Abstract
In this study, the effects of different fat levels and different particle sizes on compositional and structural characteristics of probiotic fermented sausage were investigated. In order to obtain probiotic character, Lactobacillus casei CRL431 was added. The physicochemical, microbiological and sensory analysis were done. The effect of fat level x mincer hole diameter interaction on hardness values were statistically significant (p < 0.005). At the end of the fermentation-ripening period, L.casei CRL-431 count has reached to sufficient microbial count (106 cfu/g of probiotic bacteria) to demonstrate the character of probiotic food. A significant positive correlation was found between L.casei CRL431 count and surface appearance, texture and overall acceptability scores (r = 0.60, 0.52, 0.53). The values of TBARS number of probiotic sucuk samples increased during fermentation-ripening. A significant correlation between taste-aroma scores and fat level was detected (r = −0.61,p = 0.0008). Consiquently, the best sensorial quality was determined in L3 samples and the worst sensorial quality was determined in H8 samples.
Keywords: Turkish fermented sausage, Sucuk, Probiotic, L. casei CRL 431, Fat level, Mincer hole diameter
Introduction
Probiotic bacteria, mainly lactic acid bacteria (LAB) and Bifidobacteria, are used as additives in food to provide a wide variety of health benefits, including improving the function of the immune system and prevention of cancer, atherosclerosis and coronary diseases (Wojciak et al. 2012). In general, the food industry has adopted the recommended level of 106 cfu/g of probiotic bacteria at the time of consumption (Sidira et al. 2014).
Probiotic bacteria are mainly administered through the consumption of fermented milk, yoghurts. However, probiotic LAB strains may also be used in fermented meat products. In addition, there is a reason to believe that the matrix of unheated dry-fermented sausages protects lactobacilli during their passage through the digestive tract (Wojciak et al. 2012).
LAB have been used as starters for meat fermentation due to their major role for lactic acid generation. They improve the sensory quality and the bioprotective nature of the sausage (Toldra and Reig, 2011). These bacteria present in fermented sausages constitute an integral part of the healthy gastrointestinal microflora involved in human metabolism and activaties metabolic pathways to detoxify the foreign substances (Ahmad and Srivastava 2007).
Meat has been shown to be an excellent vehicle for probiotics. Meat products with probiotics have a great future potential because consumers are increasingly paying attention to both the functional, health influence and sensory quality of meat products (Jaworska et al. 2011).
Sucuk, traditional Turkish dry-fermented sausage, is the most popular and widely consumed meat product in Turkey (Yalınkılıç et al. 2012; Akkaya et al. 2014). Generally, the fat content in the sausage formulation has a strong impact on the quality of the final product (Soyer 2005). Fat contributes to the flavor, texture, mouthfeel, juiciness and lubricity, which determine the quality and acceptability of dry sausages (Olivares et al. 2010). Particle size is another important parameter affecting the quality of fermented sausage.
The main objectives of this study were to determine the effect of different fat levels and differed size particles on the compositional and structural quality characteristics of probiotic Turkish sausage (sucuk) during fermentation-ripening.
Materials and methods
Probiotic sausage manufacturing
Probiotic Turkish sausage samples were manufactured in Research and Development Department of Pınar Meat Co., İzmir. Beef meat, fat, all ingredients were supplied by the company. Probiotic culture was purchased from Chr. Hansen Co. (Denmark).
Three different fat levels (20 %, 25 % and 30 %) were used in sausage production. Each batter were minced in mincing machine with three different mincer plate hole diameters (3 mm, 5 mm, 8 mm). Totally, nine probiotic sausage batters were prepared.
In order to give probiotic character to sausages, 0.1 g/kg L. casei CRL 431 was added in sausage formulation. The treatments were grouped as L-Low fat level (20 %), M- Medium fat level (25 %) and H- High fat level (30 %). Depending on particle size each of these groups were made as 3, 5 and 8 mm. They were coded as; L3 = 20 % fat level + 3 mm, L5 = 20 % fat level + 5 mm, L8 = 20 % fat level + 8 mm, M3 = 25 % fat level + 3 mm, M5 = 25 % fat level + 5 mm, M8 = 25 % fat level + 8 mm, H3 = 30 % fat level + 3 mm, H5 = 30 % fat level + 5 mm, H8 = 30 % fat level + 8 mm. For every treatment three replicates were maintained.
Following ingredients were added to sucuk batters; 0,15 g/kg NaNO2, 18 g/kg NaCl, 10 g/kg garlic, 4 g/kg red pepper (sweet), 7 g/kg red pepper (hot), 7 g/kg black pepper, 7,5 g/kg cumin, 2 g/kg pimento, 0,5 g/kg sodium ascorbate, 0,3 g/kg starter culture (BITEC LS-25, Germany) (Lactobacillus plantarum + Staphylococcus carnosus) and 0.1 g/kg L. casei CRL 431. 150 kg beef was used in each replication.
Prepared batters were stuffed into collagen casings (38 mm). Sausages were hung in stainless steel hangers and then placed in ripening room equipped with a process control system under the following conditions: 1 day at 24 °C and 93 ± 1%RH, 1 day at 22 °C and 93 ± 1%RH, 1 day at 20 °C and 88 ± 1%RH, 1 day at 18 °C and 85 ± 1%RH, 1 day at 18 °C and 80 ± 1%RH, 2 days at 17 °C and 78 ± 1%RH, 2 days at 17 °C and 77 ± 1%RH.
Chemical analysis
Moisture, ash, fat and protein contents of the sausage samples were determined according to AOAC (2000). The pH was determined by blending 10 g sample with 100 mL distilled water for 2 min. The pH of the homogenate was measured at 25 °C using a pHmeter (Hanna Instruments model HI 221, USA). (AOAC 1984).
Weight loss
Two strings of sausages from each treatment were weighed just before the fermented sausages were put into the fermentation room. The same strings of sausages were reweighed on days 1,3,5,7,9. The differences in weight were expressed as percentage of the first weight (Gök 2006).
TBARS (thiobarbituric acid reactive substances)
The 2-TBA (Thiobarbituric acid) test according to Tarladgis et al. (1960) was used to determine the oxidative rancidity. The concentrations were determined at 538 nm using Shimadzu (UV-Vis, Australia) Spectrophotometer.
Instrumental color measurement
The surface color measurements were carried out using a Minolta CR 300 reflectance colorimeter (Minolta Camera Co.Ltd., Osaka, Japan). L*, a*, b* color values were measured (L*: lightness; a*: redness, b*: yellowness) The Chromameter was calibrated on the Hunter lab color space system using a white tile. (C:Y = 93.6, x = 0.3130, y = 0.3193) (Minolta calibration plate).
Texture profile analysis (TPA)
Textural attributes of dry fermented sausages were measured with a TA.XT Plus Texture Analyser (Godalming, England). Dry fermented sausage slices (4x1cm) were evaluated. P/25 cylindrical probe was used. The pretest speed was 1 mm/s, test speed was 2 mm/s and the posttest speed was 2 mm/s. The samples were compressed twice and compression rate was 50 %. The textural parameters; hardness, springiness, cohesiveness, chewiness and gumminess were obtained. 50 kg load cell was assessed (Bruna et al. 2000).
Sensory evaluation
Sensory evaluation was carried out to determine the effects of fat level and mincer hole diameter on the quality of final product. Sensorial properties (surface color, surface appearance, taste and odor, texture and general acceptability) of raw sucuk samples were evaluated by 12 well trained panelists from the staff members of Celal Bayar University using 1–9 scale. The scales were 1–3 (unacceptable), 4–5 (acceptable), 6–7 (good), 8–9 (excellent) (Gök 2006).
Microbiological analysis
Lactic acid bacterial count
Drop plate method using Man Rogosa Sharpe (MRS) Agar (OXOID,CM0361, Basingstoke,UK) was used to determine the lactic acid bacteria count. Plates were incubated at 30 °C for 72 h (Pichhardt 1993).
Lactobacillus casei count
Drop plate method using MRS-IM Agar was used to determine the Lactobacillus casei CRL 431 count. Plates were incubated at 20 °C for six days (Chr Hansen 2005).
Yeast and mold count
Drop plate method using Potato Dextrose Agar (PDA) (Oxoid) was used to determine the yeast and mold count. Plates were incubated at 20–25 °C for 5–7 days.
Coliform bacterial count
Drop plate method using Violet Red Bile Glucose Agar (Merck, 1.10275) was used to determine the coliform bacteria count. Plates were incubated at 30 °C for 24 h (Harrigan, 1998).
Trial plan and statistical analysis
Trial plan was performed in three times and factorial design (3 × 3) was used to analyze the data. The factors were fat level (20 %, 25 %, 30 %) and mincer hole diameter (3 mm, 5 mm, 8 mm). Statistical analysis was carried out with the Statistical Analysis System (SAS) by using PROC GLM and PROC MIXED procedures. The effects of fat level and mincer hole diameter on compositional and structural properties of dry fermented sausage samples were evaluated. ANOVA tables were formed. Also, correlation coefficients (r) were calculated using the PROC CORR procedure of SAS software (Sante and Fernandez 2000).
Results and discussion
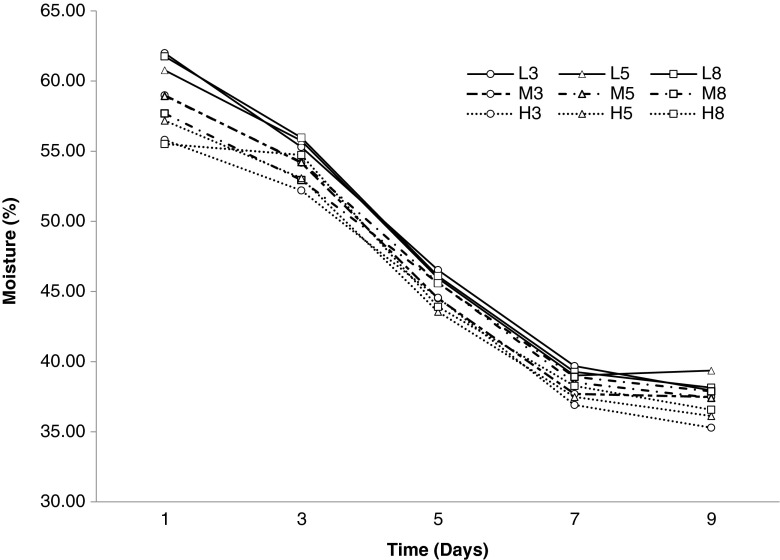
Changes in moisture contents during ripening process were shown in Fig. 1. On the first day of probiotic sausage fermentation, the highest moisture content was observed in L3 samples (62.00 %). The lowest moisture content was observed in H8 (55.53 %) samples. Moisture contents of the samples reduced due to drying, during fermentation process. The moisture contents of final products dropped to 35.28 %- 39.37 %. Moisture contents of sucuk samples during fermentation similar to the previous studies (Dalmış 2007; Gök 2006; Gönülalan et al. 2004).
Fig. 1.
Moisture values (%) of probiotic sausages over ripening period
Radulovic et al. (2011) reported that the initial moisture content of probiotic fermented sausage sample was 56.02 % and then decreased to 35.77 % at the end of fermentation process (7 days).
On the first day of fermentation, fat level affected the moisture content of probiotic sausages significantly (p < 0.05). On the third day, the moisture contents ranged 52.20 %-55.97 % values and the fat level affected these values significantly (p < 0.05). The moisture values of probiotic sausages on the 5th day were significantly affected by the fat level (p < 0.05). The values has changed between 43.55 %-46.53 %. On the 7th day, the moisture values were ranged between 36.90 %-39.69 % and affected by fat level (p < 0.0001) and mincer hole diameter (p < 0.05) significantly. At the end of the fermentation process the moisture values decreased to 35.28 % -39.37 % and were significantly affected by fat level (p < 0.005).
Significant correlations (r = −0.80, r = −0.73) on the 1st and 9th days were found between moisture and fat contents. As a result, during the fermentation-drying process while the moisture values were decreased, the fat contents were increased. It depends on the chemical composition.
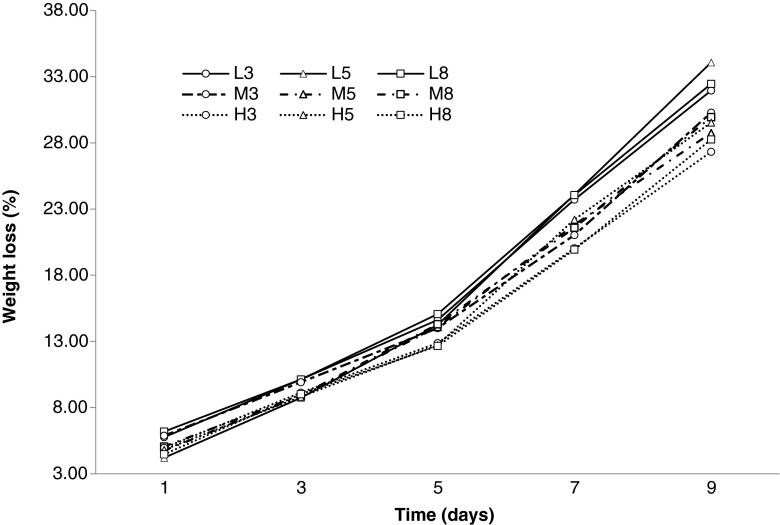
Changes in weight loss values during ripening process were shown in Fig. 2. Weight loss values increased during fermentation (9 days). Similar findings were reported by Kandemir Can (2001) and Gök (2006).
Fig. 2.
Weight loss values (%) of probiotic sausages over ripening period
The weight losses changed between 12.66 % -15.07 % on day 5 and these values were affected by fat level significantly (p < 0.05). Fat level had a very significant effect on weight loss values of 7th day (p = 0.0001). These values were determined between 19.94 %-24.09 %. At the end of the ripening, the weight loss values were increased to 27.32 %-34.08 % and affected by the fat level significantly (p < 0.005).
The fat contents of probiotic sausages were given in Table 1. The total fat contents of probiotic sucuk samples increased during the fermentation-ripening due to dehydration. The initial fat contents of batches with 20 %, 25 % and 30 % fat increased to 28.60 %-30.09 %, 32.49 %-34.82 % and 37.58 %-38.68 % in the final products, respectively. The increase in fat contents shows similarities with the previous studies by Soyer (2005), Gök (2006), Dalmış (2007), Papadima and Bloukas (1999). Papadima and Bloukas (1999) investigated the effects of different fat levels and storage conditions on the quality attributes of traditional Greek sausages. They reported that the initial fat levels 10.28 %, 19,32 %, 27,44 % increased to 21.07 %, 31.31 % and 36.36 % at the end of 7 days.
Table 1.
Fat, protein, ash values (%) of probiotic sausage samples during ripening
| Parameter | Treatments | Time (days) | |
|---|---|---|---|
| 1 | 9 | ||
| Fat (%) | L3 | 19.70c | 28.60e |
| L5 | 19.13c | 28.87e | |
| L8 | 19.41c | 30.09de | |
| M3 | 23.86b | 34.82bc | |
| M5 | 24.28b | 32.91dc | |
| M8 | 24.87b | 32.49 dc | |
| H3 | 29.68a | 37.78ab | |
| H5 | 28.73 a | 37.58ab | |
| H8 | 28.30 a | 38.68a | |
| Protein (%) | L3 | 17.04a | 23.91ab |
| L5 | 17.14 a | 23.04abc | |
| L8 | 16.89 a | 24.28a | |
| M3 | 15.33bc | 21.89abc | |
| M5 | 16.07 ab | 21.65 abc | |
| M8 | 16.08 ab | 23.45abc | |
| H3 | 14.41c | 20.65c | |
| H5 | 14.48c | 21.48 abc | |
| H8 | 14.17c | 20.98bc | |
| Ash (%) | L3 | 2.85a | 4.22b |
| L5 | 2.71ab | 4.31b | |
| L8 | 2.70 ab | 4.14b | |
| M3 | 2.66 ab | 4.87a | |
| M5 | 2.30d | 4.20 b | |
| M8 | 2.58 abc | 4.10b | |
| H3 | 2.51 abc | 3.97b | |
| H5 | 2.77ab | 3.96b | |
| H8 | 2.45bc | 3.54c | |
Values are mean of triplicates
a-eAny two means in the same column having the same letters in the same section are not significantly different (p < 0.05) according to Duncan’s test
According to the results of variance analysis, fat level used in sausage production (L-Low fat level (20 %), M- Medium fat level (25 %) and H- High fat level (30 %)) had a very significant effect on fat contents of sucuk samples on the first and 9th day (p < 0.0001). The mincer hole diameter had no significant effect on fat content on days 1, 9 (p > 0.05).
The protein contents of probiotic sausages were presented in Table 1. The fat level had a significant effect on protein contents (%) on the first day of fermentation (p < 0.0001). While the fat levels increased, the protein contents decreased due to chemical composition on the first day. Hence, a significant correlation (r = −0.81,p < 0.0001) was found between protein and fat contents. The protein contents of sucuk samples increased at the end of the fermentation-drying. Also, a significant correlation was determined between protein contents and weight losses (r = 0.73,p < 0.0001). At the end of the sucuk production, the fat level had a significant effect on protein contents (p < 0.05). As a result of statistical analysis, the mincer hole diameter had no significant effect on the protein contents on 1st and 9th days (p > 0.05).
Radulovic et al. (2011) assessed that the protein contents of probiotic fermented sausages were 18.02 % and 20.16 % on 1st and 7th days, respectively. Also, close findings were reported by Dalmış (2007), Ergönül and Kundakçı (2011).
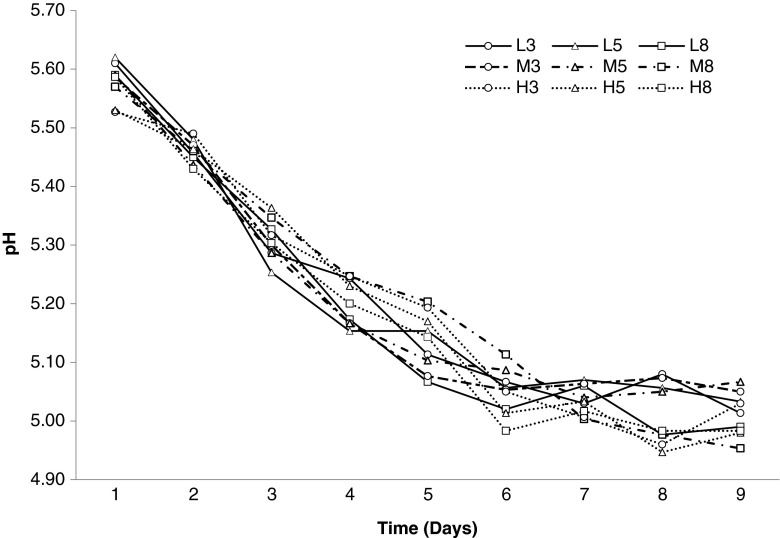
Ash is an indicator of mineral and salt content in meat and meat products. Meat has an average 1 % ash, naturally. However in meat products ash content increases due to additional curing agents, salt and spices (Vural and Öztan 1996). As shown in Table 1, ash contents increased during fermentation-drying. The interaction of fat level x mincer hole diameter affected the ash values on the first day, significantly (p < 0.05). At the end of the fermentation, it was determined that the fat level had a very significant effect on ash content (p < 0.001). Also, analysis of variance showed that the mincer hole diameter had a significant effect on ash contents (p < 0.005). The ash content decreased with high level of fat, ash content is low in H8 when compared L3. Changes in pH values during ripening process were shown in Fig. 3. The pH values of sucuk samples were on the decline during fermentation-ripening. This was probably due to ammonia and amine production as a result of enzymatic activity during ripening. According to results of variance analysis, fat level, mincer hole diameter and interaction of fat level x mincer hole diameter had non-significant effect on pH values every day of production (p > 0.05). The decline in pH values resulted from starter and probiotic culture activation in sausage batters. The results showed similarity with following previous studies. Radulovic et al. (2011) indicated that the pH values of probiotic fermented sausage samples were 5.58 and 5.20 on days 0 and 7, respectively. Kandemir Can (2001) found that the pH values of probiotic sausage samples decreased from 5.76–5.84 to 5.13–5.22 on 0. and 8th days of production, respectively. Also, Gönülalan et al. (2004) determined the initial pH values of sucuk samples ranged from 5.78 to 5.93. At the end of the fermentation, the pH values ranged from 4.94 to 5.46.
Fig. 3.
pH values (%) of probiotic sausages over ripening period
Colour values of probiotic sausages were presented in Table 2. In all samples, lightness (L*) values decreased during ripening. Similar findings were reported by Ercoşkun and Özkal (2011), Papadima and Bloukas (1999), Olivares et al., (2010). As a result of variance analysis, the fat level affected the L* values significantly (p < 0.005). Additionally, a significant correlation (r = 0.65,p = 0.0002) was determined between L* values and initial fat contents. While the fat level in sausage formulation increased, L* values increased. On the 5th and 9th days, fat level had a significant effect on L* values (p < 0.05). A significant correlation (r = −0.70, p < 0.0001) between L* values and weight loss values was determined in final products. While weight losses increased, L* values decreased, due to drying. L* values of meat products are related mainly to moisture (Sayas-Barbara et al. 2012). Wojciak et al. (2012) reported that the initial mean L* values of probiotic fermented sausage inoculated with L. casei LOCK 0900 was 59.41 and decreased gradually to 51.12 at the end of the fermentation (6th day). In another study, Sayas-Barbara et al. (2012) found that the L* belongs to fermented sausages produced with L.casei CECT 475 and dietary fiber decreased from 50.30 to 36.90.
Table 2.
L*(lightness), a*(redness) b*(yellowness), TBARS (Thiobarbituric acid reactive substances) values of probiotic sausage samples during ripening
| Parameter | Treatments | Time (days) | ||
|---|---|---|---|---|
| 1 | 5 | 9 | ||
| L* | L3 | 45.66 abc | 43.25abc | 38.96ab |
| L5 | 44.69bc | 41.49bc | 37.86b | |
| L8 | 43.62c | 40.60c | 38.18ab | |
| M3 | 46.02abc | 44.19ab | 41.19ab | |
| M5 | 45.17 abc | 43.15abc | 41.39ab | |
| M8 | 45.10 abc | 42.41bc | 40.83ab | |
| H3 | 47.39ab | 43.62abc | 39.87ab | |
| H5 | 46.80ab | 42.98abc | 40.55ab | |
| H8 | 47.68a | 45.97a | 41.43a | |
| a* | L3 | 14.66 | 14.73 | 14.19 |
| L5 | 15.08 | 14.74 | 13.48 | |
| L8 | 14.41 | 14.25 | 14.11 | |
| M3 | 14.76 | 14.72 | 15.74 | |
| M5 | 14.24 | 14.65 | 14.81 | |
| M8 | 14.77 | 13.54 | 13.86 | |
| H3 | 12.96 | 14.31 | 14.86 | |
| H5 | 13.34 | 13.76 | 13.93 | |
| H8 | 13.86 | 13.39 | 13.97 | |
| b* | L3 | 17.17 | 13.21 | 11.42 |
| L5 | 16.45 | 14.15 | 10.99 | |
| L8 | 16.25 | 13.37 | 10.42 | |
| M3 | 18.7 | 14.13 | 12.22 | |
| M5 | 16.82 | 14.7 | 13.17 | |
| M8 | 17.52 | 14.74 | 11.74 | |
| H3 | 15.03 | 13.58 | 12.22 | |
| H5 | 15.32 | 13.3 | 11.1 | |
| H8 | 16.75 | 13.69 | 11.22 | |
| TBARS (malondialdehyde/kg) | L3 | 0.38bc | 0.49d | 0.75bc |
| L5 | 0.40bc | 0.54dc | 0.82abc | |
| L8 | 0.36bc | 0.48d | 0.66c | |
| M3 | 0.46abc | 0.56dc | 0.77bc | |
| M5 | 0.42bc | 0.62bc | 0.75bc | |
| M8 | 0.52abc | 0.68b | 0.93ab | |
| H3 | 0.55ab | 0.72ab | 1.06a | |
| H5 | 0.51abc | 0.72ab | 0.82abc | |
| H8 | 0.60a | 0.81a | 0.97ab | |
Values are mean of triplicates
a-dAny two means in the same column having the same letters in the same section are not significantly different (p < 0.05) according to Duncan’s test
In this study, redness (a*) values of M3, M5, H3, H5 and H8 samples increased during ripening (Table 2). This result probably arose from the formation of nitrosomyoglobin and the increase of heme pigment concentration due to moisture loss. (Papadima and Bloukas 1999, Sayas-Barbera et al. 2012). It was observed that, redness (a*) values of L3, L5, L8 and M8 samples decreased during sausage production. This decline was probably caused by microbial and enzymatic degradation of nitrosomyoglobin (Papadima and Bloukas 1999). While, pH values of sucuk samples become closer to isoelectric point (pH = 5.3), myoglobin denaturation increased. It was reported to be related to the decrease of a* values (Ercoşkun and Özkal 2011). Also, water loss in meat might have led to the excessive accumulation of myoglobin pigment on the surface of meat (Bağdatli and Kayaardi 2015).
According to results of variance analysis, fat level, mincer hole diameter and interaction of fat level x mincer hole diameter had no significant effect on redness (a*) values on days 1, 5 and 9 (p > 0.05). In contrast to this study, redness (a*) values of fermented sausages with different fat levels were affected by fat level, significantly (Papadima and Bloukas, 1999; Soyer et al. 2005).
Sayas-Barbera et al. (2012) reported that a* values of Longaniza de Pascua (Spanish fermented sausage) were 4.11, 6.49, 6.68, 13.04 and 8.82 on days 0,1,3, 6 and 8, respectively.
A significant correlation (r = 0.43) between a* values and surface color scores was determined. Also, a significant correlation (r = 0.48) was found between a* values and surface appearance scores. Consequently, it was observed that instrumental color was related with the sensorial acceptance.
Yellowness (b*) values of all sucuk samples decreased during the ripening period (Table 2). This study is in agreement with the previous studies (Muguerza et al. 2002; Olivares et al. 2010; Wojciak et al. 2012; Dalmış 2007; Sayas-Barbera 2012; Papadima and Bloukas, 1999; Gök 2006; Kayaardı et al. 2003; Ercoşkun and Özkal 2011; Bozkurt and Bayram 2006). According to results of variance analysis, fat level, mincer hole diameter and interaction of fat level x mincer hole diameter had no significant effect on yellowness (b*) values on the 1st,5th and 9th days of production (p > 0.05). Olivares et al.(2010) also found that different fat levels (10 %,20 %,30 %) had no significant effect on yellowness (b*) values of fermented sausages.
TPA parameters of probiotic fermented sausage samples at the end of the ripening period were shown in Table 3. Hardness, springiness, cohesiveness, chewiness and gumminess parameters were obtained from Texture Profile Analysis. The highest hardness value was observed in H5 samples and the lowest hardness value was observed in L3 samples. Analysis of variance showed very significant effect of interaction of fat levelxmincer hole diameter on hardness values (p < 0.0005). A significant correlation (r = −0.73,p < 0.0001) was found between hardness values obtained from Texture Profile Analysis and moisture contents.
Table 3.
Texture Profile Analysis (TPA) parameters of probiotic sausage samples at the end of the ripening
| Treatments | Hardness (N) | Springiness | Cohesivness | Chewiness (N) | Gumminess (N) |
|---|---|---|---|---|---|
| L3 | 35.275d | 0.271 | 0.653b | 6.238 | 23.039d |
| L5 | 38.658cd | 0.286 | 0.787a | 8.695 | 30.419ab |
| L8 | 42.235bc | 0.263 | 0.634b | 7.053 | 26.790bdc |
| M3 | 46.494b | 0.249 | 0.642b | 7.438 | 29.853abc |
| M5 | 38.428cd | 0.25 | 0.597b | 5.729 | 22.959d |
| M8 | 44.981b | 0.295 | 0.573b | 7.6 | 25.771dc |
| H3 | 52.550a | 0.27 | 0.623b | 8.833 | 32.739a |
| H5 | 54.652a | 0.302 | 0.606b | 10.009 | 33.099a |
| H8 | 45.638b | 0.305 | 0.565b | 7.871 | 25.803dc |
Values are mean of triplicates
a-dAny two means in the same column having the same letters in the same section are not significantly different (p < 0.05) according to Duncan’s test
Gök (2006) observed the hardness values of sucuk samples between 3.151–3.707 kg. These findings were lower than our findings. Ergönül (2009) determined the hardness values of Turkey probiotic sausage as 6.12–6.29-7.99 kg at the end of the ripening period.
The highest springiness value was observed in H8 sample and the lowest springiness value was observed in M3 sample. Olivares et al. (2010) determined that the springiness values of sucuk samples changed between 0.696–0.730 at the end of the ripening (42 days). These values were higher than our findings.
Fat level, mincer hole diameter and interaction of fat level x mincer hole diameter had no significant effect on springiness values of probiotic sucuk (p > 0.05). There was a significant correlation (r = 0.68) between springiness and cohesiveness values. On the other hand, it was determined that a significant correlation (r = 0.56) between springiness value and general acceptability score was found. A significant correlation (r = 0.65) between springiness and sensorial texture scores was also observed.
At the end of the ripening, cohesiveness values were ranged from 0.565 to 0.787. This study is supported by the previous findings by Olivares et al. (2010) and Ergönül (2009). Sausage formulation and fat level were the most important factors affecting cohesiveness (Ergönül 2009).
The interaction of fat level x mincer hole diameter significantly (p < 0.05) affected cohesiveness values of sucuk samples. A significant correlation was determined between cohesiveness and chewiness values of sucuk samples (r = 0.82, p < 0.0001).
Fat level, mincer hole diameter and interaction of fat level x mincer hole diameter had no significance on chewiness values (final product) (p > 0.05). A significant correlation was determined between chewiness and springiness (r = 0.67). Also, a significant correlation was determined between chewiness values and sensorial texture scores (r = 0.59) (p = 0.0012).
The interaction of fat level x mincer hole diameter showed a significant difference in gumminess values (p < 0.0005). It is well known that the gumminess relevant to the product formulation. Correspondingly, a significant correlation was found between gumminess values and fat level (r = 0.72) (p < 0.0001). This study is in agreement with the previous study of Olivares et al. (2010) who found the mean gumminess values of dry fermented sausages with 10 %, 20 % and 30 % fat levels were 32.4 N, 32.0 N and 28.5 N, respectively. Also, Ergönül (2009) reported that the gumminess values of Turkey sausages added with different probiotic cultures changed between 3.20–4.51 kg.
The mean values of TBARS number of sausage samples were given in Table 2. The values of TBARS number of probiotic sucuk samples increased during fermentation-ripening. Soyer (2005) reported that the TBARS values of dry fermented sausages with 20 % and 30 % fat levels were higher than the sausage with 10 % fat level. Similar findings were observed in our study. Yalınkılıç et al. (2012) found that different fat levels had no significant effect on the quality characteristics of citrus fiber added sausage. Ercoşkun and Özkal (2011) determined that the mean TBARS number of traditional Turkish sausage increased from 0.26 mg malondialdehyde/kg to 0.52 mg malondialdehyde/kg during 9 days. Significant correlations (r = −0.45 and r = −0.41) between TBARS values with taste-aroma and texture scores were found. Fat level had a very significant effect on the TBARS number of probiotic sucuk samples on day 1 and 5 (p < 0.005, p < 0.0001). Also, mincer hole diameter and the interaction of fat level x mincer hole diameter had no significant effect on days 1, 5 and 9 (p > 0.05). At the end of the fermentation, the TBA values changed between 0.66–1.06 mg malondialdehyde/kg and the fat level affected these values significantly (p < 0.05). A significant correlation was found between TBA number and fat level of probiotic sucuk samples on the 1st and 5th days (r = 0.59 and r = 0.45) (p = 0.0012 and p = 0.0187). TBA values of sausages increased depending on processing time and fat content.
The lactic acid bacteria count of sucuk samples were given in Table 4. The lactic acid bacteria count were on the raise during fermentation-ripening. Similar findings were reported by Gençcelep (2006), Ercoşkun and Özkal (2011), Ergönül (2009), Sayas-Barbera et al. (2012), Wojciak et al. (2012), Gönülalan et al. (2004). High populations of lactic acid bacteria inhibit the growth of spoilage and pathogenic bacteria, especially Staphylococcus aureus (Papadima and Bloukas 1999). Papadima and Bloukas (1999) determined that fat level did not have a significant effect on the lactic acid bacteria number of fermented Greek sausage.
Table 4.
Microbiological count of probiotic sausage samples (log cfu/g)
| Microbiological analysis | Treatments | Time (days) | ||
|---|---|---|---|---|
| 1 | 5 | 9 | ||
| Lactic acid bacteria count | L3 | 7.49a | 8.1 | 8.58 |
| L5 | 7.34ab | 8.02 | 8.43 | |
| L8 | 6.87abc | 7.85 | 8.6 | |
| M3 | 6.73bc | 7.85 | 8.33 | |
| M5 | 7.00abc | 7.98 | 8.73 | |
| M8 | 6.55c | 7.47 | 8.13 | |
| H3 | 6.58c | 7.54 | 8.21 | |
| H5 | 6.77bc | 7.86 | 8.54 | |
| H8 | 7.24abc | 7.85 | 8.55 | |
| L. casei CRL-431 count | L3 | 6.51 | 6.87a | 7.15 |
| L5 | 6.47 | 6.76a | 7.24 | |
| L8 | 5.65 | 6.60ab | 6.91 | |
| M3 | 6.02 | 6.35ab | 6.68 | |
| M5 | 6.37 | 6.23ab | 6.94 | |
| M8 | 5.89 | 5.98b | 6.62 | |
| H3 | 6.36 | 6.54ab | 6.81 | |
| H5 | 6.58 | 6.68ab | 7 | |
| H8 | 6.49 | 6.73ab | 6.62 | |
Values are mean of triplicates
cfu/g colony forming unit per gram
a-cAny two means in the same column having the same letters in the same section are not significantly different (p < 0.05) according to Duncan’s test
Analysis of variance showed significant effect of fat level on the initial lactic acid bacteria count (p < 0.05). Also, mincer hole diameter and interaction of fat level x mincer hole diameter had no significant effect on the initial lactic acid bacteria count of probiotic sausage (p > 0.05). On 5th and 9th days, the fat level, mincer hole diameter and interaction of fat level x mincer hole diameter had no significant effect on the lactic acid bacteria count (p > 0.05). It was demonstrated that approximately 1.50 log cfu/g increase was occurred in lactic acid bacteria count of all sausage samples during fermentation-drying. A significant correlation (r = 0.45) (p = 0.0176) was found between the counts of lactic acid bacteria and L. casei CRL-431.
The survivability and number of probiotic bacteria in food products, and the stability of their probiotic activity depend on the product properties and production process (Trzasowska et al. 2014). The L. casei CRL-431 count of sucuk samples were given in Table 4. It was observed that the L. casei CRL-431 number of probiotic sucuk samples increased during fermentation-ripening. According to the results of variance analysis, fat level, mincer hole diameter and interaction of fat level x mincer hole diameter had no significant effect on the L. casei CRL-431 number, on the 1st and 9th days (p > 0.05). Analysis of variance showed significant effect of fat level on the L. casei CRL-431 number on the 5th day (p < 0.05). Mincer hole diameter and interaction of fat level x mincer hole diameter had no significant effect on the L. casei CRL-431 number (p > 0.05). Consequently, it was determined that L. casei CRL431 number reached the sufficient microbial number to obtain probiotic character at the end of the fermentation process. Thus, Kolozyn-Krajewska and Dolatowski (2009) reported that meat products should contain at least 106 cfu cells of lactic acid bacteria with probiotic properties in one gram product. Significant positive correlations were found between L. casei CRL431 count and surface appearance, texture and overall acceptability scores (r = 0.60, r = 0.52, r = 0.53) (p = 0.001, p = 0.0054, p = 0.0044).
As a result, Lactobacillus casei maintained its vitality in probiotic sausage production, during the 9-day fermentation-ripening time. It was indicated that L. casei CRL 431 can be used as probiotic culture in sausage production.
Ergönül (2009), reported that L. casei CRL 431 count of Turkey sausage produced with L. casei CRL 431 reached from 7.56 log cfu/g to 8.18 log cfu/g at the end of the ripening process. Sayas-Barbera et al. (2012) determined that the L.casei count of fermented sausage samples produced with L. casei CECT 475 reached to about 8.6–8.8 log cfu/g at the end of the ripening (8 days).
In this study, yeast and mold counts of probiotic fermented sausage samples with different fat levels (20 %,25 %,30 %) and different particle sizes (3 mm,5 mm,8 mm) were evaluated on 1st, 5th and 9th days during fermentation-ripening. Samples showed absence of yeast and mold counts. There was no growth of yeast and mold. Because of the microbiological quality was improved. Also, LAB became dominant flora and inhibit the other pathogenic and spoilage microorganism (Ahmad and Srivastava 2007).
Similar findings were obtained by Ahmad and Srivastava (2007) who found that yeast and mold counts of twelve samples of semi dry fermented sausages showed absence of yeast and mold counts till 15 days during storage at +4 °C. The results from this study support the findings of Ergönül (2009) who reported that there was no growth of yeast and mold in probiotic Turkey sausages added Lactobacillus casei CRL 431 at the first day of the production, at the end of the 8 month storage(−18 °C).
Coliform bacteria counts of probiotic fermented sausage samples with different fat levels (20 %, 25 %, 30 %) and different particle sizes (3 mm, 5 mm, 8 mm) were evaluated on 1 st, 5th and 9th days during fermentation-ripening time. Samples showed absence of coliform bacteria. No coliforms, therefore no fecal coliforms were determined throughout 9 days of production in treatments. Similarly, Ergönül (2009) found that there was no growth of coliforms in probiotic Turkey sausages produced with different probiotic cultures in any stage of production and storage. This was very important for raw consumption of probiotic sausage. Coliform bacteria inhibition probably arose from lactic acid bacteria that became dominant flora and production of acidic metabolite (Dalmış 2007).
Breakdown products of lipolysis and proteolysis, i.e. peptides, amino acids, carbonyls, and volatile flavor compounds contribute to the characteristic flavor and texture of fermented meats. Starter cultures affect the aroma and taste of fermented meat products. The quality of the final product is closely related to the ripening period (Rouhi et al. 2013).
In this study, sensory analysis were performed using 1–9 scale. The results of Duncan’s multiple range test for sensorial properties scores of sausage samples were shown in Table 5. Similar findings were reported by Radulovic et al. (2011) who found that the mean scores of appearance, color, taste, aroma and texture of probiotic fermented sausage were above 7 (9-scale) at the end of the fermentation (14 days).
Table 5.
Sensory evaluation scores of probiotic sausage samples (9 point scale) at the end of the ripening
| Treatments | Surface color | Surface appearance | Taste-aroma | Texture | Overall acceptability |
|---|---|---|---|---|---|
| L3 | 7.38 | 7.4 | 8.16a | 8.10a | 7.97 |
| L5 | 6.07 | 6.6 | 6.58d | 7.38abc | 6.96 |
| L8 | 7 | 6.87 | 7.62ab | 7.93a | 7.52 |
| M3 | 7.13 | 7.07 | 7.56abc | 7.78ab | 7.56 |
| M5 | 6.8 | 7.33 | 6.76dc | 6.65bc | 7.25 |
| M8 | 7.4 | 7.2 | 7.04bdc | 7.16abc | 6.93 |
| H3 | 7.07 | 7.2 | 6.53d | 7.60ab | 7.32 |
| H5 | 6.27 | 6.53 | 6.69d | 7.38abc | 7.09 |
| H8 | 6.4 | 5.93 | 5.62e | 6.22c | 5.89 |
Values are mean of triplicates
Sensory scale: 1–3 = unacceptable, 4–5 = acceptable, 6–7 = good, 8–9 = excellent
a-eAny two means in the same column having the same letters in the same section are not significantly different (p < 0.05) according to Duncan’s test
Analysis of variance showed non-significant effect of fat level, mincer hole diameter and interaction of fat level x mincer hole diameter on surface color, surface appearance and overall acceptability (p > 0.05). Also a significant correlation was found between surface color and surface appearance (r = 0.75, p < 0.0001). A significant effect of mincer hole diameter on texture scores (p < 0.05) was found. In contrast, there was a non-significant effect of fat level (p = 0.0572) and interaction of fat level x mincer hole diameter (p = 0.1645) on texture scores. According to the variance analysis, a significant effect of interaction of fat level x mincer hole diameter on taste-aroma scores of probiotic sausages was determined (p < 0.05).
A significant correlation between taste-aroma scores and fat level was detected (r = −0.61,p = 0.0008). According to this result, using fat above a certain adversely affected taste-aroma of sausage. The highest scores for overall acceptability, texture, taste-aroma and surface appearance were determined in L3 samples (%20 fat level + 3 mm). The highest surface color score was detected in M8 samples. In contrast, the lowest scores for overall acceptability, texture, taste-aroma and surface appearance were determined in H8 samples (%30 fat level + 8 mm). Also the lowest surface color score was found in L5 samples. Similarly, Soyer (2005) determined that the fat level affected the sensorial quality significantly and the highest overall acceptability score was found in sausage batters with 10 % and 20 % fat levels. The sausages containing 30 % fat showed undesirable sensorial attributes. Also, Yalınkılıç et al. (2012) detected that fat level (10 %-15 %-20 %) affected the taste significantly (p < 0.05). The highest taste score was found in sausage samples with %15 fat level. Finally, the least acceptable sample was H8 and the most preferred sample was L3.
Conclusions
The lactic acid bacteria number of sucuk samples were on the raise during fermentation-ripening. A significant correlation was found between the counts of lactic acid bacteria and L. casei CRL-43. The fat level, mincer hole diameter and interaction of fat level x mincer hole diameter had no significant effect on the L. casei CRL-431 number of probiotic sausage, on the 1st and 9th days. Consequently, it was determined that L. casei CRL 431 number reached the sufficient microbial number to obtain probiotic character at the end of the fermentation. Significant positive correlations were found between L. casei CRL 431 count and surface appearance, texture and overall acceptability scores was observed. In this study, it was determined that the growth of probiotic L. casei CRL 431 improve the sensorial quality. As a result, Lactobacillus casei seen to be maintained its vitality in probiotic sausage production, during the 9-day fermentation-ripening. It was indicated that L. casei CRL 431 can be used as probiotic culture in sausage production.
A significant effect of mincer hole diameter on texture scores was found. A significant correlation between taste-aroma scores and fat level was detected. According to the sensorial analysis, the least acceptable sample was H8 (30 % fat level + 8 mm) and the most preferred sample was L3 (20 % fat level + 3 mm).
In this study, the results confirmed that traditional probiotic Turkish sausage (sucuk) with high physicochemical, microbiological and sensorial quality characteristics and also standard compositional and structural properties can be produced under industrial conditions.
Acknowledgments
This work was supported by “Celal Bayar University Research Project Funds,” Turkey under Grant Project number 2011-022. The authors thank PINAR Integrated Meat and Feed Industries Inc., Kemalpaşa, İzmir for the manufacture of the sucuk.
References
- Ahmad S, Sristava PK. Quality and shelf life evaluation of fermented sausages of buffalo meat with different levels of heart and fat. Meat Sci. 2007;75:603–609. doi: 10.1016/j.meatsci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Akkaya L, Gök V, Kara R, Yaman H. Enterotoxin production by Staphylococcus aureus (a, B, C, D) during the ripening of sucuk (Turkish dry-fermented Sausage) CyTA-J Food. 2014;12(2):127–133. doi: 10.1080/19476337.2013.804124. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. Centennial. USA: Association of Official Analytical Chemists Washington D.C; 1984. [Google Scholar]
- AOAC (2000) Official Methods of Analysis of AOAC International (17.Edition). USA
- Bağdatli A, Kayaardi S. Influence of storage period and packaging methods on quality attributes of fresh beef steaks. CyTA - Journal of Food. 2015;13(1):124–133. doi: 10.1080/19476337.2014.919029. [DOI] [Google Scholar]
- Bozkurt H, Bayram M. Color and textural attributes of sucuk during ripening. Meat Sci. 2006;73:344–350. doi: 10.1016/j.meatsci.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bruna JM, Fernandez M, Hierro EM, Ordonez JA, de la Hoz L. Combined use of pronase and fungal extract (Penicillium aurantiogriseum) to potantiate the sensory characteristics of dry fermented sausages. Meat Sci. 2000;54:135–145. doi: 10.1016/S0309-1740(99)00076-5. [DOI] [PubMed] [Google Scholar]
- Chr Hansen (2005) L.acidophilus, L.casei and Bifidobacteria in Fermented Milk Products. Guidelines method for counting probiotic bacteria. Bulletin F-6 LA LC BB March2005/3:8, Chr. Hansen, Denmark
- Dalmış Ü. Sucukta üretim ve depolama sırasında meydana gelen mikrobiyolojik ve biyokimyasal değişmeler. Ankara: Doktora tezi Ankara Üniversitesi Fen Bilimleri Enstitüsü Gıda Mühendisliği Anabilim Dalı; 2007. pp. 1–123. [Google Scholar]
- Ercoşkun H, Özkal SG. Kinetics of traditional Turkish sausage quality aspects during fermentation. Food Control. 2011;22:165–172. doi: 10.1016/j.foodcont.2010.06.015. [DOI] [Google Scholar]
- Ergönül B, Kundakçı A. Microbiological attributes and biogenic amine content of probiotic Turkish fermented sausage. J Verbr Lebensm. 2011;6(1):49–56. doi: 10.1007/s00003-010-0584-0. [DOI] [Google Scholar]
- Ergönül B. Farklı probiyotik kültürler kullanılarak Hindi sucuğu üretimi ve kalite üzerine etkileri. Manisa: Doktora tezi Celal Bayar Üniversitesi Fen Bilimleri Enstitüsü Gıda Mühendisliği Anabilim Dalı; 2009. [Google Scholar]
- Gençcelep H. Sucuk üretiminde değişik starter kültürler ve farklı nitrit seviyelerinin biyojen Amin oluşumu üzerine etkileri. Erzurum: Doktora Tezi Atatürk Üniversitesi Fen Bilimleri Enstitüsü Gıda Mühendisliği ABD; 2006. [Google Scholar]
- Gök V. Antioksidan kullanımının fermente sucukların bazı kalite özellikleri üzerine etkileri. Ankara: Doktora Tezi Ankara Üniversitesi Gıda Mühendisliği Anabilim Dalı; 2006. pp. 1–110. [Google Scholar]
- Gönülalan Z, Arslan A, Köse A. Farklı starter kültür kombinasyonlarının fermente sucuklardaki etkileri. Turk J Vet Anim Sci. 2004;28:7–16. [Google Scholar]
- Harrigan WF. Laboratory methods in food microbiology. London: Academic Press; 1998. pp. 1–532. [Google Scholar]
- Jaworska D, Neffe K, Kolozyn-Krajewska D, Dolatowski Z. Survival during storage and sensory effect of potential probiotic lactic acid bacteria Lactobacillus acidophilus Bauer and Lactobacillus casei Bif3’/IV in dry fermented pork loins. Int J Food Sci Technol. 2011;1365-2621:2011.02772. [Google Scholar]
- Kandemir Can BD. Fermente türk sucuğu üretiminde probiyotik bakterilerin kullanımı. Ankara: Yüksek Lisans Tezi; 2001. pp. 1–56. [Google Scholar]
- Kayaardı S, Gök V. Effect of replacing beef fat with olive oil on quality characteristics of Turkish soudjouk (sucuk) Meat Sci. 2003;66:249–257. doi: 10.1016/S0309-1740(03)00098-6. [DOI] [PubMed] [Google Scholar]
- Kołożyn-Krajewska D, Dolatowski ZJ. Probiotics in fermented meat products. Acta Sci Pol Technol Aliment. 2009;8(2):61–74. [Google Scholar]
- Muguerza E, Fista G, Ansorena D, Astiasaran I, Bloukas JG (2002) Effect of fat level and partial replacement of pork backfat with olive oil on processing and quality characteristics of fermented sausages. Meat Sci 61/4:397–404 [DOI] [PubMed]
- Olivares A, Navarro JL, Salvador A, Flores M. Sensory acceptability of slow fermented sausages based on fat content and ripening time. Meat Sci. 2010;86:251–257. doi: 10.1016/j.meatsci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Papadima SN, Bloukas JG. Effect of fat level and storage conditions on quality characteristics of traditional Greek sausages. Meat Sci. 1999;51:103–113. doi: 10.1016/S0309-1740(98)00103-X. [DOI] [PubMed] [Google Scholar]
- Pichhardt K. Lebensmittel-mikrobiologie. Berlin: Springer Verlag; 1993. [Google Scholar]
- Radulovic Z, Zivkovic D, Mirkovic N, Petrusic M, Stajic S, Perunovic M, Paunovic D. Effect of probiotic bacteria on chemical composition and sensory quality of fermented sausages. Procedia Food Science. 2011;1:1516–1522. doi: 10.1016/j.profoo.2011.09.224. [DOI] [Google Scholar]
- Rouhı M, Sohrabvandı S, Mortazavıan AM. Probiotic fermented sausage: viability of probiotic microorganisms and sensory characteristics. Crit Rev Food Sci Nutr. 2013;53:331–348. doi: 10.1080/10408398.2010.531407. [DOI] [PubMed] [Google Scholar]
- Sante V, Fernandez X. The measurement of pH in raw and frozen Turkey pectoralis supercialis muscle. Meat Sci. 2000;55:503–506. doi: 10.1016/S0309-1740(99)00174-6. [DOI] [PubMed] [Google Scholar]
- Sayas-Barbera E, Viuda-Martos M, Fernandez-Lopez F, Perez-Alvarez JA, Sendra E. Combined use of a probiotic culture and citrus fiber in a traditional sausage ‘longaniza de Pascua’. Food Control. 2012;27:343–350. doi: 10.1016/j.foodcont.2012.04.009. [DOI] [Google Scholar]
- Sidira M, Karapetsas A, Galanis A, Kanellaki M, Kourkoutas Y. Effective survival of immobilized Lactobacillus casei during ripening and heat treatment of probiotic dry-fermented sausages and investigation of the microbial dynamics. Meat Sci. 2014;96:948–955. doi: 10.1016/j.meatsci.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Soyer A. Effect of fat level and repining temperetaure on biochemical and sensory characteristics of naturally fermented Turkish sausages (sucuk) Eur Food Res Technol. 2005;221:412–415. doi: 10.1007/s00217-005-1192-6. [DOI] [Google Scholar]
- Tarladgis BG, Watts BM, Yonathan M. Distillation method for the determination of malonaldehyde in rancid foods. J Am Oil Chem Soc. 1960;37(1):44–48. doi: 10.1007/BF02630824. [DOI] [Google Scholar]
- Toldra F, Reig M. Innovations for healthier processed meats. Trends Food Sci Technol. 2011;22:517–522. doi: 10.1016/j.tifs.2011.08.007. [DOI] [Google Scholar]
- Trzasowska M, Kolozyn-Krajewska D, Wojciak KM, Dolatowski Z. Microbiological quality of raw-fermented sausages with Lactobacillus casei LOCK 0900 probiotic strain. Food Control. 2014;35:184–191. doi: 10.1016/j.foodcont.2013.07.002. [DOI] [Google Scholar]
- Vural H, Öztan A (1996) Et ve Et Ürünleri Kalite Kontrol Laboratuarı Uygulama Klavuzu. Hacettepe Üniversitesi Mühendislik Fak. Yayınları Yayın No: 36, Ankara, 1–235
- Wojciak KM, Dolatowski ZJ, Kolozyn-Krajewska D, Trzasowska M. The effect of the Lactobacillus casei lock0900 probiotic strain on the quality of dry-fermented sausage during chilling storage. J Food Qual. 2012;35:353–365. doi: 10.1111/j.1745-4557.2012.00458.x. [DOI] [Google Scholar]
- Yalınkılıç B, Kaban G, Kaya M. The effects of different levels of orange fiber and fat on microbiological, physical, chemical and sensorial properties of sucuk. Food Microbiol. 2012;29:255–259. doi: 10.1016/j.fm.2011.07.013. [DOI] [PubMed] [Google Scholar]