Abstract
Oxidative stress is an important pathomechanism of neurological disorders such as Alzheimer disease and Parkinson disease, cardiovascular disorders and many others. This study sought to verify whether extra-virgin olive oil (EVOO), lipophilic fraction (OOLF) and hydrophilic fraction (OOHF) exerted a brain protective effect against the oxidative stress caused by 2,4-dichlorophenoxyacetic acid (2,4-D) pesticide at a dose of 5 mg/kg body weight. 2,4-D, EVOO and its fractions were administered to rats by gavages for four consecutive weeks. Oxidative stress was assessed by measuring brain lipid peroxide level, acetylcholinesterase (AChE), antioxidant enzyme activities and fatty acid composition. 2,4-D induced a decrease in both plasma and brain acetylcholinesterase activity and a rise in Brain TBARS (Thiobarbituric acid reactive substances) level and antioxidant enzyme activities compared with the control group. These changes were partly reversed by either EVOO or its fractions oral administration to 2,4-D treated rats. EVOO enhanced a neuroprotective effect evaluated by the restoration of brain fatty acid composition especially the level of docosahexaenoic acid (DHA). Our results indicate that EVOO exerts a neuroprotective activity against oxidative damage in brain induced by 2,4-D, which could be attributed to its antioxidative property.
Keywords: Extra virgin olive oil; Oxidative stress; Acetylcholinesterase; Rat; Brain and 2,4-D
Introduction
The central nervous system (CNS) is mainly susceptible to oxidative stress. This is mainly due to its high amounts of polyunsaturated fatty acids (PUFA) content which constitutes easily- oxidizable substrates and an inherently high flux of reactive oxygen species (ROS) (Bongiovanni et al. 2007, 2011). Another reason of oxidative stress is the low level of endogenous antioxidant enzymes in CNS relative to other tissues, and its high oxygen consumption (Somani et al. 1996). In fact, under physiological O2 levels, 1–2 % of the O2 consumed is converted to ROS mostly through the mitochondrial respiratory chain (Emerit et al. 2004). Therefore, the CNS is potentially sensitive to oxidative damage (Bongiovanni et al. 2007, 2011) which may play a role in dementia and age-associated neurodegenerative diseases such as Alzheimer’s disease (Behl 1999; Pratico and Delanty 2000).
Some previous studies have indicated that the CNS is one target organ for chlorophenoxyherbicide toxic effects in different animal species (Brusco et al. 1997). 2,4-Dichlorophenoxyacetic acid (2,4-D) is a selective herbicide of the phenoxyacetic acid group, with weak aromatic acid properties. Its herbicidal activity is mediated by an auxin like capacity to alter normal protein synthesis and cell division in plant meristems and leaves (Stevens and Breckenridge 2001). Exposure of humans and animals occurs through contaminated air, drinking water, soil and foodstuff or during production of the herbicide. It has been shown that 2,4-D produce a wide range of adverse effects on health from embryotoxicity and teratogenicity to neurotoxicity of animals and humans, partly due to the generation of free radicals (Venkov et al. 2000; Barnekow et al. 2000; Madrigal-Bujaidar et al. 2001).
There is now sufficient scientific evidence supporting the notion that increasing adherence to the dietary pattern characteristic of Mediterranean countries is associated with a reduction of overall, cardiovascular, cancer and neurodegenerative diseases (Agudo et al. 2007; Sofi et al. 2008). The benefits of the Mediterranean diet have been attributed, in part, to the antioxidant effect of some of its components, mainly virgin olive oil (VOO), which contains polyphenolic compounds with a clear anti-inflammatory and antioxidant effects (Visioli et al. 2002) and is a rich source of monounsaturated fatty acids (MUFA). Both of these components mediate the protective effects associated with olive oil in a number of ways. The nutritional benefits of MUFA-rich diets are well accepted. Nevertheless, the health benefits of phenolic compounds (non-nutritive dietary components) should not be overlooked. Polyphenol compounds have shown a number of biological effects both in vitro and in vivo, including antioxidation, anti-apoptosis, anti-aging, anti-carcinogenesis, anti-inflammation, anti-neurodegeneration and anti-atherosclerosis (Chang 2013). Historically, the biological actions of phenols, including those on the brain, have been attributed to their ability to exert antioxidant actions (Rice-Evans et al. 1996), through their ability to scavenge reactive species, or through their possible influences on intracellular redox status (Pollard et al. 2006). In addition, olive oil phenols have been shown to be some of neuroprotective effects against cerebral ischemia (Mohagheghi et al. 2010), spinal cord injury (Impellizzeri et al. 2012), Huntington’s disease (Tasset et al. 2011), Alzheimer’s diseases (Monti et al. 2011), Parkinson’s disease (Jones 2011), aging (Pitozzi et al. 2010), and peripheral neuropathy (Ristagno et al. 2012). On the other hand, some findings suggested that olive oil has beneficial effects on learning and memory deficits found in aging by reversing oxidative damage in the brain (Farr et al. 2012).
Consequently, extra virgin olive oil (EVOO) could be of great interest in the prevention of various aspects of neurodegenerative diseases. The aim of the present study was to investigate the effects of virgin olive oil and its fractions on 2,4-D induced oxidative stress in wistar rat brain slices, who lipid peroxidation and antioxidant defence systems and particularly brain lipid profile and fatty acid composition were determined.
Materials and methods
Materials
A commercial 2, 4-D formulation (Désormone Lourd) consisting of 600 g/L 2,4-D ester butylglycol, register number H.96064 (SEPCM), available in Tunisia, was used in the experimentation. 2-Thiobarbituric acid (TBA) was obtained from Sigma Chemicals Company (Taufkirchen, Germany). Folin-Ciocalteu phenol reagent was purchased from Fluka Biochemika (Buchs, Switzerland). All other used chemicals were of analytical grade and were obtained from Sigma Chemicals Company or Merck (Darmstadt, Germany).
Oil sample analysis
The extra virgin olive oil (EVOO) used in this experiment was obtained from Chétoui cultivar grown in the North of Tunisia. The olive oil hydrophilic fraction (OOHF) were extracted from EVOO by Montedoro et al. (1992) method using water instead of methanol to avoid its toxic effect in rats. Briefly, 10 g of EVOO was homogenized with 10 ml of water by a mixer (Ultra-Turrax T25 [IKA Labortechnik, Janke & Kunkel, Staufen, Germany]; 15 000 g/min) and centrifuged at 5000 g for 10 min. The extraction was performed two times.
The olive oil lipophilic fraction (OOLF) was obtained from EVOO as follows: the EVOO was homogenized for 1 min with water (1:1, v/v), and the oil was separated by centrifugation; this procedure was repeated six times. Then, the oil fraction (OOLF) was filtered through a cellulose acetate membrane. The procedure employed to produce the OOLF, at variance with a washing process, has been developed to selectively eliminate hydrophilic substances, such as phenolic compounds, leaving unmodified the other olive oil components such as tocopherols and fatty acids. Different fractions were daily and freshly prepared and their composition was checked at the end of the treatment.
Fatty acids were converted into fatty acid methyl esters (FAMEs) prepared by dissolving 0.1 g of EVOO or OOLF in 2 ml of heptane and 0.2 ml of KOH (0.2 N) in methanol and incubated for 1 h. Individual FAMES were separated and quantified by gas chromatography using model 5890 series II instrument (Hewelett-Packard Ca Palo Alto, CA, USA) equipped with a flame ionisation detector and a fused silica capillary column HP - INNOWAX ([J & W Scientific, Agilent, Palo Alto, CA, USA]; 30 m length × 0.25 mm i.d. and 0.25 μm of film thickness). The temperature was programmed to increase from 170 to 270 °C at a rate of 5 °C per minute. Nitrogen ultra was used as carrier gas. Results were expressed as relative area percent of the total FAMES (Dabbou et al. 2009).
Oxidation stability was evaluated by the Rancimat apparatus (Model 743, Metrohm Schweiz AG, Zofingen, Switzerland) using 3 g of oil sample heated to 120 °C with 20 l/h air flow (Tura et al. 2007). Stability was expressed as oxidation induction time (hours).
Carotenoids and chlorophylls (mg/kg oil) were determined at 470 and 670 nm, respectively, in cyclohexane using the specific extinction values according to the method of Minguez-Mosquera et al. ( 1991 ).
Phenolic compounds were extracted, estimated colorimetrically at 765 nm using the Folin–Ciocalteau reagent, and expressed as hydroxytyrosol equivalents as reported by Montedoro et al. (1992).
For the α-tocopherol analysis, the sample was diluted with n-hexane (1:10), the mixture was vortexed and 200 μl was transferred to a test tube containing 600 μl of methanol and 200 μl of internal standard (300 μg/ml). HPLC separation was carried out on a Hewlett-Packard system (Waldbronn, Germany) comprising a HP-1100 pump, a Rheodyne model 7725 injector (Cotati, CA, USA, loop volume 20 μl), a HP-1200 M multi-array detector and a Supelcosil ODS-2 column (150 × 4.5 mm id., film thickness 5 μm) (Gimeno et al. 2000).
Animals and treatment
This study was carried out using healthy male adult Wistar rats weighing 200–230 g and aged 7 to 8 weeks. The animals were housed under standard laboratory conditions of light (12- h light-dark periods), temperature (22 ± 3 °C) and relative humidity of 40 %. They were given standard diet (SICO, Sfax Tunisia) and tap water ad libitum. According to our previous paper (Nakbi et al. 2010), the animals were randomly divided into 8 experimental groups, each group consisting of 10 rats (Table 1).
Table 1.
Dietary intake of control and experimental rats
| Groups | Standard diet (g/day/rat) | EVOO (ml/day) | OOHF (ml/day) | OOLF (ml/day) | 2,4-D (mg/kg b.w./day) | Fat intake (g/day/rat) |
|---|---|---|---|---|---|---|
| C | 17.18 | - | - | - | - | 2.66 |
| 2,4-D | 18.42 | - | - | - | 5 | 2.85 |
| 2,4-D/EVOO | 18.02 | 0.3 | - | - | 5 | 3.09 |
| 2,4-D/OOHF | 18.82 | - | 1 | - | 5 | 2.91 |
| 2,4-D/OOLF | 17.94 | - | - | 0.3 | 5 | 3.08 |
| EVOO | 17.55 | 0.3 | - | - | - | 3.02 |
| OOHF | 18.97 | - | 1 | - | - | 2.94 |
| OOLF | 17.47 | - | - | 0.3 | - | 3 |
C control group, 2,4-D 2, 4-D treated group at dose 5 mg/kg BW, 2,4-D/EVOO 2, 4-D (5 mg/kg BW) plus extra virgin olive oil, 2,4-D/OOHF 0.2, 4-D plus hydrophilic fraction, 2,4-D/OOLF 0.2, 4-D plus lipophilic fraction, EVOO extra virgin olive oil treated group alone, OOHF group treated with hydrophilic fraction of olive oil, OOLF group treated with lipophilic fraction of olive oil
At the end of experimental period of 4-week, the animals were sacrificed. The plasma samples were collected and stored at −80 °C for analysis.
The brains of all dissected animals were carefully removed, washed in an isotonic solution, blotted on a filter paper and weighed. Then, some samples (cerebral cortex) were put in a suitable fixative (Bouin liquid) for histological studies. The others were minced and homogenized (10 % w/v) in an appropriate buffer (pH 7.4) and centrifuged. The resulting supernatants were collected and stored at −80 °C until used for enzyme assays.
The wet weight of the brain was defined as the absolute brain weight (g). The relative brain weight was calculated using the final body weight (bw) and absolute brain weight according to the following equation to reduce individual body weight differences:
Biochemical determinations
Thiobarbituric acid reactive substance measurements
Lipid peroxidation in homogenates of Cerebral cortex was assessed by measuring the thiobarbituric acid reactive substances (TBARS) according to the method of Yagi (1976). Briefly, an aliquot of tissue homogenate was mixed with 50 μl of tris-buffered saline (TBS, pH 7.4), 125 μl of trichloroacetic acid containing butylated hydroxytoluene in order to precipitate the proteins and then was centrifuged (1000 g, 10 min, 4 °C). 200 μl of supernatant were mixed with 40 μl of HCl (0.6 M) and 160 μl of thiobarbituric acid dissolved in Tris and the mixture was heated at 80 °C for 10 min. The absorbance of the resultant supernatant was read at 530 nm. The TBARS amount was calculated using a 156 mM−1 cm−1 extinction coefficient.
Determination of enzyme activities
The antioxidant enzyme activities were analyzed using a UV-Visible spectrophotometer (BioRad Mares la Coquette, France), with a “kinetics” program. The measurements of superoxide dismutase (SOD) and glutathione peroxidase(GSH-Px) activities in the brain were performed by the commercially available diagnostic kits supplied by Randox Laboratories.
Serum and brain homogenates acetylcholinesterase activity (AChE) was quantified using the cholinesterase kit according to Ellman et al. (1961).
Brain fatty acid composition
The fatty acids were analyzed as fatty acid methyl esters (FAMEs) by gas chromatography analysis, as previously described by Giacometti et al. (2002). Briefly, total lipids were extracted from the brain by a modified method of Folch et al. (1957) using a chloroform-methanol (2:1, v/v) solvent system containing 0.01 % butylated hydroxytoluene as antioxidant. The aliquots of the total lipids were converted into the methyl esters through a mixture of methanol-hexane-H2SO4 (75:25:1, v/v) as the methylation reagent, at 90 °C for 90 min. FAMEs were analyzed in duplicate, and 1 μl of each sample was injected into the gas chromatography system (Hewlett Packard, Palo Alto, CA, USA) equipped with a flame ionization detector and a polar fused silica capillary column HP-Innowax [J & W Scientific, Agilent, Palo Alto, CA, USA] with cross-linked PEG, Carbowax 20 M (30 m × 0.25 mm ID and 0.25 μm as film thickness). The oven temperature was programmed to increase from 180 to 250 °C at a rate of 10 °C/min, and the injector and detector temperatures were 220 and 280 °C, respectively. Fatty acid methyl esters were identified by comparing their retention times with those of individual standards.
Histopathological studies
For the qualitative analysis of the brain histology, the tissue samples were fixed for 48 h in Bouin liquid. The specimens were washed and dehydrated through a graded series of ethanol and were sequentially embedded in paraffin wax blocks. The blocks were made and sectioned at 5 μm thickness using a rotary microtome. The sections were rehydrated in distilled water and stained with Haematoxylin-Eosin (H-E) then examined under light microscopy. The images were obtained by a digital camera system attached to the microscope. A minimum of three fields for each brain slide were examined and scored semiquantitatively for the severity of changes by a pathologist unaware of the type of treatment.
Statistical analysis
The data in the text, tables, and figures are expressed as the mean ± standard error of the mean for 10 animals and were analyzed using the Statistical Package for Social Sciences (SPSS) programme, release 11.0 for Windows (SPSS, Chicago, IL, USA). The Tukey’s test was used to determine any significant differences between the means. The statistical significance was set at p < 0.05.
Results
Analytical parameters of EVOO and its compounds
The EVOO analytical parameters (fatty acid, oxidative stability, and antioxidant composition) are presented in Table 2. EVOO and OOLF presented approximately the same fatty acid composition with 17 % of SFA, 65 % of MUFA and 15 % of PUFA. Some significant differences were noted in the amount of phenols of the oil tested. In fact, EVOO and OOHF contained high amounts of phenols (579.2 and 384 mg/kg, respectively) while OOLF was deprived from phenols and presented the same amount of α-tocopherol as the EVOO (Table 2).
Table 2.
Mean values of analytical parameters, fatty acid composition (%), oxidative stability and antioxidant content of extra virgin olive oil (EVOO), lipophilic fraction (OOLF), hydrophilic fraction (OOHF) and standard diet fed to rat
| EVOO | OOLF | OOHF | Standard diet | |
|---|---|---|---|---|
| Fatty acid [%] | ||||
| Palmitic acid | 10.28 ± 0.04a | 10.40 ± 0.01a | - | 11.62 ± 0.85a |
| Palmitoleic acid | 0.77 ± 0.30a | 0.79 ± 0.60a | - | 0.10 ± 0.04 a |
| Stearic acid | 3.39 ± 0.14a | 3.56 ± 0.37a | - | 1.15 ± 0.32 b |
| Oleic acid | 64.80 ± 1.99 a | 62.58 ± 3.71 a | - | 29.73 ± 1.12 b |
| Linoleic acid | 14.34 ± 0.90 a | 15.04 ± 0.54 a | - | 53.89 ± 1.49a |
| Linolenic acid | 0.64 ± 0.04a | 0.68 ± 0.03 a | - | 0.61 ± 0.02 a |
| Arachidic acid | 0.74 ± 0.05a | 0.78 ± 0.02a | - | 0.09 ± 0.04 b |
| Gadoleic acid | 0.62 ± 0.03a | 0.57 ± 0.03a | - | 0.14 ± 0.04 b |
| Behenic acid | 2.84 ± 0.37a | 2.87 ± 0.80a | - | 0.22 ± 0.10 b |
| SFA | 17.28 ± 0.22a | 17.62 ± 0.42 a | - | 13.08 ± 0.21 b |
| MUFA | 66.20 ± 2.34 a | 63.95 ± 3.07 a | - | 29.97 ± 1.32 b |
| PUFA | 14.99 ± 0.94a | 15.72 ± 0.57a | - | 54.50 ± 2.12a |
| MUFA/PUFA | 4.43 ± 0.43 a | 4.07 ± 0.04a | - | 0.54 ± 0.32 b |
| OSI (h) | 9.6a | 4.7 b | - | - |
| Chlorophylls (mg/kg) | 12.65 ± 0.29a | 5.4 ± 0.76 b | - | ND |
| β-carotene (mg/kg) | 7.15 ± 0.20 a | 5.43 ± 0.08 b | - | ND |
| Total polyphenols (mg/kg)* | 579.17 ± 71.40a | - | 384 ± 23.18 b | ND |
| α- tocopherol (mg/kg) | 484.56 ± 11.11a | 491.64 ± 10.36a | - | 224 ± 11.86 b |
Data are representative of three independent experiments and values are expressed in mean ± SD. SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids, OSI oxidative stability Index. ND not determined
Values followed by same letters are not significantly different. (Tukey’s test, p < 0.05)
*As hydroxy tyrosyl equivalents
Evaluation of body and brain weights
After 28 days of treatment, there were no statistically significant differences between the test and the control groups of rats in body weight gain (Table 3). Compared to the control group, absolute and relative brain weights were significantly decreased by 8 and 14 % in 2,4-D treated group, respectively. However, the supplementation of EVOO and its fractions to 2,4-D treated rats restored the brain weights to control levels. The EVOO and its fractions alone had no significant effect on absolute and relative brain weights compared to the control group.
Table 3.
Weight gain, absolute and relative brain weights of control and experimental rats
| Weight gain (%) | Absolute brain weight (g) | Relative brain weight (g/100 g b.w.) | |
|---|---|---|---|
| C | 26.16 ± 4.05a | 1.58 ± 0.14ab | 0.63 ± 0.04a |
| 2,4-D | 25.62 ± 5.66a | 1.45 ± 0.17b | 0.54 ± 0.07b |
| 2,4-D/EVOO | 26.21 ± 6.15a | 1.63 ± 0.19a | 0.65 ± 0.07a |
| 2,4-D/OOHF | 25.32 ± 5.67a | 1.70 ± 0.15a | 0.65 ± 0.05a |
| 2,4-D/OOLF | 26.69 ± 5.90a | 1.64 ± 0.16a | 0.61 ± 0.05a |
| EVOO | 22.81 ± 6.10a | 1.59 ± 0.12ab | 0.60 ± 0.06a |
| OOHF | 27.08 ± 4.33a | 1.63 ± 0.15a | 0.61 ± 0.06a |
| OOLF | 29.82 ± 6.85a | 1.57 ± 0.16ab | 0.60 ± 0.04ab |
Data are expressed as means ± SD (n = 10 rats per group). Tukey’s test was used to determine any significant differences between means
C control group, 2,4-D 2, 4-D treated group at dose 5 mg/kg (Brain weight) BW, 2,4-D/EVOO 2, 4-D (5 mg/kg BW) plus extra virgin olive oil, 2,4-D/OOHF 0.2, 4-D plus hydrophilic fraction, 2,4-D/OOLF 0.2, 4-D plus lipophilic fraction, EVOO extra virgin olive oil treated group alone, OOHF group treated with hydrophilic fraction of olive oil, OOLF group treated with lipophilic fraction of olive oil
Acetyl cholinesterase activity
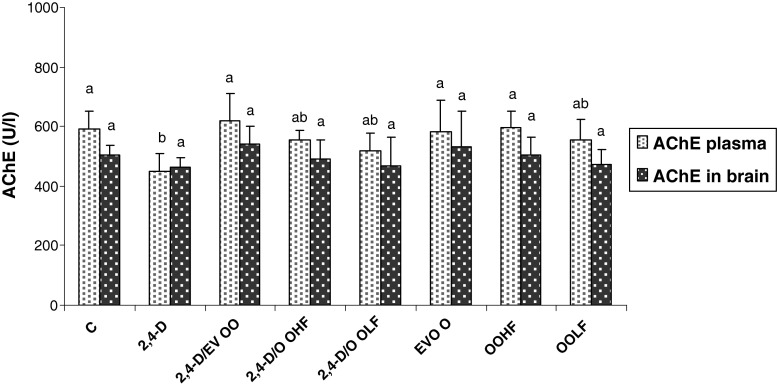
An inhibition of AChE was found both in the plasma (24 %) and brain homogenates (9 % not significant) of the 2,4-D treated rats (Fig. 1). The administration of EVOO and its fractions could attenuate the decreased level of AChE in 2,4-D treated rats. In fact, the best attenuation was detected after EVOO supplementation to 2,4-D treated rats. The feeding of EVOO and its fractions alone did not result in a significant alteration of either brain or plasma AChE activities.
Fig. 1.
Plasma and brain acetyl cholinesterase activities of treatment groups over 4 wk. C: controls group, 2,4-D: 2, 4-D treated group at dose 5 mg/kg BW, 2,4-D/EVOO: 2, 4-D (5 mg/kg BW) plus extra virgin olive oil, 2,4-D/OOHF: 0.2, 4-D plus hydrophilic fraction, 2,4-D/OOLF: 0.2, 4-D plus lipophilic fraction; EVOO: extra virgin olive oil treated group alone, OOHF: group treated with hydrophilic fraction of olive oil, OOLF: group treated with lipophilic fraction of olive oil. Data are expressed as means ± SD (n = 10 rats per group). Tukey’s test was used to determine any significant differences between means
Brain lipid peroxidation levels and antioxidant enzyme activities
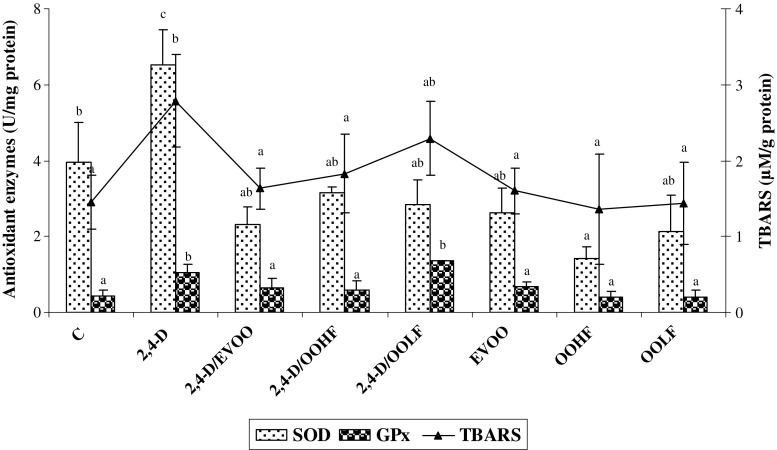
2,4-D caused a marked rise in brain homogenates levels of TBARS compared to controls demonstrating a marked brain lipid peroxidation. The treatment with EVOO and its fractions (OOHF and OOLF) decreases the elevated levels of TBARS in brain of 2,4-D treated rats by 41, 34 and 17 %, respectively (Fig. 2).
Fig. 2.
Brain antioxidant enzyme activities (SOD, GPx) and TBARs levels in control and experimental groups. C: controls group, 2,4-D: 2, 4-D treated group at dose 5 mg/kg BW, 2,4-D/EVOO: 2, 4-D (5 mg/kg BW) plus extra virgin olive oil, 2,4-D/OOHF: 0.2, 4-D plus hydrophilic fraction, 2,4-D/OOLF: 0.2, 4-D plus lipophilic fraction; EVOO: extra virgin olive oil treated group alone, OOHF: group treated with hydrophilic fraction of olive oil, OOLF: group treated with lipophilic fraction of olive oil. Data are expressed as means ± SD (n = 10 rats per group). Tukey’s test was used to determine any significant differences between means
Exposure of male rats to 2,4-D produced a significant increase of antioxidants enzyme activities such as SOD and GPx in brain homogenates (Fig. 2). The co-administration of EVOO and OOHF prevented the rise in SOD activities by 64 and 51 %, respectively and GPx by 38 and 43 %, respectively, compared to the 2,4-D-treated group. The supplementation of OOLF significantly decreased the levels of SOD enzymes but failed to improve the levels of GPx in the brains of 2,4-D treated rats.
Twenty eight day oral feeding of EVOO and its fractions alone did not result in a significant alteration of any of the antioxidant enzymes and TBARS levels in the brain except for OOHF that led to a decrease in SOD activity. This finding may be due to the antioxidant compounds in this fraction (Fig. 2). In fact, the presence of these compounds allows the body to have a powerful defence system without the intervention of SOD activity.
Fatty acid composition of brain
Brain lipids are remarkable for their high content of SFA and very low content of ‘essential’ fatty acids relative to other organs. Table 4 shows the fatty acid composition of rat brain from all the groups. The total levels of SFA and MUFA did not differ among control and treatment groups. While, a significant increase of C18:0 level by 16.10 % was observed in 2,4-D-treated rats, which may be due in parallel to the decrease in the rate of C18:1 by 15.24 % in this group. This variation has been corrected in the groups of rats treated with EVOO or its fractions with 2,4-D returning their rate close to that of control group. However, a significant decrease of PUFA level was shown in the brain of 2,4-D-treated rats caused by a significant reduction of C20:2 (ω6), C22:4 (ω6) and C22:6(ω3-DHA) by 35.57, 84.18 and 66.80 % respectively. Consequently, a significant decrease of PUFA (ω6) and PUFA (ω3) was observed in brain of 2,4-D- treated rats. These changes resulted in a + 31 and +36 % increase in the MUFA/PUFA and SFA/UFA ratios, respectively and a − 52 and −35 % decrease in the ω3/ω6 and PUFA/SFA ratios, respectively in the 2,4-D treated group compared to controls (Table 4). EVOO or its fractions supplemented to 2,4-D-treated rats prevented the decline of PUFA by maintaining its level close to that of the control group. This effect was mainly due to an increase in C18:2, C20:2(ω6), C22:4(ω6) and C22:6(ω3-DHA) in brain of animals administered olive oil or its extracts in combination with 2,4-D compared to 2,4-D treated group. As a consequence, a restoration in the saturation indexes was revealed in these groups compared to 2,4-D-treated rats (Table 4). The re-establishment of the rate of these fatty acids was more pronounced in brain of rat supplemented EVOO with 2,4-D treatment. The total levels of SFA, MUFA, and PUFA were not altered in the group treated only with EVOO or its fractions.
Table 4.
Effects of extra virgin olive oil and its fractions on brain fatty acid composition of rat treated or not with 2,4-D
| C | 2,4-D | 2,4-D/EVOO | 2,4-D/OOHF | 2,4-D/OOLF | EVOO | OOHF | OOLF | |
|---|---|---|---|---|---|---|---|---|
| C16: 0 | 25.21 ± 1.97abc | 22.66 ± 0.95c | 24.28 ± .20bc | 26.30 ± 0.59ab | 27.26 ± 1.80a | 25.10 ± 0.69abc | 26.63 ± 1.47ab | 27.34 ± 0.68a |
| C18: 0 | 24.22 ± 2.05c | 28.12 ± 0.98a | 25.60 ± 1.09bc | 25.93 ± 1.05abc | 24.29 ± 0.45c | 25.51 ± 0.88bc | 24.07 ± 1.13c | 25.98 ± 1.65abc |
| C20: 0 | 0.83 ± 0.14bc | 1.07 ± 0.17ab | 1.08 ± 0.11ab | 1.20 ± 0.12a | 1.03 ± 0.18ab | 0.99 ± 0.01abc | 0.82 ± 0.19bc | 0.75 ± 0.08c |
| C22: 0 | 0.50 ± 0.08a | 0.46 ± 0.08a | 0.47 ± 0.02a | 0.54 ± 0.08 a | 0.51 ± 0.17 a | 0.47 ± 0.01 a | 0.60 ± 0.17 a | 0.54 ± 0.10 a |
| C24: 0 | 0.61 ± 0.07ab | 0.74 ± 0.19a | 0.55 ± 0.17ab | 0.48 ± 0.03ab | 0.72 ± 0.14a | 0.58 ± 0.21ab | 0.57 ± 0.13ab | 0.41 ± 0.06b |
| Σ SFA | 51.38 ± 1.75a | 53.06 ± 1.62a | 51.99 ± 1.79a | 54.46 ± 1.22a | 53.88 ± 1.86a | 52.66 ± 1.1a | 52.70 ± 1.53a | 54.47 ± 2.27a |
| C14: 1 | 0.43 ± 0.05c | 0.46 ± 0.04c | 0.87 ± 0.06a | 0.72 ± 0.15b | 0.70 ± 0.07b | 0.80 ± 0.04ab | 0.71 ± 0.01b | 0.72 ± 0.01b |
| C16: 1 | 0.44 ± 0.11 abc | 0.30 ± 0.05 c | 0.48 ± 0.09 abc | 0.61 ± 0.22a | 0.45 ± 0.06abc | 0.55 ± 0.07ab | 0.50 ± 0.10abc | 0.40 ± 0.04bc |
| C18: 1n-9 | 15.02 ± 1.60a | 12.73 ± 0.99 a | 14.59 ± 1.20 a | 14.50 ± 3.57 a | 14.76 ± 1.84 a | 14.08 ± 0.68 a | 13.92 ± 0.99 a | 13.20 ± 2.08 a |
| C18: 1n-7 | 2.22 ± 0.23 a | 2.52 ± 0.38 a | 2.78 ± 1.06 a | 2.93 ± 0.13 a | 2.36 ± 0.79 a | 2.64 ± 0.52 a | 2.65 ± 0.31 a | 2.63 ± 0.51 a |
| C20: 1n-9 | 1.82 ± 0.14ab | 1.45 ± 0.21b | 2.57 ± 0.10a | 2.29 ± 0.85 a | 1.10 ± 0.40b | 1.43 ± 0.42b | 1.11 ± 0.05b | 1.37 ± 0.44b |
| Σ MUFA | 19.93 ± 1.64a | 17.46 ± 1.05a | 21.31 ± 1.82a | 20.99 ± 3.95a | 19.38 ± 1.95a | 19.51 ± 1.05a | 18.89 ± 1.08a | 18.33 ± 2.25a |
| C18: 2n-6 | 0.70 ± 0.20ab | 0.55 ± 0.09b | 0.78 ± 0.02a | 0.75 ± 0.08a | 0.87 ± 0.006 a | 0.70 ± 0.13ab | 0.76 ± 0.06a | 0.85 ± 0.07a |
| C20: 2n-6 | 1.04 ± 0.02b | 0.67 ± 0.17c | 1.68 ± 0.08a | 0.91 ± 0.18bc | 0.75 ± 0.24c | 0.91 ± 0.20bc | 1.10 ± 0.02b | 0.87 ± 0.15bc |
| C20: 3n-6 | 0.23 ± 0.01b | 0.41 ± 0.12a | 0.17 ± 0.01b | 0.18 ± 0.04 b | 0.16 ± 0.05 b | 0.18 ± 0.05 b | 0.20 ± 0.04 b | 0.15 ± 0.05 b |
| C20: 4n-6 | 8.79 ± 0.36b | 8.76 ± 0.34b | 9.56 ± 1.14ab | 10.71 ± 0.98a | 9.90 ± 0.65ab | 9.82 ± 0.81ab | 8.97 ± 0.74b | 9.07 ± 0.51b |
| C22: 4n-6 | 3.54 ± 0.46a | 0.56 ± 0.02c | 2.59 ± 0.94ab | 1.88 ± 0.34b | 2.44 ± 0.74ab | 3.25 ± 0.39a | 3.03 ± 0.32a | 3.27 ± 0.89a |
| C22: 5n-6 | 3.14 ± 0.24ab | 2.74 ± 0.01b | 2.88 ± 0.23b | 2.94 ± 0.21b | 2.75 ± 0.87b | 3.15 ± 0.65ab | 2.71 ± 0.75b | 3.97 ± 0.52a |
| C18: 3n-3 | 0.27 ± 0.04ab | 0.43 ± 0.12a | 0.32 ± 0.09ab | 0.26 ± 0.06b | 0.31 ± 0.09ab | 0.34 ± 0.11 ab | 0.35 ± 0.06 ab | 0.28 ± 0.01 ab |
| C22: 6n-3 | 7.14 ± 1.09ab | 2.37 ± 0.34c | 5.94 ± 0.98b | 3.65 ± 0.49c | 5.14 ± 0.52b | 8.34 ± 0.34a | 7.95 ± 0.76a | 6.06 ± 0.37a |
| Σ PUFA | 24.86 ± 1.02ab | 16.50 ± 0.44c | 23.94 ± 2.12ab | 21.30 ± 1.19b | 22.34 ± 2.09b | 26.70 ± 1.09a | 25.09 ± 1.73ab | 24.53 ± 1.53ab |
| Σ PUFA n-6 | 17.45 ± 0.74a | 13.70 ± 0.23b | 17.66 ± 1.82 a | 17.38 ± 0.77 a | 16.88 ± 1.63 a | 18.02 ± 1.20 a | 16.78 ± 1.27 a | 18.19 ± 1.28 a |
| Σ PUFA n-3 | 7.41 ± 1.12ab | 2.80 ± 0.42c | 6.26 ± 0.98b | 3.91 ± 0.48c | 5.45 ± 0.59b | 8.68 ± 028a | 8.30 ± 0.77a | 6.34 ± 0.38b |
| Σ UFA | 44.80 ± 1.67 ab | 33.96 ± 0.98 c | 45.23 ± 3.78 ab | 39.94 ± 6.02 b | 41.72 ± 3.42 ab | 46.22 ± 1.16 a | 43.99 ± 2.80 ab | 42.87 ± 2.79 ab |
| Σn-3PUFA/Σn-6PUFA ratio | 0.42 ± 0.07ab | 0.20 ± 0.03c | 0.35 ± 0.06b | 0.22 ± 0.02c | 0.32 ± 0.02b | 0.48 ± 0.04a | 0.49 ± 0.04a | 0.34 ± 0.02b |
| MUFA/PUFA ratio | 0.80 ± 0.08b | 1.05 ± 0.07a | 0.89 ± 0.04ab | 0.88 ± 0.32ab | 0.87 ± 0.08ab | 0.73 ± 0.05a | 0.75 ± 0.01a | 0.74 ± 0.09a |
| PUFA/SFA ratio | 0.48 ± 0.02ab | 0.31 ± 0.01c | 0.46 ± 0.04ab | 0.38 ± 0.02b | 0.41 ± 0.04b | 0.50 ± 0.02a | 0.47 ± 0.02ab | 0.44 ± 0.03b |
| SFA/UFA ratio | 1.14 ± 0.05c | 1.56 ± 0.09a | 1.15 ± 0.10c | 1.38 ± 0.21ab | 1.29 ± 0.10bc | 1.13 ± 0.01c | 1.20 ± 0.05bc | 1.27 ± 0.14bc |
Data are expressed as means ± SD (n = 10 rats per group). Tukey’s test was used to determine any significant differences between means
C control group, 2,4-D 2,4-D treated group at dose 5 mg/kg BW, 2,4-D/EVOO 2, 4-D (5 mg/kg BW) plus extra virgin olive oil, 2,4-D/OOHF 0.2,4-D plus hydrophilic fraction, 2,4-D/OOLF 0.2, 4-D plus lipophilic fraction, EVOO extra virgin olive oil treated group alone, OOHF group treated with hydrophilic fraction of olive oil, OOLF group treated with lipophilic fraction of olive oil.
Brain histoarchitecture
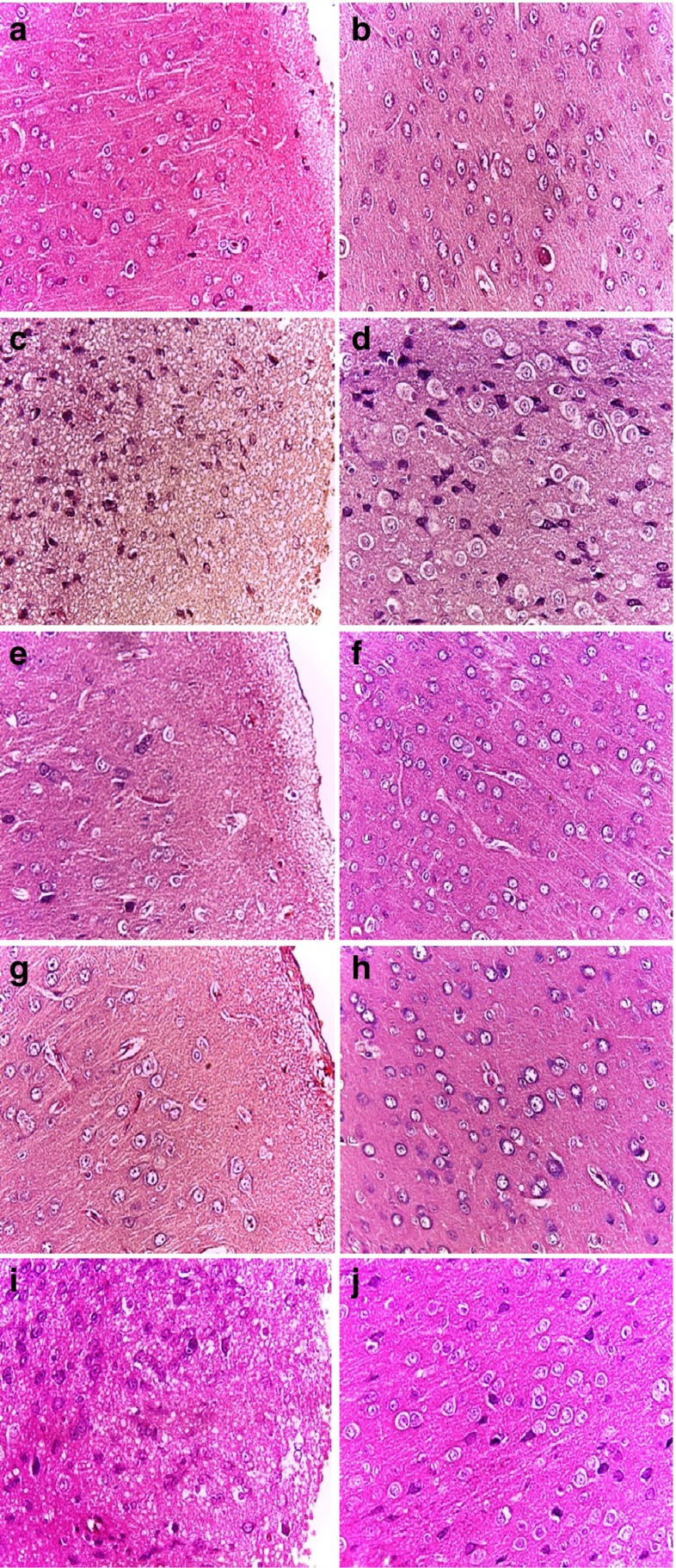
The histological findings of brains from various treatment groups were presented in Fig. 3. Figure 3a, b illustrate the cerebral cortex of normal controls showing a molecular layer (a) and a pyramidal cell layer (b). Following pesticide exposure, disorganization in both layers was observed (Fig. 3c, d). A spongiest aspect and severe necrotic degeneration were observed in the molecular layer (Fig. 3c). In the pyramidal cell layer, a prominent vacuolation (characterized by enlargement of perikaryon filled with vacuoles) could be seen around the pyramidal cells (Fig. 3d).
Fig. 3.
Effects of EVOO and its fractions (OOHF and OOLF) on the brain histological studies of rat treated or not with 2,4-D. (Haematoxylin and eosin staining, ×400). (A and B) Normal control group; (C and D) 2,4-D-treated group(5 mg/kg); (E and F) 2,4-D plus EVOO treated group; (G and H) 2,4-D plus OOLF treated group; (I and J) 2,4-D plus OOHF treated group
In 2,4-D treated group supplemented with olive oil (Fig. 3e, f) a normalisation of the brain microstructural elements in both layers was observed. The same result was obtained when only the lipophilic fraction was administrated to 2,4-D treated rats. The cortex layers were quite intact as can be observed in Fig. 3g, h. However, treatment with the hydrophilic fraction plus 2,4-D was only able to reduce the vacuolar spaces around the pyramidal cells (Fig. 3i) but had no improvement on spongiest and necrotic degeneration (Fig. 3j).
The histological examination of the cerebral cortex from the group treated only with EVOO or its fractions did not reveal any dissolution of the granular cell layer, showing the same histological structure of cerebral cortex of controls.
Discussion
For years, researchers have shown that ROS are implicated in the pathophysiology of a large number of diseases, and can be harmful because of their high reactivity and deleterious effects on cell structures, especially in the brain (Halliwell 1992). Scientists have considered these unstable molecules as a culprit of neurodegenerative disorders such as Lou Gehrig’s disease, Parkinson’s disease and Huntington’s disease.
The herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) has been widely used in agriculture and forestry since the 1940s. Some previous studies suggested an association between the exposure to 2,4-D and a variety of neurological effects (Venkov et al. 2000; Barnekow et al. 2000; Madrigal-Bujaidar et al. 2001; Bongiovanni et al. 2011). A developmental neurotoxicity study demonstrated severe neurotoxicity in young rats exposed to 2,4-D from postnatal days 12 to 25 at 70 mg/kg/day doses. These pups showed a decrease in myelin deposition and alterations in all behavioural tests at all doses (Rosso et al. 2000; Bortolozzi et al. 2001). This herbicide specifically appears to impair normal deposition of myelin in the developing brain (Duffard et al. 1996). Exposure of newborn rats to 2,4-D was shown to affect astroglial cytoarchitecture and the neuronal function (Brusco et al. 1997). Moreover, Ferri et al. (2007) reported selective oxidative stress in brain areas of neonate rats exposed to 2,4-D through the mother’s milk.
In our experimental model, we demonstrated an alteration in the brain of rats treated with 2,4-D for four weeks manifested in the loss of weight of the brain and the AChE activities in the plasma and the brain of 2,4-D-treated rats. Our results are in agreement with the findings of Bukowska and Hutnik (2006) which showed a decrease of AChE activity in human erythrocytes (in vitro) observed under the highest applied dose of 2,4-D- 500 and 1000 ppm. The changes in AChE activity, which is a good indicator of membrane damage, may be induced indirectly by ROS, formed by xenobiotic reactions. It is known that 2,4-D provokes changes in the neural system that are based on the complex formation of herbicide with acetylcholine (Sastry et al. 1997; Kalab and Skladal 1997).
The brain is especially vulnerable to oxidative damage because of its high content of easily peroxidizable unsaturated fatty acids, its high oxygen consumption rate, and its relative paucity of antioxidant enzymes (Nunomura et al. 2006). 2,4-D was identified as a chemical that could induce alterations in the activity of enzymes involved in oxidative stress and lipid peroxidation (Bongiovanni et al. 2007). Some changes in protective enzymes in human tissues have also been described by other studies (Bukowska et al. 2000; Bukowska 2003). Our results suggest that 2,4-D exposure can cause an increase of the antioxidant enzyme activities (SOD and GPx) and lipid peroxidation (TBARS). A possible explanation for this effect can be due to the different proportion of the lipid contents of the brain. In this way, we have showed that 2,4-D induced a decrease in the level of PUFA in the brain of the studied rats.
Except for GPx activity, all these changes induced by the pesticide, are counteracted by the concomitant addition of EVOO or its fractions. Therefore, the neuroprotective effect of EVOO and its fractions could be summarized in the restoration of the relative and absolute weight brain and the stimulation of AChE activity. In fact, EVOO greatly improves the toxic effect of 2,4-D on AChE activity which aims at the normal value of controls. In addition, the supplementation of EVOO or its fraction and 2,4-D restored the antioxidant enzyme activities and the lipid peroxidation to basal levels; however no change in GPx activity was detected with the supplementation of the OOLF. In fact, most researchers who have investigated the effect of different types of oil and its components on the glutathione system agree that changes in this system are not dependent on the fatty acid composition of the oil, but rather on non-glyceride components, especially vitamin E and polyphenols (Ochoa-Herrera et al. 2001; Hunkar et al. 2002). Other works have related the neuroprotective effect of EVOO mainly to the antioxidant properties of polyphenols (Simonyi et al. 2005). Hydroxytyrosol (HT) is the main antioxidant compound in olives and is responsible for attributing several beneficial properties to olive oil. An HT extract prepared from olive mill wastewater reduces neuronal damage induced by chemical oxidative stress (induced by ferrous salts or sodium nitroprusside administration in mice) (Schaffer et al. 2007). Some previous studies have shown that both HT and HT acetate protect brain slices subjected to hypoxia-reoxygenation (González-Correa et al. 2008). In vitro studies with olive leaf extract have shown that its main component, oleuropein may have neuroprotective and neurorestorative properties which could be of interest as a potential treatment of Parkinson’s disease (Pasban-Aliabadi et al. 2013). Furthermore, the potential neuroprotective effects of HT and its precursor oleuropein have also been reported against brain damages such as brain hypoxia-reoxygenation, cerebral ischemia and spinal cord injury (Khalatbary and Ahmadvand 2012; Cabrerizo et al. 2013). HT and tyrosol were able to protect neuronal cells against amyloid-β peptide induced toxicity and prevented the nuclear translocation of transcription factor NF-κB (St-Laurent-Thibault et al. 2011). Mohagheghi et al. (2009, 2010) have shown that pre-treatment with various dietary doses of virgin olive oil induces ischemic tolerance and confers different degrees of neuroprotection in the rat brain. Previous studies have revealed that diet contain olive oil is capable of inducing the generation of new cells in the adult brain, and of strengthening the neural networks which become affected with age and in neurodegenerative processes such as Alzheimer’s disease, as well as protecting neurons from oxidative and neural damage (Bawazir 2011). The beneficial health effects of olive oil are due to its high content of antioxidative substances, as well as its high content of monounsaturated fatty acids, i.e., oleic acid. Oleic acid and linoleic acid (Olive oil) induced increase in free intracellular calcium concentrations Ca2+ by recruiting calcium from endoplasmic reticulum pool via inositol 1, 4, 5-triphosphate production followed by calcium influx via opening of store-operated calcium channels (Bawazir 2011).
Brain tissue is particularly susceptible to oxidative damage, as it is rich in PUFA which easily undergoes peroxidation. Compared with the fatty acid composition of brain membranes of control rats, the membranes of 2,4-D-treated rats showed high decreases of UFA and PUFA contents. The total SFA levels were not altered after the exposure of rats to 2,4-D. The administration of EVOO or its fractions and 2,4-D produced a lipid profile close to that of the control group manifested by a restoration of the PUFA contents especially DHA level in the brain of rats that enhances neuroprotection observed for EVOO. It is clear from the literature that DHA is involved in a variety of processes in neural cells and that its role is far more complex than simply influencing cell membrane properties (Sinclair et al. 2007). Unsaturated fatty acids are important constituents of neuronal cell membranes and have neuroprotective, antioxidant, and anti-inflammatory properties (De Lau et al. 2005). From these analyses, we can also observe the neuroprotective effect of EVOO or its fractions revealed by the restoration of the fatty acid composition of the brain. In fact, the specific fatty acid distribution in the brain membranes indicates its role in regulating the fluidity and functional activity of these structures. Changes in fatty acid composition lead to biochemical and functional alterations, such as lipid-protein interaction, protein distribution, membrane permeability, ion transport, etc.
Conclusion
Based on the experimental findings of this study, it can be suggested that the sub-acute administration of 2,4-D leads to brain oxidative stress induction. However, olive oil or its fractions represents a possible natural protective agent against risk factors of acute neurotoxicity induced by 2,4-D. Accordingly, olive oil or its fractions was found to reduce the accumulation of ROS in the brain, such as lipid peroxidation and to increase the antioxidant enzyme and AChE activities. In addition, EVOO enhanced a neuroprotective effect evaluated by the restoration of brain fatty acid composition. This study indicates that olive oil will provide further insights for neuroprotection and may lead to the development of effective therapeutic strategies against pesticides.
Acknowledgments
This research was supported by a grant from the ‘Ministère de l’Enseignement Supérieur et de la Recherche Scientifique LR12ES05 “Nutrition-Functional Foods & Vascular Health”.
Contributor Information
Nakbi Amel, Phone: +216 73 462 200, Email: nakbia@yahoo.fr.
Hammami Mohamed, Phone: +216 73 462 200, Email: Mohamed.hammami@fmm.rnu.tn.
References
- Agudo A, Cabrera L, Amiano P, Ardanaz E, Barricarte A, Berenguer T, et al. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Am J Clin Nutr. 2007;85:1634–1642. doi: 10.1093/ajcn/85.6.1634. [DOI] [PubMed] [Google Scholar]
- Barnekow DE, Hamburg AW, Puvanesarajah V, Guo M. Metabolism of 2,4-dicholorophenoxyacetic acid in laying hens and lactating goats. J Agric Food Chem. 2000;49(1):156–163. doi: 10.1021/jf000119r. [DOI] [PubMed] [Google Scholar]
- Bawazir AE. Chronic effect of olive oil on some neurotransmitter contents in different brain regions and physiological, histological structure of liver and kidney of male albino rats. World J Neurosci. 2011;1:31–37. doi: 10.4236/wjns.2011.13005. [DOI] [Google Scholar]
- Behl C. Alzheimer’s disease and oxidative stress: implications for novel therapeutic approaches. Prog Neurobiol. 1999;57:301–323. doi: 10.1016/S0301-0082(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Bongiovanni B, De Lorenzi P, Ferri A, Konjuh C, Rassetto M, Evangelista de Duffard AM, Cardinali DP, Duffard R. Melatonin decreases the oxidative stress produced by 2,4-dichlorophenoxyacetic acid in rat cerebellar granule cells. Neurotox Res. 2007;11:93–99. doi: 10.1007/BF03033388. [DOI] [PubMed] [Google Scholar]
- Bongiovanni B, Ferri A, Brusco A, Rassetto M, Lopez LM, Evangelista de Duffard AM, Duffard R. Adverse effects of 2,4-Dichlorophenoxyacetic acid on rat cerebellar granule cell cultures were attenuated by amphetamine. Neurotox Res. 2011;19:544–555. doi: 10.1007/s12640-010-9188-9. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Evangelista de Duffard AM, Dajas F, Duffard R, Silveira R. Intracerebral administration of 2,4-diclorophenoxyacetic acid induces behavioral and neurochemical alterations in the rat brain. Neurotoxicology. 2001;22:221–232. doi: 10.1016/S0161-813X(01)00014-6. [DOI] [PubMed] [Google Scholar]
- Brusco A, Pecci Saavedra J, Garcia G, Tagliferro P, Evangelista DE Duffard AM, Duffard R. 2,4-Dichlorophenoxyacetic acid through lactation induces astroghos s m rat brain. Mol Chem Neuropathol. 1997;30:175–185. doi: 10.1007/BF02815096. [DOI] [PubMed] [Google Scholar]
- Bukowska B. Effects of 2,4-D and its metabolite 2,4 dichlorophenol on antioxidant enzymes and level of glutathione in human erythrocytes. Compa Biochem Physiol Part C: Toxicol Pharmacol. 2003;135:435–441. doi: 10.1016/S1095-6433(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Bukowska B, Hutnik K. 2,4-D and MCPA and their derivatives: effect on the activity of membrane erythrocytes acetylcholinesterase (in vitro) Pestic Biochem Physiol. 2006;85:174–180. doi: 10.1016/j.pestbp.2005.11.009. [DOI] [Google Scholar]
- Bukowska B, Chajdys A, Duda W, Duchnowicz P. Catalase activity in human erythrocytes: effect of phenoxy herbicides and their metabolites. Cell Biol Int. 2000;24:705–711. doi: 10.1006/cbir.2000.0553. [DOI] [PubMed] [Google Scholar]
- Cabrerizo S, De La Cruz JP, López-Villodres JA, Muñoz-Marín J, Guerrero A, Reyes JJ, Labajos MT, González-Correa JA. Role of the inhibition of oxidative stress and inflammatory mediators in the neuroprotective effects of hydroxytyrosol in rat brain slices subjected to hypoxia reoxygenation. J Nutr Biochem. 2013;24:2152–2157. doi: 10.1016/j.jnutbio.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Chang Y Lee (2013) Challenges in providing credible scientific evidence of health benefits of dietary polyphénols. J Fun Food 5: 524-526.
- Dabbou S, Issaoui M, Servili M, Taticchi A, Sifi S, Montedoro GF, Hammami M. Characterisation of virgin olive oils from European olive cultivars introduced in Tunisia. Eur J Lipid Sci Technol. 2009;111:392–401. doi: 10.1002/ejlt.200800032. [DOI] [Google Scholar]
- De Lau LML, Bornebroek M, Witteman JCM, Hofman A, Koudstaal PJ, Breteler MMB. Dietary fatty acids and the risk of Parkinson disease. Neurology. 2005;64:2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F. [DOI] [PubMed] [Google Scholar]
- Duffard R, Garcia G, Rosso S, Bortolozzi A, Madariaga M, Di Paolo O, et al. Central nervous system myelin deficit in rats exposed to 2,4-dichlorophenoxyacteic acid throughout lactation. Neurotoxicol Teratol. 1996;18:691–696. doi: 10.1016/S0892-0362(96)00087-6. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Bio Chem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Farr SA, Price TO, Dominguez LJ, Motisi A, Saiano F, Niehoff ML, et al. Extra virgin olive oil improves learning and memory in SAMP8 mice. J Alzheimers Dis. 2012;28:81–92. doi: 10.3233/JAD-2011-110662. [DOI] [PubMed] [Google Scholar]
- Ferri A, Duffard R, Evangelista de Duffard AM. Selective oxidative stress in brain areas of neonate rats exposed to 2,4- dichlorophenoxyacetic acid through mother’s milk. Drug Chem Toxicol. 2007;30:17–30. doi: 10.1080/01480540601017629. [DOI] [PubMed] [Google Scholar]
- Folch J, Lee M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Giacometti J, Milosevic A, Milin C. Gas chromatography determination of fatty acids contained in different classes after their separation by solid-phase extraction. J Chromatogr A. 2002;976:47–54. doi: 10.1016/S0021-9673(02)01159-7. [DOI] [PubMed] [Google Scholar]
- Gimeno E, Castellote AI, Lamuela-Raventos RM, de la Torre MC, Lopez-Sabater MC. Rapid determination of vitamin E in vegetable oils by reversed-phase high-performance liquid chromatography. J of Chromatogr A. 2000;881:251–254. doi: 10.1016/S0021-9673(00)00219-3. [DOI] [PubMed] [Google Scholar]
- González-Correa JA, Navas MD, Lopez-Villodres JA, Trujillo M, Espartero JL, De La Cruz JP. Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia–reoxygenation. Neurosci Lett. 2008;446:143–146. doi: 10.1016/j.neulet.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Hunkar T, Aktan F, Ceylan A, Karasu C. Effects of cod liver oil on tissue antioxidant pathways in normal and streptozotocin- diabetic rats. Cell Biochem Funct. 2002;20:297–302. doi: 10.1002/cbf.977. [DOI] [PubMed] [Google Scholar]
- Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P, et al. The effects of a polyphenol present in olive oil, oleuropein aglycone, in an experimental model of spinal cord injury in mice. Biochem Pharmacol. 2012;83:1413–1426. doi: 10.1016/j.bcp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Jones A. Can a Mediterranean-type diet prevent Parkinson’s disease. Neurol Rev. 2011;19:1–21. [Google Scholar]
- Kalab T, Skladal P. Disposable multichannel immunosensors for 2,4-dichlorophenoxyacetic acid using acetylcholinesterase as an enzyme label. Electroanalysis. 1997;9:293–297. doi: 10.1002/elan.1140090406. [DOI] [Google Scholar]
- Khalatbary AR, Ahmadvand H. Neuroprotective effect of oleuropein following spinal cord injury in rats. Neurol Res. 2012;34:44–51. doi: 10.1179/1743132811Y.0000000058. [DOI] [PubMed] [Google Scholar]
- Madrigal-Bujaidar E, Hernandez-Ceruelos A, Chamorro G. Induction of sister chromatid exchanges by 2,4-dichlorophenoxyacetic acid in somatic and germ cells of mice exposed in vivo. Food Chem Toxicol. 2001;39(9):941–946. doi: 10.1016/S0278-6915(01)00037-0. [DOI] [PubMed] [Google Scholar]
- Minguez-Mosquera MI, Rejano-Navarro L, Gandul-Rojas B, Sanchez-GomezAH G-FJ. Color-pigment correlation in virgin olive oil. JAOCS. 1991;68:332–336. doi: 10.1007/BF02657688. [DOI] [Google Scholar]
- Mohagheghi F, Bigdeli MR, Rasoulian B, Zeinallou AA. Dietary virgin olive oil reduces ischemia-reperfusion injury in rat brain in vivo. J Cereb Blood Flow Metab. 2009;29:514–522. doi: 10.1038/jcbfm.2009.164. [DOI] [Google Scholar]
- Mohagheghi F, Bigdeli MR, Rasoulian B, Zeinanloo AA, Khoshbaten A. Dietary virgin olive oil reduces blood brain barrier permeability, brain edema, and brain injury in rats subjected to ischemia-reperfusion. Sci World J. 2010;10:1180–1191. doi: 10.1100/tsw.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montedoro GF, Servili M, Baldioli M, Miniati E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J Agric Food Chem. 1992;40:1571–1576. doi: 10.1021/jf00021a019. [DOI] [Google Scholar]
- Monti MC, Margarucci L, Tosco A, Riccio R, Casapullo A. New insights on the interaction mechanism between tau protein and oleocanthal, an extra-virgin olive-oil bioactive component. Food Funct. 2011;2:423–428. doi: 10.1039/c1fo10064e. [DOI] [PubMed] [Google Scholar]
- Nakbi A, Tayeb W, Grissa A, Issaoui M, Dabbou S, Chargui I, Ellouz M, Miled A, Hammami M. Effects of olive oil and its fractions on oxidative stress and the liver’s fatty acid composition in 2,4-Dichlorophenoxyacetic acid- treated rats. Nutr Metab. 2010;7:80–91. doi: 10.1186/1743-7075-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Honda K, Takeda A, Hirai K, Zhu X, Smith MA, Perry G (2006) Oxidative damage to RNA in neurodegenerative diseases. J Biomed Biotechno l: 1–6. [DOI] [PMC free article] [PubMed]
- Ochoa-Herrera JJ, Huertas JR, Quiles JL, Mataix J. Dietary oils high in oleic acid, but with different non-glyceride contents, have different effects on lipid profiles and peroxidation in rabbit hepatic mitochondria. J Nutr Biochem. 2001;12:357–364. doi: 10.1016/S0955-2863(01)00150-4. [DOI] [PubMed] [Google Scholar]
- Pasban-Aliabadi H, Esmaeili-Mahani S, Sheibani V, Abbasnejad M, Mehdizadeh A, Yaghoobi MM. Inhibition of 6-hydroxydopamine-induced PC12 cell apoptosis by olive (Olea europaea L.) leaf extract is performed by its main component oleuropein. Rejuvenation Res. 2013;16:134–142. doi: 10.1089/rej.2012.1384. [DOI] [PubMed] [Google Scholar]
- Pitozzi V, Jacomelli M, Zaid M, Luceri C, Bigagli E, Lodovici M, et al. (2010) Effects of dietary extra-virgin olive oil on behaviour and brain biochemical parameters in ageing rats. Br J Nutr103:1674–1683. [DOI] [PubMed]
- Pollard SE, Kuhnle GG, Vauzour D, VafeiAdou K, Tzounis X, Whiteman M, Rice-Evans C, Spencer JPE. The reaction of flavonoid metabolites with peroxynitrite. Biochem Biophys Res Commun. 2006;350:960–968. doi: 10.1016/j.bbrc.2006.09.131. [DOI] [PubMed] [Google Scholar]
- Pratico D, Delanty N. Oxidative injury in diseases of the central nervous system: focus on Alzheimer’s disease. Am J Med. 2000;109(7):577–585. doi: 10.1016/S0002-9343(00)00547-7. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Ristagno G, Fumagalli F, Porretta-Serapiglia C, Orrù A, Cassina C, Pesaresi M, et al. Hydroxytyrosol attenuates peripheral neuropathy in streptozotocin-induced diabetes in rats. J Agric Food Chem. 2012;60:5859–5865. doi: 10.1021/jf2049323. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Garcia GB, Madariaga MJ, Evangelista de Duffard AM, Duffard RO. 2,4-Dichlorophenoxyacetic acid in developing rats alters behaviour, myelination and regions brain gangliosides pattern. Neurotoxicology. 2000;21(1–2):155–163. [PubMed] [Google Scholar]
- Sastry BVR, Janson VE, Clark CP, Owens LK. Cellular toxicity of 2,4,5-trichlorophenoxyacetic acid; formation of 2,4,5-trichlorophenoxyacetylcholine. Cell Mol Biol. 1997;43:549–557. [PubMed] [Google Scholar]
- Schaffer S, Podstawa M, Visioli F, Bogani P, Müller WE, Eckert GP. Hydroxytyrosol-rich olive mill wastewater protects brain cells in vitro and ex vivo. J Agric Food Chem. 2007;55:5043–5049. doi: 10.1021/jf0703710. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol. 2005;31:135–147. doi: 10.1385/MN:31:1-3:135. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Begg D, Mathai M, Weisinger RS. Omega 3 fatty acids and the brain: review of studies in depression. Asia Pac J Clin Nutr. 2007;16:391–397. [PubMed] [Google Scholar]
- Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somani SM, Husain K, Diaz-Phillips L, Lanzotti DJ, Kareti KR, Trammell GL. Interaction of exercise and ethanol on antioxidant enzymes in brain regions. Alcohol. 1996;13(6):603–610. doi: 10.1016/S0741-8329(96)00075-4. [DOI] [PubMed] [Google Scholar]
- Stevens JT, Breckenridge CB. Crop protection chemicals. In: Hayes WA, editor. Principles and methods of toxicology. Philadelphia p: Taylor & Francis; 2001. pp. 565–648. [Google Scholar]
- St-Laurent-Thibault C, Arseneault M, Longpre F, Ramassamy C. Tyrosol and hydroxytyrosol, two main components of olive oil, protect N2a cells against amyloid-beta-induced toxicity. Involvement of the NF-kappaB signaling. Curr Alzheimer Res. 2011;8:543–551. doi: 10.2174/156720511796391845. [DOI] [PubMed] [Google Scholar]
- Tasset I, Pontes AJ, Hinojosa AJ, de la Torre R, Túnez I. Olive oil reduces oxidative damage in a 3-nitropropionic acid-induced Huntington’s disease-like rat model. Nutr Neurosci. 2011;14:106–111. doi: 10.1179/1476830511Y.0000000005. [DOI] [PubMed] [Google Scholar]
- Tura D, Gigliotti C, Pedo S, Failla O, Bassi D, Serraiocco A. Influence of cultivar and site of cultivation on levels of lipophilic and hydrophilic antioxidants in virgin olive oils (Olea europea L) and correlations with oxidative stability. HortSci. 2007;112:108–119. [Google Scholar]
- Venkov P, Topashka-Ancheva M, Georgieva M, Alexieva V, Karanov E. Genotoxic effect of substituted phenoxyacetic acids. Arch Toxicol. 2000;74:560–566. doi: 10.1007/s002040000147. [DOI] [PubMed] [Google Scholar]
- Visioli F, Poli A, Galli C. Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev. 2002;22:65–75. doi: 10.1002/med.1028. [DOI] [PubMed] [Google Scholar]
- Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biomark Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]