Abstract
Active antioxidant food packaging films were developed by incorporation of apple pomace (AP) with 1, 5, 10, and 30 % (w/w) into polyvinyl alcohol (PVA) matrix. A complete thermal, structural, mechanical and functional characterization was carried out. The findings of this study showed that the incorporation of AP into PVA films enhanced the total phenolic content and antioxidant properties. As regards the physical properties, higher AP content incorporated into PVA films revealed significantly lower tensile strength, elongation at break and increase in thickness. PVA-AP films exhibited lower transparency value compared to control film. The thermal stability of PVA-AP films was improved and grew with the increasing concentration of AP. FTIR spectra indicated that protein–polyphenol interactions were involved in the PVA-AP films. Rough surface and compact-structure were observed in PVA-AP films. The storage study of soybean oil at 60 °C in PVA-AP pouch showed the antioxidant activity and the effectiveness for delaying its lipid oxidation.
Keyword: Apple pomace, Bio composite film, Mechanical properties, Antioxidant, Active packaging
Introduction
Antioxidant properties in active packages is a research area with high interest due to the increasing consumer’s demands, market trends and substitute to traditional food packaging where antioxidants are incorporated into or coated on food packaging materials to reduce oxidation of the food, which is one of the main causes of food spoilage. Currently focused is on use of natural antioxidants in packaging material than the synthetic additives. Natural antioxidants including tocopherol, plant extracts, and essential oils from herbs such as rosemary, oregano and tea that are safer and in most cases offer multiple health benefits (López-de-Dicastillo et al. 2012).
In recent years, much effort has been devoted on to developing environmentally compatible products by incorporating bio-composite materials as a potential alternative for petroleum-based synthetic polymers (Avella et al. 2009). Bio-composite materials have many advantages such as being comparatively less expensive, more environmental friendly and naturally biodegradable. The natural products of crop residues, agricultural wastes, and by-products are good bio-composite organic materials (Narayan 1994). The recent utilization of agricultural by-products, fruit pomace provides a value added composite material when incorporated with the polymeric material as it mostly retains major components of pectin, proteins, organic acids, and sugars (Jiang and Simonsen 2011).
Apple pomace as by-product in the apple juice industry generates about 30 % of the weight of the fresh apple processed (Joshi et al. 2006). Only a small amount of apple pomace has been utilized as fertilizer, while most is disposed of (Reis et al. 2012). To recover such by-products a universal recovery process is well established which includes, maximize the yield of compound, demands of industrial processing, clarifying the high added-value ingredients from impurities, avoiding deterioration and loss of functionality during processing and ensuring the food grade nature of the final product (Galanakis 2012). The solid residues in apple pomace consist of carbohydrates, pectin, crude fiber, and minerals. As a component of dietary fiber in apple pomace, pectin is composed of mainly one, 4-linked D-galacturonic acid units and its methyl ester, and is a potential film-forming material and miscible with other polymers. The dietary fiber content in apple pomace ranged between 33 to 35 %. Apple pomace also contains the healthy and beneficial compounds with high phytochemicals such as quercetin glycosides and procyanidins, and represents antioxidant capacity (Bhushan and Kalia 2008).
Polyvinyl alcohol (PVA) is a water soluble polymer with highly polar forming by the hydrolysis and polymerization of vinyl acetate. PVA is a good biodegradable synthetic material with high strength property and well suited for blends with natural polymeric materials. It is widely used in diverse applications of adhesives, plastics, and various binders (Kim and Netravali 2010). Recent researches have been conducted on development of the polymer composite blends of PVA with starch (Iman et al. 2005, Guohua et al. 2006). An incorporation of starch as fillers into PVA matrix was challenged to develop the market products such as agricultural mulching films or water-soluble laundry film bags (Chiellini et al. 2001a, b).
As the incorporation of other natural materials into the PVA polymer film, several studies have been shown to develop the casting films of PVA/gellan (Sudhamani et al. 2003) and PVA/konjac glucomannan (Xiao et al. 2000), as well as PVA composites containing proteins such as wheat gluten (Dicharry et al. 2006), collagen (Alexy et al. 2003, Sarti and Scandola 1995), and gelatin (Chiellini et al. 2001a, b, Bergo et al. 2006). However, the development of composite films of PVA polymer with the produce pomace has not yet been reported.
The objectives of this study were to develop the active film from apple pomace and PVA by casting for food packaging application and to determine the morphology properties, mechanical, thermal and gas permeability of developed films. This study also evaluates the capacity of antioxidant activity of the developed composite films. Knowledge developed from this study would be a good precursor for the useful application of apple pomace that may be suitable for bio-composite packaging materials.
Materials and methods
Materials
Apple pomace (AP) as the residue left after juice extraction was supplied by Hanul Food Co., (Sang-ju, Korea). The wet apple pomace was frozen at −18 °C in the refrigerator before use. Polyvinyl alcohol (99 % hydrolyzed grade, molecular weight range of 89,000–98,000) was obtained from OCI Company Ltd., (Seoul, Korea). Two, 2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma Chemical Co., (St. Louis, MO, USA).
Preparations of PVA composite films added with AP
Viscosity of PVA/AP solution
The viscosity of PVA/AP solutions was measured using a Brookfield Viscometer (Model DV- II, Brookfield Engineering Labs Inc., Stoughton, MA, USA). The PVA solution at 10 % (w/w) was prepared by dissolving PVA in distilled water. Then the PVA solutions were put into a jacketed container to measure the viscosity. A circulating water bath was connected to this jacketed container for maintaining the temperature at 25 °C. Before adding different concentrations of apple pomace, a suitable spindle was mounted and activated with a desired rotating speed, and measurements were taken after the designated temperature was reached and a stable reading was recorded. The viscosity was recorded at predetermined time intervals. An average of three replicates were reported for each time point.
Film preparations
The PVA/AP films were prepared by using a casting method. The temperature conditions of the experiment were based on the curing temperature of PVA reported in a previous study (Lui and Peng 2005). A known amount of PVA resin was placed into a 150 mL volumetric flask. 90 mL of distilled water was added and allowed to dissolve in an aqueous solution at 90 °C for 120 min. The weighed apple pomace powder was then added to the aqueous PVA solution. The mixture was blended to form a homogeneous gel-like solution with a mechanical stirrer (200 rpm, Hot plate & Stirrer, Seoul, Korea) at room temperature for 80 min. The prepared gel-like solution was poured on the glass plate (200 mm × 200 mm × 1 mm) with 500 mm thickness. The sample was dried in a ventilated heating oven (Sambo Inc., Seoul, Korea) at 50 °C for 24 h. Each composite film was prepared with the apple pomace contents of 1, 5, 10, and 30 % on the weight basis of the PVA (Fig. 1).
Fig. 1.
The prepared PVA/AP composite films containing the different apple pomace contents
Determination of properties of PVA/AP composite films
Film thickness
Film thickness was measured using a digital micrometer (Digimatic Micrometer MDC-25SB, Mitutoyo Co., Japan). Five replications were conducted for each sample treatment. Five measurements were taken at random positions around the film sample and the mean values were calculated.
Mechanical properties
Mechanical properties including tensile strength (TS) and percentage of elongation at break (%E) were measured with a texture analyzer (TA-XT2, Stable Micro System Ltd., UK) following the ASTM Standard Test Method D 882-91 (ASTM 2010). Each film strip (15 × 2.5 cm) was mounted between the grips and tested with an initial grip separation of 5 cm and crosshead speed of 5 mm/min. TS was calculated by dividing the maximum load by the initial cross sectional area of the film sample expressed as Mpa. Elongation at break was calculated as the ratio of the film extension at the point of sample rupture to the initial length of a sample and expressed as a percentage. Measurements represent an average of five samples.
Oxygen transmission rate (OTR)
Oxygen transmission rates of the developed films were measured using the oxygen permeation tester (8001 Oxygen Permeation Analyzer, Illinois Instruments Co., USA). Oxygen transmission rates were set with an auto stop program when the rate of error was ±1 %. Each sample was tested in triplicate. The flow rates of oxygen and nitrogen were 20 and 10cm3/min, respectively. The test samples were measured at the temperature of 23 °C and a relative humidity of 50 ± 5 % according to ASTM D 3985 (ASTM 2010).
Color and opacity
Color of film was determined using a Minolta Chroma Meter (CR-300, Minolta Camera Co., Osaka, Japan).Color of film was expressed as L*- (lightness), a* - (redness/greenness) and b*- (yellowness/blueness) values. Total difference in color (∆E*) was calculated according to the Eq. 1 (Gennadios et al. 1996).
Opacity was determined by measuring the film absorbance at 600 nm using a UV spectrophotometer (UV/Vis spectrometer Lambda 25, PerkinElmer, Inc., USA). The films were cut into a rectangle piece and directly placed in a spectrophotometer test cell. An empty test cell was used as reference. The opacity of the films was calculated by the Eq. 2.
| 1 |
| 2 |
where ΔL = Lstandard − Lsample , Δa = astandard − asample , and Δb = bstandard − bsample, T is the transparency, Abs600 is the value of absorbance at 600 nm, and x is the film thickness (mm). According to this equation, the high values of T indicate lower transparency and higher degree of opacity.
Characterization of the selected films
Thermo-gravimetric analysis (TGA)
Films were scanned using a thermo-gravimetric analyzer (TGA-4000, Perkin Elmer Co., Netherlands) from 25 to 800 °C at a rate of 20 °C/min (ASTM E1131). Nitrogen was used as the purge gas at a flow rate of 20 mL/min.
Scanning electron microscopy (SEM)
Morphology of surface and freeze-fractured cross-section of film samples were visualised using a scanning electron microscope (SEM) (LEICA S 360, Leica Cambridge Ltd., USA) at an accelerating voltage from 10 to 15 kV. Prior to visualization, the film samples were cut with a sharp scalpel, and were mounted on aluminum stubs and sputtered with gold in order to make the sample conductive. For cross-section, freeze-fractured films were mounted around stub using carbon adhesive tape, coated with gold.
Fourier-transform infrared (FTIR) spectroscopy
Fourier-transform infrared (FTIR) spectroscopy was carried out to observe the structural interactions between PVA films incorporated with apple pomace polyphenols. The FTIR spectra of the films were recorded from 400 and 4000 cm−1 at resolution of 4 cm−1 using an FTIR spectrometer (FTIR) (PerkinElmer, spectrum 65, Netherlands).
X-ray diffraction
X-ray patterns of PVA/AP composite films were analyzed using an X-ray diffractometer (D2 PHASER, Bruker, Germany) with Cu Kα radiation at a voltage of 40 kV and 30 mA. The samples were scanned between 2θ = 3–40° with a scanning speed of 2° min−1. Prior to testing, the samples were dried and stored in a desiccator.
Analysis of the selected films
Total phenolic (TP) content
25 mg of each film sample was dissolved in 5 mL of distilled water. Total phenolic content of the films was determined according to the Folin–Ciocalteu method as described by Siripatrawan and Harte (2010) Briefly, 0.1 ml of film extract solution were mixed with 7 ml distilled water and 0.5 ml of Folin & Ciocalteu reagent (0.5 ml) (Kanto Chemical Co. Inc. Tokyo, Japan) The mixture was incubated for 8 min at room temperature before addition of 1.5 ml of sodium carbonate solution and 0.9 ml of distilled water. The mixture was stored in a dark chamber at room temperature for 2 h. The absorbance of the mixture was then measured at 765 nm using a spectrophotometer (UV/Vis spectrometer Lambda 25, PerkinElmer, Inc., USA) Gallic acid solutions (Kanto Chemical Co. Inc. Tokyo, Japan) in the specific concentration range were used to construct a calibration curve. The concentration of total phenolic compounds in the samples is expressed as gallic acid equivalents (GAE), which reflect the phenolic content as the amount of gallic acid in mg per gram dry weight of the sample. The results were expressed as mg gallic acid equivalents (GAE) per gram of dried film according to the Eq. 3.
| 3 |
where, Cg is the concentration of gallic acid obtained from the calibration curve (mg/mL), V is the volume of the film extract (mL). Ws is the weight of dried film (g).
Determination of antioxidant activity
The antioxidant activity of the film samples was evaluated using DPPH (2, 2-diphenyl-1-picrylhydrazyl) free radical scavenging assay (Blois 1958). 3 mL of film extract solution were mixed with 1 mL of 1 mM methanolic solution of DPPH. The mixture was blended using a vortex-Genie2 (Scientific Industries, Inc., USA) and incubated in the dark at ambient temperature for 30 min. When the DPPH solution was mixed with the sample mixture acting as a hydrogen atom donor, a stable nonradical form of DPPH is obtained with simultaneous change of the violet color to pale yellow. The absorbance was then measured at 517 nm. The percentage of DPPH free radical quenching activity was determined using the following equation:
| 4 |
where, AbsDPPH is the absorbance value at 517 nm of the methanolic solution of DPPH. Absextract is the absorbance value at 517 nm for the sample extracts. Each sample was assayed at least five times.
Storage study of soybean oil for lipid oxidation
Soybean oil packaged with PVA/AP composite films was used to evaluate the oxidation of lipids during storage. The oxidation of lipid in the film sample was determined by thiobarbituric acid reactive substances (TBARS) analysis. PVA/AP films (50 × 50 mm) were immersed in 3 mL of oil. The extent of oxidation was determined at 23 and 60 °C for periods of up to 12 days. 23 °C is considered as ambient temperature and 60 °C is the commonly employed temperature in oil stability test since the rancidity develops at 60 °C in soybean oil (Jarvi et al. 1971). Selection of range of temperatures is not useful in the present study since 60 °C was the concern temperature in the stability test of soybean oil. At the end of the storage for 12 days, 0.1 mL of the oil sample was mixed with 0.9 mL water and 2.0 mL TBA reagent (5.2 mg/mL solution of TBA reagent) in test tubes and heated in a water bath for 15 min. The tubes were cooled to room temperature for 10 min and then vortexed for 2 min. The absorbance was measured at 532 nm. Concentrations of TBARS were evaluated from a standard calibration curve prepared using malondialdehyde (MDA) standard.
Statistical analysis
Data analysis for the experimental results was performed with the SPSS software (SPSS 10.0 for windows, SPSS Inc. Chicago, IL). Data were subjected to analysis of variance (ANOVA). The statistical significance of differences between mean values was established at p < 0.05 and the Duncan’s New Multiple Range Test was applied for all statistical analysis.
Results and discussion
Viscosity of film forming solution
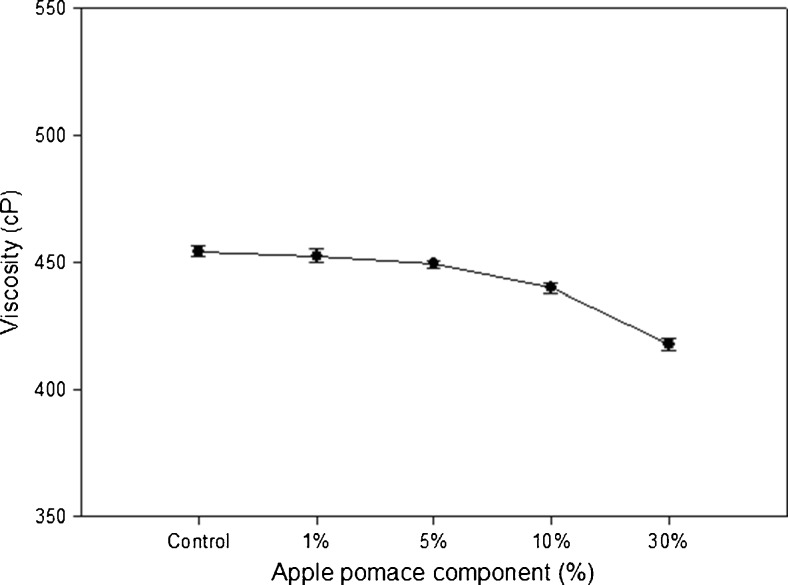
To observe the influence of the PVA solution with addition of AP on physical properties of the PVA/AP composite film, the viscosity of the PVA/AP solution was determined by the AP concentrations of 1, 5, 10, and 30 % (Fig. 2). The viscosity values of the PVA solutions with 1, 5, 10 and 30 % AP were 451, 448, 440 and 425 cP, respectively. There was no clear difference between pure PVA solution and the PVA solutions with 5 % AP. However, the PVA solutions with 10 and 30 % AP had lower values of viscosity as compared to the pure PVA solution. The results indicate that the increase of AP contents, decrease the viscosity of solution, because of interfering the hydrogen-bond interaction with PVA molecules. PVA polymer interaction with AP depends on the solution viscosity and may affect the film surface tension (Alexy et al. 2003).
Fig. 2.
Viscosity of film forming solutions with different concentrations of AP
Properties of PVA/AP composite films
Thickness
The thickness of PVA film and PVA/AP films containing 1, 5, 10, 30 % AP is shown in Table 1. Thickness of film increased with increasing AP concentration. It was plausibly due to the protruded structures mediated by interaction between chemical components present in apple pomace and PVA matrix. The results suggested that PVA could not form the compact and ordered film network in the presence of AP as indicated by increased thickness. Moreover, the increases in thickness with the addition of AP could be due to the increase in solid content in films. Arfat et al. (2014) reported the thickness of fish protein isolate/fish skin gelatin film is increased with increasing basil leaf oil due to the increase in solid content in films. Film containing 30 % AP had higher thickness, compared with control PVA film. All PVA/AP film was somewhat thicker than the control. However, there were no significant differences (p > 0.05) between each film.
Table 1.
Mechanical properties and OTR of PVA films formulated with AP
| Sample | Thickness (mm) | Tensile strength (MPa) | Elongation (%) | OTR (g/m2/day) |
|---|---|---|---|---|
| Control | 0.037 ± 0.001a | 11.24 ± 1.27a | 1.78 ± 0.25a | 1792 ± 70c |
| AP-1 | 0.042 ± 0.001a | 10.85 ± 0.88a | 1.68 ± 0.13ab | 1781 ± 82c |
| AP-5 | 0.041 ± 0.008a | 10.31 ± 1.80a | 1.44 ± 0.25bc | 1878 ± 33bc |
| AP-10 | 0.039 ± 0.007a | 7.24 ± 1.80b | 1.12 ± 0.25d | 1995 ± 66b |
| AP-30 | 0.045 ± 0.009a | 2.0 ± 0.76c | 0.88 ± 0.28e | 2211 ± 59a |
Mechanical property
The tensile strength (TS) is the measurement of maximum strength of a film to withstand against applied tensile stress and percentage of elongation (EL) represents the ability of a film to stretch (Park and Zhao 2004). TS and EL of the PVA/AP film containing did not change significantly when AP concentration increased from 0 to 5 % as shown in Table 1. Films containing 5 and 30 % AP showed significant changes, indicating that an increase in AP content in the films decreased their TS from 10.31 ± 1.80 to 2.0 ± 0.76 MPa and EL from 1.44 ± 0.25 to 0.88 ± 0.28 %. The results showed that the PVA/AP films had hard and fragile properties at AP contents greater than 10 %. This is in agreement with studies by Chiellini et al. (2001a, b), in which the composite films were too brittle and not suitable for the mechanical test at 70 % fiber content in the polymer matrix. Presence of sugar in AP is an effective plasticizer which promotes a considerable change in the mechanical properties of film (Teixeira and Da Róz 2007) and higher molecular weight of individual compounds such as phenolic compounds constituents present in the AP as well as hydrophilic properties of AP might have contributed to the similar tensile strength of PVA films by participating to increase the inter-chain interactions (Rababah et al. 2004). Flexibility can be related to the higher elongation values at breaking point. The addition of AP formulated film reduced the flexibility of the PVA films.
Oxygen transmission rate (OTR)
The oxygen transmission rate of the respective PVA/AP films containing AP of 1, 5, 10, and 30 % are shown in Table 1. The results exhibit that the oxygen transmission rate of PVA/AP film containing 5 % AP was 1792 ± 70 g/m2/day and not significantly different from its value of PVA film. However, over 10 % AP levels of PVA/AP film showed high oxygen transmission rates 1995 ± 66 to 2211 ± 59 g/m2/day compared to the PVA film. It assumes that an increase in the oxygen transmission rate with an increase of AP content in the films because the AP particles in the PVA film structure induce the porous micro-structural properties. This is due to the different tortuosity of the diffusion paths of the film systems, which becomes higher OTR when the particle is scatter. Similar results were obtained by Altiok et al. (2010), where films containing high concentrations of thyme oil increased the oxygen transmission rate. Jang and Lee (2003) reported that PVA has an excellent gas barrier due to it small, dense and closely packed monoclinic crystal structure.
Color and opacity of PVA/AP films
The effect of AP concentration on film color and opacity are shown in Table 2. PVA/AP film containing 1, 5, 10, 30 % AP were measured for surface color and transparency. Values of L, a, b, and ΔE on PVA/AP film containing 30 % AP were 92.08 ± 1.27, 0.32 ± 0.68, 19.17 ± 1.73, and 21.37 ± 2.14, respectively. L value of PVA/AP film decreased from 97.50 ± 0.25 to 92.08 ± 1.27, but the value of increased from 0.13 ± 0.20 to 0.32 ± 0.68 and b increased from 1.68 ± 0.02 to 19.17 ± 1.73 indicating the tendency towards redness and yellowness compared to the control (PVA film). These results indicated that an addition of the 30 % AP content in PVA films significantly affected the changes of L, a, and b values (p < 0.05). An increase of AP content in PVA/AP film also leads to high color and increased value of ΔE from 1.10 ± 0.24 to 21.37 ± 2.14, indicating the total color changes of films. The results indicated the polyphenol compounds present in AP contents can be attributed to the color change in PVA/AP film. Similar patterns were reported in aqueous green tea extract incorporated in chitosan films by Siripatrawan and Harte (2010). Chitosan film incorporated with aqueous green tea extract containing high content of polyphenols decreased the lightness.
Table 2.
Color values of PVA/AP films containing different concentrations of AP
| Sample | L | a | b | ΔE | T |
|---|---|---|---|---|---|
| Control | 97.50 ± 0.25a | 0.13 ± 0.20a | 1.68 ± 0.02a | 1.10 ± 0.24a | 1.36 ± 0.13a |
| AP-1 | 96.62 ± 0.46a | 0.21 ± 0.20b | 3.43 ± 0.49b | 2.66 ± 0.54ab | 2.58 ± 0.34b |
| AP-5 | 96.53 ± 0.65a | 0.22 ± 0.37b | 4.73 ± 0.55bc | 3.70 ± 0.69bc | 3.30 ± 0.23c |
| AP-10 | 94.92 ± 0.15b | 0.23 ± 0.32b | 5.88 ± 0.25c | 5.58 ± 0.14c | 4.33 ± 0.27d |
| AP-30 | 92.08 ± 1.27c | 0.32 ± 0.68c | 19.17 ± 1.73d | 21.37 ± 2.14d | 6.40 ± 0.59e |
The transparency values of all film sample are presented in Table 2. The control film had the lowest transparency value, compared with those films added with AP. The lower transparency values represents the greater transparency of the film. The transparency value of films increased from 1.36 ± 0.13 to 6.40 ± 0.59 as the level of AP incorporated increased (p < 0.05). This result was in agreement with Arfat et al. (2014) who reported that the films containing basil leaf essential oil became less transparent. The decrease in transparency of films were related with the decrease in light transmission when basil leaf essential oil was incorporated. Similarly, Wu et al. (2013) reported that the increases in transparency value of gelatin films incorporated with green tea extract were observed, compared with the control film. Thus, the incorporation of AP had the profound impact on light transmittance and transparency of films.
Characteristics of PVA/AP composite films
Thermogravimetric analysis (TGA)
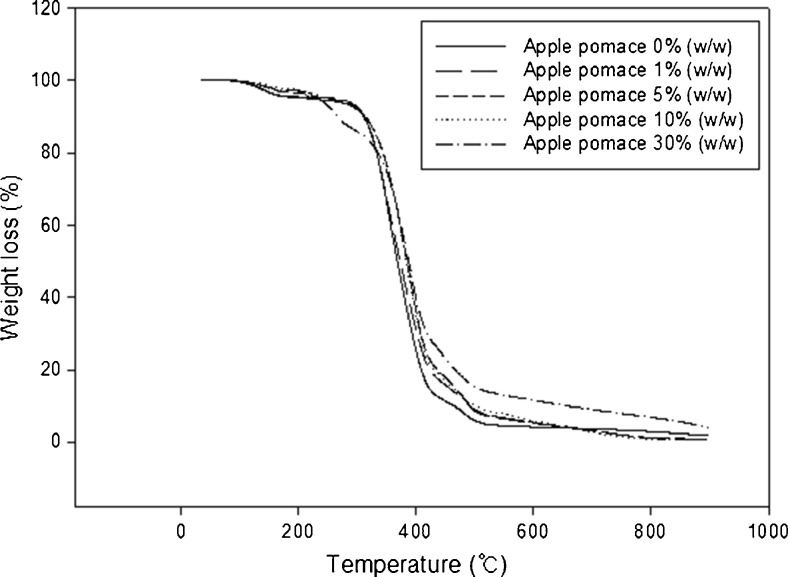
Figure 3 shows the weight remains versus temperature as measured by TGA for various PVA/AP blend films. The behavior of the mass loss curves was much similar for several films. Three distinct regions can be seen in these thermogravimetric curves, The initial weight loss is generally due to the loss of water in the blend system (25–150 °C); the second stage is the main degradation zone of both the apple pomace and the polyvinyl alcohol (predominantly due to dehydration of hydroxyl groups and the subsequent formation of low molecular weight unsaturated and aliphatic carbon species) (300–500 °C), and the final stage is generally carbonization (above 500 °C) (Luo et al. 2012).
Fig. 3.
TGA Curves of PVA/AP films containing different concentrations of apple pomace
Significant weight losses (%) occurred when the PVA film and PVA/AP films were between 294.39–330.03 °C. These temperatures corresponded to the beginning of thermal degradation. The PVA film underwent the thermal decomposition with an initial degradation temperature of 294.39 °C in a single-stage decomposition. A slightly higher temperature of degradation on PVA/AP films was observed because of the relatively poor thermal stability of PVA compared to the PVA/AP composite. An addition of apple pomace into the PVA film showed that the temperature of degradation shifted to a higher temperature. Similar finding was observed by Luo et al. (2012) in the study on effect of gelatinization and additives on morphology and thermal behavior of corn starch/PVA blend films. In general, the thermal degradation of fiber can be assumed to be a composite of the thermograms of the individual components.
Scanning electron microscopy (SEM)
SEM micrographs of the surfaces and freeze-fractured cross section of PVA/AP films containing 1, 5, 10, and 30 % AP are illustrated in Fig. 4. The SEM images indicate that the surface of the film without addition of AP was smooth and homogenous. The incorporation of AP films showed a rough surface without cracks compared to the control PVA film. The roughness of the surface on PVA/AP film increased with the increase of the AP contents because of the agglomeration of AP on the upper surface of film. This might associated with the coexisting of protruded film structure as indicated by the increased thickness of resulting film (Table 1). Arfat et al. (2014) reported similar result roughness of surface structure was more pronounced in films incorporated with BEO and ZnONP than that found in the control sample. The fracture of PVA/AP film illustrated that the agglomeration of the AP particles appeared as dark features in the micrographs, which resulted predominantly within the main PVA polymer phase. In addition, the results showed that small dark spots of AP particles were uniformly dispersed in the PVA polymer matrix as a filler material. This indicates that the interaction of AP bounding within the PVA matrix occurs during the casting process. Chiellini et al. (2001a, b), observed that pictures of the freeze-fractured surface revealed a uniform and close-packed distribution of fibers in a homogeneous smooth polymeric matrix. This is in agreement with the film sample surfaces in terms image analysis of the optical micrographs.
Fig. 4.
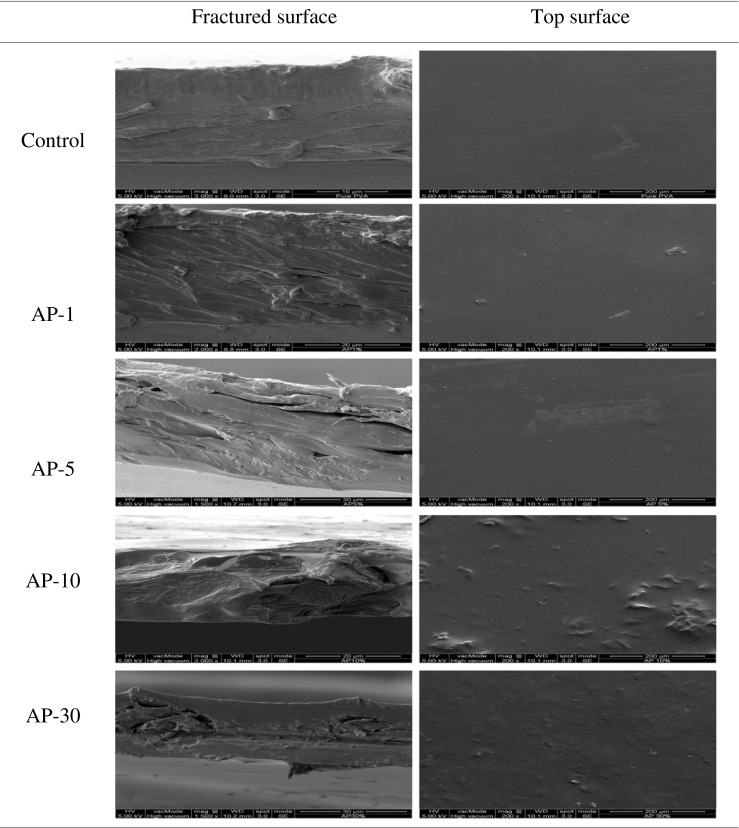
SEM photographs of the fractured and surface of PVA/AP films
Fourier-transform infrared (FT-IR) spectroscopy
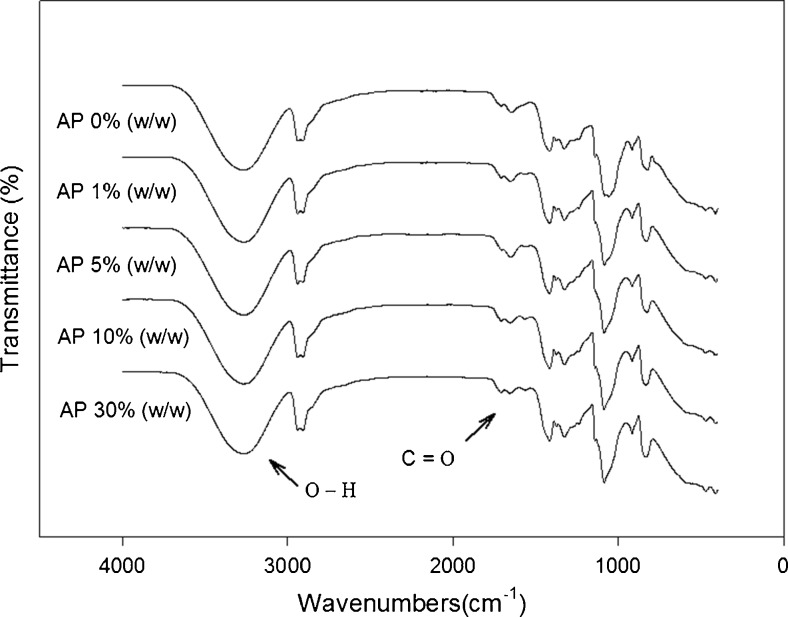
FTIR spectroscopy was used to investigate the interactions between PVA and apple pomace polyphenols by measuring the absorbent during wavenumber range of 4000–400 cm−1 at resolution of 4 cm−1.
The FTIR spectra of the control film and respective films containing 1, 5, 10 and 30 % of AP concentrations are shown in Fig. 5. The peaks between 3500 and 3000 cm−1, corresponding to stretching vibration of free hydroxyl and to asymmetric and symmetric stretching of the N-H bonds in amino group, respectively, similar in control film when compared to those incorporated with AP. It corresponds to the N-H bending band at 1560 cm−1 and the C=O stretching band for a small peak near to 1655 cm−1. A peak at 1740 cm−1 suggests the presence of a carbonyl groups. An increase in the absorption bands at 1700 and 1660 cm−1 in the FTIR spectra was coincidental with the decrease in peaks at 3500–3000, 1530 and 1440 cm−1. This observation supported the assumption that there could be a specific arrangement in the PVA/AP films due to the interactions of apple pomace polyphenolic compounds with hydroxyl and amino groups in PVA matrix. Siripatrawan and Harte (2010) reported similar effect on particular arrangement in the films due to the interactions of green tea polyphenolic compounds with hydroxyl and amino groups in chitosan matrix.
Fig. 5.
FTIR Spectrum of PVA films incorporated with AP
Each spectrum of the film samples showed no significant differences and represented very similar peak patterns. These results were similar to the results of the chitosan-PVA blended film spectrum reported by Miya and Iwamoto (1984).
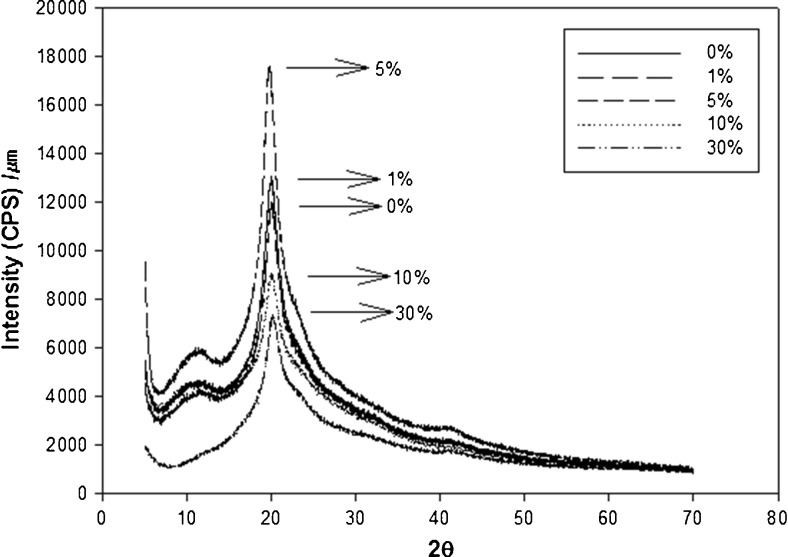
X-ray diffraction
X-ray diffraction is a proven tool to study crystal lattice arrangements and yields very useful information on degree of sample crystallinity (Bourbon et al. 2011). X-ray patterns of PVA and PVA/AP films containing AP of 0, 1, 5, 10, and 30 % are shown in Fig. 6. X-ray diffraction patterns of the PVA film showed the diffractive peak in the region from 2θ = 5° to 47° with the intensity of about 13,074 counts at 2θ = 19.9°. The PVA/AP film containing 1, 5, 10, and 30 % AP showed the peaks with the highest intensity about 12,071 counts at 2θ = 20.0°, about 17,612 counts at 2θ = 19.8°, about 9148 counts at 2θ = 20.0°, about 7095 counts at 2θ = 20.1°, respectively. The XRD patterns of PVA/AP films represented a typical property of partially crystalline materials with a characteristic peak at 2θ = 20° and is similar to the results reported by Kaczmarek and Podgorski (2007) indicating the XRD peak with PVA film without montmorillonite shows the broad signal characteristic for amorphous phase occurs at about 2θ = 19°. XRD pattern was observed for PVA containing 10 and 30 % AP, the signal intensity was proportionally lower than 1 and 5 % AP. The intensity of this peak is decreased when the amount of AP increased. From these diffractograms, it is obvious that PVA containing 5 % apple pomace is more crystalline than other film samples.
Fig. 6.
X-ray diffraction patterns of the respective PVA/AP films containing different AP contents of 1, 5, 10, and 30 %
Analysis of the selected PVA/AP films
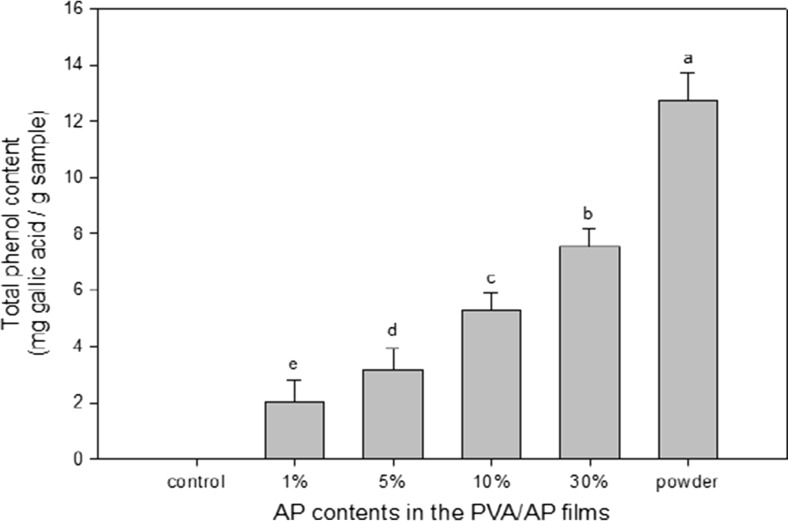
Total phenolic (TP) content
The control films contained no phenolics (data not shown). As shown in Fig. 7, the total phenolic contents in the PVA/AP films containing 1, 5, 10, 30 were 2, 3.7, 5.3, and 7.9 mg/g, respectively. The total phenolic content in the AP powder was 12.3 mg/g. The results showed that total phenolic content in the PVA/AP films were significantly increased with an increase of AP content. Similar finding was observed by Siripatrawan and Harte (2010) who found that total phenolic content in the chitosan film significantly increased with increasing green tea extract concentration. The total phenolic content of films directly correlated with the antioxidant activity measured by DPPH radical scavenging activity. Phenolic compounds are the effective hydrogen donors and possess ideal structures for scavenging free radicals, indicating a good antioxidant property. Arcan and Yemenicioglu (2011) reported that the incorporation of phenolic compounds such as catechin, gallic acid, p-hydroxy benzoic acid and ferulic acid in zein films may have the antioxidant function of the films because a considerable portion of the phenolic compound in the films were existed in soluble form.
Fig. 7.
Total polyphenolic contents on the respective PVA/AP films containing different AP contents. Values indicate the mean and standard deviation. Different letters show significantly different (p < 0.05) according to the analysis of Duncan’s new multiple range test
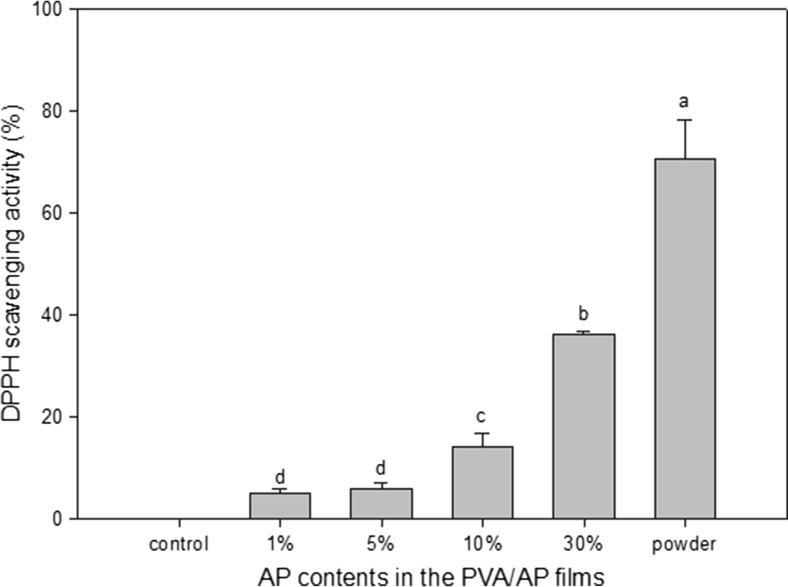
Antioxidant activity
The antioxidant activity of the PVA/AP film was determined by DPPH scavenging assay. The antioxidants can reduce the DPPH radical to a yellow-colored compound of diphenylpicrylhydrazine in the DPPH method. This reaction depends on the hydrogen-donating ability of the antioxidants (Blois 1958). The results showed that DPPH scavenging activities of the PVA/AP films were significantly increased (p < 0.05) with increasing AP content as shown in Fig. 8. The scavenging activities of PVA/AP film containing AP of 30 % was 39.8 %, compared to the control PVA film, which showed no scavenging activity value. The scavenging activities of AP powder was approximately 70 %. These results indicate that the AP powder had a high antioxidant capacity. As the AP increased in the film formulation, so did the expected antioxidant property of the active film. This could be explained by AP compositions containing plenty of phenolic compounds. Similar results were found in the composite films based on gelatin containing oregano and rosemary extracts (Gomez-Estaca et al. 2009).
Fig. 8.
DPPH scavenging activity of PVA/AP films containing AP of the different concentrations. Different letters indicate a statistically significant difference (P < 0.05)
The major components of the apple polyphenols, procyanidins have long been recognized as having high antioxidant activity (Chinnici et al. 2004, Tsao et al. 2005) and derivatives (Oszmiański et al. 2008). The antioxidant activity of hydroxycinnamic and benzoic acids and flavonoids as other phenols have also been ascertained (Tsao et al. 2005, Kim et al. 2002). Interactions between PLA and polyphenolic compounds from AP may play a major role on the modification of film properties. From these results we conclude that the, use of the apple pomace with the PVA improve the antioxidant property of the film as well as increase in the amount of AP increasing in the antioxidant properties of the film.
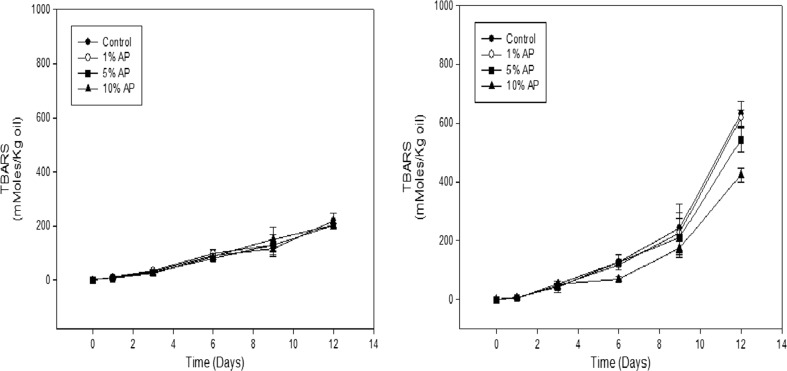
Storage study of soybean oil for lipid oxidation
The effect of the inclusion of apple pomace on the oxidative stability of oil during storage at 23 and 60 °C was reflected by the TBARS values which is an index of lipid peroxidation are shown in Fig. 9. Soybean oil packaged in the respective PVA/AP films began to increase their TBARS value during storage on day 3. However, the TBARS value in the soybean oil packaged in the PVA/AP film was not significantly different (P > 0.05) compared to that in the control PVA film as a function of the storage time at 23 °C. The highest values of TBARS were observed in the soybean oil packaged in the control during storage at 60 °C. It indicates that the effect of the PVA/AP film containing 10 % AP was much greater than that of the control and PVA/AP films containing 1 and 5 % AP in retarding lipid oxidation, this is probably due to oxygen permeability characteristic of PVA/AP film. Increasing the amount of AP in the film oxygen permeability of film is increase (Xu et al. 2005). The antioxidant activities of the AP polyphenols have been attributed to assorted mechanisms, including prevention of radical chain initiation, binding of transition metal ion catalysts, and interaction with the free radicals to inhibit lipid oxidation (Yalcin et al. 2011). The results reported by Siripatrawan and Harte (2010) for chitosan films containing the total phenolic compounds in green tea extract showed similar free radical scavenging ability of the films with an increase of green tea extract content. Antioxidant properties of apple pomace is very high (Yalcin et al. 2011). This result suggested that lipid oxidation in soybean oil samples could be minimized by incorporation of AP in the PVA film due to the antioxidant activity of AP.
Fig. 9.
Effect of the respective PVA/AP film pouch on the oxidative stability for soybean oil at 23 and 60 °C
Conclusion
This study indicated that an active film from PVA/AP prepared by a cast process had a great potential application in consuming apple pomace as the natural byproduct of apple. The PVA/AP film also showed function of antioxidant activities. FTIR spectra demonstrated good interaction between functional groups of PVA with AP. The compatibility of PVA with AP film was also confirmed. In addition, apple pomace imparted excellent antioxidant activities to the films. Therefore, this film can be of great potential for being developed into functional packaging material for food to ensure food safety and to extend the shelf-life of packaged foods and is a promising alternative to synthetic packaging materials.
Footnotes
Research highlights
• Bio-composite polyvinyl alcohol (PVA) films with apple pomace for food packaging were developed and characterized
• Physico-mechanical properties of polyvinyl alcohol films were governed by apple pomace.
• PVA/AP film had improved antioxidant property.
• PVA film incorporated with apple pomace has well efficient to retard lipid oxidation.
• PVA film added with apple pomace could be used as active packaging material.
References
- Alexy P, Bakos D, Hanzelova LS, Kukol LL, Kupec J, Charva Ltova LK, Chiellini E, Cinelli P. Poly (vinyl alcohol)-collagen hydrolysate thermoplastic blends: I. Experimental design optimization and biodegradation behavior. Polym Test. 2003;22:801–809. doi: 10.1016/S0142-9418(03)00016-3. [DOI] [Google Scholar]
- Altiok D, Altiok E, Tihminlioglu F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J Mater Sci Mater Med. 2010;21:2227–2236. doi: 10.1007/s10856-010-4065-x. [DOI] [PubMed] [Google Scholar]
- Arcan I, Yemenicioglu A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res Int. 2011;44:550–556. doi: 10.1016/j.foodres.2010.11.034. [DOI] [Google Scholar]
- Arfat YA, Benjakul S, Prodpran T, Sumpavapol P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocolloids. 2014;41:265–273. doi: 10.1016/j.foodhyd.2014.04.023. [DOI] [Google Scholar]
- ASTM (American Society for Testing and Materials) (2010) Standard test method for oxygen gas transmission rate through plastic film and sheeting using a coulometric sensor, D-3985, Annual book of ASTM. Philadelphia, PA, USA
- Avella M, Buzarovska A, Errico ME, Gentile G, Grozdanov A. Eco-challenges of bio-based polymer composites. Materials. 2009;2:911–925. doi: 10.3390/ma2030911. [DOI] [Google Scholar]
- Bergo PV, Carvalho RA, Sobral PJA, Bevilacqua FRS, Pinto JKC, Souza J P. Microwave insertion loss measurements in gelatin-based films. Meas Sci Technol. 2006;17:3261–3264. doi: 10.1088/0957-0233/17/12/010. [DOI] [Google Scholar]
- Bhushan S, Kalia K. Processing of apple pomace for bioactive molecules. Crit Rev Biotechnol. 2008;181:1199–1200. doi: 10.1080/07388550802368895. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of as table free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bourbon AI, Pinheiro AC, Cerqueira MA, Rocha CMR, Avides MC, Quintas MAC, Vicente AA. Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. J Food Eng. 2011;106:111–118. doi: 10.1016/j.jfoodeng.2011.03.024. [DOI] [Google Scholar]
- Chiellini E, Cinelli P, Fernandes EG, Kenawy ES, Lazzeri A. Gelatin-based blends and composites. Morphological and thermal mechanical characterization. Biomacromolecules. 2001;2:806–811. doi: 10.1021/bm015519h. [DOI] [PubMed] [Google Scholar]
- Chiellini E, Cinelli P, Imam SH, Mao L. Composite films based on biorelated agro-industrial waste and poly (vinyl alcohol) preparation and mechanical properties characterization. Biomacromolecules. 2001;2:1029–1037. doi: 10.1021/bm010084j. [DOI] [PubMed] [Google Scholar]
- Chinnici F, Bendini A, Gaiani A, Riponi C. Radical scavenging activities of peels and pulps from cv. golden delicious apples as related to their phenolic composition. J Agric Food Chem. 2004;52:4684–4689. doi: 10.1021/jf049770a. [DOI] [PubMed] [Google Scholar]
- Dicharry RN, Ye P, Saha G, Waxman E, Asandei AD, Parnas RS. Wheat gluten-thiolated poly(vinyl alcohol) blends with improved mechanical properties. Biomacromolecules. 2006;7:2837–2844. doi: 10.1021/bm060432n. [DOI] [PubMed] [Google Scholar]
- Galanakis C M. Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci Technol. 2012;26:68–87. doi: 10.1016/j.tifs.2012.03.003. [DOI] [Google Scholar]
- Gennadios A, Weller CL, Hanna MA, Froning GW. Mechanical and barrier properties of egg albumin films. J Food Sci. 1996;61:585–589. doi: 10.1111/j.1365-2621.1996.tb13164.x. [DOI] [Google Scholar]
- Gomez-Estaca J, Bravo L, Gomez-Guillen MC, Aleman A, Montero P. Antioxidant properties of tuna-skin and bovine-hide gelatin films induced by the addition of oregano and rosemary extracts. Food Chem. 2009;112:18–25. doi: 10.1016/j.foodchem.2008.05.034. [DOI] [Google Scholar]
- Guohua Z, Ya L, Cuilian F, Min Z, Caiqiong Z, Zongdao C. Water resistance, mechanical properties of methylated-corn starch/poly(vinyl alcohol) blend film. Polym Degrad Stab. 2006;91:703–711. doi: 10.1016/j.polymdegradstab.2005.06.008. [DOI] [Google Scholar]
- Iman SH, Cinelli P, Gordon SH, Chiellini E. Characterization of biodegradable composite films prepared from blends of poly (vinyl alcohol), cornstarch, and lignocellulosic fiber. J Polym Environ. 2005;13:47–55. doi: 10.1007/s10924-004-1215-6. [DOI] [Google Scholar]
- Jang J, Lee DK. Plasticizer effect on the melting and crystallization behavior of polyvinyl alcohol. Polymer. 2003;44:8139–8146. doi: 10.1016/j.polymer.2003.10.015. [DOI] [Google Scholar]
- Jarvi PK, Lee GD, Erickson DR, Butkus EA. Determination of the extent of rancidity of soybean oil by gas chromatography compared with peroxide value. J Am Oil Chem Soc. 1971;48:121–124. doi: 10.1007/BF02545733. [DOI] [Google Scholar]
- Jiang Y, Simonsen J. Compression-molded biocomposite boards from red and white wine grape pomaces. J Appl Polym Sci. 2011;119:2834–2846. doi: 10.1002/app.32961. [DOI] [Google Scholar]
- Joshi VK, Parmar M, Rana NS. Pectin esterase production from apple pomace in solid-state and submerged fermentation. Food Technol Biotechnol. 2006;44:253–256. [Google Scholar]
- Kaczmarek H, Podgorski A. The effect of UV-irradiation on poly(vinyl alcohol) composites with montmorillonite. J Photochem Photobiol. 2007;191:209–215. doi: 10.1016/j.jphotochem.2007.04.025. [DOI] [Google Scholar]
- Kim JT, Netravali AN. Mercerization of sisal fibers: effect of tension on mechanical properties of sisal fiber and fiber-reinforced composites. Appl Sci Manuf. 2010;41:1245–1252. doi: 10.1016/j.compositesa.2010.05.007. [DOI] [Google Scholar]
- Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- López-de-Dicastillo C, Gómez-Estaca J, Catalá R, Gavara R, Hernández-Muñoz P. Active antioxidant packaging films: development and effect on lipid stability of brined sardines. Food Chem. 2012;131:1376–1384. doi: 10.1016/j.foodchem.2011.10.002. [DOI] [Google Scholar]
- Lui WB, Peng J. Physical, mechanical, biodegradable properties and energy absorption behavior of corn grit-polyvinyl alcohol cushioning extrudates. J Food Eng. 2005;71:73–84. doi: 10.1016/j.jfoodeng.2004.10.021. [DOI] [Google Scholar]
- Luo X, Li J, Lin X. Effect of gelatinization and additives on morphology and thermal behavior of corn starch/PVA blend films. Carbohydr Polym. 2012;90:1595–1600. doi: 10.1016/j.carbpol.2012.07.036. [DOI] [PubMed] [Google Scholar]
- Miya M, Iwamoto R. FT-IR study of intermolecular interactions in polymer blends. J Polym Sci Part B Polym Phys. 1984;22:1149–1151. doi: 10.1002/pol.1984.180220615. [DOI] [Google Scholar]
- Narayan R. Polymeric materials from agricultural feedstocks. In: Fishman ML, Friedman RB, Huang SJ, editors. Polymers from agricultural coproducts, ACS symposium series. American Chemical Society: Washington, DC; 1994. pp. 2–28. [Google Scholar]
- Oszmiański J, Wolniakb M, Wojdyłoa A, Wawerb A. Influence of apple purée preparation and storage on polyphenol contents and antioxidant activity. Food Chem. 2008;107:1473–1484. doi: 10.1016/j.foodchem.2007.10.003. [DOI] [Google Scholar]
- Park S, Zhao Y. Incorporation of a high concentration of mineral or vitamin into chitosan-based films. J Agric Food Chem. 2004;52:1933–1939. doi: 10.1021/jf034612p. [DOI] [PubMed] [Google Scholar]
- Rababah TM, Hettiarachchy NS, Horax R. Total phenolics and antioxidant activities of fenugreek, green tea, black tea, grape seed, ginger, rosmary, gotu kola, ginkogo extracts, vitamin E and tert-butylhdroquinon. J Agric Food Chem. 2004;52:5183–5186. doi: 10.1021/jf049645z. [DOI] [PubMed] [Google Scholar]
- Reis SF, Raib DK, Ghannama AB. Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chem. 2012;135:1991–1998. doi: 10.1016/j.foodchem.2012.06.068. [DOI] [PubMed] [Google Scholar]
- Sarti B, Scandola M. Viscoelastic and thermal properties of collagen/poly (vinyl alcohol) blends. Biomaterials. 1995;16:785–792. doi: 10.1016/0142-9612(95)99641-X. [DOI] [PubMed] [Google Scholar]
- Siripatrawan U, Harte BR. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010;24:770–775. doi: 10.1016/j.foodhyd.2010.04.003. [DOI] [Google Scholar]
- Sudhamani SR, Prasad MS, Sankar KU. DSC and FTIR studies on gellan and polyvinyl alcohol (PVA) blend films. Food Hydrocoll. 2003;17:245–250. doi: 10.1016/S0268-005X(02)00057-7. [DOI] [Google Scholar]
- Teixeira EM, Da Róz AL. The effect of glycerol/sugar/water and sugar/water mixtures on the plasticization of thermoplastic cassava starch. Carbohydr Polym. 2007;69:619–624. doi: 10.1016/j.carbpol.2007.01.022. [DOI] [Google Scholar]
- Tsao R, Yang R, Xie S, Sockovie E, Khanizadeh S. Which polyphenolic compounds contribute to the total antioxidant activities of apple? J Agric Food Chem. 2005;53:4989–4995. doi: 10.1021/jf048289h. [DOI] [PubMed] [Google Scholar]
- Wu J, Chen S, Ge S, Miao J, Li J, Zhang Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013;32:42–51. doi: 10.1016/j.foodhyd.2012.11.029. [DOI] [Google Scholar]
- Xiao C, Liu H, Gao S, Zhang L. Characterization of poly (vinyl alcohol)-konjac glucomannan blend films. J Macromol Sci Pure Appl Chem. 2000;34:1009–1021. doi: 10.1081/MA-100101137. [DOI] [Google Scholar]
- Xu YX, Kim KM, Hanna MA, Nag D. Chitosan–starch composite film: preparation and characterization. Ind Crop Prod. 2005;21:185–192. doi: 10.1016/j.indcrop.2004.03.002. [DOI] [Google Scholar]
- Yalcin H, Karaman S, Ozturk I. Evaluation of antioxidant efficiency of potato and orange peel and apple pomace extract in sunflower oil. Ital J Food Sci. 2011;23:55–61. [Google Scholar]