Abstract
Objectives. To test whether text message reminders sent to caregivers improve the effectiveness of a home micronutrient fortification program in western China.
Methods. We carried out a cluster-randomized controlled trial in 351 villages (clusters) in Shaanxi Province in 2013 and 2014, enrolling children aged 6 to 12 months. We randomly assigned each village to 1 of 3 groups: free delivery group, text messaging group, or control group. We collected information on compliance with treatments and hemoglobin concentrations from all children at baseline and 6-month follow-up. We estimated the intent-to-treat effects on compliance and child anemia using a logistic regression model.
Results. There were 1393 eligible children. We found that assignment to the text messaging group led to an increase in full compliance (marginal effect = 0.10; 95% confidence interval [CI] = 0.03, 0.16) compared with the free delivery group and decrease in the rate of anemia at end line relative to the control group (marginal effect = −0.07; 95% CI = −0.12, −0.01), but not relative to the free delivery group (marginal effect = −0.03; 95% CI = −0.09, 0.03).
Conclusions. Text messages improved compliance of caregivers to a home fortification program and children’s nutrition.
Anemia is one of the most prevalent nutritional disorders among preschool-aged children in developing countries.1 Despite overall improvements in child health in China over the past 3 decades, the prevalence of anemia among children younger than 5 years in rural areas is still high.2–4 One national survey demonstrated that although the prevalence of anemia had decreased among infants aged 6 to 12 months from 35.0% in 2005 to 28.2% in 2010, the disease’s prevalence is still nontrivial.4,5 Other studies have found similarly high rates (e.g., 23% in Guangxi Province; 58% in Gansu).6,7
Although anemia is influenced by many factors, iron deficiency is the major cause, accounting for about half of the disease’s global incidence.8–10 The development of daily-use micronutrient packets containing microencapsulated iron offers a way to provide key micronutrients to iron-deficient children.11
In China, research shows that 90% of anemia in children stems from iron deficiency.12 Research on the effectiveness of micronutrient supplementation, however, is mostly limited to studies based on small, nonrandomized samples.13–18
Although supplementation has potential if caregivers systematically comply with supplement programs, there are indications that, internationally, lack of compliance has been a barrier to its success.19–21 Unfortunately, almost no research has been conducted on how supplementation can best be delivered or how caregivers can be persuaded to regularly give their children micronutrient supplements. One study evaluated household compliance to a micronutrient supplement distribution program in Kenya.20 Another study in Bangladesh compared the relative effectiveness of different approaches to administering micronutrient supplements to individuals.21 Although both studies found low compliance, researchers concluded that the nature of the delivery strategy was associated with decreased rates of anemia in children.20,21 The authors of both studies, however, suggested that the relatively low rates of use of the micronutrient supplement indicated generally poor project design, and that systematic research was needed to evaluate the impact of alternative delivery strategies on caregiver compliance and infant outcomes.
We studied how text messaging could influence the effectiveness of micronutrient supplement programs. Text messaging technology has changed the face of communications globally and in China. A large percentage (67%) of households in the world have mobile phones.22 An even larger percentage (> 90%) of households in China—even in rural areas—have mobile phones.23
Mobile technology is increasingly used as a way to promote health and prevent disease,24,25 although text message–based programs have often met with mixed results. Internationally, the application of text messaging for behavioral change in smoking cessation, antiobesity behavior modification, and diabetes management has shown positive results.26–28 In China, a short message service (SMS) intervention was found to promote longer duration of exclusive breastfeeding for mothers. The study, however, did not follow a randomized controlled trial (RCT) design and was underpowered.29
There are also examples of text messaging programs that do not work. Factors identified as leading to the programs’ failure included limited phone access, privacy concerns, phone maintenance, and text message content.30–32 A recent literature review of text messaging’s effect on health concluded that most studies were not carried out systematically.33
Using a cluster RCT design, we aimed to test whether text message reminders sent to caregivers’ mobile devices would improve the effectiveness of a home micronutrient fortification program. To meet this goal, we had 3 specific objectives. We examined caregiver compliance to a home fortification program. We evaluated the impact of adding a program that included text message reminders sent to caregivers. Finally, we examined whether text messaging had any impact on child nutrition, focusing on the prevalence of anemia.
METHODS
Our research team carried out a cluster RCT in rural China that used villages as the clusters. Our study included 3 experimental groups: 2 treatment groups and a control group. We enrolled 2 cohorts of children aged 6 to 12 months. The intervention period lasted for 6 months for both cohorts.
We conducted our baseline survey in 2 waves; one began in April 2013 and one in October 2013. From 11 nationally designated poverty counties in Southern Shaanxi, we selected 174 townships to participate in the study. We included all townships in each county except the one that housed the county seat and those that did not have any villages with at least 800 people.
To meet the power requirements of an RCT, we required a minimum of 5 infants per village (prior to attrition). We used official government data to compile a list of all villages in each township. We then randomly selected 2 villages from the list in each township, using a random numbers generator. We selected an additional 3 villages by randomly selecting 3 townships and 1 village in each selected township. Our final sample consisted of 351 villages. We obtained a list of all registered births over the past 12 months from the local family planning office. We enrolled all infants in the desired age range (6–12 months).
We selected sample villages at the time of the initial wave (April 2013). At the time of each of the first home visits (April 2013 for wave 1; October 2013 for wave 2), we sampled all children in the desired age range (6–12 months) living in the village. Overall, the baseline sample included 1818 children.
Once the sample selection was complete, one of the authors (R. L.) randomly assigned villages by computer-generated random numbers into 2 treatment groups and a control group. Assignment was cluster randomized, with 117 villages in each treatment group. We randomly assigned 619 children to the control group, 600 children to treatment group 1, and 599 children to treatment group 2. After assignment, the caregivers did not know whether or not they were in an RCT or were being evaluated.
Interventions
The caregivers of children in treatment group 1 received one-on-one health education training on nutrition and feeding practices. Sample caregivers also received a free 6-month supply of micronutrient supplement packets containing a home fortification powder along with instructions on how to use the powder. This group served as our free delivery group (FDG).
The caregivers of children in treatment group 2 (text messaging group, or TMG) received the same treatment as the FDG; however, the TMG caregivers also were enrolled in a daily text message reminder program. In partnership with a cellular communications provider based in Shaanxi Province (China Mobile Communications Corporation), daily reminder messages were sent to the TMG for 6 months. The messages are shown in Table A (available as a supplement to the online version of this article at http://www.ajph.org).
In both the FDG and TMG, we used a Heinz-produced micronutrient powder called “NurtureMate.” The powder is tasteless and contains a mix of iron; zinc; vitamins A, C, D, B1, B2, B6, and B12; and folic acid (online Table B). Approved by China’s government, NurtureMate is recommended for infants aged 6 to 36 months. It is recommended that caregivers give infants 5 packets per week or 1 packet per day.
In both intervention groups, enumerators gave each household a plastic storage envelope in which to store the NurtureMate packets, with instructions to return the empty packets to the survey team at the end of the study. Enumerators tallied unused and empty packets to assess compliance.
Data Collection
The research team conducted 2 rounds of surveys for each cohort: one at baseline and one at follow-up. For cohorts 1 and 2, the team conducted the baseline survey in April and October 2013 and the follow-up survey in October 2013 and April 2014, respectively.
At each baseline, survey nurses from Xi’an Jiaotong Medical School collected hemoglobin concentrations from all infants and caregivers. They measured the hemoglobin concentrations by a HemoCue Hb 201+ finger prick system (HemoCue Inc, Ängelholm, Sweden). Teams of enumerators collected socioeconomic data from study households. They identified each infant’s primary caregiver and administered a survey on infant, parental, and household characteristics, including each child’s gender, age and birth order, maternal age and education, and whether the family was receiving Minimum Living Standard Guarantee payments (a poverty indicator). Each family identified the primary caregiver as the individual most responsible for the infant’s care (typically, the child’s mother or grandmother). Enumerators administered to the primary caregiver a survey on child feeding practices and recorded the caregiver’s mobile phone number. All baseline tests and surveys were readministered at the end line by nurses and enumerators.
Nurses determined anemia status by finger blood analysis of hemoglobin concentrations. Following international standards for our sample age group, we defined anemia as a hemoglobin count of less than 110 grams per liter.34,35
To collect the information needed to assess compliance, survey enumerators counted the total number of opened and unopened packets and divided the number of opened packets by the number of days that had elapsed between the passing out of the packets and the follow-up survey, and then multiplied by 7. We kept careful records of when we distributed the packets and when we counted them. We also asked households to self-report the number of packets used per week. Analysis of the differences among the 3 approaches is shown in online Table C; the 3 approaches yielded statistically similar results. We used opened packages as our main compliance variable. Following the manufacturer’s recommendation, we counted a caregiver as being fully compliant if she gave her infant 5 to 7 packets per week.
Statistical Analysis
We determined the study’s sample size by power calculations performed before enrollment using Optimal Design, a software developed by University of Michigan.36 The power to detect a difference in anemia rates between the treatment and control groups in a cluster RCT depends on 5 factors:
number of children per village,
number of villages,
intracluster correlation of anemia prevalence,
minimum effect size that we would expect to be able to detect, called minimum detectable effect, and
how anemia rates within villages correlate over time (R2).
On the basis of previous studies,37–40 we assumed an intracluster correlation of 0.1 and an R2 of 0.5. We then assumed 4 infants per village. On the basis of these parameters, we calculated that we required 112 villages per group to detect a standardized effect of 0.2 at 80% power given a significance level of .05. We added 5 villages to each group to overpower the study when the budget allowed.
We conducted statistical analyses using Stata version 12.0 (StataCorp, College Station, TX). We considered P values less than .05 to be statistically significant. We used analysis of variance (ANOVA) and the χ2 test to test the balance of the control variables in the baseline. We examined caregiver compliance to the fortification program through a histogram showing the frequency distribution of the fully compliant by intervention arms. Our primary outcome variable was compliance with the home fortification program, as measured by a caregiver being fully compliant (5–7 packets per week). Our secondary outcome variable was an infant nutritional indicator (anemia status).
We estimated the treatment effects on both caregiver compliance and anemia status of infants using an intent-to-treat analysis. To estimate the intent-to-treat impacts, we used a multivariate logistic regression model and controlled for observable baseline infant, caregiver, and household characteristics (child’s age, gender, low birth weight, premature birth, birth order, baseline anemia status of child, whether the family received social security support, relationship of primary caregiver to child, maternal education, maternal micronutrient supplementation during pregnancy, maternal hemoglobin concentration, breastfeeding duration, formula feeding duration, complementary feeding after 6 months, infant iron supplementation, meat consumption) and fixed-effects at county and cohort levels. In all analyses, we accounted for clustering within villages using Huber–White cluster-adjusted standard errors.
To accommodate partial compliance (because not all caregivers administered nutrient packets 5–7 days/week) and to measure the impact of full compliance on children’s anemia status, we estimated the average treatment effect on the treated41 by using a 2-stage least-squares approach to instrument a variable indicating full compliance (this variable is 1 if nutrient packets were given 5–7 days/week, 0 otherwise) with variables indicating random assignment.
RESULTS
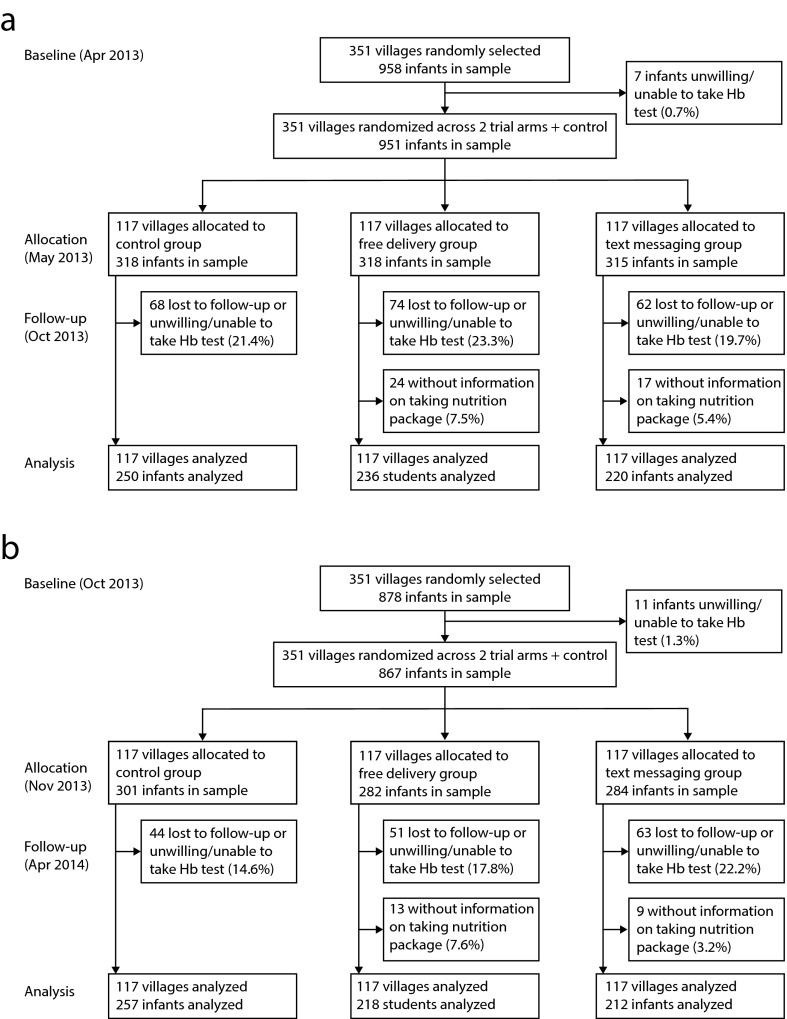
A total of 1818 infants aged 6 to 12 months in 351 villages were enrolled at baseline. Although we tracked each infant with valid contact information who was still living in the sample villages, we had an attrition rate of 23.4% between baseline and follow-up surveys. This attrition was due either to incomplete data (n = 130) or to relocation of the household (infant with mother) out of our sample areas (n = 295). Incomplete data occurred for 2 reasons: caregivers refused permission for their infants to be given the hemoglobin test (n = 67) or there were incomplete data on home fortification compliance (n = 63). After accounting for all attrition, we followed up with 1393 children (end line). No villages (clusters) were lost (Figure 1). As shown in online Table D, infants lost through attrition had individual and family characteristics that were statistically identical to those of tracked children. We compared the attrition rates of those lost to follow-up (variable 1) and those lost because the families were unwilling or unable to let their child take the hemoglobin test (variable 2) across the 3 groups (FDG, TMG, and control). We also compared the attrition rates due to not having information on taking nutrient packages (variable 3) across the 2 treatment groups. The analyses demonstrated that there were no statistical differences across the 3 groups (for variables 1 and 2, P = .38; online Table E) or between the 2 treatment groups (for variable 3, P = .20; online Table F). According to our data, the study’s power reached 94.5% (minimum detectable effect = 0.24; intracluster correlation = 0.058; R2 = 0.5; number of villages = 351; number of children per village = 4; number of groups = 3). The 3 groups were balanced at baseline across socioeconomic status, caregiver and mother characteristics, feeding practices, and child nutritional indicators (P values > .05; Table 1).
FIGURE 1—
Trial of Home Micronutrient Fortification Program Against Child Anemia, Comprising Free Micronutrient Plus Text Messaging Group, Free Micronutrient Only Group, and Control Group by (a) cohort 1 (April 2013–October 2013) and (b) cohort 2 (October 2013–April 2014): Rural Shaanxi Province, China, 2013–2014
Note. Hb = hemoglobin.
TABLE 1—
Baseline Characteristics of Sample Children and Their Caregivers in 3 Randomized Groups: Rural Shaanxi Province, China, 2013–2014
| Characteristics | Control Group (n = 507), Mean ±SD or % (No.) | Free Micronutrient Delivery Group (n = 438), Mean ±SD or % (No.) | Free Micronutrient Plus Text Messaging Group (n = 448), Mean ±SD or % (No.) | Pa |
| Socioeconomic | ||||
| Age, mo | 9.0 ±1.9 | 9.1 ±1.9 | 9.1 ±1.8 | 0.94 |
| Girls, % | 50.5 (256) | 48.4 (212) | 45.5 (204) | 0.31 |
| Premature birth, % | 9.7 (49) | 12.1 (53) | 10.9 (49) | 0.49 |
| Low birth weight, % | 4.3 (22) | 4.3 (19) | 4.7 (21) | 0.96 |
| First birth, % | 60.6 (307) | 61.0 (267) | 61.0 (267) | 0.91 |
| Families received social security support, % | 24.1 (122) | 22.2 (97) | 22.8 (102) | 0.77 |
| Caregivers’ and mothers’ characteristics | ||||
| Mother is primary caregiver, % | 84.2 (427) | 81.7 (358) | 86.4 (387) | 0.17 |
| Maternal education above 9 y, % | 78.3 (397) | 76.9 (337) | 80.6 (361) | 0.41 |
| Maternal micronutrient supplement during pregnancy,b % | 82.5 (418) | 80.4 (352) | 79.2 (355) | 0.44 |
| Maternal anemia rate at baseline, % | 22.1 (112) | 22.8 (100) | 23.0 (103) | 0.94 |
| Infant feeding practice | ||||
| Period of breastfeeding, mo | 6.8 ±3.6 | 6.6 ±3.7 | 6.9 ±3.5 | 0.37 |
| Supplementary feeding after 6 mo, % | 65.9 (334) | 69.0 (302) | 67.6 (303) | 0.60 |
| Iron supplement for children, % | 12.0 (61) | 13.2 (58) | 13.0 (58) | 0.84 |
| Infant ate meat yesterday, % | 32.4 (164) | 37.7 (165) | 35.9 (161) | 0.21 |
| Period of taking formula, mo | 4.4 ±4.7 | 4.8 ±4.1 | 4.2 ±3.9 | 0.14 |
| Infant nutrition status | ||||
| Anemia rate, % | 47.9 (243) | 46.1 (202) | 49.8 (223) | 0.63 |
Note. Total sample was n = 1393.
Analysis of variance (ANOVA) test for means comparison and χ2 test for rate or proportion comparison.
About 70% of mothers reported taking folic acid during pregnancy.
Among caregivers in the TMG, 92.4% caregivers (414 of 448) reported that they had regularly received our daily text messages and were reading them. Only 3.6% caregivers (16 of 448) changed their cellphone numbers.
The data presented in Table 1 also show that baseline anemia prevalence was just under 50% for each of the 3 groups. There were no differences in anemia rates among the 3 groups (P = .63).
Overall, 42.7% of caregivers were fully compliant with the program (i.e., on average, caregivers administered 5–7 NurtureMate packets/week). A higher relative frequency of children in the TMG consumed 5 to 7 packets per week (201/448 = 44.9%), compared with children in the FDG (177/438 = 40.4%), although the difference was not statistically significant (P = .09). The share of all children that were anemic at baseline was balanced in terms of indicators for socioeconomic, caregiver, and maternal characteristics and for feeding practices (P values > .05; online Table G). However, among caregivers with children anemic at baseline who administered 5 to 7 packets per week, there was a higher percentage in the TMG (45.3%) than in the FDG (36.7%; P = .05; online Table H).
Using the intent-to-treat model, we show the impact of text message reminders on caregiver compliance (using full compliance as dependent variable) in Table 2. After adjusting for other covariates, we found that assignment to the TMG led to an increase in the likelihood that caregivers had full compliance to the program (marginal effect = 0.10; 95% confidence interval [CI] = 0.03, 0.16).1
TABLE 2—
Intent-to-Treat Analysis of the Impact of the Intervention on Caregiver Compliance and Child Anemia: Rural Shaanxi Province, 2013–2014
| Marginal Effect (95% CI) | P | |
| Caregiver compliancea | 0.10 (0.03, 0.16) | < .001 |
| Child anemiaa | ||
| Free delivery group (yes = 1, no = 0)b | −0.03 (−0.09, 0.03) | .38 |
| Text messaging group (yes = 1, no = 0)b | −0.07 (−0.12, –0.01) | .02 |
Note. CI = confidence interval. Sample for caregiver compliance analysis was n = 886. Sample for child anemia analysis was n = 1393.
Adjusted for age, gender, low birth weight, premature birth, birth order, families received social security support, whether primary caregiver is mother, maternal education, maternal micronutrients supplement during pregnancy, maternal hemoglobin concentration, period of breastfeeding, food supplement feeding after 6 months, infant iron supplement, taking meat, period of taking formula and infant hemoglobin concentration in baseline, as well as county fixed effect and cohort fixed effect. Clustering is at the village level. Caregiver compliance was measured by administration to child of 5 to 7 micronutrient packets per week (yes = 1, no = 0), and Text messaging group = 1, Free delivery group = 0 in this model.
The marginal effects measure impact of the treatments relative to the control group.
Table 2 also summarizes the effects of the treatment condition on infant anemia status. Using our intent-to-treat model, we found no significant impact of the FDG on anemia status at end line relative to the control group (P > .05). We did, however, find that, relative to the control group, assignment to the TMG reduced anemia rates by 7 percentage points at end line (marginal effect = −0.07; 95% CI = −0.12, −0.01). There were no differences in the marginal effects of the FDG and TMG (marginal effect = −0.09; 95% CI = −0.20, 0.01). The differences between the baseline and end-line anemia rates for the different groups (online Table I) were similar to those shown in Table 2.
Using our model for average treatment effect on the treated, we estimated that among fully compliant households in the intervention groups (FDG and TMG together), anemia rates fell by 13 percentage points (marginal effect = −0.13; 95% CI = −0.25, −0.002) relative to the control group (online Table J). Therefore, micronutrient packets were effective at reducing anemia if administered 5 to 7 times per week.
DISCUSSION
We found that among families participating in a micronutrient fortification program for children aged 6 to 12 months, caregivers who received a daily text message reminder showed better compliance relative to the FDG. The children in the TMG also experienced lower levels of anemia at end line relative to the control group. However, we found no significant impact of the FDG on anemia status at end line relative to the control group. It appears that the additional lowering of the anemia rate resulted from the presence of a TMG, rather than only an FDG. Compared with the control group, those in the TMG had compliance rates that reached statistical significance.
We have 2 main theories about the mechanism behind our results. First, we believe that the text messages may have raised the salience of (i.e., impressed on caregivers the importance of) providing nutrition through the intervention. Second, we believe that the text messages may have addressed caregivers’ forgetfulness. The reminder function could be especially important when caregivers encountered a challenge to their regular compliance, such as when their child came down with an illness. When children became sick, there was often a disruption in the household’s daily routine, including administration of the nutrient packages. Text message reminders may have improved overall compliance by reminding caregivers to return to their prechallenge routine.
Another finding is that text messages helped more caregivers who were raising anemic children to persist in being fully compliant with the home fortification program. This is indicated because, among caregivers of children anemic at baseline who persisted in administering 5 to 7 packets per week, a significantly higher proportion were in the TMG than in the FDG. We have 2 theories for why text message reminders were more effective among caregivers of anemic infants, both of which are directly related to the 2 mechanisms discussed in the previous paragraph. First, text messages may have had a larger effect on those caregivers through the first mechanism (raising salience) because they tended to be poorer and less educated than the caregivers of nonanemic children. Studies have shown that the poor tend to face more distractions and obstacles in their daily life.42 Because the amount of attention that individuals have to devote to different considerations is limited and the attention of the poor is more constrained, an intervention that raises the salience of a given activity (such as text reminders) may have a larger effect on the behavior of the poor. Second, text message reminders may have had more of an effect on caregivers of anemic children through the second mechanism: because anemic infants are more likely than other infants to experience an illness episode that disrupts the family’s routine, reminders to their caregivers are more important.
One objective of this study was to examine the impact of texting on the anemia rate of sample children. Our findings confirmed that assignment to the TMG led to a fall in children’s anemia rate at end line. The result is consistent with results from western Kenya20 and Bangladesh21 in which the rates of anemia fell in children who were given nutrient packets. It appears that text messages can play a role in public programs that try to promote health.
Our study makes important contributions. First, our trial is the largest cluster RCT to evaluate the impact of alternative delivery strategies on adherence of caregivers to a home micronutrient fortification program. Previous studies had limited sample sizes, did not include a control group, or only measured impacts on outcomes after (at most) 2 months.21
Another strength of this study is its policy relevance. To our knowledge, no other study has attempted to measure the impact of a home fortification study outside of a tightly controlled researcher-implemented environment. In our study, households were left alone to resume their regular routines for 6 months, during which time they had no contact with any researcher. Most studies of home fortification programs employed weekly or biweekly visits from researchers to ensure high levels of compliance.43–46 This level of engagement is unrealistic in areas of the world where health resources are constrained and infrastructure is underdeveloped.
Several limitations should be recognized. First, the observed attrition rate was relatively high (23.4%), because of either incomplete data or relocation of the household. From interviews with family members of the children lost to attrition, we know that this relocation was spurred by a search for job opportunities. Incomplete data occurred for several reasons. Even allowing for attrition, the statistical power of the study reached 95%. High rates of attrition also appear to be a common problem in other studies.47 Therefore, we do not believe that our findings are invalidated by missing data. We also were unable to conduct test paneling of whole blood for nutritional deficiencies; therefore, hemoglobin level was our sole indicator of micronutrient deficiency. The study’s measured impact should therefore be considered as a lower-bound estimate.
In conclusion, our findings show that daily text message reminders can lead to sustained improvements in the compliance of caregivers to home fortification programs. The increases in compliance are also associated with improvements in child nutrition. We therefore recommend that low-cost text message reminders be used to complement existing program delivery strategies.
ACKNOWLEDGMENTS
We are grateful for project funding from the International Initiative for Impact Evaluation (3ie), the UBS Optimus Foundation, the China Medical Board, the Bank of East Asia, the H. J. Heinz Company Foundation, the Huaqiao Foundation, and Noblesse.
We also thank the dedicated leaders and local cadres at the National Health and Family Planning Commission for their unparalleled assistance in implementing this study.
HUMAN PARTICIPANT PROTECTION
This study received ethical approval from the Stanford University institutional review board and from the Sichuan University Medical Ethical Review Board. The trial is registered with ISRCTN (ISRCTN44149146). All participating caregivers gave their oral consent for both their own and their infant’s involvement in the study.
REFERENCES
- 1.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 2.The World Health Report 2005: Make Every Mother and Child Count. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 3.Chen YC, Tang SC, Le H, Yu XM, Wang DG, Hao M. Compare health development of rural areas in different districts of China: about social economy and inhabitant’s health condition [in Chinese] Chin Health Econ. 2006;25:37–38. [Google Scholar]
- 4.Chang S, Wu H, Jia FM, Chen CM. Analysis on the changes of nutritional status in China anemia status of children under 5 in China [in Chinese] Wei Sheng Yan Jiu. 2007;36(2):210–212. [PubMed] [Google Scholar]
- 5.The Nutrition Development Report of Chinese Children Aged 0–6 (2012) Beijing, China: Ministry of Health; 2012. pp. 1–18. [Google Scholar]
- 6.Ma L, Zeng G, Zhao L et al. Growth and anemia status investigation of 0–2 years infant and child in poor rural areas of Guangxi Autonomous Region [in Chinese] Wei Sheng Yan Jiu. 2010;39(1):65–67. [PubMed] [Google Scholar]
- 7.Dong CX, Ge PF, Zhang CJ et al. Effects of different feeding practices at 0–6 months and living economic conditions on anemia prevalence of infants and young children [In Chinese] Wei Sheng Yan Jiu. 2013;42(4):596–599. [PubMed] [Google Scholar]
- 8.Balarajan Y, Ramakrishnan U, Öaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378(9809):2123–2135. doi: 10.1016/S0140-6736(10)62304-5. [DOI] [PubMed] [Google Scholar]
- 9.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CLJ, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004. pp. 163–209. [Google Scholar]
- 10.Black RE, Victora CG, Walker SP et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 11.Guideline: Use of Multiple Micronutrient Powders for Home Fortification of Foods Consumed by Infants and Children 6–23 Months of Age. Geneva, Switzerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 12.Lin LM, Song XF, Liu YL et al. Relationship between vitamin A deficiency and anemia for Chinese children [in Chinese] Chin J Child Health Care. 2003;11:242–244. [Google Scholar]
- 13.Xu ZK, Wang LJ, Chang F et al. Effectiveness of nutrition interventions about infants aged 6–24 months in Ningqiang County affected by Wenchuan earthquake [in Chinese] Chin J Child Health Care. 2012;20(8):728–730. [Google Scholar]
- 14.Wang L, Huo J, Sun J et al. Nutrition effectiveness of infants and young children aged 6 to 23 months by Yingyangbao in Lixian County affected by Wenchuan earthquake in Sichuan Province [in Chinese] Wei Sheng Yan Jiu. 2011;40(1):61–64. [PubMed] [Google Scholar]
- 15.Wang Y, Chen C, Jia M, Fang J. Effect of complementary food supplements on anemia in infant and young children [in Chinese] Wei Sheng Yan Jiu. 2004;33(3):334–336. [PubMed] [Google Scholar]
- 16.Sun J, Dai Y, Zhang S et al. Implementation of a programme to market a complementary food supplement (Ying Yang Bao) and impacts on anaemia and feeding practices in Shanxi, China [in Chinese] Matern Child Nutr. 2011;7(suppl 3):96–111. doi: 10.1111/j.1740-8709.2011.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng HY, Zhu SH. Effects of Heinz nutrition package complementary food supplement on hemoglobin levels for infants and young children [in Chinese] Med J Chin Peoples Health. 2014;26(8):26–27. [Google Scholar]
- 18.Xu YQ, Wang HS, Gong LM, Hong M, Zhao J, Huang XN. Effect of complementary food supplements on incidence of anemia among children in rural areas of China [in Chinese] Chin J Woman Child Health Res. 2011;22(2):128–131. [Google Scholar]
- 19.Galloway R, McGuire J. Determinants of compliance with iron supplementation: supplies, side effects, or psychology? Soc Sci Med. 1994;39(3):381–390. doi: 10.1016/0277-9536(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 20.Suchdev PS, Ruth LJ, Woodruff BA et al. Selling sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in western Kenya: a cluster-randomized controlled trial. Am J Clin Nutr. 2012;95(5):1223–1230. doi: 10.3945/ajcn.111.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ip H, Hyder SMZ, Haseen F, Rahman M, Zlotkin SH. Improved adherence and anaemia cure rates with flexible administration of micronutrient Sprinkles: a new public health approach to anaemia control. Eur J Clin Nutr. 2009;63(2):165–172. doi: 10.1038/sj.ejcn.1602917. [DOI] [PubMed] [Google Scholar]
- 22.International Telecommunication Union. The world in 2010: ICT facts and figures. Available at: http://www.itu.int/ITU-D/ict/material/FactsFigures2010.pdf. Accessed March 20, 2016.
- 23.Information Research Department. State Information Center. China digital divide report [in Chinese] Inf Res. 2013. p. 9. Available at: http://www.sic.gov.cn/News/258/2135.htm. Accessed March 20, 2016.
- 24.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health. 2009;15(3):231–240. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- 25.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior changer in disease prevention and management. Epidemiol Rev. 2010;32:56–69. doi: 10.1093/epirev/mxq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers A, Corbett T, Riddell T, Wills M, Lin R, Jones M. Do u smoke after txt? Results of a randomized trial of smoking cessation using mobile phone text messaging. Tob Control. 2005;14(4):255–261. doi: 10.1136/tc.2005.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joo N, Kim B. Mobile phone short message service messaging for behavior modification in a community-based weight control programme in Korea. J Telemed Telecare. 2007;13(8):416–420. doi: 10.1258/135763307783064331. [DOI] [PubMed] [Google Scholar]
- 28.Franklin V, Waller A, Pagliari C, Greene S. A randomized controlled trial of SweetTalk, a text-messaging system to support young people with diabetes. Diabet Med. 2006;23(12):1332–1338. doi: 10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H, Li M, Wen LM et al. Effect of short message service on infant feeding practice: findings from a community-based study in Shanghai, China. JAMA Pediatr. 2014;168(5):471–478. doi: 10.1001/jamapediatrics.2014.58. [DOI] [PubMed] [Google Scholar]
- 30.Chong A, Karlan D, Shapiro J, Zinman J. (Ineffective) messages to encourage recycling: evidence from a randomized evaluation in Peru. World Bank Econ Rev. 2015;29(1):180–206. [Google Scholar]
- 31.Jamison J, Karlan DS, Raffler P. Mixed method evaluation of a Passive mHealth Sexual Information Texting Service in Uganda. Inf Technol Int Dev. 2013;9(3):1544–7529. [Google Scholar]
- 32.Bruxvoort K, Festo C, Kalolella A et al. Cluster randomized trial of text message reminders to retail staff in Tanzanian drug shops dispensing artemether-lumefantrine: effect on dispenser knowledge and patient adherence. Am J Trop Med Hyg. 2014;91(4):844–853. doi: 10.4269/ajtmh.14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kannisto KA, Koivunen MH, Välimäki MA. Use of mobile phone text message reminders in health care services: a narrative literature review. J Med Internet Res. 2014;16(10):e222. doi: 10.2196/jmir.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. Morb Mortal Recomm Rep. 1998;47(RR-3):1–29. [PubMed] [Google Scholar]
- 35.Life Sciences Research Office. Assessment of the Iron Nutrition Status of the US Population Based on Data Collected in the Second National Health and Nutrition Survey, 1976–1980. Bethesda, MD: Federation of American Societies for Experimental Biology; 1984. [Google Scholar]
- 36.Spybrook J, Raudenbush S, Liu XF, Congdon R. Optimal Design for Longitudinal and Multilevel Research: Documentation for the Optimal Design Software. Ann Arbor, MI: University of Michigan Press; 2008. [Google Scholar]
- 37.Ermis B, Demirel F, Demircan N, Gurel A. Effect of three different iron supplementations in term health infants after 5 months of life. J Trop Pediatr. 2002;48(5):280–284. doi: 10.1093/tropej/48.5.280. [DOI] [PubMed] [Google Scholar]
- 38.Berger J, Ninh NX, Khan NC et al. Efficacy of combined iron and zinc supplementation on micronutrient status and growth in Vietnamese infants. Eur J Clin Nutr. 2006;60(4):443–454. doi: 10.1038/sj.ejcn.1602336. [DOI] [PubMed] [Google Scholar]
- 39.Pasricha S, Hayes E, Kalumba K, Biggs B. Effect of daily iron supplementation on health in children aged 4–23 months: a systematic review and meta-analysis of randomised controlled trials. Lancet Glob Health. 2013;1(2):e77–e86. doi: 10.1016/S2214-109X(13)70046-9. [DOI] [PubMed] [Google Scholar]
- 40.Luo R, Shi Y, Zhang L et al. Nutrition and educational performance in rural China’s elementary schools: results of a randomized control trial in Shaanxi Province. Econ Dev Cult Change. 2012;60(1):735–772. [Google Scholar]
- 41.Duflo E, Glennerster R, Kremer M. Using randomization in development economics research: a toolkit. In: Behrman J, Srinivasan TN, editors. Handbook of Development Economics. Vol 4. Amsterdam, Netherlands: North-Holland; 2007. pp. 3895–3962. [Google Scholar]
- 42.World Development Report 2015: Mind, Society, and Behavior. Washington, DC: World Bank; 2015. [Google Scholar]
- 43.Zlotkin S, Arthur P, Schauer C, Antwi KY, Yeung G, Piekarz A. Home-fortification with iron and zinc sprinkles or iron sprinkles alone successfully treats anemia in infants and young children. J Nutr. 2003;133(4):1075–1080. doi: 10.1093/jn/133.4.1075. [DOI] [PubMed] [Google Scholar]
- 44.Adu-Afarwuah S, Lartey A, Brown KH. Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. Am J Clin Nutr. 2008;87(4):929–938. doi: 10.1093/ajcn/87.4.929. [DOI] [PubMed] [Google Scholar]
- 45.Kounnavong S, Sunahara T, Mascie-Taylor CGN et al. Effect of daily versus weekly home fortification with multiple micronutrient powder on haemoglobin concentration of young children in a rural area, Lao People’s Democratic Republic: a randomised trial. Nutr J. 2011;10:129. doi: 10.1186/1475-2891-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menon P, Ruel MT, Loechl CU et al. Micronutrient Sprinkles reduce anemia among 9- to 24-mo-old children when delivered through an integrated health and nutrition program in rural Haiti. J Nutr. 2007;137(4):1023–1030. doi: 10.1093/jn/137.4.1023. [DOI] [PubMed] [Google Scholar]
- 47.Soofi S, Cousens S, Iqbal SP et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet. 2013;382(9886):29–40. doi: 10.1016/S0140-6736(13)60437-7. [DOI] [PubMed] [Google Scholar]