Abstract
Powders obtained from three grape pomaces (Barbera, Chardonnay before distillation, Chardonnay after distillation) were added at two concentration levels (0.8 and 1.6 % w/w) into semi-hard and hard cheeses (Italian Toma-like and Cheddar, respectively) to increase their polyphenol content. Effects on physicochemical characteristics, total phenolic content (TPC), radical scavenging activity (RSA), proteolysis, organic acids content, starter and non-starter bacteria were evaluated during ripening. The amount and the type of powder used did not significantly affect the physicochemical parameters of cheese with the exception of pH their values. Italian Toma-like and Cheddar cheeses fortified with Chardonnay after distillation powder showed at the end of ripening (30 days and 120 days respectively) the highest TPC and RSA values. Proteolysis and microbial counts did not show statistically significant differences between fortified and control cheeses. This study demonstrated that grape pomace powder can be a functional ingredient to increase TPC and RSA in consumers' diets and the addition of this by-product to cheese is an environmentally friendly way to manage winemaking wastes.
Keywords: Grape pomace, Cheese, Antioxidant activity, Proteolysis, Polyphenols
Introduction
Grape pomace (GP), composed of a mix of grape seeds and skins, corresponds to approximately 62 % of total waste generated during the winemaking process. Although part of GP is distilled for ethanol extraction, the majority of this by-product is discarded with several environmental and economic consequences (Ruggieri et al. 2009). Contrary to fresh GP, distilled GP is not currently used by industries, therefore the need of disposal still present and need to be solved. Nevertheless fresh and distilled GP are a rich source of high-value products like tartaric, malic and citric acid, bioactive compounds such as dietary fibre and, especially, polyphenols (Teixeira et al. 2014) with anti-inflammatory, anticancer, antimicrobial and cardiovascular protective properties to benefit human health (Teixeira et al. 2014).
In previous studies, GP powder (GPP) was successfully used as ingredient in pasta (Sant’Anna et al. 2014), tomato puree (Lavelli et al. 2014), tea infusion (Cheng et al. 2010), minced fish (Sánchez-Alonso et al. 2007), salad dressing (Tseng and Zhao 2013), biscuits (Mildner-Szkudlarz et al. 2013) and bread (Mildner-Szkudlarz et al. 2011). Similarly, grape seed powder was employed in the production of different foodstuffs, such as cereal bars, pancakes and noodles (Rosales Soto et al. 2012), frankfurters (Özvural and Vural 2011), and bread (Hoye and Ross 2011).
The use of fresh or distilled GPP as ingredient in foods combines the need for green production, minimizing by-product treatment costs, creating a new source of income to grape producers and increasing consumer interest in healthier foods. Furthermore, the addition of grape by-products to foods may represent a novel strategy for the production of functional foods with a natural source of antioxidants.
In dairy products, GPP was only used in yogurt formulation by Tseng and Zhao (2013), leading to increased antioxidant activity and dietary fiber content. Therefore, among dairy products, cheese could be an interesting way of GPP addition, owing to its contribution to protein, calcium, phosphorus and magnesium intake in human daily diet (Apostolidis et al. 2007). To our knowledge, no studies regarding the addition of fresh or distilled GPPs to cheese and its impact on ripening are present in literature. The aims of this work were to evaluate the feasibility of adding these by-products into semi-hard and hard cow’s milk cheeses (Italian Toma-like and Cheddar, respectively) and to define the effect of this addition on their ripening.
Material and methods
Grape pomace powder (GPP) production
White grape pomaces of Chardonnay cultivar were provided by a distillation factory (Bonollo, Padova, Italy) while the red grape pomaces of Barbera cultivar were provided by a winemaking factory (Cantina Clarea, Turin, Italy). For Chardonnay grape pomaces were used fresh and after distillation.
Grape pomaces were sieved to remove grape seeds and the skins obtained were vacuum packed before being stored at −20 °C until drying.
Grape pomaces were dried with a Memmert UFE 550 oven (ENCO, Spinea, Italy) at 54 °C for 48 h and milled with a ZM200 grinder (Retsch Gmbh, Haan, Germany) to a particle size of 250 μm. The powders obtained were stored in sealed polyethylene bags at 4 °C until cheesemaking.
Toma-like cheese production
Raw cow’s milk (fat 3.6 %; protein 3.1 % and lactose 4.8 %) was pasteurised at 63 °C for 30 min, cooled to 38 °C and then inoculated at a level of 2 % (w/v) with Choozit star 2 starter culture (Santamaria, Burago di Molgora, Italy) and left for 30 min prior to addition of liquid calf rennet Extra 10,000 (Santamaria, Burago di Molgora, Italy) at a level of 0.35 mL L−1.
Coagulum was cut and the curd/whey mixture was stirred continuously for 10 min. The whey was drained and curd was subdivided in seven batches then GPPs were added and manually mixed. The addition levels of GPPs were (w/w): 0 % (Control); 0.8 % of Chardonnay before distillation (ChBD0.8); 1.6 % of Chardonnay before distillation (ChBD1.6); 0.8 % of Chardonnay after distillation (ChAD0.8); 1.6 % of Chardonnay after distillation (ChAD1.6); 0.8 % of Barbera (BAR0.8) and 1.6 % of Barbera (BAR1.6).
Curds were placed in round moulds (0.5 kg), pressed (12 h), brine salted (8 h) then ripened at 4–6 °C for 30 days. Three independent cheesemaking trials were performed.
Cheddar production
Raw cow’s milk was standardized to a protein to fat ratio of 0.70:1, pasteurised at 73.5 °C for 20 s, cooled to 30 °C and inoculated at a level of 0.03 % (w/v) with R-604Y starter culture (Chr. Hansen Ltd., Little Island, Cork, Ireland) and left for 30 min prior to the addition of 0.9 mL L−1 of 1 mol calcium chloride solution (Fluka-Sigma Aldrich, Milan, Italy). After 2 min, liquid rennet Chymax Plus (200 IMCU mL−1, Chr. Hansen Ireland Ltd) was added to milk at a level of 0.3 mL L−1.
Coagulum was cut and the curd/whey mixture was allowed to heal for 10 min and then stirred continuously. Curd was heated from 31 to 39 °C over 30 min. The whey was drained at pH 6.15, curd was cheddared to pH 5.35 and then milled. The curd was subdivided into seven batches, dry-salted at 2.5 % (w/w) and GPPs were added as previously described for Toma-like cheese. Cheeses were mellowed for 20 min, placed in round moulds (2 kg), prepressed at 0.13 kPa for 30 min then pressed overnight at 2.5 kPa. The cheeses were vacuum packaged and ripened at 8 C for a period of 120 days. Three independent cheesemaking trials were performed.
Chemicals
All reagents, standards and solvents were purchased from Sigma-Aldrich (Milano, Italy). All chemicals were of analytical or higher grade, and ultrapure water was produced with a Milli-Q System (Millipore, Milan, Italy).
Chemical composition
GPPs and both cheese types at the end of ripening were analysed for gross composition. Moisture was evaluated by an oven drying method at 102 °C, total protein and pH 4.6-soluble nitrogen were determined by Kjeldhal method, and ash was determined according to AOAC 942.05 (AOAC 2000). Cheese fat was determined by the FIL-IDF Standard 5A method (FIL-IDF 1969) and GPP fat was determined by AOAC 996.06 (2001).
Cheese pH was measured electrometrically (against two reference buffer solutions) by means of a penetration electrode Microph 2002 (Crison Strumenti SpA, Carpi, Italy) inserted directly into cheese. All analyses were performed in triplicate.
Proteolysis
The pH 4.6-soluble and -insoluble fractions were extracted according to the method of Hayaloglu et al. (2004). Urea-polyacrylamide gel electrophoresis was carried out on the insoluble fraction using the procedure described by Bertolino et al. (2011). Densitometric analysis was performed on the scanned image using gel analysis software TotalLab 1D (Nonlinear Dynamix, Newcastle upon Tyne, UK).
Toma-like cheeses were sampled at 5, 10, 20, 30 days over the ripening period. Cheddar cheeses were sampled at 14, 30, 60, 120 days over the ripening period.
Antioxidant capacity of GPPs and cheeses
Extraction of bioactive compounds
The bioactive compounds extraction was carried out as describe by Apostolidis et al. (2007) with slight modifications. Briefly, 5 g of cheese or 0.040 g of GPP were added to 10 mL of ultrapure water and mixed in a stomacher LAB Blender 400 (PBI, Milan, Italy) for 5 min at 230 rpm. The slurries were centrifuged (16.800 × g, 10 min, 4 °C) and the supernatants were collected, filtered through 0.45 μm polypropylene membrane filter (VWR, Milan, Italy), and stored at −18 °C in amber glass vials until further analyses. Extraction was conducted in triplicate for each trial.
Total phenolic content
The total phenolic content (TPC) was assayed using the method of Apostolidis et al. (2007) with some modifications. Briefly, 500 μL of extract was mixed with 500 μL of 95 % ethanol and 2.5 mL of ultrapure water. To each sample, 250 μL of 50 % (v/v) water solution of Folin-Ciocalteau reagent was added and mixed. After 5 min, 500 μL of 5 % (w/v) Na2CO3 were added to the reaction mixture and allowed to stand for 60 min in the dark. The sample mixture was centrifuged (16,800 × g, 10 min, 20 °C) and the absorbance was read at 725 nm. The absorbance values were converted to total phenolic and were expressed as milligrams equivalents of gallic acid per gram (GAE mg g−1) of sample. Standard curve was established using various concentrations of gallic acid in water (0–750 μmol). The assay was conducted in triplicate for each trial.
DPPH radical scavenging capacity
The free radical scavenging activity (RSA) of the extracts was determined according to the method proposed by Apostolidis et al. (2007) using the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH•). Briefly the extract (250 μL) was added to 3 mL of 60 μM DPPH• ethanol solution and incubated for 1 h at room temperature in the dark. Samples mixture were centrifuged (16,800 × g, 10 min, 20 °C), before reading the absorbance decrease at 517 nm against ethanol as a control and ethanol solution of DPPH• as a blank. The inhibition percentage of the DPPH• by the extracts was calculated according to the formula:
where A0min is the absorbance of the control at t = 0 min, and A60min is the absorbance of the samples at 60 min. The results were expressed as μmol Trolox equivalents (TE) per gram of sample by means of a dose-response curve for Trolox (0–350 μmol). The assay was conducted in triplicate for each trial.
Organic acids content
Organic acids content was determined according to the method of Bertolino et al. (2011). Cheese samples (5 g) were added to 25 mL of 0.013 N H2SO4 and mixed in a stomacher LAB Blender 400 (PBI, Milan, Italy) for 10 min at 230 rpm. The slurry was subsequently centrifuged (4000 × g, 10 min, 10 °C) and the supernatant was filtered through a 0.45 μm polypropylene membrane filter (VWR, Milan, Italy). Ion-exchange high-performance liquid chromatography was used to determine organic acids in all samples.
The HPLC system (Thermo Quest, San Jose, CA) was equipped with a P4000 isocratic pump, a multiple autosampler AS3000 fitted with a 20-μL loop, an UV detector (Spectra Focus UV100, Thermo Quest) set at 210 nm. ChromQuest software version 3.0 (Thermo Quest) was used for instrument control and UV-data collection and processing.
The analyses were performed isocratically at 0.8 mL min−1 and 65 °C with a 300 × 7.8 mm i.d. cation exchange column (Aminex HPX-87 H) equipped with a cation H+ microguard cartridge (Bio-Rad Laboratories, Hercules, CA, USA). Mobile phase was 0.013 N H2SO4. Identification was achieved by comparing the retention times and spectra with those of authentic standards. Two analyses were performed for each sample.
Microbial counts
Cheese samples (10 g) were added to 90 mL of sterile 2 % (w/v) trisodium citrate solution and mixed in a stomacher LAB Blender 400 (PBI, Milan, Italy) for 4 min at 230 rpm.
Decimal dilutions in quarter-strength Ringer’s solution were prepared, and aliquots of 1 mL of the appropriate dilutions were spread in triplicate on the following media: (i) De Man Rogosa and Sharpe agar (MRS, Oxoid, Milan, Italy) for non-starter (NSLAB), incubated in microaerophilic conditions at 30 °C for 48 h; (ii) M17 agar (Oxoid) for Streptococcaceae (SLAB) incubated at 30 °C for 48 h. Results were calculated as the means of log colony forming units (CFU) for three independent determinations. Samples were analyzed in duplicate.
Data analysis
A one-way analysis of variance (ANOVA) with Duncan’s test for mean comparison was used to highlight significant differences among the cheese samples. All calculations were performed with the STATISTICA software for Windows (Release 7.0; StatSoft Inc., Tulsa, OK, USA).
Results and discussion
Chemical composition of GPPs
Table 1 shows the chemical composition of GPPs and the results of variance analysis. Barbera GPP had the lowest moisture content and it was also characterized by the lowest protein content. The high Barbera ash value was probably due to the winemaking process where the crystallization and precipitation of tartrates occur.
Table 1.
Gross composition, total phenolic content (TPC) and radical scavenging activity (RSA) of grape pomace powders (GPPs) and results of variance analysisW
| Ashesx | Fatx | Proteinx | Moisture (%) | TPCy | RSAz | ||
|---|---|---|---|---|---|---|---|
| GPPs | BAR | 8.26 ± 0.06b | 5.18 ± 0.5b | 10.37 ± 0.4a | 4.66 ± 0.37a | 3.64 ± 0.04a | 27.49 ± 0.61a |
| ChBD | 5.74 ± 0.12a | 6.88 ± 0.28b | 12.68 ± 0.76b | 6.26 ± 0.42b | 5.73 ± 0.42b | 38.69 ± 2.20b | |
| ChAD | 6.07 ± 0.13ab | 3.85 ± 0.20a | 13.43 ± 0.81b | 6.47 ± 0.29b | 16.06 ± 1.06c | 44.99 ± 1.77c | |
| Sig. | ** | * | * | * | *** | *** | |
WData are expressed as mean ± SD (n = 3)
BAR Barbera, ChBD Chardonnay before distillation, ChAD Chardonnay after distillation, TPC total phenolic content, RSA radical scavenging activity
Different letters within a column indicate significant differences (Duncan test, p < 0.05) between mean values
Significance: * p < 0.05; ** p < 0.01; *** p < 0.001;
x% (w/w) dry matter
ymg GAE g−1
zInhibition % (at a concentration of GPP 0.04 g/10 mL)
The distillation process allows the extraction of fat, therefore Chardonnay after distillation GPP showed the lowest fat content. Among the other parameters no differences were highlighted for Chardonnay due to the distillation process.
The TPC of the GPP ranged between 3.64 and 16.06 GAE mg g−1 with the highest value assessed for ChAD (Table 1). The TPC of ChBD was very close to those reported recently by Sri Harsha et al. (2014) in grape pomace samples of Chardonnay recovered after the winemaking process (4.6 and 6.7 GAE mg g−1). Contrary to other reports (Deng et al. 2011; Sri Harsha et al. 2013), the phenolic content of BAR pomace recovered from red winemaking was lower than that of white Chardonnay. Sri Harsha et al. (2013) reported value of TPC in Barbera grape pomace around 8 times higher than that determined in our samples. This different behaviour could be explain by the different extraction technique adopted, where a soft extraction with water applied in this work was replaced with an exhaustive extraction with 80 % acidified methanol, the most common solvent system used for extraction of anthocyanins (Cheynier 2006).
As expected, the radical scavenging activity of GPP extracts was well related to TPC and followed the same order, with ChAD characterized by the highest value. The highest values detected in ChAD for both assays highlighted the important role played by distillation process in the release of bioactive compounds. This result was in agreement with Parejo et al. (2002) who evaluated different extracts of herbs and aromatic plants before and after being distilled for essential oils production.
Cheese gross composition
Table 2 shows the gross composition of cheeses and the results of variance analysis. Despite the addition of GPPs, the gross composition of Toma-like cheeses was comparable to that reported by Ambrosoli et al. (1998) and also for Cheddar, its gross composition is similar to that previously reported (Bansal et al. 2009).
Table 2.
Gross composition in control and cheeses fortified with grape pomace powder and results of analysis of variance W
| Ashesx | Fatx | Proteinx | Moisture (%) | TNy | SNz | pH | ||
|---|---|---|---|---|---|---|---|---|
| Toma (30 days) | Control | 2.80 ± 0.20 | 27.81 ± 2.23 | 20.05 ± 1.42 | 49.41 ± 1.71 | 3.14 ± 0.22 | 8.98 ± 0.63 | 5.25 ± 0.03d |
| BAR0.8 | 2.80 ± 0.21 | 26.93 ± 0.77 | 21.81 ± 0.34 | 47.75 ± 0.46 | 3.42 ± 0.05 | 10.51 ± 0.92 | 5.21 ± 0.02bcd | |
| ChBD0.8 | 3.18 ± 0.24 | 28.07 ± 0.85 | 21.50 ± 1.30 | 45.30 ± 3.91 | 3.37 ± 0.20 | 10.07 ± 1.26 | 5.22 ± 0.01cd | |
| ChAD0.8 | 3.07 ± 0.36 | 26.58 ± 1.38 | 20.77 ± 0.22 | 51.52 ± 2.64 | 3.26 ± 0.04 | 9.87 ± 2.03 | 5.24 ± 0.03cd | |
| BAR1.6 | 3.01 ± 0.17 | 24.61 ± 1.79 | 22.46 ± 0.85 | 47.71 ± 0.84 | 3.52 ± 0.13 | 10.77 ± 2.29 | 5.13 ± 0.01a | |
| ChBD1.6 | 2.96 ± 0.57 | 26.52 ± 1.37 | 22.13 ± 1.38 | 46.39 ± 5.81 | 3.47 ± 0.22 | 8.75 ± 2.90 | 5.19 ± 0.04bc | |
| ChAD1.6 | 3.32 ± 0.58 | 25.32 ± 2.20 | 21.64 ± 1.61 | 47.59 ± 2.07 | 3.39 ± 0.25 | 8.92 ± 1.23 | 5.16 ± 0.03ab | |
| Sig. | ns | ns | ns | ns | ns | ns | *** | |
| Cheddar (120 days) | Control | 3.78 ± 0.18 | 31.20 ± 1.68 | 25.10 ± 1.63 | 36.40 ± 0.92b | 3.93 ± 0.26 | 17.87 ± 1.54ab | 5.07 ± 0.01d |
| BAR0.8 | 4.10 ± 0.17 | 30.62 ± 1.97 | 23.77 ± 1.02 | 37.94 ± 0.53d | 3.73 ± 0.16 | 18.49 ± 0.73b | 5.00 ± 0.02b | |
| ChBD0.8 | 3.98 ± 0.07 | 30.53 ± 0.90 | 25.52 ± 0.95 | 36.45 ± 0.72b | 4.00 ± 0.15 | 16.76 ± 1.43a | 5.04 ± 0.01c | |
| ChAD0.8 | 3.96 ± 0.55 | 30.13 ± 0.33 | 24.40 ± 1.15 | 36.53 ± 0.70b | 3.82 ± 0.18 | 17.62 ± 1.11ab | 5.04 ± 0.01c | |
| BAR1.6 | 4.19 ± 0.09 | 29.99 ± 2.24 | 24.32 ± 0.67 | 37.08 ± 0.73bc | 3.81 ± 0.10 | 18.52 ± 0.42b | 4.95 ± 0.01a | |
| ChBD1.6 | 4.08 ± 0.14 | 31.35 ± 0.75 | 24.95 ± 1.42 | 35.36 ± 0.46a | 3.91 ± 0.22 | 16.78 ± 1.24a | 5.03 ± 0.02c | |
| ChAD1.6 | 4.10 ± 0.03 | 30.78 ± 0.98 | 24.80 ± 1.48 | 37.69 ± 1.24cd | 3.89 ± 0.23 | 17.33 ± 0.76a | 4.96 ± 0.01a | |
| Sig. | ns | ns | ns | *** | ns | * | *** | |
WData are expressed as mean ± SD (n = 9)
BAR Barbera, ChBD Chardonnay before distillation, ChAD Chardonnay after distillation; 0.8: 0.8 % grape pomace powder (w/w); 1.6: 1.6 % grape pomace powder (w/w)
Different letters within a column indicate significant differences (Duncan test, p < 0.05) between mean values Significance: ns = not significant; * p < 0.05; *** p < 0.001;
x% (w/w) dry matter
y% Total Nitrogen (w/w)
zpH 4.6-soluble Nitrogen as % of Total Nitrogen
Most parameters showed no statistical differences in composition between Control cheeses and fortified cheeses due to GPP addition. GPP addition produced a significant pH decrease (p < 0.001) due to the presence of organic acids (tartaric, malic and citric acids) in grape powder, therefore the lowest values were measured with the 1.6 % of GPPs addition.
Due to the precipitation of tartrate during winemaking, the acid concentration of GPPs increases, therefore cheeses with added Barbera powder showed lowest pH values. For Toma-like cheese the pH decreases from 5.25 (Control) to 5.13 (BAR1.6) while for Cheddar the pH decreases from 5.07 (Control) to 4.95 (BAR1.6).
For Cheddar cheeses statistically significant differences within samples were found only for moisture (p < 0.001) and soluble nitrogen (p < 0.05). Moisture of Cheddar fortified cheeses was generally higher than Control (+4 % for BAR0.8). These differences are probably due to the water absorption by the GPPs, since Cheddar ripening is performed under vacuum, moisture differences are significant, whereas no differences were found for Toma-like cheeses where ripening is performed exposed to the air.
Also nitrogen content is higher for cheeses added with GPPs but only for Cheddar there are significant differences against Control cheeses.
Proteolysis
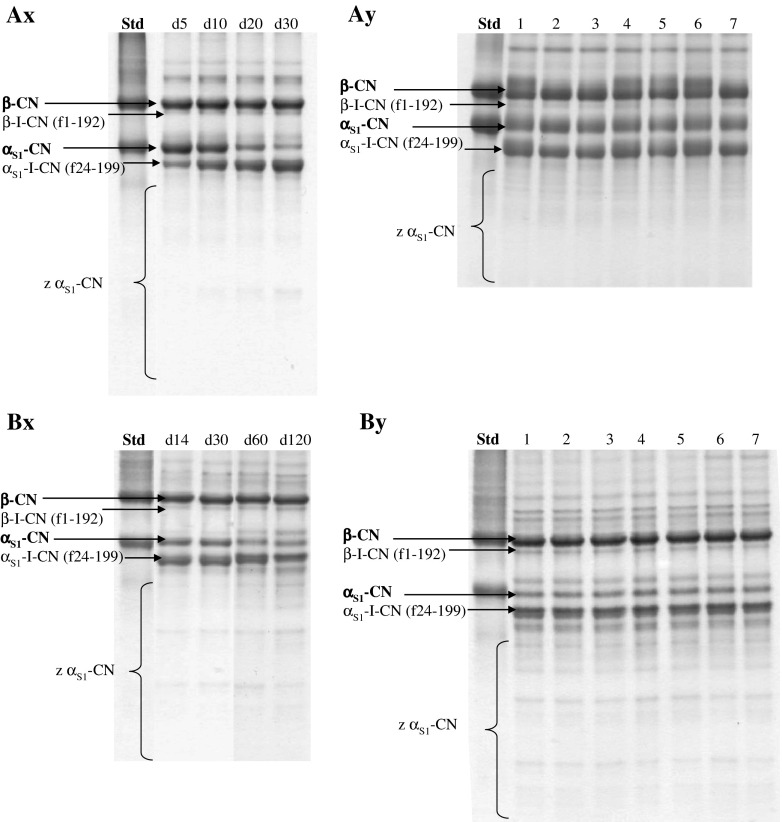
Urea-PAGE gel electrophoretograms of Toma-like and Cheddar cheeses are reported in Fig. 1A and B respectively. Densitometric analysis of electrophoretograms (data not shown) showed no significant differences in casein degradation between control and fortified cheeses for Toma-like or Cheddar cheeses.
Fig. 1.
Urea-polyacrylamide gel electrophoretograms of a Toma-like and b Cheddar cheeses. ChAD1.6 Toma-like cheese during ripening (Ax); Control and fortified cheeses at day 30 of ripening (Ay). ChAD1.6 Cheddar cheese during ripening (Bx); Control and fortified cheeses at day 120 of ripening (By). Abbrev: (Std) Sodium caseinate; (1) Control; (2) ChBD0.8; (3) ChBD1.6; (4) ChAD0.8; (5) ChAD1.6; (6) BAR0.8; (7) BAR1.6
In particular, during the ripening (Fig. 1Ax and Bx), the typical breakdown patterns of β-CN and αs1-CN were observed. Neither β- nor αs1-CN were totally degraded at the end of the ripening and αs1-CN hydrolysed more than β-CN, as previously studied on Toma Piemontese cheese by Bertolino et al. (2008) and on Cheddar by McMahon et al. (2014).
The band corresponding to the αs1-I-casein (αs1-CN f24-199) was present in electrophoretograms of all samples which is the first product of rennet action on αs1-casein. The intensity of this band increased until the end of the ripening in Toma-like cheeses, and until day 60 for Cheddar cheeses. Other bands corresponding to other peptides (marked as z αs1-CN) and characterised to have a faster mobility than αs1-I-casein (αs1-CN f24-199) were also present and their intensity increased during the ripening.
The action of chymosin on β-CN corresponds to a faint band of β-I-CN (β-CN f1-192) present in all cheeses and its intensity increased until the end of ripening.
Concerning β-CN degradation in Toma-like cheeses at the end of ripening (Fig. 1Ay), ChBD and Control showed a similar decrease whereas the other fortified cheeses were characterised by a slightly lower degradation. αs1-CN breakdown of ChBD cheeses was higher than the others that had have a similar trend, which could be attributed to a greater microbial activity as demonstrated by the higher lactic acid concentration (Table 4).
Table 4.
Organic acid concentrations (g kg−1) in control and cheeses fortified with grape pomace powder during ripening and results of analysis of varianceW
| Toma | ||||||
|---|---|---|---|---|---|---|
| Days | 5 | 10 | 20 | 30 | Significance | |
| Citric acid | Control | 1.22 ± 0.13ABb | 1.26 ± 0.25ABb | 1.26 ± 0.15Ab | 0.66 ± 0.06Aa | ** |
| BAR0.8 | 1.09 ± 0.06A | 1.13 ± 0.15A | 1.09 ± 0.12A | 0.88 ± 0.16AB | ns | |
| ChBD0.8 | 2.79 ± 0.21D | 2.89 ± 0.07D | 2.96 ± 0.47D | 2.68 ± 0.36E | ns | |
| ChAD0.8 | 1.45 ± 0.08BCa | 1.55 ± 0.24Ba | 1.55 ± 0.37ABa | 1.32 ± 0.19BCa | ns | |
| BAR1.6 | 1.74 ± 0.32C | 2.06 ± 0.03C | 1.87 ± 0.04B | 1.40 ± 0.53CD | ns | |
| ChBD1.6 | 2.75 ± 0.24Db | 2.66 ± 0.27Db | 2.41 ± 0.22Cb | 1.86 ± 0.20Da | *** | |
| ChAD1.6 | 1.21 ± 0.06AB | 1.22 ± 0.06A | 1.12 ± 0.14A | 1.06 ± 0.05ABC | ns | |
| Significance | *** | *** | *** | *** | ||
| Tartaric acid | Control | ndA | ndA | ndA | ndA | ns |
| BAR0.8 | 0.68 ± 0.02B | 0.66 ± 0.07B | 0.59 ± 0.05BC | 0.47 ± 0.17B | ns | |
| ChBD0.8 | ndAa | 1.65 ± 0.23Db | 1.63 ± 0.25Db | 1.36 ± 0.36Db | *** | |
| ChAD0.8 | 0.84 ± 0.10C | 0.92 ± 0.11C | 0.91 ± 0.32C | 0.67 ± 0.01BC | ns | |
| BAR1.6 | ndA | nd | 0.26 ± 0.24AB | ndA | ns | |
| ChBD1.6 | 1.71 ± 0.16Db | 1.74 ± 0.25Db | 1.45 ± 0.22Db | 0.89 ± 0.10Ca | ** | |
| ChAD1.6 | 0.71 ± 0.10BC | 0.71 ± 0.03BC | 0.58 ± 0.19BC | 0.52 ± 0.13B | ns | |
| Significance | *** | *** | *** | *** | ||
| Piruvic acid | Control | nd | nd | ndA | ndA | ns |
| BAR0.8 | 0.01 ± 0.02 | 0.01 ± 0.01 | ndA | ndA | ns | |
| ChBD0.8 | 0.06 ± 0.10 | 0.06 ± 0.06 | 0.10 ± 0.02C | 0.08 ± 0.02D | ns | |
| ChAD0.8 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.03 ± 0.03A | 0.03 ± 0.01B | ns | |
| BAR1.6 | 0.01 ± 0.02 | 0.05 ± 0.05 | 0.01 ± 0.02A | ndA | ns | |
| ChBD1.6 | 0.08 ± 0.03 | 0.08 ± 0.06 | 0.06 ± 0.01B | 0.05 ± 0.01C | ns | |
| ChAD1.6 | 0.03 ± 0.04 | 0.02 ± 0.02 | 0.01 ± 0.02A | ndA | ns | |
| Significance | ns | ns | *** | *** | ||
| Lactic acid | Control | 8.74 ± 0.63A | 8.84 ± 0.48A | 9.24 ± 0.80A | 8.12 ± 0.86A | ns |
| BAR0.8 | 8.91 ± 0.61Ab | 8.11 ± 0.87Aab | 7.96 ± 0.68Aab | 6.68 ± 0.90Aa | * | |
| ChBD0.8 | 22.45 ± 0.62Dc | 21.34 ± 1.09Dbc | 19.44 ± 1.42Cb | 15.66 ± 2.14Ca | ** | |
| ChAD0.8 | 12.98 ± 2.75Bb | 12.37 ± 1.28Bab | 14.34 ± 2.58Bb | 9.31 ± 1.20Aa | ** | |
| BAR1.6 | 18.18 ± 0.93Cb | 15.88 ± 1.54Cab | 15.20 ± 2.67Bab | 13.03 ± 2.64BCa | * | |
| ChBD1.6 | 23.05 ± 1.67Dc | 22.46 ± 0.38Dc | 19.31 ± 1.55Cb | 12.19 ± 1.43Ba | *** | |
| ChAD1.6 | 10.16 ± 0.39A | 9.66 ± 1.17A | 9.78 ± 0.62A | 8.71 ± 0.95A | ns | |
| Significance | *** | *** | *** | *** | ||
| Cheddar | ||||||
| Days | 14 | 30 | 60 | 120 | Significance | |
| Citric acid | Control | 1.45 ± 0.32B | 1.38 ± 0.19 | 1.42 ± 0.07D | 1.51 ± 0.18C | ns |
| BAR0.8 | 0.84 ± 0.09A | 0.98 ± 0.16 | 0.85 ± 0.10AB | 0.98 ± 0.18AB | ns | |
| ChBD0.8 | 1.04 ± 0.14A | 0.84 ± 0.66 | 1.11 ± 0.04C | 1.22 ± 0.12B | ns | |
| ChAD0.8 | 0.96 ± 0.07A | 1.18 ± 0.23 | 1.07 ± 0.05BC | 1.12 ± 0.15AB | ns | |
| BAR1.6 | 0.75 ± 0.10A | 0.86 ± 0.22 | 0.80 ± 0.06A | 0.88 ± 0.15A | ns | |
| ChBD1.6 | 0.89 ± 0.04A | 1.06 ± 0.10 | 1.10 ± 0.25C | 1.15 ± 0.24AB | ns | |
| ChAD1.6 | 0.88 ± 0.14A | 1.02 ± 0.20 | 1.01 ± 0.12ABC | 1.11 ± 0.08AB | ns | |
| Significance | ** | ns | *** | ** | ||
| Tartaric acid | Control | ndA | ndA | ndA | ndA | ns |
| BAR0.8 | 1.03 ± 0.09C | 1.09 ± 0.18D | 1.08 ± 0.16CD | 1.19 ± 0.14CD | ns | |
| ChBD0.8 | 0.19 ± 0.33A | 0.14 ± 0.25A | 0.21 ± 0.24A | 0.23 ± 0.25A | ns | |
| ChAD0.8 | 0.54 ± 0.03B | 0.52 ± 0.02B | 0.50 ± 0.03B | 0.77 ± 0.33B | ns | |
| BAR1.6 | 1.17 ± 0.02C | 1.16 ± 0.10D | 1.27 ± 0.03D | 1.31 ± 0.09D | ns | |
| ChBD1.6 | 0.69 ± 0.04B | 0.55 ± 0.09BC | 0.60 ± 0.07B | 0.68 ± 0.24B | ns | |
| ChAD1.6 | 0.75 ± 0.14B | 0.79 ± 0.17C | 0.95 ± 0.15C | 0.85 ± 0.22BC | ns | |
| Significance | *** | *** | *** | *** | ||
| Piruvic acid | Control | 0.06 ± 0.01Ca | 0.06 ± 0.00BCa | 0.06 ± 0.00Ca | 0.10 ± 0.01CDb | ** |
| BAR0.8 | 0.03 ± 0.00ABa | 0.03 ± 0.00Aa | 0.03 ± 0.01ABab | 0.04 ± 0.00Ab | * | |
| ChBD0.8 | 0.07 ± 0.02Ca | 0.06 ± 0.01Ca | 0.06 ± 0.00Ca | 0.11 ± 0.02Db | * | |
| ChAD0.8 | 0.04 ± 0.00Ba | 0.04 ± 0.00ABa | 0.05 ± 0.01BCa | 0.07 ± 0.01BCb | * | |
| BAR1.6 | 0.03 ± 0.00Aa | 0.02 ± 0.00Aa | 0.03 ± 0.00Aa | 0.04 ± 0.01Ab | * | |
| ChBD1.6 | 0.06 ± 0.00Ca | 0.05 ± 0.00BCa | 0.06 ± 0.01Ca | 0.10 ± 0.01CDb | * | |
| ChAD1.6 | 0.05 ± 0.01B | 0.05 ± 0.02BC | 0.06 ± 0.02C | 0.06 ± 0.02AB | ns | |
| Significance | *** | * | * | *** | ||
| Lactic acid | Control | 19.12 ± 1.36Da | 25.46 ± 1.08Ea | 27.89 ± 1.71Da | 28.06 ± 1.68Eb | *** |
| BAR0.8 | 10.15 ± 0.90ABa | 14.93 ± 0.47Bb | 13.95 ± 0.84Ab | 14.81 ± 0.65Bb | *** | |
| ChBD0.8 | 19.57 ± 1.02D | 22.66 ± 1.82D | 22.19 ± 2.18C | 23.30 ± 2.42D | ns | |
| ChAD0.8 | 12.30 ± 0.79Ca | 17.48 ± 0.88Cb | 17.30 ± 1.16Bb | 18.42 ± 0.34Cb | *** | |
| BAR1.6 | 9.41 ± 0.29Aa | 12.35 ± 1.12Ab | 12.59 ± 0.18Ab | 12.01 ± 1.06Ab | ** | |
| ChBD1.6 | 11.81 ± 1.05BCa | 18.85 ± 0.60Cb | 20.56 ± 0.90Cc | 20.38 ± 0.12Cc | *** | |
| ChAD1.6 | 11.57 ± 1.06BCa | 14.59 ± 1.30Bb | 14.60 ± 0.33Ab | 15.47 ± 0.67Bb | ** | |
| Significance | *** | *** | *** | *** | ||
WData are expressed as mean ± SD (n = 6)
BAR Barbera, ChBD Chardonnay before distillation, ChAD Chardonnay after distillation; 0.8: 0.8 % grape pomace powder (w/w); 1.6: 1.6 % grape pomace powder (w/w); nd: not detected
Different uppercase letters in the same column indicate significant statistical differences (Duncan test, p < 0.05) within cheeses
Different lowercase letters in the same row indicate significant statistical differences (Duncan test, p < 0.05) within time
Significance: ns = not significant; * p < 0.05; ** p < 0.01; *** p < 0.001
Regardless GPPs varieties, when comparing the effect of percentage used, it was observed that major addition determined higher β- and αs1-CN breakdown, probably due to the lower pH.
Concerning β- and αs1-CN degradation in Cheddar cheeses at the end of ripening (Fig. 1By), ChBD and Control showed a similar decrease whereas the other fortified cheeses were characterised by a slightly higher degradation.
Regardless GPPs varieties, when comparing the effect of percentage used, the trend was the same observed for Toma-like cheeses.
Total phenolic content and antioxidant capacity of cheese
Total phenolic content (TPC) and free radical scavenging activity (RSA) of cheeses are reported in Table 3. The phenolic compounds detected in control cheeses were endogenous phenolic compounds in bovine milk (Kuhnen et al. 2014). In addition, a portion of TPC values might be derived from the reaction of protein and sugar components of the milk with the Folin-Ciocalteau reagent (Singleton et al. 1999).
Table 3.
Total phenolic content (TPC) and radical scavenging activity (RSA) in control and cheeses fortified with grape pomace powder during ripening and results of analysis of variance W
| Toma | ||||||
|---|---|---|---|---|---|---|
| Days | 5 | 10 | 20 | 30 | Significance | |
| TPCx | Control | 90.44 ± 4.13Aa | 128.90 ± 23.13ABCb | 137.03 ± 26.69b | 127.90 ± 22.67Ab | *** |
| BAR0.8 | 97.04 ± 13.60ABa | 118.40 ± 16.70ABb | 134.63 ± 11.51c | 151.80 ± 13.77ABCd | *** | |
| ChBD0.8 | 92.36 ± 9.60Aa | 137.64 ± 20.63BCb | 140.99 ± 15.55b | 133.58 ± 23.37ABb | *** | |
| ChAD0.8 | 108.37 ± 15.82BCa | 110.65 ± 7.20Aa | 134.42 ± 36.22ab | 142.81 ± 33.75ABb | * | |
| BAR1.6 | 109.17 ± 8.20BCa | 114.89 ± 9.73Aa | 151.80 ± 33.27b | 155.98 ± 20.33BCDb | *** | |
| ChBD1.6 | 113.79 ± 8.46CDa | 138.00 ± 21.28BCb | 138.60 ± 25.11b | 179.94 ± 25.54Dc | *** | |
| ChAD1.6 | 126.95 ± 32.40Da | 147.66 ± 20.20Ca | 142.42 ± 25.01a | 174.71 ± 24.63CDb | ** | |
| Significance | *** | * | ns | *** | ||
| RSAy | Control | 7.64 ± 2.66Aa | 25.31 ± 18.92b | 24.90 ± 10.91b | 22.26 ± 7.63Ab | ** |
| BAR0.8 | 36.61 ± 17.44B | 36.47 ± 26.75 | 37.16 ± 14.43 | 27.51 ± 8.88A | ns | |
| ChBD0.8 | 11.56 ± 7.22A | 27.93 ± 26.32 | 27.77 ± 26.20 | 35.09 ± 21.87AB | ns | |
| ChAD0.8 | 37.77 ± 11.72B | 37.27 ± 14.36 | 41.98 ± 20.16 | 56.16 ± 14.91BC | ns | |
| BAR1.6 | 33.06 ± 9.41Ba | 35.92 ± 3.63a | 53.13 ± 17.32b | 53.57 ± 10.57CDb | *** | |
| ChBD1.6 | 36.03 ± 25.87Ba | 43.81 ± 29.41a | 30.88 ± 8.95a | 79.64 ± 12.80Eb | ** | |
| ChAD1.6 | 36.63 ± 10.89Ba | 55.27 ± 15.14b | 50.96 ± 16.05b | 65.29 ± 12.19Db | ** | |
| Significance | *** | ns | ns | *** | ||
| Cheddar | ||||||
| Days | 14 | 30 | 60 | 120 | Significance. | |
| TPCx | Control | 145.77 ± 5.96Abc | 134.19 ± 4.15Aa | 140.67 ± 4.92Ab | 149.98 ± 6.99Ac | *** |
| BAR0.8 | 157.84 ± 8.34BCb | 142.96 ± 7.80Ba | 145.99 ± 8.75Aa | 160.05 ± 5.58Bb | *** | |
| ChBD0.8 | 158.58 ± 12.80ABCc | 137.45 ± 4.97Aa | 146.51 ± 4.80Ab | 164.07 ± 6.14BCd | *** | |
| ChAD0.8 | 185.27 ± 14.14Db | 157.47 ± 8.31Ca | 160.58 ± 6.51Ba | 166.96 ± 3.78BCa | *** | |
| BAR1.6 | 152.23 ± 7.92ABb | 143.71 ± 3.27Ba | 164.08 ± 6.01Bc | 169.24 ± 9.56Cc | *** | |
| ChBD1.6 | 164.42 ± 4.89Cc | 136.79 ± 6.10Aa | 155.22 ± 6.44Bb | 161.49 ± 7.69Bbc | *** | |
| ChAD1.6 | 175.24 ± 7.14Db | 149.15 ± 7.14Ba | 170.23 ± 12.37Cb | 171.81 ± 8.77Cb | *** | |
| Significance | *** | *** | *** | *** | ||
| RSAy | Control | 16.07 ± 3.18Ab | 11.11 ± 5.54Aa | 10.53 ± 4.06Aa | 13.54 ± 1.60Aab | * |
| BAR0.8 | 28.17 ± 3.60Bc | 24.97 ± 1.39Bb | 18.76 ± 2.03Ba | 26.49 ± 1.25Bbc | *** | |
| ChBD0.8 | 26.59 ± 1.48B | 24.45 ± 2.82B | 23.40 ± 2.07C | 25.43 ± 1.84B | ns | |
| ChAD0.8 | 33.49 ± 3.92Cb | 25.21 ± 4.46Ba | 21.00 ± 3.07BCa | 24.91 ± 6.22Ba | *** | |
| BAR1.6 | 40.17 ± 2.32D | 37.70 ± 4.46D | 40.31 ± 6.25F | 40.00 ± 3.64D | ns | |
| ChBD1.6 | 46.04 ± 4.80Ec | 29.98 ± 2.63Ca | 35.33 ± 1.91Db | 36.87 ± 2.43Cb | *** | |
| ChAD1.6 | 52.16 ± 1.92Fc | 35.17 ± 8.60CDa | 38.40 ± 5.95Eab | 43.32 ± 5.19Db | *** | |
| Significance | *** | *** | *** | *** | ||
WData are expressed as mean ± SD (n = 9)
BAR Barbera, ChBD Chardonnay before distillation, ChAD Chardonnay after distillation; 0.8: 0.8 % grape pomace powder (w/w); 1.6: 1.6 % grape pomace powder (w/w)
Different uppercase letters in the same column indicate significant statistical differences (Duncan test, p < 0.05) within cheeses
Different lowercase letters in the same row indicate significant statistical differences (Duncan test, p < 0.05) within time
Significance: ns = not significant; * p < 0.05; ** p < 0.01; *** p < 0.001.
xμg GAE/g of cheese
yInhibition % (of extract at a concentration of 5 g cheese/10 mL, corresponding to a theoretical concentration of 0.04 or 0.08 GPP/10 mL)
The TPC of Toma-like cheeses showed statistically significant differences for GPP type at each sampling time, except for values at day 20. During ripening, the TPC of the control increased to around 41 % between day 5 and 10 and then remained constant until the last sampling time (p < 0.001). In cheeses fortified with 0.8 % of GPP, the TPC increased to about 45 %, 32 % and 57 % in ChBD, ChAD and BAR, respectively. In cheeses fortified with the highest GPP addition (1.6 %), the TPC increased by approximately 58 % for ChBD, 37 % for ChAD and 43 % for BAR. Although the TPC of Chardonnay GPP before and after distillation were very different, the differences reported between the added cheeses, during the ripening and for both addition percentages, were not likewise significant. However, each type of Chardonnay addition tested showed a similar trend, with early TPC increasing in ChBD samples (day 10) and later in ChAD (day 30). In cheeses with added Chardonnay GPPs, the percentage of addition significantly affected the TPC assessed at the end of ripening, with mean increase value of about 8 % and 39 % in 0.8 and 1.6 % of addition respectively. For BAR GPPs, the percentage of addition did not significantly affect the TPC of the cheeses.
Considering values at day 30, all fortified cheeses had higher TPC values compared to the control. For 1.6 % GPP addition, samples were statistically different from the control with values of 41 %, 37 % and 22 % for ChBD, ChAD and BAR respectively.
TPC of Cheddar showed highly significant differences (p < 0.001) within cheese type and ripening time. As mentioned above, fortified cheeses had higher TPC values than the control. Generally, after an initial decrease between day 14 and 30, the TPC increased until the end of the ripening; however, Chardonnay samples reached values lower or near to those of the beginning, while BAR-fortified cheeses were characterized by higher increases (1.4 % for BAR0.8 and 11 % for BAR1.6). This trend was also observed for the control.
Unlike Toma-like cheeses, comparing Cheddar samples at the end of the ripening, the mean value of TPC for ChAD was higher than for ChBD. The differences among Chardonnay samples were more evident at the 1.6 % of addition. The percentage of addition also affected the TPC of BAR.
All fortified Toma-like cheeses, at each sampling time, showed higher RSA values than the control; however, no differences during ripening were detected for cheeses with 0.8 % added GPPs, whereas the RSA changed in control and in all 1.6 % fortified cheeses (p < 0.01). At the end of ripening, the RSA values of control, BAR, ChBD and ChAD were 3, 1.6, 2 and 1.8 fold higher, respectively, than that of the same cheeses at the beginning of ripening. As reported for the TPC, the RSA values of cheeses with 1.6 % Chardonnay GPP added increased throughout ripening; nevertheless, the behaviour of the two parameters assessed in ChBD1.6 and ChAD1.6 did not follow similar trends. Probably due to the complexity of the matrices analysed, it was difficult to establish an effective relationship between grape pomace distillation and higher increase of the phenolics contribution in cheeses. A lower contribution of red grape pomace was confirmed. According to a previous study (Giroux et al. 2013), ripening time affected Cheddar RSA in the control (p < 0.05) and fortified cheeses (p < 0.001). The values of control and most of fortified cheeses decreased significantly during ripening, except for ChBD0.8 and BAR1.6, where the slight decrease was not significant. This trend was partially in accordance with those of the TPC. The RSA decrease was most abundant in ChAD cheeses in comparison of those with added ChBD. Considering the values at last sampling time, each sample was at least twofold higher than control, and the highest values belong to ChAD1.6 and BAR1.6, with BAR1.6 characterised by the highest stability.
The lack of previously published data made it difficult to compare our results with other experiments in order to establish a tendency of phenolics to persist in fortified cheeses during ripening. In agreement with results reported by Rashidinejad et al. (2013), that studied the effect of catechin addition on the phenolic content and antioxidant properties of low-fat cheese, the total phenolic content of all cheeses, including both controls, increased during the ripening period. The higher values of TPC could be explained by the presence of milk-derived compounds and reduced analytical accuracy, as well as to the lack of selectivity of the Folin–Ciocalteau reagent used for TPC analysis which reacts not only with phenols but also with other reducing compounds such as carotenoids, amino acids, sugars and vitamin C (Rondeau et al. 2013). At the end of ripening, the phenolic content of Toma-like cheeses fortified at 0.8 %, without the phenolic contribution of the control, corresponded, theoretically, to values of 2.99, 0.71 and 1.86 mg GAE g−1 GPP in BAR0.8, ChAD0.8 and ChBD0.8 cheeses, respectively. In 0.8 % Cheddar fortified cheeses values of 1.26, 1.76 and 2.12 mg GAE g−1 GPP were calculated. All these values were lower compared to those found for the GPP extracts, with lower losses in BAR fortified cheeses. Complex metabolic mechanisms could be involved in this evolution, starting from the protein-phenolic interactions that lead changes in the structural, functional and nutritional properties of both compounds (O’Connel and Fox 2001). Typically, milk-containing food products have low antioxidant capacities (Ozdal et al. 2013), and RSA value determined in 0.8 % fortified cheeses confirmed this tendency.
Organic acids
Data for the organic acids content of cheeses and results of variance analysis are shown in Table 4. Citric and lactic acids were the most abundant acids found in Toma-like cheeses. Regardless of sampling time, citric acid concentrations were highly significantly different between samples (p < 0.001) and the highest values were found in ChBD-fortified cheeses. Mean values during ripening were not statistically different in most fortified cheeses, except for ChBD1.6 where values were quite similar until day 20 followed by a decrease at day 30.
Lactic acid content of fortified Toma-like cheeses was significantly higher than control cheese, except for BAR0.8 and ChAD1.6. Comparing non-distilled varieties regardless of sampling time, Chardonnay has shown the highest values.
Taking into consideration the average lactic acid content during ripening, both ChBD0.8 and ChBD1.6 cheeses had twofold higher values than the control. Such a trend might be expected since ChBD sugar content could be higher than the control, ChAD (fermented and distilled) and Barbera (fermented). Lactic acid values decreased throughout the ripening in all samples. Comparing organic acids values between ChBD and ChAD, was notable that at any sampling time for any organic acids, the distillation process reduce their content.
Data concerning tartaric acid content of fortified cheeses confirmed the expected behaviour, since the highest values were found among cheeses containing 1.6 % of GPPs. Cheddar cheeses BAR0.8 and BAR1.6 had the highest tartaric acid content, ranging from 1.08 to 1.31 g kg−1. These data could explain the lowest pH values observed in chemical composition of these cheeses (see Table 2).
In this study, citric acid content in control Cheddar cheese was similar to that reported by Mullin and Emmons (1997) with a mean value of 1.5 g kg−1 for commercial Cheddar at 120 days of ripening, whereas Bouzas et al. (1991) found a level of approximately 2.2 g kg−1 that kept constant throughout the ripening period. Regarding fortified cheeses, no significant differences were seen for citric acid throughout ripening. At day 120, citric acid values in fortified cheeses were significantly lower when compared to the control cheese (p < 0.01). Between non-distilled varieties the reduction rates were more relevant in Barbera than Chardonnay, the distillation had no effect on citric acid values among cheeses added with Chardonnay GPPs.
In contrast to that observed in Toma-like cheeses, the lactic acid content of fortified Cheddar cheeses was significantly lower than Control cheese independent of sampling time. Overall, after an initial increase, lactic acid content in cheeses was rather constant until the end of maturation. Comparing values from day 14 to day 120, the lactic acid content increased of 47 % in Control cheese, similarly to ChBD and ChAD, hence, the distillation showed no effect. As expected, a minor increase was found for Barbera due to its low sugar content which is reduced by microbial fermentation during winemaking.
Microbial counts
Microbial counts (data not shown), evaluated in Toma-like cheeses showed that no statistical differences within cheeses or ripening times were found for SLAB populations. In the control cheese, the SLAB started at log 9.0 CFU g−1 of cheese, reached a maximum at day 10 (log 9.34 CFU g−1) followed by a slow decrease towards the end of ripening reaching log 9.09 CFU g−1.
Also NSLAB counts were not statistically different among cheese types whereas significant differences were observed during ripening time. In all cheese types, values at 5 days were lower and statistically different from those at 10, 20 and 30 days. Control cheese contained an initial log 3.69 CFU g−1 of NSLAB that increased reaching log 7.83 CFU g−1 at 30 days.
Regarding Cheddar cheeses, SLAB populations decrease significantly throughout ripening, according to Fenelon and Guinee (2000) and Giroux et al. (2013). Control and fortified cheeses had an initial population of log 9.55 and 9.62 CFU g−1 of cheese that decreased by about 1 log cycle during ripening (p < 0.05).
No remarkable differences caused by GPP addition were detected in cheese samples at any sampling time, therefore GPP polyphenols did not affect their growth. Regarding NSLAB, no statistically significant differences within cheese types were found during ripening.
Conclusion
Results obtained by this study showed that addition of GPPs during the manufacture of Toma-like and Cheddar cheeses had no effects on gross composition of the resulting cheeses. The most important results found, attributable to GPPs addition to cheese, were a higher antioxidant activity and phenolic content in all fortified cheeses, but to obtain a significant increase of cheese antioxidant activity it is necessary to add at least 1.6 % of GPPs. Comparing samples before and after distillation, it was possible to highlight that the distillation process allows a major release of bioactive compounds, giving the highest TPC and RSA values. During ripening, the addition of non-sterile GPPs did not interfere with SLAB and NSLAB numbers and cheese proteolysis.
The use of GPPs containing antioxidants, before or after distillation, as ingredient in cheesemaking is thus a new approach to achieve a functional cheese. This study demonstrated that grape pomace powder can be a functional ingredient to increase TPC and RSA in consumer’s diet and the addition of this by-product to cheese is an environmentally friendly way to manage winemaking wastes.
However in order to obtain a real beneficial effect on human health, further studies are required to investigate in more detail the antioxidants' bioavailability in these novel products.
Acknowledgments
Research supported by AGER (project No. 2010-2222). We would kindly thank Mr. David Waldron for cheesemaking and all the staff of University College Cork (UCC).
Footnotes
Highlights
1. Grape pomace powder can be used as raw material in cheese making process.
2. Grape pomace powder increase cheese total phenolic content and antioxidant activity.
3. Grape distillation process allows a major release of bioactive compound.
4. Addition of grape pomace powder has not effect on cheese proteolysis.
5. Grape pomace powder did not interfere with lactic bacteria growth during ripening.
References
- Ambrosoli R, Gerbi V, Zeppa G, Terrone S, Tallone G. Aspetti tecnologici, microbiologici, chimici e sensoriali. In: Soster M, editor. Toma piemontese. Torino, Italy: Regione Piemonte Assessorato Agricoltura; 1998. pp. 53–73. [Google Scholar]
- Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol. 2007;8:46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- Association of Official Analytical Chemists (2000) A.O.A.C. Official methods of analysis. 17th ed., Washington DC
- Association of Official Analytical Chemists (2001) A.O.A.C. Official methods of analysis of the AOAC 996.06 Fat (total, saturated and unsaturated) in foods, Washington DC
- Bansal N, Drake MA, Piraino P, Broe ML, Harboe M, Fox PF, McSweeney PLH (2009) Suitability of recombinant camel (Camelus dromedarius) chymosin as a coagulant for cheddar cheese. Int Dairy J 19:510–517
- Bertolino M, Zeppa G, Gerbi V, McSweeney PLH. Study of proteolysis in miniature toma piemontese cheese made using wild bacteria. Italian J Food Sci. 2008;20:57–73. [Google Scholar]
- Bertolino M, Dolci P, Giordano M, Rolle L, Zeppa G. Evolution of chemico-physical characteristics during manufacture and ripening of castelmagno PDO cheese in wintertime. Food Chem. 2011;129:1001–1011. doi: 10.1016/j.foodchem.2011.05.060. [DOI] [PubMed] [Google Scholar]
- Bouzas J, Kantt CA, Bodyfelt F, Torres JA. Simultaneous determination of sugars and organic acids in cheddar cheese by high-performance liquid chromatography. J Food Sci. 1991;56:276–278. doi: 10.1111/j.1365-2621.1991.tb08034.x. [DOI] [Google Scholar]
- Cheng VJ, Bekhit AED, Sedcole R, Hamid N. The impact of grape skin bioactive functionality information on the acceptability of tea infusions made from wine by-products. J Food Sci. 2010;75:S167–S172. doi: 10.1111/j.1750-3841.2010.01576.x. [DOI] [PubMed] [Google Scholar]
- Cheynier V. Flavonoids in wine. In: Andersen ØM, Markham RM, editors. Flavonoids: chemistry, biochemistry and applications. Boca Raton, FL: CRC Press; 2006. pp. 263–318. [Google Scholar]
- Deng Q, Penner MH, Zhao Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res Int. 2011;44:2712–2720. doi: 10.1016/j.foodres.2011.05.026. [DOI] [Google Scholar]
- Fenelon MA, Guinee TP. Primary proteolysis and textural changes during ripening in cheddar cheeses manufactured to different fat contents. Int Dairy J. 2000;10:151–158. doi: 10.1016/S0958-6946(00)00040-6. [DOI] [Google Scholar]
- FIL-IDF (1969) Determinazione del tenore in materia grassa del formaggio e dei formaggi fusi - Standard 5A - Norme FIL-IDF: definizioni, metodiche di analisi e di prelievo del latte e derivati (vol. 1). La Nazionale, Parma, Italy
- Giroux HJ, Grandpré G, Fustier P, Champagne CP, St-Gelais D, Lacroix M, Britten M. Production and characterization of cheddar-type cheese enriched with green tea extract. Dairy Sci Technol. 2013;93:241–254. doi: 10.1007/s13594-013-0119-4. [DOI] [Google Scholar]
- Hayaloglu A, Guven M, Fox P, Hannon J, McSweeney PL. Proteolysis in Turkish white-brined cheese made with defined strains of lactococcus. Int Dairy J. 2004;14:599–610. doi: 10.1016/j.idairyj.2003.12.008. [DOI] [Google Scholar]
- Hoye C, Ross CF. Total phenolic content, consumer acceptance, and instrumental analysis of bread made with grape seed flour. J Food Sci. 2011;76:S428–S436. doi: 10.1111/j.1750-3841.2011.02324.x. [DOI] [PubMed] [Google Scholar]
- Kuhnen S, Moacyr JR, Mayer JK, Navarro BB, Trevisan R, Honorato LA, Maraschin M, Pinheiro Machado Filho LC. Phenolic content and ferric reducing-antioxidant power of cow’s milk produced in different pasture-based production systems in southern Brazil. J Sci Food Agric. 2014;94:3110–3117. doi: 10.1002/jsfa.6654. [DOI] [PubMed] [Google Scholar]
- Lavelli V, Sri Harsha PSC, Torri L, Zeppa G. Use of winemaking by-products as an ingredient for tomato puree: the effect of particle size on product quality. Food Chem. 2014;152:162–168. doi: 10.1016/j.foodchem.2013.11.103. [DOI] [PubMed] [Google Scholar]
- McMahon DJ, Oberg CJ, Drake MA, Farkye N, Moyes LV, Arnold MR, Ganesan B, Steele J, Broadbent JR. Effect of sodium, potassium, magnesium, and calcium salt cations on pH, proteolysis, organic acids, and microbial populations during storage of full-fat cheddar cheese. J Dairy Sci. 2014;97:4780–4798. doi: 10.3168/jds.2014-8071. [DOI] [PubMed] [Google Scholar]
- Mildner-Szkudlarz S, Zawirska-Wojtasiak R, Szwengiel A, Pacyński M. Use of grape by-product as a source of dietary fibre and phenolic compounds in sourdough mixed rye bread. Int J Food Sci Technol. 2011;46:1485–1493. doi: 10.1111/j.1365-2621.2011.02643.x. [DOI] [Google Scholar]
- Mildner-Szkudlarz S, Bajerska J, Zawirska-Wojtasiak R, Górecka D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J Sci Food Agric. 2013;93:389–395. doi: 10.1002/jsfa.5774. [DOI] [PubMed] [Google Scholar]
- Mullin WJ, Emmons DB. Determination of organic acids and sugars in cheese, milk and whey by high performance liquid chromatography. Food Res Int. 1997;30:147–151. doi: 10.1016/S0963-9969(97)00026-4. [DOI] [Google Scholar]
- O’Connel JE, Fox PF. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: a review. Int Dairy J. 2001;11:103–120. doi: 10.1016/S0958-6946(01)00033-4. [DOI] [Google Scholar]
- Ozdal T, Capanoglu E, Altay F. A review on protein–phenolic interactions and associated changes. Food Res Int. 2013;51:954–970. doi: 10.1016/j.foodres.2013.02.009. [DOI] [Google Scholar]
- Özvural EB, Vural H. Grape seed flour is a viable ingredient to improve the nutritional profile and reduce lipid oxidation of frankfurters. Meat Sci. 2011;88:179–183. doi: 10.1016/j.meatsci.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codina C. Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants. J Agric Food Chem. 2002;50:6882–6890. doi: 10.1021/jf020540a. [DOI] [PubMed] [Google Scholar]
- Rashidinejad A, Birch EJ, Sun-Waterhouse D, Everett DW. Effects of catechin on the phenolic content and antioxidant properties of low-fat cheese. Int J Food Sci Technol. 2013;48:2448–2455. doi: 10.1111/ijfs.12234. [DOI] [Google Scholar]
- Rondeau P, Gambier F, Jolibert F, Brosse N. Compositions and chemical variability of grape pomaces from French vineyard. Ind Crop Prod. 2013;43:251–254. doi: 10.1016/j.indcrop.2012.06.053. [DOI] [Google Scholar]
- Rosales Soto MU, Brown K, Ross CF. Antioxidant activity and consumer acceptance of grape seed flour-containing food products. Int J Food Sci Technol. 2012;47:592–602. doi: 10.1111/j.1365-2621.2011.02882.x. [DOI] [Google Scholar]
- Ruggieri L, Cadena E, Martínez-Blanco J, Gasol CM, Rieradevall J, Gabarrell X, Gea T, Sort X, Sánchez A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J Clean Prod. 2009;17:830–838. doi: 10.1016/j.jclepro.2008.12.005. [DOI] [Google Scholar]
- Sánchez-Alonso I, Jiménez-Escrig A, Saura-Calixto F, Borderías AJ. Effect of grape antioxidant dietary fibre on the prevention of lipid oxidation in minced fish: evaluation by different methodologies. Food Chem. 2007;101:372–378. doi: 10.1016/j.foodchem.2005.12.058. [DOI] [Google Scholar]
- Sant’Anna V, Christiano FDP, Marczak LDF, Tessaro IC, Thys RCS. The effect of the incorporation of grape marc powder in fettuccini pasta properties. LWT Food Sci Technol. 2014;58:497–501. doi: 10.1016/j.lwt.2014.04.008. [DOI] [Google Scholar]
- Singleton V, Orthofer R, Lamuela-Raventos R. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enz. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sri Harsha PSC, Gardana C, Simonetti P, Spigno G, Lavelli V. Characterization of phenolics, in vitro reducing capacity and anti-glycation activity of red grape skins recovered from winemaking by-products. Biores Technol. 2013;140:263–268. doi: 10.1016/j.biortech.2013.04.092. [DOI] [PubMed] [Google Scholar]
- Sri Harsha PSC, Lavelli V, Scarafoni A. Protective ability of phenolics from white grape vinification by-products against structural damage of bovine serum albumin induced by glycation. Food Chem. 2014;156:220–226. doi: 10.1016/j.foodchem.2014.01.104. [DOI] [PubMed] [Google Scholar]
- Teixeira A, Baenas N, Dominguez-Perles R, Barros A, Rosa E, Moreno DA, Garcia-Viguera C. Natural bioactive compounds from winery by-products as health promoters: a review. Int J Mol Sci. 2014;15:15638–15678. doi: 10.3390/ijms150915638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A, Zhao Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013;138:356–365. doi: 10.1016/j.foodchem.2012.09.148. [DOI] [PubMed] [Google Scholar]