Abstract
Background: Patients with multiple sclerosis (MS) have higher rates of fatigue, mood disturbance, and cognitive impairments than healthy populations. Disease-modifying agents may affect sleep. Although patients taking natalizumab often show improvement in fatigue during the first year of therapy, the mechanism behind this effect is unknown. The aim of the NAPS-MS study was to investigate whether natalizumab affected objective measures of sleep as determined by polysomnography (PSG) and multiple sleep latency testing (MSLT) in patients with MS with fatigue or sleepiness initiating therapy. Additional goals were to evaluate changes in measures of fatigue, mood, and cognition and to correlate these measures with objective sleep measures.
Methods: Patients underwent PSG and MSLT before their first natalizumab infusion and after their seventh. Patients completed the Modified Fatigue Impact Scale, Fatigue Severity Scale (FSS), Epworth Sleepiness Scale (ESS), and visual analogue scale for fatigue (VAS-F) at their first, fourth, and seventh natalizumab infusions. NeuroTrax cognitive tests and the Hospital Anxiety and Depression Scale (HADS) were performed at the first and seventh natalizumab infusions.
Results: Changes in sleep efficiency, wakefulness after sleep onset, and multiple sleep latency from baseline to 6 months of therapy did not reach significance. The FSS, VAS-F, ESS, and HADS scores were significantly improved after 6 months of therapy; cognitive scores were not significantly improved.
Conclusions: Although treatment with natalizumab was associated with improvements in fatigue, sleepiness, and mood, changes in objective measures of sleep were not significant.
Many studies have shown that patients with multiple sclerosis (MS) have higher incidences of fatigue and sleepiness than healthy control or chronically ill populations.1–3 Excessive sleepiness is defined as sleepiness occurring in a situation when an individual is expected to be awake and alert.4 Fatigue is defined as a subjective lack of physical or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities.5 Sleepiness and fatigue, although often occurring in the same patient, can exist independently, and either can negatively impact quality of life in MS.3 The etiology of these symptoms has not been completely elucidated, although studies have shown that sleep disturbances are associated with a higher incidence of fatigue and daytime sleepiness.6–8 Obstructive sleep apnea, often associated with sleepiness or fatigue, is prevalent in MS.9,10 Other non–therapy-related diagnoses that may affect the efficiency of sleep could include sleep fragmentation, restless leg syndrome/periodic limb movements of sleep, menopause, frequent urination, muscle spasms, depression, and pain.
Disease-modifying agents (DMAs), including interferon and glatiramer acetate, may affect sleep. One study showed that fatigue increases with interferon treatment and that this fatigue drives lower scores on quality of life measures.11 In a prospective study, glatiramer acetate had a more favorable effect on fatigue than the interferons.12 Another smaller study did not confirm this finding and also found, by actigraphy, that interferon and glatiramer were associated with reduced sleep efficiency (SE) compared with untreated patients.13
Natalizumab significantly reduces the relapse rate and progression of disability in relapsing forms of MS.14 Several studies have shown that natalizumab reduces fatigue. In the ENER-G (Evaluation of Natalizumab for thE Relief of MS Associated FatiGue) study, significant improvement in scores on fatigue scales was noted within 12 weeks, and this benefit persisted during the 48-week study.15 Likewise, the TYNERGY study found that patients with MS reported significantly less fatigue after 12 months of natalizumab treatment.16 Furthermore, the TYNERGY study found improvements in cognition, mood, and quality of life. In another study investigating patient-reported outcomes, fatigue and cognition improved during natalizumab therapy.17
The mechanism whereby natalizumab positively affects fatigue is unknown. One possibility would be an effect on sleep quality. The primary purpose of the NAPS-MS study was to investigate whether natalizumab affected objective measures of sleep as determined by polysomnography (PSG) and multiple sleep latency testing (MSLT) in patients with fatigue or sleepiness who are initiating therapy. Additional goals were to evaluate changes in measures of fatigue, mood, and cognition during 6 months of natalizumab treatment and to correlate these measures with objective measures of sleep.
Patients and Methods
Patients
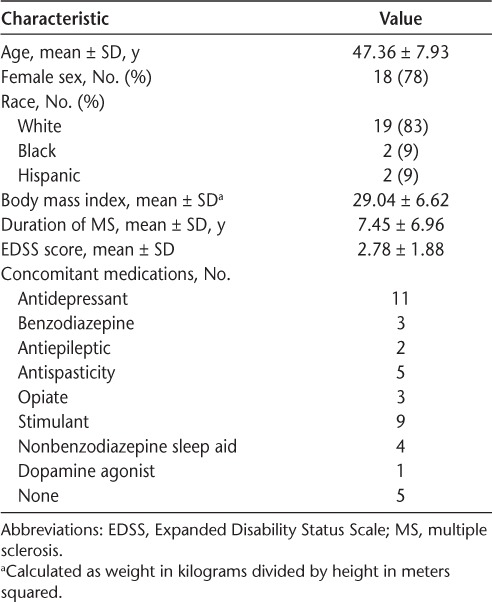
The NAPS-MS study was approved by the Aspire Institutional Review Board (Santee, CA). A cohort of 37 patients planning to initiate natalizumab therapy was recruited from three sites. For inclusion, individuals had to be naive to natalizumab, be able to give valid informed consent, have an Expanded Disability Status Scale18 score of 0 to 6.0, and be aged 18 to 65 years. Patients were excluded if they did not speak English, had severe depression (score >32 on the Beck Depression Inventory-II [BDI-II]),19 or had severe cognitive impairment. To select for a population with either fatigue or sleepiness, all the patients needed to score greater than 9 on the Epworth Sleepiness Scale (ESS),20 greater than 30 on the Modified Fatigue Impact Scale (MFIS),21 or greater than 4 on the Fatigue Severity Scale (FSS).22 No patients were taking DMAs at the time of baseline PSG. Patients found to have obstructive sleep apnea on the baseline sleep study who were planning to initiate CPAP or other therapy were withdrawn to eliminate this confounding factor. Of the 37 patients who were recruited, 33 passed the screening (3 patients did not meet the fatigue or sleepiness criteria and 1 patient had severe depression). One patient withdrew consent before the sleep studies were performed. Correlations between baseline PSG findings, symptoms, and cognition for these 32 patients have been reported elsewhere.9 Two patients withdrew from the study to initiate obstructive sleep apnea therapy, three patients stopped taking natalizumab before the second PSG, and four patients withdrew consent during the study. The remaining 23 patients completed the study and were analyzed. Patient demographic information and concomitant medications are reported in Table 1. Patients were requested to continue taking their symptomatic medications without change during the study.
Table 1.
Baseline characteristics of the 23 study patients
Evaluations
During the screening visit, patient demographic data, medical records, and MS history were obtained (Table 1). The Expanded Disability Status Scale score was determined by an individual trained in this evaluation. The MFIS, FSS, visual analogue scales for fatigue and pain (VAS-F and VAS-P), and ESS were completed to assess fatigue and sleepiness. Depression was assessed using the BDI-II.
Overnight PSG was performed by certified technicians and interpreted by board-certified physicians. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications was used to perform the studies and report the results.23 The absolute time and percentage of stage NI, N2, N3, and rapid eye movement (REM) sleep were reported. Sleep onset latency, SE, and wakefulness after sleep onset (WASO) were determined. Respiratory measures, including the apnea-hypopnea index, respiratory disturbance index, and oxygenation nadir, were measured. The limb movement index, limb movement arousal index, respiratory arousal index, spontaneous arousal index, and total arousal index were determined. The morning after the overnight PSG, patients underwent MSLT.4 The mean sleep latency of each of the four or five naps and whether REM sleep was observed were reported.
Patients completed the Hospital Anxiety and Depression Scale (HADS-D and HADS-A) during the first and seventh natalizumab infusion visits.24 The HADS-D was chosen over the BDI-II to measure mood over time because it does not have questions related to sleep or fatigue that may have overemphasized the magnitude of change with treatment. Patients completed the MFIS, FSS, and VAS-F fatigue scales and the VAS-P pain scale during the first, fourth, and seventh infusion visits. Patients took the NeuroTrax neurocognitive battery during the screening visit and the first and seventh infusion visits. This test has been validated for use in MS.25 Because of a possible training effect of repeated neurocognitive testing,26 the scores obtained during the first infusion were used as the baseline for comparison with the tests during treatment. After the seventh infusion, patients returned to the sleep laboratories for the on-treatment PSG and MSLT.
Statistical Analysis
Values are reported as mean ± SD. For comparison of data over time, the paired-samples t test was used for normally distributed data and the Wilcoxon matched-pairs signed rank test was used for non-normally distributed data. The Shapiro-Wilk test of normality was used to test for a violation of normal distribution. For between-group analysis, the nonparametric Mann-Whitney U test was used. Significance was determined using a 2-tailed test. All the statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp, Armonk, NY).
Results
Objective Measures of Sleep
The results of PSG and MSLT performed after the seventh infusion were compared with the baseline results (Table 2). No significant improvements were noted in SE (P = .523), WASO (P = .108), sleep onset latency (P = .574), percentage of stage N2 sleep (P = .08), percentage of stage N3 sleep (P = .117), or percentage of stage REM sleep (P = .114). There was a significant decrease in percentage of stage N1 sleep (P = .016). Also, there was no difference in average sleep latency measured on MSLT (P = .661).
Table 2.
Polysomnography and MSLT data before and after 6 months of natalizumab therapy
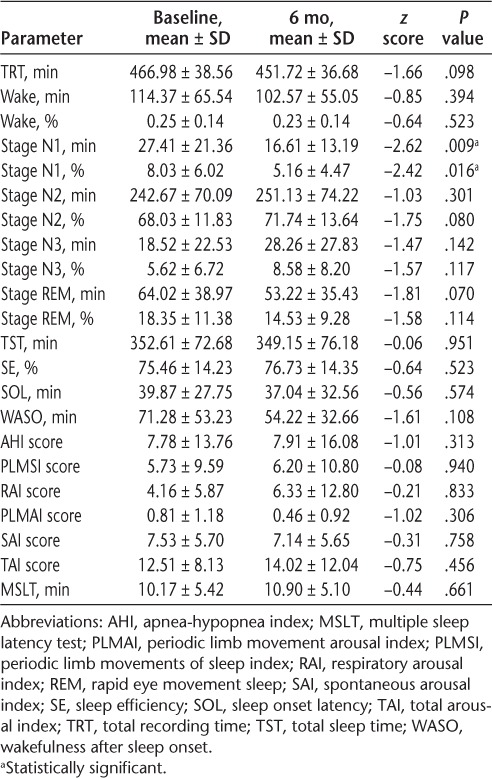
In addition, there were no significant differences in apnea-hypopnea index, periodic limb movements of sleep index, total arousal index, respiratory arousal index, periodic limb movement arousal index, or spontaneous arousal index scores (all P > .1).
Fatigue and Sleepiness
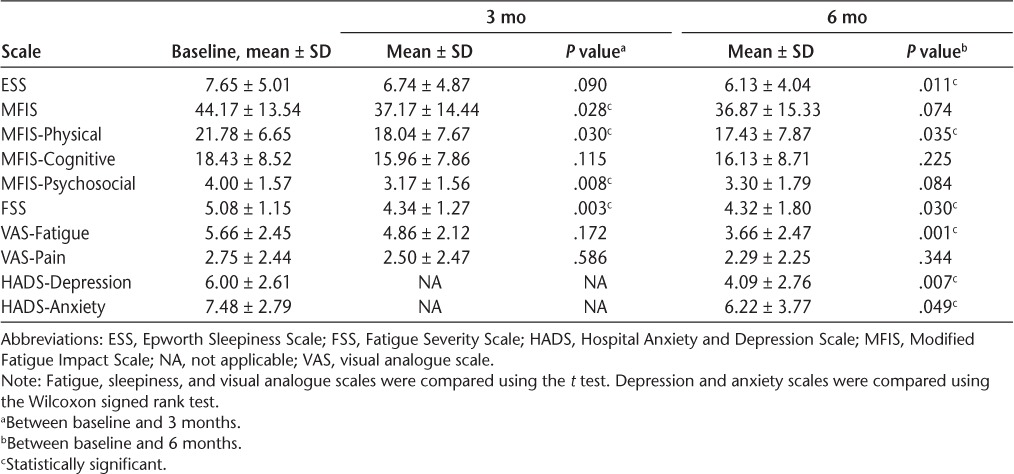
The FSS (P = .03) and VAS-F (P = .001) fatigue scale scores were significantly improved from baseline to month 6, and the MFIS score showed a trend toward improvement (P = .074) (Table 3). Improvement in fatigue occurred rapidly, with MFIS (P = .028) and FSS (P = .003) scores showing significant differences between baseline screening and month 3. Significant improvements in the physical and psychosocial subscales of the MFIS were noted over 6 months, and improvements in the cognitive subscale did not reach significance. The ESS also showed that patients reported less sleepiness between screening and 6 months (P = .011). Patients who improved by more than 1 SD on the FSS, MFIS, or VAS-F did not show significant changes in SE or WASO (Mann Whitney U > 0.05).
Table 3.
Self-reported scales of sleepiness, fatigue, pain, depression, and anxiety
Mood and Cognition
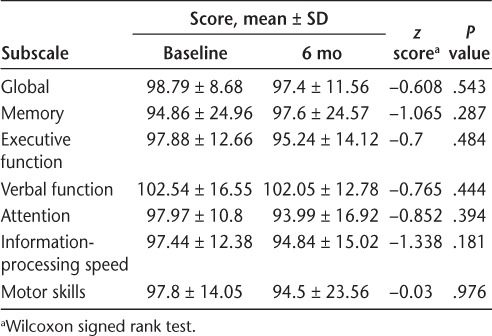
The HADS-A and HADS-D were administered during the first and seventh natalizumab infusions (Table 3). The HADS-D (P = .007) and HADS-A (P = .049) scores were significantly improved between these two points. Improvement in mood did not correlate to improvement in SE or WASO. Patients took the NeuroTrax cognitive tests during the first and seventh infusions (Table 4). During this period, there was no significant improvement in the global measure or in the memory, executive function, verbal function, attention, information-processing speed, or motor skills subscales.
Table 4.
NeuroTrax cognitive testing at baseline and after 6 months of natalizumab therapy
Discussion
This is the first study to evaluate the effect of natalizumab on objective measures of sleep as determined by PSG and MSLT. Less stage N1 sleep was observed, but the difference was only 11 minutes, or approximately 3.7% of sleep time. Because this decrease was accompanied by a similar increase in stage N2 sleep and no significant change in total arousal index, this finding is unlikely to be meaningful. Sleep efficiency and WASO were not significantly improved. In addition, the mean sleep latency was not significantly different.
Very few data are available quantifying the effect of DMAs on sleep. One actigraphy study of patients with MS treated with interferon found reduced SE the night after injection and also found that patients treated with glatiramer had lower SE compared with untreated patients.13 A second actigraphy study comparing sleep at baseline with sleep after starting interferon beta use found that treatment reduced SE.27 In an unrelated disease state, interferon alpha decreased SE and increased WASO in patients with hepatitis C.28 To our knowledge, the present study is the first to compare PSG findings at baseline and after starting a specific DMA for MS.
The present finding that patients receiving natalizumab therapy report improved fatigue as measured by the MFIS, FSS, and VAS-F is similar to that in other reports.15–17,29,30 Improvement occurred rapidly and was significant by the third month. Improvement in sleepiness, as measured by the ESS, was also observed. Metz et al.12 found that 24.8% and 12.9% of patients with MS treated with glatiramer acetate and interferon, respectively, improved 1 SD or more on the Fatigue Impact Scale over 6 months. When a similar evaluation was performed with the present population, we found that 39.1%, 43.4%, and 47.8% of patients improved 1 SD or more on the related MFIS, the FSS, and the VAS-F, respectively.
We also found that patients improved on depression and anxiety scales after 6 months of natalizumab therapy. This finding contrasts with a study showing that interferon or glatiramer acetate treatment has no effect on depression as measured by the BDI.31 Furthermore, subcutaneous interferon beta-1a was not found to have an effect on depression in the PRISMS-4 (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis-4) study.32
The finding that changes in sleep do not seem to play a prominent role in the improved reports of fatigue could be due to the small sample size in this study. Alternatively, another non–sleep-related mechanism may be responsible. Reduction of proinflammatory cytokines or other proteins in the central nervous system might explain some of the observed improvement in fatigue and other symptoms. Proinflammatory cytokines, including interleukin (IL)-1 beta, tumor necrosis factor, and IL-6, are found at elevated levels in the cerebrospinal fluid (CSF) of patients with MS.33,34 Khademi et al.35 showed that natalizumab therapy decreases lymphocyte counts in the CSF. In that study, intrathecal cells from natalizumab-treated patients expressed less messenger RNA for the proinflammatory interferon gamma and IL-23 cytokines and more messenger RNA for the anti-inflammatory IL-10 cytokine.35 Mellergård et al.36 confirmed that natalizumab-treated patients had reduced numbers of cells in the CSF and also found lower levels of the proinflammatory cytokines IL-1b, IL-6, and IL-8 and several chemokines. Other studies in patients without MS found that IL-6 is increased in the CSF of patients with depression or failed suicide attempts.37,38 Iaffaldano et al.30 found that natalizumab decreases serum osteopontin levels. The decrease correlated with improvement in cognitive impairment but not improvement in the FSS score observed during natalizumab treatment.
In summary, this study found that significant improvement in objective measures of sleep did not occur during 6 months of natalizumab therapy despite improvements in fatigue, reported sleepiness, depression, and anxiety. This exploratory study is limited by a small sample size and lack of a placebo or an active comparator arm. In addition, the duration of the study may be insufficient to demonstrate slow improvement over a longer period. However, changes in sleep quality are unlikely to fully explain the observed improvements in fatigue and other symptoms during natalizumab therapy.
PracticePoints.
Six months of therapy with natalizumab was associated with improvement in fatigue, sleepiness, depression, and anxiety.
Natalizumab therapy was not associated with significant changes in sleep efficiency, wakefulness after sleep onset, or multiple sleep latency as measured by polysomnography.
Acknowledgments
We thank Melinda Merrill, PhD, for reviewing the manuscript.
Footnotes
From Cornerstone Neurology, High Point, NC, USA (RAS, PS); South Shore Neurologic Associates, Patchogue, NY, USA (MG); Providence Multiple Sclerosis Center, Portland, OR, USA (KK-R); and Hope Neurology, Knoxville, TN, USA (DWB).
Financial Disclosures: Dr. Sater has served as a speaker or consultant for Acorda, Biogen Idec, EMD Serono, Genzyme, and Novartis. Dr. Gudesblatt has served as a speaker or consultant for Acorda, Biogen Idec, EMD Serono, Genzyme, and Teva. Dr. Kresa-Reahl has served as a speaker or consultant for Acorda, Biogen Idec, EMD Serono, Genzyme, Pfizer, Novartis, and Teva. Dr. Brandes has served as a speaker or consultant for Acorda, Avanir, Biogen Idec, Genzyme, Novartis, Questcor, and Teva. Ms. Sater has no conflicts of interest to disclose.
Funding/Support: This study was supported by an investigator-initiated grant from Biogen Idec (US-TYS-11-10221).
References
- 1.Bamer AM, Johnson KL, Amtmann D, Kraft GH. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler. 2008;14:1127–1130. doi: 10.1177/1352458508092807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kos D, Kerckhofs E, Nagels G, D'hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil Neural Repair. 2008;22:91–100. doi: 10.1177/1545968306298934. [DOI] [PubMed] [Google Scholar]
- 3.Stanton B, Barnes F, Silber E. Sleep and fatigue in multiple sclerosis. Mult Scler. 2006;12:481–486. doi: 10.1191/135248506ms1320oa. [DOI] [PubMed] [Google Scholar]
- 4.Arand D, Bonnet M, Hurwitz T, Mitler M, Rosa R, Sangal RB. The clinical use of the MSLT and MWT. Sleep. 2005;28:123–144. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]
- 5.Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-Based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998. [Google Scholar]
- 6.Attarian HP, Brown KM, Duntley SP, Carter JD, Cross AH. The relationship of sleep disturbances and fatigue in multiple sclerosis. Arch Neurol. 2004;61:525–528. doi: 10.1001/archneur.61.4.525. [DOI] [PubMed] [Google Scholar]
- 7.Kaynak H, Altintaş A, Kaynak D et al. Fatigue and sleep disturbance in multiple sclerosis. Eur J Neurol. 2006;13:1333–1339. doi: 10.1111/j.1468-1331.2006.01499.x. [DOI] [PubMed] [Google Scholar]
- 8.Pokryszko-Dragan A, Bilińska M, Gruszka E, Biel Ł, Kamińska K, Konieczna K. Sleep disturbances in patients with multiple sclerosis. Neurol Sci. 2013;34:1291–1296. doi: 10.1007/s10072-012-1229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sater RA, Gudesblatt M, Brandes DW, Sater PA. The relationship between objective parameters of sleep and measures of fatigue, depression, and cognition in multiple sclerosis. MSJ-ETC. 2015;1:1–8. doi: 10.1177/2055217315577828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaminska M, Kimoff RJ, Benedetti A et al. Obstructive sleep apnea is associated with fatigue in multiple sclerosis. Mult Scler. 2012;18:1159–1169. doi: 10.1177/1352458511432328. [DOI] [PubMed] [Google Scholar]
- 11.Simone IL, Ceccarelli A, Tortorella C et al. Influence of interferon beta treatment on quality of life in multiple sclerosis patients. Health Qual Life Outcomes. 2006;4:96. doi: 10.1186/1477-7525-4-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metz LM, Patten SB, Archibald CJ et al. The effect of immunomodulatory treatment on multiple sclerosis fatigue. J Neurol Neurosurg Psychiatry. 2004;75:1045–1047. doi: 10.1136/jnnp.2002.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendozzi L, Tronci F, Garegnani M, Pugnetti L. Sleep disturbance and fatigue in mild relapsing remitting multiple sclerosis patients on chronic immunomodulant therapy: an actigraphic study. Mult Scler. 2010;16:238–247. doi: 10.1177/1352458509354551. [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, O'Connor PW, Havrdova E et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 15.Wilken J, Kane RL, Sullivan CL et al. Changes in fatigue and cognition in patients with relapsing forms of multiple sclerosis treated with natalizumab: the ENER-G study. Int J MS Care. 2013;15:120–128. doi: 10.7224/1537-2073.2012-043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svenningsson A, Falk E, Celius EG et al. Natalizumab treatment reduces fatigue in multiple sclerosis: results from the TYNERGY trial: a study in the real life setting. PLoS One. 2013;8:e58643. doi: 10.1371/journal.pone.0058643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson JJ, Kern DM, Agarwal SS et al. Impact of natalizumab on patient-reported outcomes in multiple sclerosis: a longitudinal study. Health Qual Life Outcomes. 2012;10:155. doi: 10.1186/1477-7525-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79–S83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 22.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson AQS. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.Zigmond A, Snaith R. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Achiron A, Doniger GM, Harel Y, Appleboim-Gavish N, Lavie M, Simon ES. Prolonged response times characterize cognitive performance in multiple sclerosis. Eur J Neurol. 2007;14:1102–1108. doi: 10.1111/j.1468-1331.2007.01909.x. [DOI] [PubMed] [Google Scholar]
- 26.Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J Int Neuropsychol Soc. 2003;9:419–428. doi: 10.1017/S1355617703930074. [DOI] [PubMed] [Google Scholar]
- 27.Melamud L, Golan D, Luboshitzky R, Lavi I, Miller A. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci. 2012;314:37–40. doi: 10.1016/j.jns.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Raison CL, Rye DB, Woolwine BJ et al. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Putzki N, Yaldizli O, Tettenborn B, Diener HC. Multiple sclerosis associated fatigue during natalizumab treatment. J Neurol Sci. 2009;285:109–113. doi: 10.1016/j.jns.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Iaffaldano P, Viterbo RG, Paolicelli D et al. Impact of natalizumab on cognitive performances and fatigue in relapsing multiple sclerosis: a prospective, open-label, two years observational study. PLoS One. 2012;7:e35843. doi: 10.1371/journal.pone.0035843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirzinger SS, Jones J, Siegwald A, Crush AB. Relationship between disease-modifying therapy and depression in multiple sclerosis. Int J MS Care. 2013;15:107–112. doi: 10.7224/1537-2073.2012-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patten SB, Fridhandler S, Beck CA, Metz LM. Depressive symptoms in a treated multiple sclerosis cohort. Mult Scler. 2003;9:616–620. doi: 10.1191/1352458503ms960oa. [DOI] [PubMed] [Google Scholar]
- 33.Hauser SL, Doolittle TH, Lincoln R, Brown RH, Dinarello CA. Cytokine accumulations in CSF of multiple sclerosis patients: frequent detection of interleukin-1 and tumor necrosis factor but not interleukin-6. Neurology. 1990;40:1735–1739. doi: 10.1212/wnl.40.11.1735. [DOI] [PubMed] [Google Scholar]
- 34.Maimone D, Gregory S, Arnason BG, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol. 1991;32:67–74. doi: 10.1016/0165-5728(91)90073-g. [DOI] [PubMed] [Google Scholar]
- 35.Khademi M, Bornsen L, Rafatnia F et al. The effects of natalizumab on inflammatory mediators in multiple sclerosis: prospects for treatment-sensitive biomarkers. Eur J Neurol. 2009;16:528–536. doi: 10.1111/j.1468-1331.2009.02532.x. [DOI] [PubMed] [Google Scholar]
- 36.Mellergård J, Edström M, Vrethem M, Ernerudh J, Dahle C. Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler. 2010;16:208–217. doi: 10.1177/1352458509355068. [DOI] [PubMed] [Google Scholar]
- 37.Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- 38.Lindqvist D, Janelidze S, Hagell P et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]


