Abstract
Biological active compounds, 1-O-alkyl-sn-glycerols (AG), were isolated from liver oil of the squid Berryteuthis magister. The main components of the initial lipids were 1-O-alkyl-2,3-diacyl-sn-glycerols (38.50 %) and triacylglycerols (24.26 %). The first step of separation was the alkaline hydrolysis of oil to form a lipid mixture consisting of AG, free fatty acids and cholesterol. AG were separated by double recrystallization from acetone at −20 °C and 1 °C. A simple procedure is proposed for obtaining AG with a purity of 99.22 %, the main component of which is chimyl alcohol (94.39 %). Purity and structure of the obtained products were confirmed by GC and GC-MS technique. Isolated AG may be used in nutrition and cosmetics.
Keywords: 1-O-alkyl-sn-glycerols, Biologically active compounds, Low temperature crystallization, Marine lipids
Introduction
Marine lipids are the source of many biologically active compounds. Together with the n-3 polyunsaturated fatty acids (PUFA), the positive effects of which have been extensively studied (Schmitz and Ecker 2008), AG, ethers of glyceroal and fatty alcohols, arouse interest of many researchers. AG exhibit a variety of biological activities (Iannitti and Palmieri 2010). These compounds are precursors in the biosynthesis of plasmalogens, phospholipids with an alkenyl bond at sn-1 position of glycerol. The first steps of the plasmalogens biosynthesis, namely, the formation of the alkyl bond in the dihydroxyacetone phosphate molecule takes place in the peroxisomes (Nagan and Zoller 2001). Therefore peroxisomal dysfunction is one of the main reasons of neurodegenerative diseases and reduction plasmalogens in cells, for instance the level decline of plasmenyl phosphatidylethanolamine with docosahexaenoic acid (DHA, C22:6 n-3) in sn-2 position of glycerol in brain cells in Alzheimer’s disease (Braverman and Moser 2012). The addition to the diet of plasmalogens precursors - AG and DHA – can promote the increase of their level in brain cells. In recent years, various synthetic plasmalogens precursors (Wood et al. 2011) or semisynthetic plasmalogens (Chen and Liu 2013) have been used in investigations of neurodegenerative disorders. At the same time, the inclusion of DHA in the sn-2 position of the glycerol was the most important condition for such preparations. However, this approach is associated with complicated chemical or biochemical synthesis and this may prevent using them in practice. Dietary lipids are known to be exposed to the lipolytic enzymes with elimination of DHA, and it significantly reduces therapeutic efficiency of preparations. Human acyl-CoA transferase in the endoplasmic reticulum of the hepatocytes has been shown to include DHA in the sn-2 position of AG (Watschinger and Werner 2013); it generates formation of plasmalogens; which are then transported into the brain by the blood lipoproteins (Braverman and Moser 2012).
Marine lipids are the best known and abundant in natural sources of AG and n-3 PUFA. Liver of cartilaginous fish and some mollusks contain a high amounts of ether lipids, 1-O-alkyl-2,3-diacyl-sn-glycerols (DAGE), the content of which in the total lipids reaches 50.0 % (Bakes and Nichols 1995; Hayashi and Kishimura 2002). Diversity of molecular species of neutral ether lipids is determined by the length and double bond number of the alkyl fragments in sn-1 position, and also by differences in the fatty acid composition. Alkyl fragments C18:1 (30.0–61.4 %) are the most common in the DAGE composition in the shark liver lipids (Magnusson and Haraldsson 2011), while chimyl alcohol C16:0 (70.2 %) is the main component of liver oil of the gonatid squid B. magister (Hayashi and Kishimura 2002). Moreover, the lipids of these marine organisms contain up to 25.0 % of n-3 PUFA, DHA and eicosapentaenoic acid (EPA, C20:5 n-3) being the main fatty acids (Bakes and Nichols 1995; Hayashi and Kishimura 2002).
Despite the B. magister squid liver lipids are the source of AG, EPA and DHA, the high content of free fatty acids (FFA) and cholesterol (CHOL) imposes restrictions on the use of the oil and requires a special approach to processing it.
Separation of highly purified AG is a rather difficult problem, despite the simple structure and high concentration in the marine oils. The proximity of the physicochemical properties of triacylglycerols (TAG) and DAGE complicates a single-stage separation. Lipase-catalysed transesterification (Fernández et al. 2013), supercritical fluid extraction and vacuum distillation (Tenllado et al. 2011) was allowed to obtain products rich in DAGE and AG. However these methods do not permit to isolate highly purified AG. The column chromatography on silica gel used for isolation of AG from soft coral lipids (Martinez Diaz et al. 2015), shark liver oil (Bordier et al. 1996) is limiting stage for technological application.
The purpose of this study was to design a technological approach of separation of highly purified AG from B. magister squid liver oil.
Material and methods
Squid was fished in the Bering Sea in September 2013. After squid processing, the liver was separated and stored for 3 months at −12 ° C. All chemical reagents used in this study are of analytical grade (Sigma–Aldrich, USA). All solvents were of HPLC grade and supplied by Sigma–Aldrich (USA).
Separation and hydrolysis of squid liver oil
Extraction of total lipids was conducted according to Bligh and Dyer (1959). Lipids (120 g) were mixed with 300 ml of 2 M KOH solution in 70 % aqueous ethanol with following heating to 70°С under stirring during 1 h. The resulting solution was diluted with 400 ml water and acidified with 400 ml of 1 N H2SO4 aqueous solution. Mixture of FFA, CHOL and AG was extracted from aqueous ethanol solution with hexane (3 × 300 ml). The hexane extract was washed with water and dried with anhydrous Na2SO4; the solvent was evaporated.
Analysis of the obtained lipid mixture was conducted by thin layer chromatography (TLC). AG was isolated by preparative TLC in the system hexane:diethyl ether:acetic acid in the ratios of 50:50:1 (v/v/v) followed by determination of the composition of AG as trimethylsilyl derivaties (TMS-AG) using gas chromatography (GC) and gas chromatography–mass spectrometry (GC-MS). The composition FA as methyl esters was determined by GC.
Isolation of AG
Precipitation of AG was carried out by double crystallization in acetone at different temperatures. The mixture of saponified lipids (30 g) was dissolved in acetone at a ratio of 1:5 (w/w). The solution was kept at −20 °C for 24 h. The sediment 1 was separated by filtration at a temperature of crystallization followed by dissolving in acetone at a ratio of 1:5 (w/w). The second crystallization was carried out at 1 °C for 24 h. The sediment 2 was separated by filtration at 1 °C, washed with cold acetone, dried and weighed. The AG yield was 57.47 % of the AG content in saponified lipids. Lipid composition analysis of the sediments and filtrates was performed by TLC. Composition of AG as TMS-AG was determined by GC and GC-MS.
Analytical methods
Determination of lipid composition
The lipid composition was determined by TLC on the precoated Merck Kieselgel 60 silica gel G plates (10 × 10 cm) according to Imbs et al. (2006). The plates were developed in the system hexane:diethyl ether:acetic acid in the ratios of 80:20:1 (v/v/v) for analysis of total lipid composition and 70:30:1 (v/v/v) for analysis of saponified lipids, filtrates and sediments. After drying in a stream of air, plates were sprayed with 7 % solution of H2SO4/EtOH, with following heating. The chromatograms were scanned using a scanner Epson Perfection 2400 PHOTO (Japan) in grayscale mode. The percentage of lipid components was determined by the intensity of the spots by an image processing program Sorbfil TLC Videodensitometer DV (Russia). The units were calibrated using the known standards for each lipid class.
Determination of AG composition
TMS-AG were prepared as follows: 50 μl of N,O-Bis(trimethylsilyl)trifluoroacetamide were added to 5 mg AG; the mixture was heated to 80 °C for 1 h. After addition of 200 μl of hexane 1 μl of each silylated fraction was injected into the GC. The composition of TMS-AG was determined by GC using a chromatograph Shimadzu GC-2010 plus with a flame ionization detector (Japan) and a capillary column Supelco SLB™-5 ms 30 m × 0.25 mm i.d. (USA). Separation of mixture components was carried out under the following conditions: initial temperature 200 °C, heating rate of 2 °C/min to 290 °C, which was held at this temperature for 35 min. Injector temperature was 270 °C, detector temperature 250 °C. AG identification was performed by comparison with available known standards. GC-MS was used to identify the structure of TMS-AG. Electronic impact spectra were recorded using an instrument Shimadzu TQ-8040 (Japan) with the column Supelco SLB™-5 ms (USA) at 70 eV under the same temperature conditions as during GC.
Determination of FA composition
Methyl esters of FA from squid oil were prepared according to the accepted procedure (Carreau and Dubacq 1978). An analysis of FA esters was conducted by gas chromatography on a Shimadzu GC-17A chromatograph (Japan) with a flame-ionization detector, a capillary column 30 m × 0.25 mm i.d. Supelcowax 10 (USA). An analysis was performed under the following conditions: column temperature 190°С, the injector and detector temperature 240 °C. Helium was used as a carrier gas. The peaks of methyl esters of FA were identified by retention times of the individual FA esters through comparison of their equivalent carbon length numbers with the authentic standards (PUFA-3 mix from menhaden oil was purchased from Supelco, USA) (Stransky et al. 1997).
Results and discussion
Lipid composition of B. magister squid liver oil
The total lipid content was 53 % of the wet weight of the liver. TLC analysis demonstrated that the squid liver oil consisted of CHOL and its esterified form, DAGE, TAG, FFA, mono- and diacylglycerols (MAG and DAG), as well as minor amounts of polar lipids (Table 1). The contents of the main components (DAGE, TAG and FFA) were 38.49, 24.26 and 20.24 %, respectively. CHOL, and its esterified form, and polar components were present in small amounts, which is in consistency with the previously published data for oil of the B. magister liver (Hayashi and Kishimura 2002). Considerable amounts of FFA, MAG and DAG were found in the lipids, which is not characteristic of normal tissues. The high levels of the above compounds may be produced by enzymatic hydrolysis of the TAG and DAGE during a prolonged storage at −12 °C and thawing (Hayashi and Kishimura 2002). Digestive systems of marine invertebrates, as it was shown before, contain highly active lipases and phospholipases (Patton 1975).
Table 1.
Composition of the original squid liver oil and the saponified lipids after alkaline hydrolysis
| Class of lipids | Liver oil | Saponified lipids |
|---|---|---|
| Cholesterol esters | 2.12 ± 0.32a | ND |
| 1-O-alkyl-2,3-diacyl-glycerol | 38.49 ± 2.71 | ND |
| Triacylglycerols | 24.26 ± 2.14 | ND |
| Free fatty acids | 20.24 ± 4.16 | 74.51 ± 0.91 |
| Cholesterol | 5.45 ± 0.41 | 6.35 ± 0.55 |
| Mono-diacylglycerols | 6.87 ± 2.19 | ND |
| 1-O-alkyl-sn-glycerols | tr | 19.14 ± 0.51 |
| Polar components | 2.57 ± 0.25 | ND |
tr in traces (less than 0.05 %), ND not detected
amean % of sum peak areas ± standard deviation (n = 5)
All acyl-containing lipids were completely deacylated after alkaline hydrolysis, and the composition of the obtained lipid mixture is given in Table 1.
Composition of AG in saponified squid liver oil
oil and sylilation of preliminary isolated AG determination of TMS-AG composition was performed by GC (Table 2) TMS-AG were identified by GC-MS. Identification was performed by characteristic mass peaks of molecular ion M+, (M-15)+ and base peak of m/z 205 indicating a TMS-AG structure (Bordier et al. 1996).
Table 2.
AG composition of saponified lipids, AG after double crystallization
| Alkyl chain | Saponified lipids | AG after double crystallization |
|---|---|---|
| C14:0a | 1.77 ± 0.47b | 1.26 ± 0.16 |
| C15:0 | 0.28 ± 0.01 | ND |
| C16:0 | 76.36 ± 0.81 | 94.39 ± 0.03 |
| C16:1 | 0.53 ± 0.11 | ND |
| C17:0 | 0.22 ± 0.01 | ND |
| C17:1 | 0.31 ± 0.03 | ND |
| C18:0 | 4.77 ± 0.50 | 4.35 ± 0.37 |
| C18:1 | 7.01 ± 0.04 | ND |
| C18:1 | 5.45 ± 0.55 | ND |
| C18:1 | 0.65 ± 0.02 | ND |
| C18:2 | 0.12 ± 0.08 | ND |
| C20:1 | 0.55 ± 0.05 | ND |
| C20:1 | 0.99 ± 0.05 | ND |
| C22:1 | 0.19 ± 0.01 | ND |
| ΣBranchedc | 0.80 ± 0.10 | |
| Σsat | 84.20 ± 0.77 | 100 ± 0.00 |
| Σunsat | 15.80 ± 0.77 |
ND not detected, sat saturated AG, unsat unsaturated AG
aindicated by chain length and double bond of alkyl chain in 1-O-alkyl-sn-glycerols (AG)
bmean weigh % ± standard deviation (n = 5)
cbranched - 16:0i, 17:0i, 18:0i
Analysis of the AG composition has shown that the main component is chimyl alcohol (75.36 %) in the saponified lipids (Table 2). Unsaturated AG (15.80 %) were represented mostly by isomers of C18:1 and C20:1, which is in consistency with previously published results (Hayashi and Yamamoto 1987).
AG isolation
Saponification of squid liver oil
Alkaline hydrolysis of lipids was used in the first stage to isolate the most significant components (AG and PUFA) from squid liver oil. The formation of AG and fatty acid salts was occurred in the result of DAGE cleavage. The other acyl-containing components were also deacylated. The procedure made it possible to obtain a mixture of FA salts and unsaponifiable lipids (CHOL and AG). Extraction of unsaponifiable compounds from the hydrolysis reaction mixture is an important stage in the separation of AG. Hayashi (1986) separated unsaponifiable lipids from FA salts by extraction with diethyl ether. However, the use of diethyl ether is permitted only to a limited extent because of the risk of working with large amounts of this solvent. In addition, the FA salts and the AG form stable emulsions during the nonpolar solvent extraction of unsaponifiable compounds from the mixture obtained after hydrolysis; this complicates the extraction process and does not permit to separate unsaponifiable compounds quantitatively. According to the approach of Bordier et al. (1996), the FA salts were converted into FA methyl esters after hydrolysis followed by the separation of a mixture of squalene, FA methyl esters, CHOL and AG by the column chromatography on silica gel. The use of column chromatography makes it possible to obtain preparative amounts of AG. In this work, just after hydrolysis, the FA salts were transferred in FFA by acidification. As a result, we have obtained the fractions of FFA (74.51 %), CHOL (6.35 %) and AG (19.14 %) (Table 1).
AG precipitation from acetone
Crystallization of the organic solvent at low temperatures is a simple method for isolating FA. This method is widely used, in particular for pre-enrichment of PUFA (Shahidi and Wanasundara 1998).
As it has been shown before, squid liver lipids are abundant in saturated AG (84.20 %). First of all, we had to remove the maximum FFA in the stage of AG separation. Acetone was used as a solvent for crystallization. The lipid mixture after the hydrolysis and acidification was dissolved in acetone in a ratio of 1:5 at room temperature and incubated for 24 h at −20 °C. Saturated FA and CHOL precipitated together with AG in the sediment 1 at low temperature crystallization, while PUFA remained in the filtrate 1. Recrystallization of sediment 1 was conducted for a complete separation of AG from free saturated FA and CHOL residues at 1:5 and temperature of 1 °C for 24 h. As a result, analysis of the obtained product by TLC and GC revealed that the AG content was 99.22 %, the main component being chimyl alcohol (Table 2). The total yield of AG was 57.47 % from initial AG content in saponified lipids. The filtrate 2 contained mostly saturated FFA, CHOL and AG.
Separation of AG and PUFA
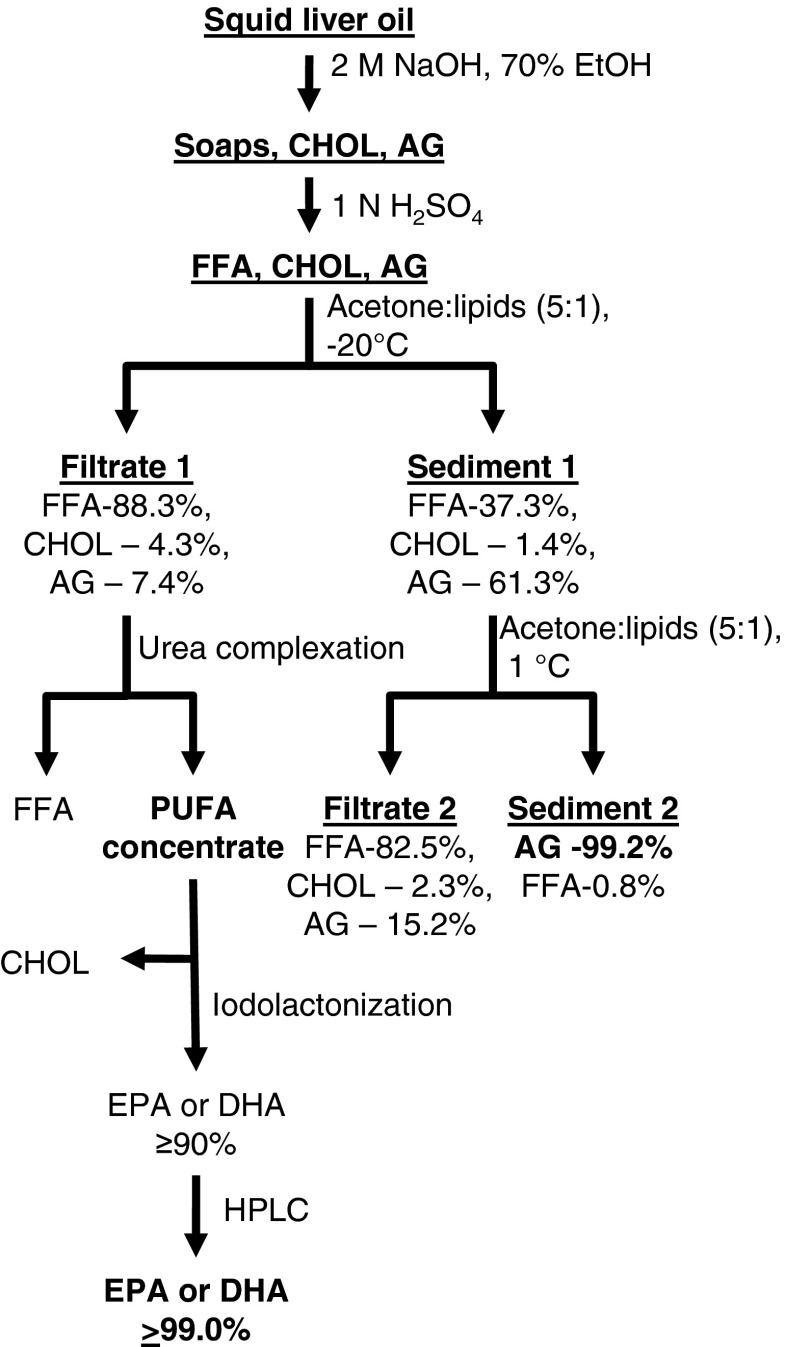
Based on previous work (Latyshev et al. 2014) and the results obtained in this study, we have designed a new technological approach of isolation AG and n-3 PUFA from lipids of squid liver (Fig. 1). It is important that full removal of CHOL from preparations of AG, EPA and DHA occurs during oil processing. The proposed methods of separation and concentration of biologically active components allow to obtain highly purified preparations for plasmalogens-replacement nutritional therapy of neurodegenerative diseases and for the cosmetic use (Johnson 2011).
Fig. 1.
The scheme of separation of AG and PUFA from squid liver oil. CHOL cholesterol, AG 1-O-alkyl-sn-glycerols, FFA free fatty acids, PUFA polyunsaturated fatty acids, EPA eicosapentaenoic acid, DHA docosahexaenoic acid
Conclusion
Separation of highly purified AG from liver lipids of the squid B. magister was carried out. Chimyl alcohol (94.39 %) was the main component of the obtained preparation. The proposed method does not require any sophisticated equipment and costly reagents; the method allows the use of low-grade oil obtained from wastes of the squid processing; this significantly reduces the cost of the final products.
Acknowledgments
We are grateful to I. A. Barsegova for translation of our manuscript from Russian and Dr. V. G. Rybin for assistance in the GC-MS
Compliance with ethical standards
Study of the composition of obtained preparations was supported by grant of Russian Science Foundation (project №14-50-00034)
Conflict of interest
The authors declare no conflict of interest
Footnotes
Research highlights
1 Highly purified 1-O-alkyl-sn-glycerols was isolated from squid processing waste
2 The proposed method does not require any sophisticated equipment
3 The obtained product may be used in nutrition and cosmetics
References
- Bakes MJ, Nichols PD. Lipid, fatty acid and squalene composition of liver oil from six species of deep-sea sharks collected in southern Australian waters. Comp Biochem Physiol. 1995;110B:267–275. doi: 10.1016/0305-0491(94)00083-7. [DOI] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Bordier CG, Sellier N, Foucault AP, Le Goffic F. Purification and characterization of deep sea shark Centrophorus squamosus liver oil 1-O-alkylglycerol ether lipids. Lipids. 1996;31:521–528. doi: 10.1007/BF02522646. [DOI] [PubMed] [Google Scholar]
- Braverman N, Moser A. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Carreau JP, Dubacq JP. Adaptation of a macro-scale method to the micro-scale for fatty acid methyl transesterification of biological lipid extracts. J Chromatogr А. 1978;151:384–390. doi: 10.1016/S0021-9673(00)88356-9. [DOI] [Google Scholar]
- Chen S, Liu C. Ether glycerophospholipids and their potential as therapeutic agents. Curr Org Chem. 2013;17:802–811. doi: 10.2174/1385272811317080006. [DOI] [Google Scholar]
- Fernández Ó, Vázquez L, Reglero G, Torres CF. Discrimination against diacylglycerol ethers in lipase-catalysed ethanolysis of shark liver oil. Food Chem. 2013;136:464–471. doi: 10.1016/j.foodchem.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Hayashi K. Isolation of alkyl glyceryl ethers from liver oil unsaponifiables by recrystallization. Bull Jpn Soc Sci Fish. 1986;52:1475. doi: 10.2331/suisan.52.1475. [DOI] [Google Scholar]
- Hayashi K, Kishimura H. Amount and composition of diacyl glyceryl ethers in various tissue lipids of the deep-sea squid Berryteuthis magister. J Oleo Sci. 2002;51:523–529. doi: 10.5650/jos.51.523. [DOI] [Google Scholar]
- Hayashi K, Yamamoto S. Content and composition of alkyl glyceryl ethers in liver of gonatid squid Berryteuthis magister from the Northwestern Pacific. Nippon Suisan Gakkaishi. 1987;53:137–140. doi: 10.2331/suisan.53.137. [DOI] [Google Scholar]
- Iannitti T, Palmieri B. An update on the therapeutic role of alkylglycerols. Mar Drugs. 2010;8:2267–2300. doi: 10.3390/md8082267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbs AB, Demina OA, Demidkova DA. Lipid class and fatty acid composition of the boreal soft coral Gersemia rubiformis. Lipids. 2006;41:721–725. doi: 10.1007/s11745-006-5023-8. [DOI] [PubMed] [Google Scholar]
- Johnson W Jr (2011) On the safety assessment of alkyl glyceryl ethers as used in cosmetics. Cosmetic Ingredient Review. http://www.cir-safety.org/supplementaldoc/final-safety-assessment-alkyl-glyceryl-ethers-used-cosmetics. Accessed 19 Dec 2011
- Latyshev NA, Ermolenko EV, Kasyanov SP. Concentration and purification of polyunsaturated fatty acids from squid liver processing wastes. Eur J Lipid Sci Technol. 2014;116:1608–1613. doi: 10.1002/ejlt.201400083. [DOI] [Google Scholar]
- Magnusson CD, Haraldsson GG. Ether lipids. Chem Phys Lipids. 2011;164:315–340. doi: 10.1016/j.chemphyslip.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Martinez Diaz Y, Leverde GV, Gamba LR et al (2015) Biofilm inhibition activity of compounds isolated from two Eunicea species collected at the Caribbean Sea. Rev Bras Farm. doi:10.1016/j.bjp.2015.08.007
- Nagan N, Zoller R. Plasmalogen: biosynthesis and functions. Prog Lip Res. 2001;40:199–229. doi: 10.1016/S0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- Patton J. High levels of pancreatic nonspecific lipase in rattlesnake and leopard shark. Lipids. 1975;10:562–564. doi: 10.1007/BF02532361. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Wanasundara U. Omega-3 fatty acid concentrates: nutritional aspects and production technologies. Trends Food Sci Technol. 1998;9:230–240. doi: 10.1016/S0924-2244(98)00044-2. [DOI] [Google Scholar]
- Stransky K, Jursik T, Vitek A. Standard equivalent chain length values of monoenic and polyenic (methylene-interrupted) fatty acids. J High Resolut Chromatogr. 1997;20:143–158. doi: 10.1002/jhrc.1240200305. [DOI] [Google Scholar]
- Tenllado D, Reglero G, Torres CF. A combined procedure of supercritical fluid extraction and molecular distillation for the purification of alkylglycerols from shark liver oil. Sep Purif Technol. 2011;83:74–81. doi: 10.1016/j.seppur.2011.09.013. [DOI] [Google Scholar]
- Watschinger K, Werner ER. Orphan enzymes in ether lipid metabolism. Biochimie. 2013;95:59–65. doi: 10.1016/j.biochi.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PL, Smith T, Lane N, Khan MA, Ehrmantraut G, Goodenowe DB. Oral bioavailability of the ether lipid plasmalogen precursor, PPI-1011, in the rabbit: a new therapeutic strategy for Alzheimer’s disease. Lipids Health Dis. 2011;10:227. doi: 10.1186/1476-511X-10-227. [DOI] [PMC free article] [PubMed] [Google Scholar]