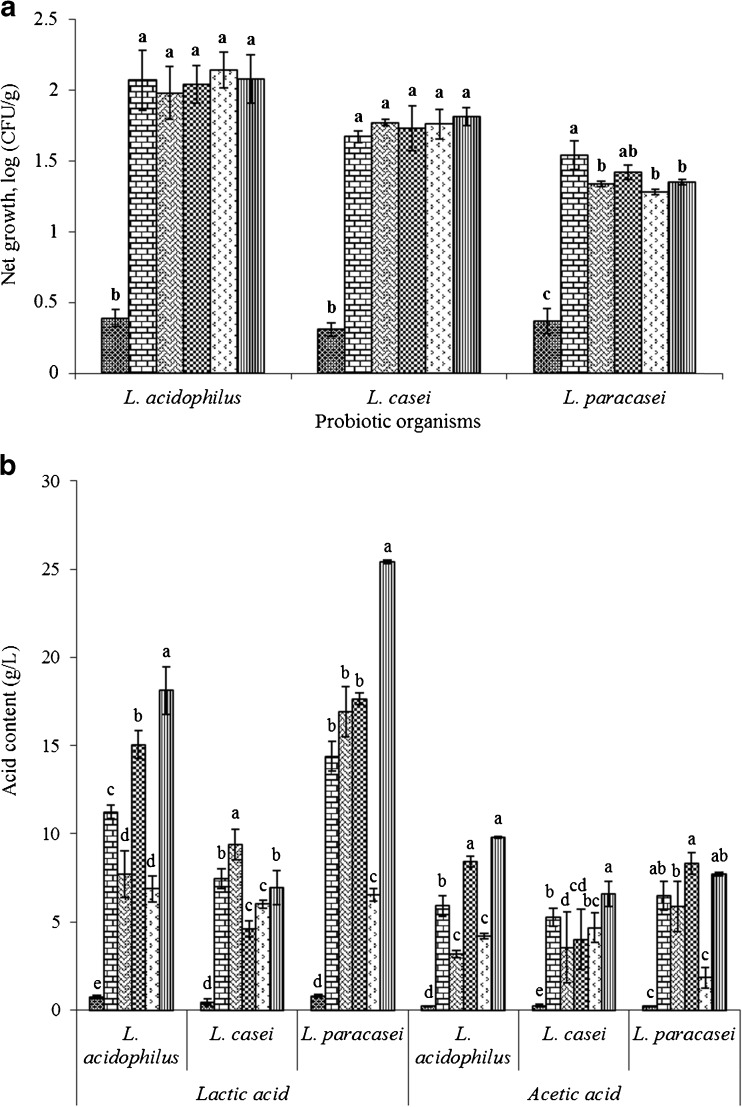
The principal characteristic of prebiotics is their ability to enhance the proliferation of beneficial microbes or probiotics that are part of human colonic microbiota (Gibson et al. 2004). However, this synbiotic association is strain specific. The net probiotic growth (in 24 h) of L. acidophilus, L. casei and L. paracasei in customized MRS broth containing freeze dried pineapple peel and pomace powders as a direct carbohydrate source were not substantially different (P ≥ 0.05) from those of customized MRS broth supplemented with glucose (Fig. 1a). In all the pineapple powders tested, bacterial growth was more than one log higher compared to the negative control (customized MRS broth without carbohydrate). Both pineapple peel and pomace powders have been reported to be rich in dietary fibre, mainly cellulose, hemicellulose, lignin, fructans, pectin, and pectic substances (Chitturi et al. 2013; Huang et al. 2011). Furthermore, several studies have reported prebiotic properties of pectin-derived oligosaccharides (Gullón et al. 2013). Thus, the enhanced growth of tested probiotic organisms in our study was likely due to prebiotic components and other growth factors present in the fibre-rich fractions of pineapple powders.
Fig. 1.
a and b display net growth of probiotic organisms (L. acidophilus, L. casei, L. paracasei), and concentration of organic acids (lactic and acetic) respectively in customized MRS broth without glucose (cMRS) separately containing freeze dried pineapple pomace powder ( ), oven dried pineapple pomace powder (
), oven dried pineapple pomace powder ( ), freeze dried pineapple peel powder (
), freeze dried pineapple peel powder ( ), oven dried pineapple peel powder (
), oven dried pineapple peel powder ( ) as a carbohydrate source compared to glucose as positive control (
) as a carbohydrate source compared to glucose as positive control ( ) and without carbohydrate as negative control (
) and without carbohydrate as negative control ( ) at 37 °C for 24 h. The monocultures were aseptically inoculated at 1 % (v/v) separately. a,b,c,d,eResults were expressed as means ± standard deviation (n = 3), values with different letters within each organisms a and each acids b were significantly different (P < 0.05)
) at 37 °C for 24 h. The monocultures were aseptically inoculated at 1 % (v/v) separately. a,b,c,d,eResults were expressed as means ± standard deviation (n = 3), values with different letters within each organisms a and each acids b were significantly different (P < 0.05)
LAB metabolize carbohydrate substrates for growth and energy and the predominant fermentation products are short chain fatty acids, mainly lactic and acetic acids (Gibson 1999). Thus, the production of lactic and acetic acids after 24 h of fermentation was also investigated to assess the growth of probiotic species in terms of release of metabolites (Fig. 1b). Espírito Santo et al. (2012) also reported similar results, where the total dietary fibre from by-products of banana, apple or passion processing increased the probiotic viability in yogurt. The peel and pomace powders in our study supported the growth of the probiotic organisms, in a manner similar to that of complex medium, as evidenced by the equivalent production of organic acids.
These observations showed the in vitro synbiotic potential of pineapple powders upon the probiotic organisms tested, which were similar to the previous reports with dietary fibres from various fruit processing by-products on probiotics (Espírito Santo et al. 2012). These synbiotic combinations were therefore applied in yogurt manufacturing for enhancing liberation of peptides through improved growth.
Synbiotic study in yogurt
The viable count as log cfu/g of S. thermophilus, L. bulgaricus, L. acidophilus, and L. casei, L. paracasei in yogurt samples supplemented with PWPs were performed selectively and reported in Table 3. The viable counts of S. thermophilus and L. bulgaricus in the yogurts supplemented with pineapple powders (Table 3) showed similar value in comparison to those in yogurts without supplementation (Sah et al. 2014). On the other hand, probiotic yogurts with PWPs (yogurts 2, 4, 6, 8) experienced enhanced growth of the probiotic species- high counts of L. acidophilus (8.13–8.71 log cfu/g; Table 3) and L. casei, L. paracasei (8.12–8.72 log cfu/g; Table 3) than non-supplemented probiotic yogurts (7.34 log cfu/g and 7.83 log cfu/g, respectively) (Sah et al. 2014). Moreover, the probiotic counts were very high compared to the minimum therapeutic count of 6 log cfu/g, if 100 g yogurt is considered as a daily serving dose (Lourens-Hattingh and Viljoen 2001).
Table 3.
Viable counts, pH, titratable acidity of control and probiotic yogurts supplemented with pineapple peel and pomace powder at ultimate pH (4.5 ± 0.05) prepared by fermenting aseptically inoculated yogurt mix with 1 % (v/v) of S. thermophilus, L. bulgaricus cultures with or without L. acidophilus, L. casei and L. paracasei cultures at 42 °C
| Yogurt types | Count (log cfu/g) | pH | Titratable acidity (% lactic acid) | ||||
|---|---|---|---|---|---|---|---|
| Cultures | Pineapple powders | S. thermophilus | L. bulgaricus | L. acidophilus | L. casei & paracasei | ||
| SC | Oven-dried peel powder | 9.32 ± 0.14a | 8.00 ± 0.15c | – | – | 4.51 ± 0.02a | 1.03 ± 0.02ab |
| SC + PC | 9.21 ± 0.08a | 8.17 ± 0.07abc | 8.25 ± 0.02c | 8.11 ± 0.03d | 4.49 ± 0.02a | 1.04 ± 0.01ab | |
| SC | Freeze-dried peel powder | 9.25 ± 0.15a | 8.17 ± 0.14abc | – | – | 4.50 ± 0.02a | 1.02 ± 0.04b |
| SC + PC | 9.13 ± 0.06a | 8.23 ± 0.13ab | 8.71 ± 0.08a | 8.73 ± 0.08a | 4.49 ± 0.01a | 1.01 ± 0.01b | |
| SC | Oven-dried pomace powder | 9.24 ± 0.03a | 8.22 ± 0.16ab | – | – | 4.49 ± 0.02a | 1.05 ± 0.04ab |
| SC + PC | 9.23 ± 0.16a | 8.08 ± 0.09bc | 8.13 ± 0.03d | 8.37 ± 0.05c | 4.48 ± 0.02a | 1.04 ± 0.03ab | |
| SC | Freeze-dried pomace powder | 9.14 ± 0.10a | 8.35 ± 0.06a | – | – | 4.48 ± 0.02a | 1.05 ± 0.03ab |
| SC + PC | 9.20 ± 0.14a | 8.28 ± 0.11ab | 8.50 ± 0.10b | 8.50 ± 0.10b | 4.49 ± 0.02a | 1.07 ± 0.01a | |
SC Starter culture (S. thermophilus + L. bulgaricus); PC: Probiotic culture (L. acidophilus + L. casei + L. paracasei)
abcdResults were expressed as means ± standard deviation (n = 6), values with different superscripts within a column were significantly different (P < 0.05)
The pH and titratable acidity of all yogurt samples were within 4.51–4.48 and 1.01 % – 1.07 %, respectively (Table 3), within 5 h 45 min. These results were in agreement with the various works on addition of fibres from fruit by-products to yogurt (Espírito Santo et al. 2012; García-Pérez et al. 2005). The pineapple powders contained dietary fibres, proteins, and minerals as well as fats (Table 2), which could have served as growth factors for probiotics and therefore improved growth. Similarly, improved growth would have resulted in high metabolic activities and hence proteolysis (Donkor et al. 2007a).
Enhancement of milk proteolysis by supplementing PWPs
The proteolytic enzymes of LAB used in our study cleaved peptide bonds of milk proteins and the extent of proteolysis determined was expressed as percentage degree of hydrolysis as presented in Table 4. There were significant effects of probiotic addition and type of pineapple fibre supplementation on degree of proteolysis in yogurt (Table 5). The probiotic yogurts (numbers 2, 4, 6, 8) with pineapple powder supplementation producing a significantly high degree of hydrolysis (13.07–14.30 %; Table 4) compared with control yogurts (numbers 1, 3, 5, 7) with pineapple powder supplementation (6.47–7.13 %; Table 4) or without the supplementation (5.38 %) (Sah et al. 2014). For probiotic yogurts, supplementation of freeze-dried peel and pomace led to higher degree of proteolysis than that of oven-dried peel and pomace (Tables 4 and 5). Freeze-drying has advantages such as nutrients preservation, solubility etc. over conventional drying techniques (Sogi et al. 2013). The principal milk proteins, caseins, are broken down first by extracellular proteinases to oligopeptides and then by intracellular peptidases into peptides (Donkor et al. 2007b). Additionally, the activity of proteolytic enzymes could have been markedly enhanced because of the increased level of divalent ions such as Ca2+, Fe2+, Co2+, Mn2+, and Mg2+ in the yogurts due to the supplementation with pineapple powders (Table 2). Divalent metal ions have been reported to enhance production and activity of proteases in microorganisms (Llorente-Bousquets et al. 2008).
Table 4.
Degree of hydrolysis (DH) of milk proteins, antioxidant activity (evaluated by measuring scavenging activities of DPPH• and •OH radicals), and antimutagenic activity of water-soluble peptide extracts from yogurts supplemented with pineapple peel and pomace powder with or without probiotic addition at ultimate pH (4.5 ± 0.05)
| Yogurt types | DH, % | Antioxidant activity | Antimutagenic activity (% Inhibition) | ||
|---|---|---|---|---|---|
| Cultures | Pineapple powders | DPPH (IC50, mg/ml) | HRSA (%) | ||
| SC | Oven-dried peel powder | 6.47 ± 0.30e | 0.37 ± 0.01a | 58.99 ± 0.72e | 22.04 ± 1.65c |
| SC + PC | 13.07 ± 0.23c | 0.23 ± 0.02d | 70.21 ± 0.54b | 27.39 ± 0.89b | |
| SC | Freeze-dried peel powder | 6.99 ± 0.45de | 0.23 ± 0.01d | 60.59 ± 0.60d | 25.42 ± 1.24b |
| SC + PC | 13.79 ± 0.21ab | 0.19 ± 0.01e | 73.55 ± 0.68a | 32.72 ± 1.98a | |
| SC | Oven-dried pomace powder | 7.02 ± 0.09de | 0.30 ± 0.02b | 58.52 ± 0.32e | 18.60 ± 1.44d |
| SC + PC | 13.43 ± 0.42bc | 0.27 ± 0.01c | 67.63 ± 0.54c | 26.27 ± 1.73b | |
| SC | Freeze-dried pomace powder | 7.13 ± 0.09d | 0.26 ± 0.01c | 58.79 ± 0.37e | 20.15 ± 0.98cd |
| SC + PC | 14.30 ± 0.51a | 0.20 ± 0.00e | 68.06 ± 0.48c | 27.85 ± 1.60b | |
SC: Starter culture (S. thermophilus + L. bulgaricus); PC: Probiotic culture (L. acidophilus + L. casei + L. paracasei)
abcdeResults were expressed as means ± standard deviation (n = 6), values with different superscripts within a column were significantly different (P < 0.05)
Table 5.
Analysis of variance (ANOVA) depicting the significance (at P < 0.05) of types of yogurt (control and probiotic yogurts), pineapple powder (oven dried and freeze dried - peel and - pomace powder) and their effect on degree of protein hydrolysis, antioxidant (evaluated by measuring scavenging activities of DPPH• and •OH radicals) and antimutagenic activities
| Source of variation | P- value | |||
|---|---|---|---|---|
| Degree of proteolysis | Antioxidant activity | Antimutagenic activity | ||
| DPPH | HRSA | |||
| Yogurt type | < 0.000 | < 0.000 | < 0.000 | < 0.000 |
| Pineapple powder type | < 0.000 | < 0.000 | < 0.000 | < 0.000 |
| Yogurt type × Pineapple powder type | 0.038 | < 0.000 | < 0.000 | 0.180 |
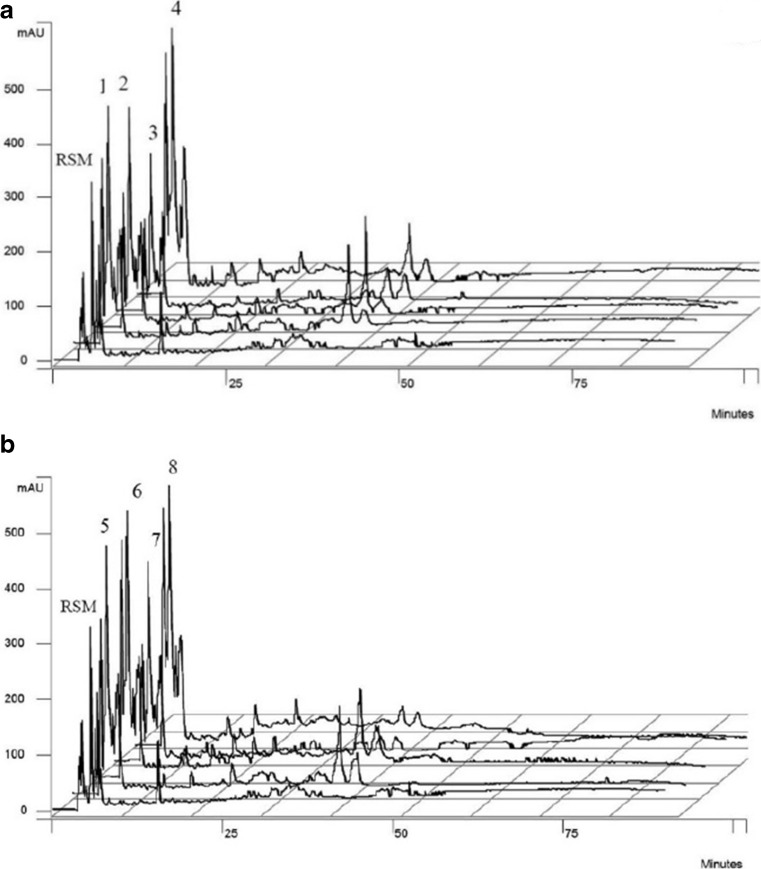
The proteolytic activities of the enzymes produced by LAB were evidenced through chromatographic profiling of water-soluble extracts from yogurt samples using a reversed-phase HPLC (Figs 2a and 2b). Control yogurts (numbers 1, 3, 5, 7) with PWPs addition and probiotic yogurts (numbers 2, 4, 6, 8) with PWPs addition displayed more peaks (peptides) than that of the reconstituted skim milk (RSM) sample only. The WSPE containing these peptides were further tested for their antioxidant and antimutagenic activities.
Fig. 2.
RP-HPLC profile of the WSPEs of yogurts: a) 1, 2, 3, 4; and b) 5, 6, 7, 8, compared with reconstituted skim milk (RSM). Yogurt samples denoted 1, 3, 5 and 7 were fermented with the starter culture only and supplemented respectively with oven-dried peel, freeze-dried peel, oven-dried pomace, and freeze-dried pomace. Yogurt samples denoted 2, 4, 6 and 8 were fermented with the starter culture and probiotics supplemented with oven-dried peel, freeze-dried peel, oven-dried pomace, and freeze-dried pomace, respectively. The peptides were eluted at a flow rate of 0.75 ml/min and detected by UV absorption at 215 nm. The flow of solvent A (0.1 % TFA in Milli-Q water) was at a linear gradient from 100 % to 0 % in solvent B (0.1 % TFA in acetonitrile) over 90 mins
Enhancement of antioxidant activities by supplementation with PWPs
In this study, the antioxidant activity was assessed by measuring scavenging capacity of WSPE for DPPH• and •OH radicals. The DPPH• scavenging capacity of WSPE was expressed as IC50 value and presented in Table 4. Probiotic yogurts (numbers 2, 4, 6, 8) with PWPs supplementation displayed strong scavenging capacity (IC50 values of 0.27–0.19 mg/ml; Table 4) compared to the control yogurts (numbers 1, 3, 5, 7) with PWPs supplementation (IC50 values of 0.37–0.23 mg/ml; Table 4) or without the supplementation (IC50 value of 2.23 mg/ml) (Sah et al. 2014). The significant interaction (Table 5) indicates that the effect of different pineapple powders on the antioxidant activity was different in probiotic yogurts compared to control yogurts. Specifically, the probiotic yogurts supplemented with freeze-dried peel or pomace powders exhibited the most potent radical scavenging activities as compared to the other yogurts. Several studies (Kudoh et al. 2001; Sah et al. 2014) also reported the generation of potent antioxidant peptides from milk proteins. It can be inferred that the generated peptides had acted as hydrogen or electron donors and could have reacted with free DPPH radicals resulting into stable products (Prior et al. 2005).
In current study, •OH scavenging capacity of WSPEs was determined and presented in Table 4. Probiotic yogurts (numbers 2, 4, 6, 8) with PWPs supplementation displayed strong scavenging capacity (67.63–73.55 %) compared to the control yogurts (numbers 1, 3, 5, 7). The significant interaction (Table 5) indicates that the effect of different pineapple powders on the •OH scavenging activity was different in probiotic yogurts compared to control yogurts. Specifically, the probiotic yogurts supplemented with freeze-dried peel powder exhibited the most potent hydroxyl radical scavenging activities as compared to the other yogurts. These results suggest that WSPEs could have the potential to safeguard human against •OH-induced damage or prevent food spoilage.
Additionally, degree of protein hydrolyses were correlated with the IC50 values of DPPH• scavenging activities (P < 0.05, r = − 0.676) and the percentage of •OH scavenging activities (P < 0.05, r = 0.942). These results were in agreement with Igoshi et al. (2008), who reported significant correlation between generated peptides in cheese ripening and antioxidative activity. Thus, the more proteins degraded during yogurt manufacturing, because of enhanced proteolysis, the more potent peptides with antioxidant activity are generated.
Enhancement of antimutagenic activities by supplementing PWPs
The antimutagenic activity of the WSPEs was determined by evaluating inhibitory activity of peptides against mutagenicity effect of sodium azide on S. typhimurium and reported in Table 4. The effect of PWPs addition on antimutagenic activity of liberated peptides was not significantly different for both control and probiotic yogurts (Table 5). However, the probiotic yogurts (numbers 2, 4, 6, 8) with PWPs supplementation showed high mutagen inhibitory activity (26.27–32.72 %; Table 4) compared to the control yogurts (numbers 1, 3, 5, 7) with PWPs supplementation (18.60–25.42 %; Table 4) or without the supplementation (15.87 %) (Sah et al. 2014). Moreover, the probiotic yogurt supplemented with freeze-dried peel (yogurt 4) exhibited a significantly higher antimutagenic activity compared to other yogurts. These results were in line with others (Espeche Turbay et al. 2012) and indicated that the probiotic yogurts containing pineapple peel may be a contributor to the dietary prevention of cancer.
Antimutagenic activities and degree of protein hydrolysis were also highly correlated (P < 0.05, r = 0.817). This result supported the outcome of Matar et al. (1997) who concluded a linkage between proteolytic activity of Lactobacillus helveticus L89 and antimutagenicity of the fermented milk. Enhanced proteolysis increases peptide production, which in turn enhances antimutagenic activity of the probiotic yogurt.
Effect of drying techniques employed during preparation of PWPs on overall properties of yogurts
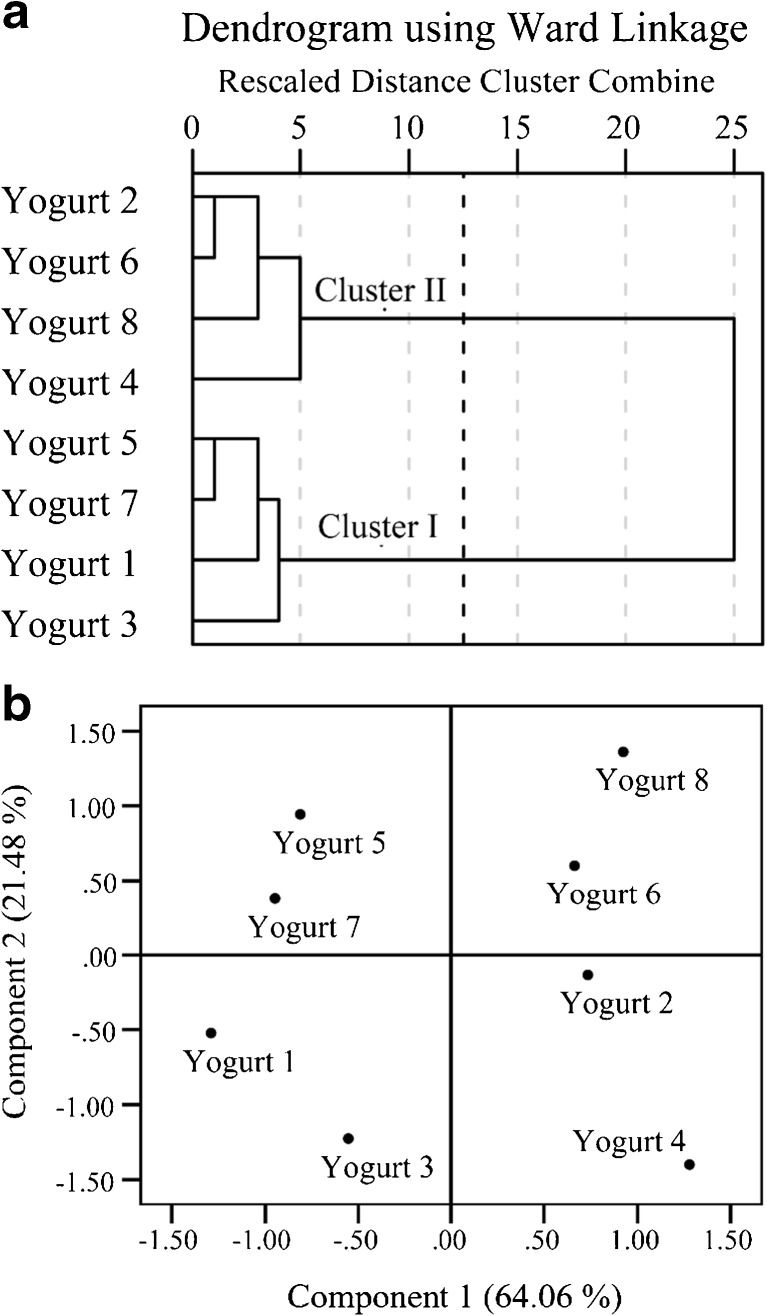
Cluster analysis (CA) was carried out using the hierarchical clustering method with Ward’s linkage and identified two clusters based on similarities in the pH, titratable acidity, degree of protein hydrolysis, antioxidant, and antimutagenic activities (Fig. 3a). Control yogurts with PWPs supplementation (numbers 1, 3, 5, 7) arranged in the first cluster and probiotic yogurts with PWPs supplementation (numbers 2, 4, 6, 8) were arranged in the second cluster. The yogurts were clustered according to yogurt types rather than peel and pomace powder supplementation.
Fig. 3.
a and b. Dendrogram a displays the clustering of yogurts according to similarities amongst measured variables. b shows score plot of principal components of measured variables in yogurts. The measured variables were pH, titratable acidity, degree of protein hydrolysis, antioxidant (evaluated by measuring scavenging activities of DPPH• and •OH radicals) and antimutagenic activities
Principal component analysis was also carried out and two interpretable components were chosen based on the Kaiser’s criterion of eigenvalues greater than 1.0. Score plot (Fig. 3b) displayed the relationships among all yogurts in two main components. Samples were grouped in four distinct groups: the yogurts enriched with pomace powder at the bottom left and right quadrants, while yogurts enriched with peel powder at top left and right. Distributions of yogurts enriched with freeze- dried and oven- dried powders in each quadrants conferred that types of drying techniques had no pronounced effects on probiotic bioactivities.
Conclusions
The pineapple peel and pomace powders were rich in nutrients such as dietary fibre, protein, and divalent cations and exhibited prebiotic effects on Lactobacillus (L.) acidophilus (ATCC® 4356™), L. casei (ATCC® 393™) and L. paracasei spp. paracasei (ATCC® BAA52™). Simultaneously, the proteolytic activities of starter and probiotic cultures were increased substantially in the presence of PWPs. PWPs addition into yogurts also resulted into increased antioxidant and antimutagenic activities compared to the non-supplemented yogurts. Moreover, the WSPE of probiotic yogurt with freeze-dried pineapple peel powder displayed greater antioxidant and antimutagenic activities. Interestingly, both oven and freeze-drying techniques employed in the preparation of powders displayed similar outcomes. Overall, PWPs inclusion in yogurt comprises an interesting approach for enhancement of probiotic growth and improvement of health benefits, and an ecological alternative to reduce fruit-processing waste. However, further works need to be performed to isolate and characterize these bioactive peptides from the WSPEs.
Acknowledgments
The authors are thankful to the Australian Government for offering an Australia Awards Scholarships and Australia Awards Leadership Program place to B. N. P. Sah.
Abbreviations
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- WSPE
Water soluble peptide extract
- CA
Cluster analysis
- PCA
Principal component analysis
Footnotes
Highlights• Pineapple by-products are a potential source of bacterial growth factors.• Pineapple fiber increased probiotic growth and production of organic acids.• Pineapple fiber addition resulted in enhanced antioxidative activity of peptide extract.• Pineapple fiber addition resulted in enhanced antimutagenic property of peptide extract.
References
- Canadian Legal Legislation Institute (2014). Regulation respecting food, CQLR c P-29, r 1. from http://www.canlii.org/en/qc/laws/regu/cqlr-c-p-29-r-1/latest/cqlr-c-p-29-r-1.html
- Chau CF, Huang YL. Characterization of passion fruit seed fibres—a potential fibre source. Food Chem. 2004;85(2):189–194. doi: 10.1016/j.foodchem.2003.05.009. [DOI] [Google Scholar]
- Chitturi S, Talatam VG, Vuppu S. Studies on protein content, protease activity, antioxidants potential, melanin composition, glucosinolate and pectin constitution with brief statistical analysis in some medicinally significant fruit peels. Der Pharm Lett. 2013;5(1):13–23. [Google Scholar]
- Chowdhury BR, Chakraborty R, Raychaudhuri U. Study on β-galactosidase enzymatic activity of herbal yogurt. Int J Food Sci Nutr. 2008;59(2):116–122. doi: 10.1080/09637480701447787. [DOI] [PubMed] [Google Scholar]
- Donkor ON, Nilmini SLI, Stolic P, Vasiljevic T, Shah NP. Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. Int Dairy J. 2007;17(6):657–665. doi: 10.1016/j.idairyj.2006.08.006. [DOI] [Google Scholar]
- Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin-converting enzyme inhibitory activity in fermented milk. Lait. 2007;87(1):21–38. doi: 10.1051/lait:2006023. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Espeche Turbay MB, de Moreno de LeBlanc A, Perdigón G, Savoy de Giori G, Hebert EM. β-casein hydrolysate generated by the cell envelope-associated proteinase of Lactobacillus delbrueckii ssp. lactis CRL 581 protects against trinitrobenzene sulfonic acid-induced colitis in mice. J Dairy Sci. 2012;95(3):1108–1118. doi: 10.3168/jds.2011-4735. [DOI] [PubMed] [Google Scholar]
- Espírito Santo AP, Cartolano NS, Silva TF, Soares FASM, Gioielli LA, Perego P, Converti A, Oliveira MN. Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase CLA content in yoghurts. Int J Food Microbiol. 2012;154(3):135–144. doi: 10.1016/j.ijfoodmicro.2011.12.025. [DOI] [PubMed] [Google Scholar]
- FAO/WHO . Guidelines for the evaluation of probiotics in food. London, Ontario, Canada: Report of a Joint FAO/WHO; 2002. [Google Scholar]
- García-Pérez FJ, Lario Y, Fernández-López J, Sayas E, Pérez-Alvarez JA, Sendra E. Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Res Appl. 2005;30(6):457–463. doi: 10.1002/col.20158. [DOI] [Google Scholar]
- Gibson GR. Dietary modulation of the human gut microflora using the prebiotics oligofructose and inulin. J Nutr. 1999;129(7 SUPPL.):1438S–1441S. doi: 10.1093/jn/129.7.1438S. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Gullón B, Gómez B, Martínez-Sabajanes M, Yáñez R, Parajó JC, Alonso JL. Pectic oligosaccharides: manufacture and functional properties. Trends Food Sci Technol. 2013;30(2):153–161. doi: 10.1016/j.tifs.2013.01.006. [DOI] [Google Scholar]
- Horwitz W, Latimer Jr GW (2006) Official methods of analysis of AOAC international. AOAC International, AOAC International, Gaithersburg, Md
- Huang YL, Chow CJ, Fang YJ. Preparation and physicochemical properties of fiber-rich fraction from pineapple peels as a potential ingredient. J Food Drug Anal. 2011;19(3):318–323. [Google Scholar]
- Igoshi K, Kondo Y, Kobayashi H, Kabata K, Kawakami H. Antioxidative activity of cheese. Milchwissenschaft. 2008;63(4):424–427. [Google Scholar]
- Kudoh Y, Matsuda S, Igoshi K, Oki T. Antioxidative peptide from milk fermented with Lactobacillus delbrueckii subsp. bulgaricus IFO13953. J Jpn Soc Food Sci. 2001;48(1):44–50. doi: 10.3136/nskkk.48.44. [DOI] [Google Scholar]
- Llorente-Bousquets A, Pérez-Munguía S, Farrés A. Novel extracellular proteolytic activity in Pediococcus acidilactici ATCC 8042. Can J Microbiol. 2008;54(8):694–699. doi: 10.1139/W08-055. [DOI] [PubMed] [Google Scholar]
- Lourens-Hattingh A, Viljoen BC. Yogurt as probiotic carrier food. Int Dairy J. 2001;11(1–2):1–17. doi: 10.1016/S0958-6946(01)00036-X. [DOI] [Google Scholar]
- Matar C, Nadathur SS, Bakalinsky AT, Goulet J. Antimutagenic effects of milk fermented by Lactobacillus helveticus L89 and a protease-deficient derivative. J Dairy Sci. 1997;80(9):1965–1970. doi: 10.3168/jds.S0022-0302(97)76139-3. [DOI] [PubMed] [Google Scholar]
- Mirhosseini H, Amid BT (2013) Effect of different drying techniques on flowability characteristics and chemical properties of natural carbohydrate-protein gum from durian fruit seed. Chem Cent J 7(1) [DOI] [PMC free article] [PubMed]
- Moreno-Vilet L, Garcia-Hernandez MH, Delgado-Portales RE, Corral-Fernandez NE, Cortez-Espinosa N, Ruiz-Cabrera MA, Portales-Perez DP. In vitro assessment of agave fructans (Agave salmiana) as prebiotics and immune system activators. Int J Biol Macromol. 2014;63:181–187. doi: 10.1016/j.ijbiomac.2013.10.039. [DOI] [PubMed] [Google Scholar]
- Mota MVT, Ferreira IMPLVO, Oliveira MBP, Rocha C, Teixeira JA, Torres D, Gonçalves MP. Trypsin hydrolysis of whey protein concentrates: characterization using multivariate data analysis. Food Chem. 2006;94(2):278–286. doi: 10.1016/j.foodchem.2005.01.016. [DOI] [Google Scholar]
- Pownall TL, Udenigwe CC, Aluko RE. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J Agric Food Chem. 2010;58(8):4712–4718. doi: 10.1021/jf904456r. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Effect of probiotics on antioxidant and antimutagenic activities of crude peptide extract from yogurt. Food Chem. 2014;156:264–270. doi: 10.1016/j.foodchem.2014.01.105. [DOI] [PubMed] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Identification of anticancer peptides from bovine milk proteins and their potential roles in management of cancer: a critical review. Compr Rev Food Sci Food Saf. 2015;14(2):123–138. doi: 10.1111/1541-4337.12126. [DOI] [PubMed] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Effect of refrigerated storage on probiotic viability and the production and stability of antimutagenic and antioxidant peptides in yogurt supplemented with pineapple peel. J Dairy Sci. 2015;98(9):5905–5916. doi: 10.3168/jds.2015-9450. [DOI] [PubMed] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT Food Sci Technol. 2016;65:978–986. doi: 10.1016/j.lwt.2015.09.027. [DOI] [Google Scholar]
- Siow HL, Gan CY. Extraction of antioxidative and antihypertensive bioactive peptides from Parkia speciosa seeds. Food Chem. 2013;141(4):3435–3442. doi: 10.1016/j.foodchem.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Sogi DS, Siddiq M, Greiby I, Dolan KD. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013;141(3):2649–2655. doi: 10.1016/j.foodchem.2013.05.053. [DOI] [PubMed] [Google Scholar]
- Thammarutwasik P, Hongpattarakere T, Chantachum S, Kijroongrojana K, Itharat A, Reanmongkol W, Tewtrakul S, Ooraikul B. Prebiotics – a review. Songklanakarin J Sci Technol. 2009;31(4):401–408. [Google Scholar]
- Tseng A, Zhao Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013;138(1):356–365. doi: 10.1016/j.foodchem.2012.09.148. [DOI] [PubMed] [Google Scholar]
- Vasiljevic T, Kealy T, Mishra VK. Effects of β-glucan addition to a probiotic containing yogurt. J Food Sci. 2007;72(7):C405–C411. doi: 10.1111/j.1750-3841.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- Zheng XQ, Wang JT, Liu XL, Sun Y, Zheng YJ, Wang XJ, Liu Y. Effect of hydrolysis time on the physicochemical and functional properties of corn glutelin by Protamex hydrolysis. Food Chem. 2015;172:407–415. doi: 10.1016/j.foodchem.2014.09.080. [DOI] [PubMed] [Google Scholar]