Abstract
Temporomandibular disorder (TMD) is a common condition that can be difficult to manage in physical therapy. A number of interventions, such as manual therapy, therapeutic exercise, and patient education have typically been used in some combination. However, the evidence regarding thrust manipulation of not only the local but also adjacent segments is sparse. Specifically, the use of cervicothoracic (CT) junction thrust manipulation has not previously been described in the management of individuals with TMD. In this case report, CT junction thrust manipulation, in addition to locally directed manual therapy, exercise, and postural education, was associated with immediate improvements in neck and jaw symptoms and function in a complex patient with TMD. The patient was seen for seven visits over the course of 2 months and demonstrated clinically significant changes in the neck disability index (NDI), the numeric rating of pain scale (NPRS), and the global rating of change (GROC) scale. The purpose of this report is to describe the successful physical therapy management of a patient with TMD utilizing manual therapy, including CT junction thrust manipulation, education, and exercise.
Keywords: Case report, Craniofacial, Manual therapy, Pressure pain threshold, TMD
Background
The temporomandibular joint (TMJ) is the most commonly used joint in the human body. The diagnosis, temporomandibular disorder (TMD), describes a number of pathologies in the craniofacial region. Epidemiological studies report that 50–75% of the population may suffer at least one symptom of TMD at some point in their life, with females most often affected.1–4 Temporomandibular disorder has a multi-factorial etiology, but may be associated with macrotrauma (direct blow or a motor vehicle accident with whiplash), microtrauma (clenching or bruxism), or degenerative changes from osteoarthritis (OA).5,6 Although a number of diagnostic tools and tests are available, their utility is questionable, and the diagnosis of TMD is largely clinically based.7,8 Clinical manifestation of TMD includes, but is not limited to, pain, limited jaw opening, joint noises, headaches, dizziness, neck symptoms, earache, tinnitus, and dysphagia.5,9 A number of interventions including patient education, postural correction, diet/oral habit modification, medication, occlusal therapies, psychotherapy, modalities, soft tissue and joint mobilization, exercise, and in some cases, surgery are commonly used in the management of TMD.5,9–14 Because of the wide range of potential symptoms reported and various structures involved, a standardized approach to management is not appropriate and frequently not effective.
Although there have been a number of studies examining evaluation and treatment of TMD, gaps in the literature still exist, particularly when patients present with multiple impairments beyond the local site of TMJ pathology. While inconclusive, a number of reports have suggested an association between pain and disability of the TMJ pain and cervical spine.15–17 Biomechanically, a forward head posture can alter the resting position of the mandibular condyle within the TMJ, pulling it inferior and posterior, and placing compressive forces on the retrodiscal tissues.18 Similarly, forward head posture can contribute to reduced cervical spine extension, as the mid/lower cervical segments assume a more flexed position. The lack of lower cervical mobility might contribute to stiffness at the cervicothoracic (CT) junction. Being unable to extend through the CT region can make it more challenging to return from a forward head posture, which may place increased stress through the TMJ and adjacent structures. Therefore, it may be appropriate for clinicians to not only examine and treat the local TMJ pathology but also address the CT region.
Manual therapy, including thrust and non-thrust mobilization, has been shown to have mechanical effects associated with improved joint mobility in addition to neurophysiological effects associated with decreased pain.19–23 In a recent case report, both thrust and non-thrust mobilization, along with exercise directed at the CT spine, was associated with positive outcomes on a patient with whiplash-associated allodynia.24 Another randomized controlled trial showed positive effects of the combination of manual therapy and exercise directed at the cervical spine on pressure pain sensitivity in patients with TMD.25 Recently, thrust manipulation of the atlanto-occipital and thoracic spine was instrumental, as a component of a larger treatment plan, in the management of patients with TMD.26,27 These studies suggest that manual therapy and exercise directed at the CT spine and adjacent segments may have a role in the rehabilitation of individuals with head, neck, and TMJ pain.
Thrust manipulation has been shown to be a useful intervention not only for local effects but also at remote sites.28–33 For example, previous studies have shown that thrust manipulation directed at the thoracic spine can have positive effects on patients with neck pain,28–30 whereas thrust manipulation to the lumbosacral spine has been associated with potential effects on quadriceps function.31–33 Similarly, another study showed that thrust manipulation of the CT region has been associated with positive improvements in individuals with shoulder pain.34 In light of the evidence for distal influences of thrust manipulation, the authors believed that manipulation of the CT junction might have both biomechanical and neurophysiological regional effects on the cervical spine and TMJ. The purpose of this report is to not only describe the successful physical therapy management of a patient with TMD using CT junction thrust manipulation, education, and exercise but also emphasize the importance of assessing the CT junction in individuals with TMD.
Patient Characteristics
The patient was a 46-year-old female presenting to a university outpatient physical therapy clinic with a medical diagnosis of left-sided facial pain for 6 months. The patient worked previously as both a mail carrier and certified nursing assistant, but was currently off work and on disability due to pain. Her medical history included a diagnosis of fibromyalgia and clinical depression that were being respectively treated with medication and psychiatric counseling. Her dental history was unremarkable. No previous treatment for her facial pain was reported. A history of chronic low back pain was also reported. This had responded well to physical therapy. The patient provided verbal consent to have her clinical data used for publication purposes, with all relevant identifying information removed.
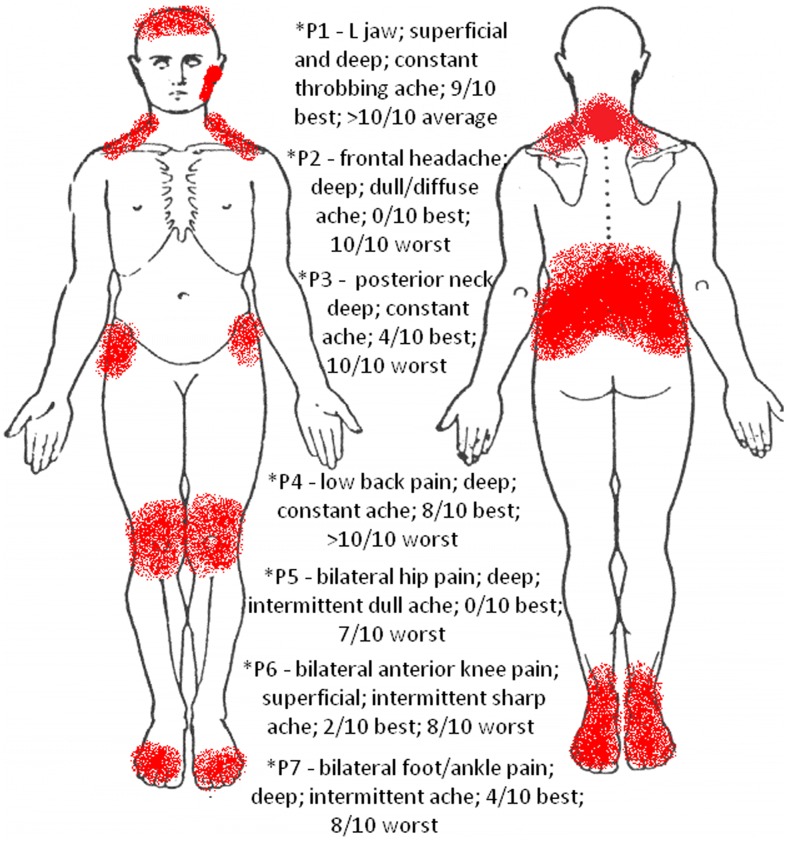
The patient reported experiencing severe facial, jaw, and neck pain with insidious onset, which she believed was due to her fibromyalgia, although she stated that the facial pain felt was different. Upon further questioning, she reported falling backward and hitting her head a year ago when she slipped on ice with resultant neck pain, but noted that her current pain was not the same. A number of painful regions were reported (Fig. 1); however, the patient's primary complaint of pain was a throbbing ache at the left TMJ radiating into her lateral mandible. On the numeric pain rating scale (NPRS), she stated that the pain at rest (and lowest) was 9/10, with intensity frequently greater than 10/10. Pain was aggravated while eating (regardless of texture), making facial expressions, and changes in weather. Rest, stretching, exercise, and medication each helped to alleviate the pain slightly. The patient was regularly taking Tramadol, Oxycodone, and Zoloft without recent changes in dosage. No other co-interventions were reported. Her neck pain, described as a sharp ache, was also reported at her lower cervical spine, and was aggravated by sustaining any posture more than 30 minutes or when her jaw pain would become intense. Infrequent bilateral headaches were also reported and would often be present when her jaw and neck pain were intense. She denied radicular pain and paresthesias in the upper extremities (UE). The patient also denied any diplopia, dysphagia, dysarthria, drop attacks, or dizziness, making the presence of cervical arterial dysfunction less likely.35 Owing to her high level of pain, the patient reported a significant decrease in her functional level and interest in social interactions with friends. The patient's primary goal was to decrease her facial pain to allow an improvement in function and quality of life.
Figure 1.
Body chart.
Examination
The patient demonstrated a number of impairments of her jaw, upper cervical spine, and CT region. She demonstrated forward head, rounded shoulders posture, and a mild left lateral flexion of the cervical spine. Upper extremity myotomes, dermatomes, and reflex assessment were normal. Cranial nerve examination as well as the Sharp-Purser and Alar ligament tests were all normal.
Cervical active range of motion (AROM) was limited by 25% upon visual observation into rotation and lateral flexion bilaterally without symptom production. Cervical extension was restricted by 50% with reports of tension and pain at the CT junction, which increased with overpressure. With active TMJ motion (measured using a ruler36), the patient demonstrated pain and limited maximal opening to 33 mm with a C-curve to the left; lateral excursion of 14 mm to the left, with pain; and lateral excursion of 8 mm to the right with a stretch. No clicks were identified upon palpation. Left-sided jaw pain was reproduced with overpressures into opening and left lateral excursion.
When assessing joint mobility as described by Maitland et al.,37 restriction was noted with a posterior–anterior (PA) force at C1-2 on the left (without headache reproduction), C5-T4 with central PA force (pain at C7 and T1), caudal distraction of the mandible, and opening mobilization of the left TMJ (where the mandibular condyle is mobilized caudally into an anterolateral position). On the left side, tenderness to palpation was noted at the TMJ line, masseter, temporalis, medial pterygoid, and suboccipitals. Tenderness was not present on the right side of the patient's face. Increased resting tension was present in the suboccipitals, left sternocleidomastoid, masseter, and pterygoids. Additional impairments included restricted pectoralis minor length on the left greater than right and 3+/5 strength of the middle and lower trapezius bilaterally, as measured by manual muscle testing. Poor recruitment of the deep neck flexors (DNF) was noted by an inability to perform a cervical spine chin tuck without compensatory sternocleidomastoid recruitment. A shoulder screen was negative for reproduction of symptoms.
Outcome measures that were used included the NPRS, pressure pain thresholds (PPTs), tampa scale for kinesiophobia (TSK), neck disability index (NDI), jaw symptom and oral habit questionnaire (JSOHQ), and global rating of change (GROC) scale. The NPRS is a self-reported pain scale with a minimal clinically important difference (MCID) of 1·2.38 Pressure pain threshold testing has been used as a measure of how much pressure an individual tolerates before experiencing pain. In individuals with pain sensitization or hyperalgesia, PPT readings tend to be lower, indicating that a smaller pressure stimulus induces a pain response.39 The TSK is a self-reported outcome measure describing an individual's fear of movement.40 Although originally developed for individuals with low back pain, the TSK has been shown to have psychometric value for individuals with neck pain.41 The NDI has been reliably used to describe the level of disability for individuals with neck pain, with a MCID of 19-percentage points.38 The JSOHQ is an outcome measure with pain and function subscales that has been validated for use in screening individuals with TMD when compared to asymptomatic controls.42 The GROC provides a measure of self-perceived change over time with a minimal important difference of 3 scale points.43
Clinical Impression
Based on the presenting signs and symptoms, it appeared that the patient's primary complaint of jaw and facial pain was due to capsular restrictions of the left TMJ. The patient exhibited increased pain with mastication and facial expressions, hyperalgesia, increased resting tension of the mastication muscles on the left, a C-curve to the left with opening, decreased jaw opening, and hypomobility of the left TMJ. A C-curve has been associated with capsular dysfunction5 as the tight capsule will pull the mandible to the affected side during opening and will limit lateral excursion to the contralateral side. No clicking or popping with TMJ AROM was noted, making a unilateral disk dysfunction less likely. Although impairments were found during the cervical spine examination, that assessment did not reproduce the patient's primary complaint of jaw and facial pain, making it less likely to be the primary cause of her symptoms. A negative neurologic screen increased the likelihood of a mechanical cause for her symptoms.
As a result of the working diagnosis of TMD, expected interventions included mobilization of the left TMJ, soft tissue mobilization of the mastication muscles, postural education, and DNF stabilization, to facilitate improved joint mobility and function. Given the patient's forward head, hypomobility and pain with joint mobility testing at the CT junction, and neck pain, the primary author believed that addressing the CT region would be beneficial in reducing compressive load at the TMJ. Considering the patient's local hyperalgesia and symptom irritability, the decision was made to treat remote sites initially, which might allow for increased tolerance to treatment.
Intervention
At initial evaluation, the primary intervention was patient education, which has been shown to be beneficial for patients with TMD.44,45 The patient was given instruction on the anatomy, pathology, pain science, and posture related to TMDs. The patient also received instruction on cervical and scapular retraction exercises, as well as an exercise for controlled jaw opening. The controlled jaw opening exercise emphasizes equal opening, without compensatory mandibular deviation to the involved side, facilitating proper motor patterns. The latter involves slowly opening the mouth while the tongue maintains contact with the roof of the mouth. These exercises were performed properly following instruction and were to be performed in a pain-free ROM, at least 10 times each day to facilitate improved postural awareness.
Visit 2 (Week 2)
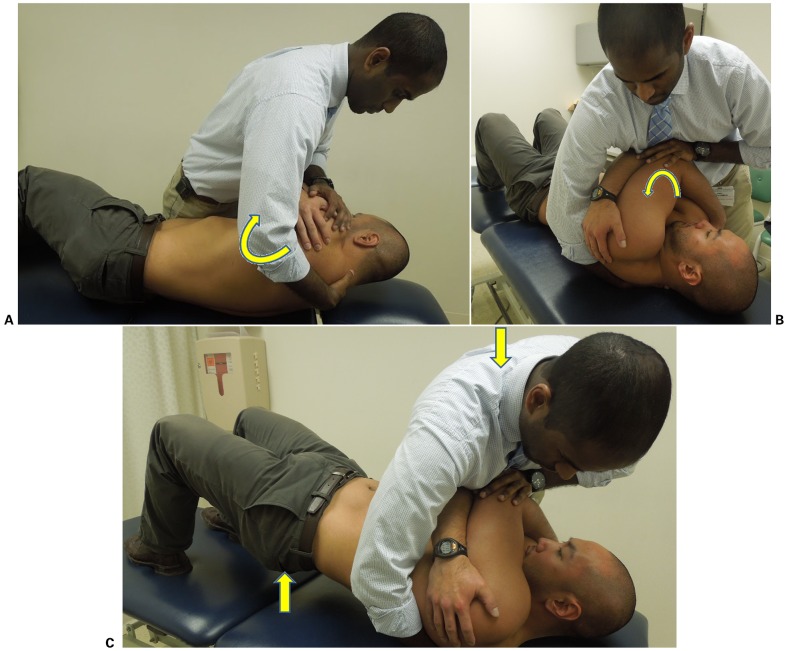
At the first follow-up visit, the patient reported mild improvement; however, the pain was unchanged. Following brief re-assessment, the therapist believed that addressing the patient's CT junction impairments would allow for improved cervical mobility, in turn decreasing excessive stress through the TMJ. Cervicothoracic thrust manipulation (Fig. 2) was performed at the onset of treatment, with PPT readings taken immediately before and after the manipulation. The masseter was used for PPT readings, as this was the most significant location of pain upon palpation. Pressure pain threshold readings were taken using a pressure algometer, as previously described by Goulet et al.,46 with the average of three trials recorded. Subjective reports of improved neck pain and mobility were reported, yet immediate PPT readings decreased. It is possible that this decrease was associated with the patient clenching her jaw during the procedure despite education regarding the application of the manipulation. Maximal opening of the jaw was unchanged but cervical AROM had improved into extension without pain. Following CT junction thrust manipulation and PPT readings, mandibular caudal distraction and intraoral opening mobilizations of the left TMJ were performed, followed by the exercises discussed at initial evaluation, with improvements noted in opening ROM afterward.
Figure 2.
(A) Initial patient position; the therapist rolls the patient toward him (arrow), palpates for the spinous processes of C7 and T1, and places his hand in an open palm lumbrical grip such that the spinous process of C7 rests between the thenar eminence and metacarpal heads. (B) The patient is rolled back into supine on top of the therapist's hand (arrow), which slides slightly inferiorly toward T1 to obtain a skin lock. (C) The patient is instructed to bridge his hips up (arrow). As the therapist feels the CT junction lock out, a high-velocity, low-amplitude thrust manipulation is performed with a line of force perpendicular to the treatment table (arrow). Source: Author.
Visits 3–5 (Weeks 3–6)
The patient returned stating that her neck felt better following the thrust manipulation at the previous visit, not only immediately after the session but for the rest of the day. Given that subjective report of improvement and persistent hypomobility at the CT junction, a CT junction thrust manipulation was performed at the onset of subsequent visits. Pressure pain threshold readings were measured before and after the thrust technique. Despite a decrease in PPT values following the first session, PPT values consistently improved at subsequent visits (Table 1). Joint mobilization of the left TMJ was performed after CT junction manipulation, followed by the previously described exercises in attempts to control the new ROM. In this patient, as well as the general population with TMD, the DNFs have been shown to be impaired.47 Therefore, DNF recruitment exercises were introduced to improve postural stability and decrease neck pain. Proper form without sternocleidomastoid compensation was noted, and this was added to the patient's home exercise program HEP.
Table 1.
Pressure pain threshold (PPT) readings taken at the center at the masseter (kilopascals)
| Visit | Pre-manipulation | Post-manipulation | ||
|---|---|---|---|---|
| Right | Left | Right | Left | |
| 1 (Evaluation) | NT | NT | NT | NT |
| 2 | 40·2 | 29·4 | 19·6 | 16·7 |
| 3 | 33·3 | 28·4 | 38·2 | 32·4 |
| 4 | 39·2 | 31·4 | 46·1 | 40·2 |
| 5 | 52·0 | 49·0 | 59·8 | 57·9 |
| 6 | 69·6 | 58·8 | 78·5 | 72·6 |
| 7 | 84·3 | 79·4 | 88·3 | 88·3 |
NT: not tested.
Visits 6 and 7 (Weeks 7 and 8)
Based on the patient's continued subjective reports of improved pain and function, improving cervical and TMJ mobility, and increasing PPT values, the remaining patient visits included a CT junction thrust manipulation. These were followed by intraoral TMJ mobilization and exercise. However, due to complaints of tenderness of the left masseter to palpation, external soft tissue mobilization of the masseter was added. Deep neck flexor recruitment was progressed to the more functional positions of sitting and standing. The patient was satisfied with progress, achieved all goals, and was discharged with a HEP for self-management of symptoms.
Outcomes
The patient was seen in physical therapy for a total of seven visits over eight weeks, including the initial evaluation, with positive functional and impairment-based outcomes noted (Table 2). Although pain intensity remained high, the MCID was met for the NDI, NPRS, and the GROC scales, indicating significant improvement. Pressure pain threshold readings more than doubled at the left masseter improving from 29·4 to 79·4 kPa and the right masseter improving from 40·2 to 84·3 kPa. Although a direct correlation cannot be made, the intrarater minimal detectable change (MDC) for PPT readings at the upper trapezius was found to be 47·2 kPa, which was achieved on the patient's symptomatic left side.48 Positive changes were also noted based on the TSK and JSOHQ scores indicating self-reported functional improvement. However, MCID values for these measures were not found through a literature search, making it difficult to determine if these improvements were statistically or clinically meaningful. Specifically on the JSOHQ, the patient's responses to questions about pain when opening wide or yawning, pain when chewing, pain when not chewing, and jaw muscle pain improved from ‘hurts a lot’ to ‘doesn't hurt at all’. Actively, the patient demonstrated maximal jaw opening of 43 mm (satisfying the MDC of 6–9 mm change),49 and no C-curve was noted indicating improved joint mobility and muscular balance. The patient was subsequently seen in physical therapy for her low back pain allowing for follow up, and improvements were maintained at two months following discharge (Table 2).
Table 2.
Outcomes
| Scoring | Evaluation | 4 weeks | Discharge | 2-month follow up | |
|---|---|---|---|---|---|
| NDI | 10 items scored 0–5; higher score indicates greater disability; MCID: 19% | 34/50 | 28/50 | 18/50* | 16/50* |
| JSOHQ total | 13 items scored 0–4; higher scores indicate greater dysfunction; MCID: NA | 19/52 | 10/52 | 7/52 | 7/52 |
| Pain subscale | 15/32 | 8/32 | 5/32 | 5/32 | |
| Function subscale | 4/20 | 2/20 | 2/20 | 2/20 | |
| TSK | 17 items scored 1–4; higher scores indicate greater fear of movement; MCID: NA | 44/68 | 30/68 | 24/68 | 23/68 |
| NPRS | 0–10 scale; 0 is no pain, 10 is worst pain imaginable; MCID: 1·2 | 9/10 | 7/10* | 6/10* | 5/10* |
| PPT (kPa)** | Higher values indicate greater pressure tolerance before pain sensation; MDC: 47·2*** | ||||
| Left | 29·4 | 31·4 | 79·4* | 107·9* | |
| Right | 40·2 | 39·2 | 84·3 | 110·8* | |
| GROC | −7 to +7 Likert scale, +7 indicates a great deal better; MCID: 3 | NT | +3* | +5* | +5* |
| Maximal opening (mm) | Functional ROM 35 mm; normal for females 45–55 mm; MDC: 6–9 | 33 | 40 | 43* | 45* |
NDI: neck disability index; JSOHQ: jaw symptom and oral health scale; TSK: tampa scale of kinesiophobia; NPRS: numeric pain rating scale; PPT: pressure pain threshold; GROC: global rating of change; MCID: minimal clinically important difference; MDC: minimal detectable change; ROM: range of motion; NT: not tested; NA: not available.
*Met MCID or MDC for improvement.
**PPT readings were taking at visit 2 (first follow-up) and not the initial evaluation, and represent pre-manipulation values.
***Intrarater testing of the upper trapezius.
Discussion
Temporomandibular disorder can be a challenging condition to manage for clinicians. While research is continuing to emerge regarding effective interventions, questions still exist regarding the best practice. This may be partly attributable to the variable patient presentations and a number of associated cervical, thoracic, and psychosocial impairments that make specific recommendations for all patients inappropriate. In the case provided here, the successful multimodal management of an individual with TMD incorporating CT junction thrust manipulation is described.
Physical therapy management of TMD has often included some combination of manual therapy, exercise, and patient education.5,11–14,44,45 Recently, thrust and non-thrust mobilizations to the cervical and thoracic spine have been associated with positive effects in individuals with TMD.25–27 Furthermore, a number of studies have shown that thoracic and lumbosacral thrust manipulations have effects not only at the site of application but also at both proximal and distal sites.28–33,50 However, interventions directed specifically to the CT junction for TMD have not yet been described in the literature. Given previous evidence and the biomechanical link between a forward head posture and TMD, it seems appropriate to evaluate and treat the CT region in individuals with similar presentations to optimize functional outcomes.
Although the patient subjectively and objectively demonstrated clinically significant improvements in pain and self-reported function and was satisfied with her outcomes, some measures continued to indicate that she still had notable symptoms. For example, although PPT values more than doubled, when compared to normative values, readings remained dramatically low.51 Depression has been correlated with chronic pain and fear avoidance behaviors,52 and could have affected these values. Additionally, given the number of painful sites at intake, hyperalgesia, and chronicity of symptoms, it is likely that the patient had a component of central sensitization and altered pain perceptions.39,53 It is also likely that the patient's fibromyalgia played a role in her continued pain perceptions. However, despite continued reports of high pain intensity, the patient reported and maintained functional improvement. She reported being able to perform her activities of daily living and socialize with friends without increased pain, which had a positive impact on her quality of life. This highlights the potential of a weak correlation existing between pain and disability.
There are a number of limitations associated with this report. As this is a single case report, it is impossible to generalize results. For example, this patient was concurrently being treated for her clinical depression. While no significant changes were reported in psychological management during her course of care, this could have been a confounding variable when determining the effectiveness of physical therapy management. It is impossible to draw definitive conclusions as to the potential effects of the CT junction thrust manipulation from a case report. Although starting at the second follow-up visit PPT readings were consistently improved following manipulation, it is difficult to determine causal relationships. A number of manual therapy techniques were employed, in addition to CT junction thrust manipulation, and each could have individually or cumulatively created a treatment effect beyond the manipulation leading to the improvements. Future investigations should be more objective in determining pre-and post-manipulation findings beyond PPT readings preferably with fewer variables. Additionally, no objective measure was used to provide information on the potential impact of fibromyalgia on the patient's pain. Given the patient's apparent altered pain perceptions and centrally mediated pain, objectifying the impact of fibromyalgia could have provided an enhanced treatment approach.
The importance of this case report is that it suggests that the CT junction should be considered in the evaluation of patients with TMD. In this case, CT junction thrust manipulation appeared to be a safe and effective intervention in the multimodal physical therapy management of an individual with TMD. It should be noted that, at each subsequent visit, CT thrust manipulation was indicated by subjective reports, joint mobility, and AROM findings. The authors do not support blindly manipulating a vertebral segment because of its potential relationship with improved PPT values. However, it is the authors' hope that this case report will encourage further research into the management of individuals with TMD incorporating CT junction thrust manipulation.
Disclaimer Statements
Contributors This case report describes the novel approach of physical therapy services for an individual with TMD. The care was provided solely by the primary author (DJJ) who evaluated and treated the patient as part of a Orthopedic Fellowship program. The secondary author (NST) is the primary author's clinical mentor and was integral in the development of treatment interventions and clinical reasoning process throughout the course of care. Both DJJ and NST were involved actively in the preparation of the manuscript being submitted.
Funding No funding was obtained for this case report.
Conflicts of interest The authors have no conflicts of interest to report.
Ethics approval The University of Illinois at Chicago's Institutional Review Board was contacted regarding whether IRB approval was required. It was determined that that this case report was exempt from IRB approval, as it is a single case report utilizing typical clinical practice and all necessary identifying information was removed.
References
- 1.Anastassaki A, Magnusson T. Patients referred to a specialist clinic because of suspected temporomandibular disorders: a survey of 3194 patients in respect of diagnoses, treatments, and treatment outcome. Acta Odontol Scand. 2004;62(4):92–183. [DOI] [PubMed] [Google Scholar]
- 2.Gremillion HA. The prevalence and etiology of temporomandibular disorders and orofacial pain. Tex Dent J. 2000;117(7):30–9. [PubMed] [Google Scholar]
- 3.Armijo-Olivo S, Magee DJ, Parfitt M, Major P, Thie NM. The association between the cervical spine, the stomatognathic system, and craniofacial pain: a critical review. J Orofac Pain. 2006;20(4):271–87. [PubMed] [Google Scholar]
- 4.Placzek JD, Boyce DA, editors. Orthopedic physical therapy secrets, 2nd edn. St Louis, MO: Mosby Elsevier; 2006. [Google Scholar]
- 5.Ho S. The temporomandibular joint: physical therapy management utilizing current evidence. Current Concepts of Orthopaedic Physical Therapy; La Crosse, WI; 2011. [Google Scholar]
- 6.Pullinger AG, Monteiro AA. History factors associated with symptoms of temporomandibular disorders. J Oral Rehabil. 1988;15(2):117–24. [DOI] [PubMed] [Google Scholar]
- 7.Greene CS; American Association for Dental Research Diagnosis and treatment of temporomandibular disorders: emergence of a new care guidelines statement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(2):137–9. [DOI] [PubMed] [Google Scholar]
- 8.Baba K, Tsukiyama Y, Yamazaki M, Clark GT. A review of temporomandibular disorder diagnostic techniques. J Prosthet Dent. 2001;86(2):184–94. [DOI] [PubMed] [Google Scholar]
- 9.Dimitroulis G. Temporomandibular disorders: a clinical update. BMJ. 1998;317(7152):190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.List T, Axelsson S. Management of TMD: evidence from systematic reviews and meta-analyses. J Oral Rehabil. 2010;37(6):430–51. [DOI] [PubMed] [Google Scholar]
- 11.Furto ES, Cleland JA, Whitman JM, Olson KA. Manual physical therapy interventions and exercise for patients with temporomandibular disorders. Cranio. 2006;24(4):283–91. [DOI] [PubMed] [Google Scholar]
- 12.Medlicott MS, Harris SR. A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Phys Ther. 2006;86(7):955–73. [PubMed] [Google Scholar]
- 13.McNeely ML, Armijo-Olivo S, Magee DJ. A systematic review of the effectiveness of physical therapy interventions for temporomandibular disorders. Phys Ther. 2006;86(5):710–25. [PubMed] [Google Scholar]
- 14.Tuncer AB, Ergun N, Tuncer AH, Karahan S. Effectiveness of manual therapy and home physical therapy in patients with temporomandibular disorders: a randomized controlled trial. J Bodyw Mov Ther. 2013;17(3):302–8. [DOI] [PubMed] [Google Scholar]
- 15.Armijo-Olivo S, Magee D. Cervical musculoskeletal impairments and temporomandibular disorders. J Oral Maxillofac Res. 2013;3(4):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivo SA, Fuentes J, Major PW, Warren S, Thie NM, Magee DJ. The association between neck disability and jaw disability. J Oral Rehabil. 2010;37(9):670–9. [DOI] [PubMed] [Google Scholar]
- 17.Bevilaqua-Grossi D, Chaves TC, de Oliveira AS. Cervical spine signs and symptoms: perpetuating rather than predisposing factors for temporomandibular disorders in women. J Appl Oral Sci. 2007;15(4):259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann DA. Kinesiology of the musculoskeletal system: foundations for physical rehabilitation. St. Louis, MI: Mosby; 2002. [Google Scholar]
- 19.Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002;2(5):357–71. [DOI] [PubMed] [Google Scholar]
- 20.Bialosky JE, Bishop MD, Robinson ME, Zeppieri G Jr, George SZ. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther. 2009;89(12):1292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maigne JY, Vautravers P. Mechanism of action of spinal manipulative therapy. Joint Bone Spine. 2003;70(5):336–41. [DOI] [PubMed] [Google Scholar]
- 22.Bialosky JE, Simon CB, Bishop MD, George SZ. Basis for spinal manipulative therapy: a physical therapist perspective. J Electromyogr Kinesiol. 2012;22(5):643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaltenborn FM. Manual mobilization of the joints: the Kaltenborn method of joint examination and treatment; the extremities. I. Oslo, Norway: Olaf Noris Bokhandel Universitetsgaten; 1999. [Google Scholar]
- 24.Lowry CD, O'Hearn MA, Courtney CA. Resolution of whiplash-associated allodynia following cervicothoracic thrust and non-thrust manipulation. Physiother Theory Pract. 2011;27(6):451–9. [DOI] [PubMed] [Google Scholar]
- 25.La Touche R, Fernandez-de-las-Penas C, Fernandez-Carnero J, Escalante K, Angulo-Diaz-Parreno S, Paris-Alemany A, et al. . The effects of manual therapy and exercise directed at the cervical spine on pain and pressure pain sensitivity in patients with myofascial temporomandibular disorders. J Oral Rehabil. 2009;36(9):644–52. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira-Campelo NM, Rubens-Rebelatto J, Marti N-Vallejo FJ, Alburquerque-Sendi NF, Fernandez-de-Las-Penas C. The immediate effects of atlanto-occipital joint manipulation and suboccipital muscle inhibition technique on active mouth opening and pressure pain sensitivity over latent myofascial trigger points in the masticatory muscles. J Orthop Sports Phys Ther. 2010;40(5):310–7. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Iglesias J, Cleland JA, Neto F, Hall T, Fernandez-de-Las-Penas C. Mobilization with movement, thoracic spine manipulation, and dry needling for the management of temporomandibular disorder: a prospective case series. Physiother Theory Pract. 2013;29(8):586–95. [DOI] [PubMed] [Google Scholar]
- 28.Cleland JA, Childs JD, McRae M, Palmer JA, Stowell T. Immediate effects of thoracic manipulation in patients with neck pain: a randomized clinical trial. Man Ther. 2005;10(2):127–35. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Iglesias J, Fernandez-de-las-Penas C, Cleland JA, Gutierrez-Vega Mdel R. Thoracic spine manipulation for the management of patients with neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2009;39(1):20–7. [DOI] [PubMed] [Google Scholar]
- 30.Cross KM, Kuenze C, Grindstaff TL, Hertel J. Thoracic spine thrust manipulation improves pain, range of motion, and self-reported function in patients with mechanical neck pain: a systematic review. J Orthop Sports Phys Ther. 2011;41(9):633–42. [DOI] [PubMed] [Google Scholar]
- 31.Suter E, McMorland G, Herzog W, Bray R. Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain. J Manipulative Physiol Ther. 1999;22(3):149–53. [DOI] [PubMed] [Google Scholar]
- 32.Hillermann B, Gomes AN, Korporaal C, Jackson D. A pilot study comparing the effects of spinal manipulative therapy with those of extra-spinal manipulative therapy on quadriceps muscle strength. J Manipulative Physiol Ther. 2006;29(2):145–9. [DOI] [PubMed] [Google Scholar]
- 33.Grindstaff TL, Hertel J, Beazell JR, Magrum EM, Ingersoll CD. Effects of lumbopelvic joint manipulation on quadriceps activation and strength in healthy individuals. Man Ther. 2009;14(4):415–20. [DOI] [PubMed] [Google Scholar]
- 34.Mintken PE, Cleland JA, Carpenter KJ, Bieniek ML, Keirns M, Whitman JM. Some factors predict successful short-term outcomes in individuals with shoulder pain receiving cervicothoracic manipulation: a single-arm trial. Phys Ther. 2010;90(1):26–42. [DOI] [PubMed] [Google Scholar]
- 35.Kerry R, Taylor AJ. Cervical arterial dysfunction assessment and manual therapy. Man Ther. 2006;11(4):243–53. [DOI] [PubMed] [Google Scholar]
- 36.Norkin CC, White DJ. Measurement of joint motion: a guide to goniometry, 3rd edn. Philadelphia, PA: F.A. Davis Company; 2003. [Google Scholar]
- 37.Maitland G, Hengeveld E, Banks K, English K, editors. Maitland's vertebral manipulation, 7th edn. Edinburgh: Elsevier; 2005. [Google Scholar]
- 38.Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil. 2008;89(1):69–74. [DOI] [PubMed] [Google Scholar]
- 39.Courtney CA, Kavchak AE, Lowry CD, O'Hearn MA. Interpreting joint pain: quantitative sensory testing in musculoskeletal management. J Orthop Sports Phys Ther. 2010;40(12):818–25. [DOI] [PubMed] [Google Scholar]
- 40.Kori SH, Miller RP, Todd DD. Kinesiophobia: a new view of chronic pain behavior. Pain Manag. 1990;3:35–43. [Google Scholar]
- 41.Hudes K. The Tampa Scale of Kinesiophobia and neck pain, disability and range of motion: a narrative review of the literature. J Can Chiropr Assoc. 2011;55(3):222–32. [PMC free article] [PubMed] [Google Scholar]
- 42.Gerstner GE, Clark GT, Goulet JP. Validity of a brief questionnaire in screening asymptomatic subjects from subjects with tension-type headaches or temporomandibular disorders. Community Dent Oral Epidemiol. 1994;22(4):235–42. [DOI] [PubMed] [Google Scholar]
- 43.Wang YC, Hart DL, Stratford PW, Mioduski JE. Baseline dependency of minimal clinically important improvement. Phys Ther. 2011;91(5):675–88. [DOI] [PubMed] [Google Scholar]
- 44.Michelotti A, de Wijer A, Steenks M, Farella M. Home-exercise regimes for the management of non-specific temporomandibular disorders. J Oral Rehabil. 2005;32(11):779–85. [DOI] [PubMed] [Google Scholar]
- 45.De Laat A, Stappaerts K, Papy S. Counseling and physical therapy as treatment for myofascial pain of the masticatory system. J Orofac Pain. 2003;17(1):42–9. [PubMed] [Google Scholar]
- 46.Goulet JP, Clark GT, Flack VF, Liu C. The reproducibility of muscle and joint tenderness detection methods and maximum mandibular movement measurement for the temporomandibular system. J Orofac Pain. 1998;12(1):17–26. [PubMed] [Google Scholar]
- 47.Armijo-Olivo S, Fuentes JP, da Costa BR, Major PW, Warren S, Thie NM, et al. . Reduced endurance of the cervical flexor muscles in patients with concurrent temporomandibular disorders and neck disability. Man Ther. 2010;15(6):586–92. [DOI] [PubMed] [Google Scholar]
- 48.Walton DM, Macdermid JC, Nielson W, Teasell RW, Chiasson M, Brown L. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J Orthop Sports Phys Ther. 2011;41(9):644–50. [DOI] [PubMed] [Google Scholar]
- 49.Kropmans T, Dijkstra P, Stegenga B, Stewart R, de Bont L. Smallest detectable difference of maximal mouth opening in patients with painfully restricted temporomandibular joint function. Eur J Oral Sci. 2000;108(1):9–13. [DOI] [PubMed] [Google Scholar]
- 50.Iverson CA, Sutlive TG, Crowell MS, Morrell RL, Perkins MW, Garber MB, et al. . Lumbopelvic manipulation for the treatment of patients with patellofemoral pain syndrome: development of a clinical prediction rule. J Orthop Sports Phys Ther. 2008;38(6):297–309. [DOI] [PubMed] [Google Scholar]
- 51.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, et al. . Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–43. [DOI] [PubMed] [Google Scholar]
- 52.Boersma K, Linton SJ. Psychological processes underlying the development of a chronic pain problem: a prospective study of the relationship between profiles of psychological variables in the fear-avoidance model and disability. Clin J Pain. 2006;22(2):160–6. [DOI] [PubMed] [Google Scholar]
- 53.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]