Abstract
Study design
Randomized clinical trial.
Objective
To investigate the effect of including manual therapy (MT) in a pulmonary rehabilitation program for patients with chronic obstructive pulmonary disease (COPD).
Background
The primary source of exercise limitation in people with COPD is dyspnea. The dyspnea is partly caused by changes in chest wall mechanics, with an increase in chest wall rigidity (CWR) contributing to a decrease in lung function. As MT is known to increase joint mobility, administering MT to people with COPD carries with it the potential to influence CWR and lung function.
Methods
Thirty-three participants with COPD, aged between 55 and 70 years (mean = 65·5±4 years), were randomly assigned to three groups: pulmonary rehabilitation (PR) only, soft tissue therapy (ST) and PR, and ST, spinal manipulative therapy (SM), and PR. Outcome measures including forced expiratory volume in the 1st second (FEV1), forced vital capacity (FVC), 6-minute walking test (6MWT), St. George's respiratory questionnaire (SGRQ), and the hospital anxiety and depression (HAD) scale were recorded at 0, 8, 16, and 24 weeks.
Results
There was a significant difference in FVC between the three groups at 24 weeks (P = 0·04). For the ST+SM+PR group versus PR only the increase was 0·40 l (CI: 0·02, 0·79; P = 0·03). No major or moderate adverse events (AE) were reported following the administration of 131 ST and 272 SM interventions.
Discussion
The increase in FVC is a unique finding. Although the underlying mechanisms responsible for this outcome are not yet understood, the most likely explanation is the synergistic effect resulting from the combination of interventions. These results support the call for a larger clinical trial in the use of MT for COPD.
Keywords: Manual therapy, Spinal manipulation, Pulmonary rehabilitation, Chronic obstructive pulmonary disease, COPD
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable, treatable disease with extra-pulmonary effects that contribute to the severity in individual patients.1 These extra-pulmonary effects include an increase in dyspnea and a loss of exercise capacity. Pulmonary rehabilitation (PR) has become a key component in managing these effects in people with moderate to severe COPD.2,3 Pulmonary rehabilitation is a multi-disciplinary approach that includes patient assessment, exercise training, health education, nutritional intervention, and psychosocial support.1–4 It reduces symptoms, disability, and hospitalizations and improves function.1 In addition to this, exercise training on its own has been shown to improve dyspnea and fatigue, increase exercise endurance, and improve health-related quality of life, emotional function, and the patient’s self-control over his/her condition.5
Regardless of the duration of the PR program, studies have failed to show any clinically significant changes in forced expiratory volume in the 1st second (FEV1) or forced vital capacity (FVC).6–9 This has been attributed to the fact that much of the morbidity from COPD results from secondary conditions such as cardiac de-conditioning, skeletal muscle dysfunction, and anxiety.10
Patients with COPD have a less active lifestyle compared to healthy people of a similar age.11 As increased exercise capacity has been linked to an overall increase in physical activity,6,12 interventions such as short-acting bronchodilators have been used to facilitate an increase in exercise capacity. This approach relies on delaying the onset of exercise limiting symptoms such as dyspnea thereby allowing the patient to gain greater benefit from each exercise session.13,14 Non-pharmacological interventions that delay the onset of dyspnea could be used to increase exercise capacity in a similar manner.
While the origin of exercise limitation in COPD is multi-factorial, the primary source of the limitation is dyspnea11,15 with the cause being attributed, in part, to changes in chest wall mechanics.16–18 Airway narrowing, impaired gas exchange, and a loss of lung elastic tissue result in airway wall collapse and lung hyperinflation.1 The hyperinflation produces a passive increase in chest wall rigidity (CWR). This process forces the respiratory muscles to operate at non-optimal lengths resulting in a shift in the resting balance between inspiratory and expiratory muscles.19 Over time there is a loss in respiratory muscle strength and an increase in the effort required for breathing leading to an increase in dyspnea.16,20–22 Alleviating CWR has therefore been suggested as a way to decrease the level of dyspnea.23
The extent of CWR is related to the nature of movements in the joints and muscles associated with the thoracic spine, sternum, and ribs. In COPD, the initial increase in CWR absorbs part of the available movement in these joints. As lung hyperinflation persists, changes occur in the thixotropic properties of the respiratory muscles.19 Thixotropy refers to the resistance of a muscle to imposed motion and describes the behavior of passive muscle to movement that is not length or velocity dependent.24 In the case of respiratory muscles, the passive response to stretching depends on their contractile length immediately before the stretch is applied.19 The distortion of the chest wall resulting from lung hyperinflation causes an increase in the resting tension of the inspiratory muscles which contributes to changes in end-expiratory lung volumes.25,26 These changes lead to a corresponding increase in CWR with some researchers suggesting that inflation and deflation of the respiratory system is due more to the history-dependent mechanical properties of the chest wall than to the properties of the lung.19
Techniques that indirectly reduce CWR such as the three step active cycle of breathing technique used to clear secretions from the lungs (breathing control followed by deep breathing exercises followed by forced expiratory technique or ‘huffing’), positive expiratory pressure (PEP) therapy or devices that combine PEP and an oscillatory vibration of the air within the airways (e.g. Flutter®) have been shown to reduce contractile respiratory muscle effort and improve dyspnea.1,16 These techniques, grouped under the heading ‘chest physiotherapy’,1,27 have not included techniques that reduce CWR through direct intervention to the chest wall.
Manual therapy (MT) has the potential to increase muscle length and joint mobility.28,29 This could be achieved by using joint-focused techniques such as mobilization and manipulation and/or soft tissue techniques such as massage and stretching.28 Applying MT to the chest wall has the potential to reverse some of the thixotropic changes in the respiratory muscles by reducing the extent of their contraction and alleviating chest wall joint restriction leading to a short term reduction in CWR. A more detailed explanation of this hypothesis has been described previously.30
There is some evidence to show that direct application of MT to the chest wall benefits patients with chronic respiratory disease. Soft tissue-focused techniques have been used with mixed success in the management of COPD31,32 and pneumonia in the elderly,33–35 while joint-focused techniques have been shown to benefit lung function and quality of life in patients with COPD.31,36–38 Two recent studies reported immediate improvements in lung function and exercise capacity following 4 weeks of MT intervention that included soft tissue and joint focused techniques plus exercise in people with moderate and severe COPD.39,40 Measuring the effect of combining these two types of MT with exercise raises the question: are both types of MT required in order to achieve the improvements in lung function and exercise capacity? Reports that some soft-tissue techniques produce a worsening in pulmonary function immediately post-intervention when applied on their own31,32 suggest that this form of MT may need to be administered in conjunction with joint-focused MT in order to produce improvements in lung function in people with COPD. Furthermore, if the improvements in lung function and exercise capacity were sustained could they affect ventilatory ability? To our knowledge, there have been no reports on the ongoing effects of combining either type of MT with exercise in people with COPD.
The aim of this trial was to investigate the medium term effects on lung function and exercise capacity of administering soft tissue and joint focused MT in conjunction with exercise to patients with moderate to severe COPD.
Methods
Eligibility for participation in this trial was dependent on a person being referred by a respiratory specialist to the PR unit at Sutherland Hospital, a medium-sized public hospital in Sydney, Australia. Inclusion criteria included age between 55 and 70 years at the time of enrollment, diagnosis of COPD, no contra-indications to MT including a bone density T score ≧−2·5 and Z score ≧−1, being a non-smoker for at least the preceding 12 months and ability to complete a 6-minute walking test (6MWT), which was used as a measure of exercise capacity.
A total of 45 participants were planned for this study. This was based on the number of people referred to this facility for PR annually and was set at one-third of the average of the preceding 2 years’ totals. This was a pragmatic decision that factored in the workload of the hospital.
Allocation to an intervention group was randomized and concealed from both participants and researchers. Each participant randomly selected 1 of 45 sealed, opaque envelopes with one of the three group numbers written inside and was assigned to a group according to that number. Block randomization was not used. Group 1 (PR) received the standard pulmonary rehabilitation program prescribed at Sutherland Hospital; group 2 (ST+PR) received soft tissue therapy described below, plus the same PR program; and group 3 (ST+SM+PR) received the same soft tissue therapy plus spinal manipulation described below, plus the same PR program.
Pulmonary rehabilitation consisted of a 24-week program made up of intervention and non-intervention phases. The intervention phase consisted of two stages: an 8-week ‘Introductory’ stage, where participants were assessed for exercise capacity and introduced to health education and exercise training, followed by an 8-week ‘Maintenance’ stage, where exercise intensity was gradually increased to a level that was considered suitable for that participant. The non-intervention phase followed completion of the ‘Maintenance’ stage and involved an 8-week period of no PR intervention. Participants were directed to continue exercising at their own discretion during this period. There were four assessment points during the 24 weeks: an initial assessment (week 0), at the end of the ‘Introductory’ stage (week 8), at the end of the ‘Maintenance’ stage (week 16) and at the end of the non-intervention phase (week 24).
The MT administered in this trial followed a manual therapy protocol (MTP) that has been reported in earlier studies involving participants with and without chronic respiratory disease.39,41 Each MT intervention session lasted 20 minutes and was administered in addition and just before the exercise component of a PR session. Soft tissue therapy (ST) consisted of gentle Effleurage, friction, and cross-fiber friction massage applied to the muscles of the posterior chest wall including the intercostal, serratus posterior and anterior, rhomboid, trapezius, latissimus dorsi, erector spinae, quadratus lumborum, and levator scapulae muscles. Spinal manipulation (SM) involved the graded delivery of high velocity low amplitude (HVLA) joint manipulation to the thoracic inter-vertebral, costo-vertebral, and costo-transverse joints (Fig. 1). Spinal manipulation was restricted to this region to maximize the effect on CWR. All HVLA manipulations were administered as non-specific, multi-joint (group) manipulations. This form of manipulation was chosen as it reduced the total number of manipulations required to manage the chest wall within a single intervention session as each manipulation was capable of affecting several spinal segments and their associated ribs simultaneously.
Figure 1.
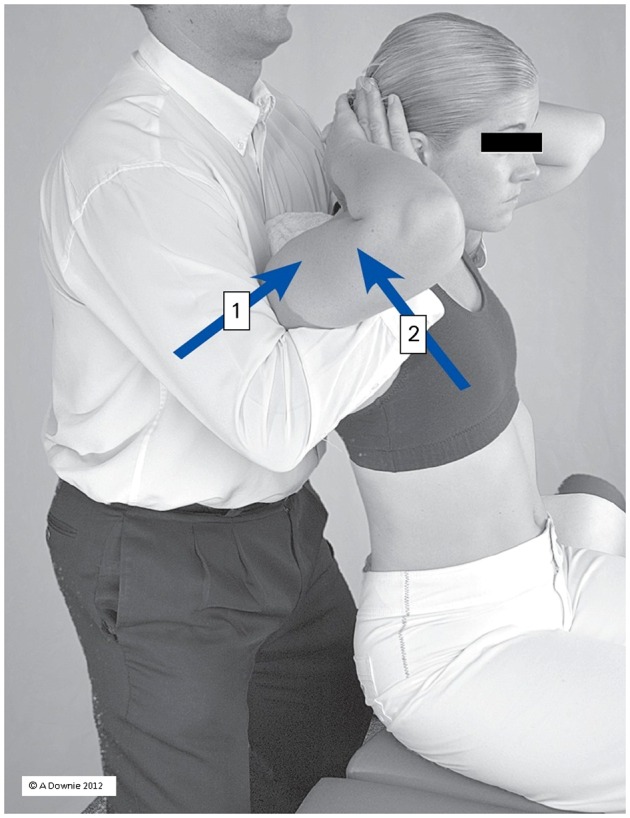
Spinal and rib manipulation (SM): High velocity low amplitude (HVLA) multi-joint (group) manipulation administered simultaneously to the thoracic spine and ribs (upper/middle shown). Box 1: posterior-anterior force; Box 2: anterior-posterior force.
In total, MT intervention was administered twice a week for 8 weeks from the middle of the ‘Introductory’ stage to the middle of the ‘Maintenance’ stage, i.e. from weeks 4 to 12 of PR. Delaying application of MT until week 4 permitted participants time to acclimatize to the exercise component of PR before having the second intervention applied.
Adverse events (AE) associated with MT have been classified as ‘mild’, ‘moderate’, and ‘severe’.42,43 A ‘mild’ AE is defined as being short-term and non-serious with the patient’s function remaining intact; a ‘moderate’ AE is medium to long term of moderate intensity; while a ‘severe’ AE is medium to long term, unacceptable and usually requiring further treatment. The presence of an AE was recorded on a session by session basis. At the beginning of each MT intervention session each participant in the ST+PR and ST+SM+PR groups was asked to complete a simple checklist that included a list of symptoms from all three categories of AEs. These were presented on a scale ranging from muscle soreness lasting less than 48 hours (mild) to symptoms that persisted beyond this period (moderate) to symptoms that required further medical attention and/or hospitalization (severe).
The trial was designed so that it could be easily integrated into the existing PR program at Sutherland Hospital. The outcome measurements used in the trial were the same as the ones used in the hospital’s existing PR program and included systolic and diastolic blood pressure, FEV1, FVC,44,45 the St. George’s Respiratory Questionnaire (SGRQ),46–48 Hospital Anxiety and Depression (HAD) scale49,50 and the 6MWT.51,52 The SGRQ is designed to measure health impairment in patients with respiratory disease. A fall in the SGRQ score represents a decrease in health impairment or an increase in quality of life. The HAD scale is a measure of the level of anxiety (HAD anx) and depression (HAD dep). A total score of between 0 and 7 on either scale represents the normal range. A score of 11 or higher indicates the presence of the relevant mood disorder.
All assessors were blinded to the participants’ intervention group and all participants were blinded to the results of their outcome measures during the trial. A single practitioner (RE), with over 27 years of experience in the application of MT, administered all SM and ST. For obvious reasons, RE could not be blinded to a participant’s group. He was, however, blinded to the results from all outcome measures until the end of the intervention phase of the trial. Participants who received SM and/or ST, while aware they were receiving MT, were not made aware of their PR assessment results until the end of the trial.
Participants in the ST and SM groups attended 16 MT sessions over an 8-week period at the rate of two sessions per week. This schedule was chosen for pragmatic reasons as it coincided with the hospital’s routine PR scheduling, but was also similar to rates used in other trials that involved the application of MT to people with COPD.37,39 Lung function measurements were taken using a Vitalograph 6000 spirometer (Ennis, Ireland) and in accordance with the guidelines set down by the Australian Lung Foundation and the Australian Physiotherapy Association.1,53 All 6MWTs were conducted using the same guidelines. Over the 16 intervention sessions, each participant in the ST and SM groups received 16 ST interventions with participants in the SM group also receiving 32 HVLA manipulations, at the rate of two per session directed at the upper/middle and middle/lower thoracic spine and ribs.
The study was approved by the Human Ethics committees of Macquarie University (HE23MAR2007-D05054) and the South Eastern Sydney and Illawarra Area Health Service (07/41) and registered with the Australian New Zealand Clinical Trials Registry (ACTRN:012607000388415). All participants gave informed consent to participate in the trial.
Statistical analysis was performed as an ANCOVA for difference between all three groups with baseline as a covariate and standard errors calculated using a non-parametric bootstrap to allow for the different error variances for each intervention group. A P value of <0·05 was set for statistical significance. For individual comparisons, a between groups Bonferroni correction was applied to correct for multiple comparisons between the three groups with adjusted P values shown. For outcomes found to be statistically significant the proportion of participants with a change greater than the minimum clinically important difference (MCID) was calculated. The number needed to treat (NNT) was calculated using Bender’s method for confidence intervals. Missing data were accounted for by using an intention-to-treat (ITT) analysis with data from subjects lost to follow-up imputed using the last observation carried forward (LOCF) method. Statistical analysis was performed using Stata Release 11 (StataCorp., 2009).54
Results
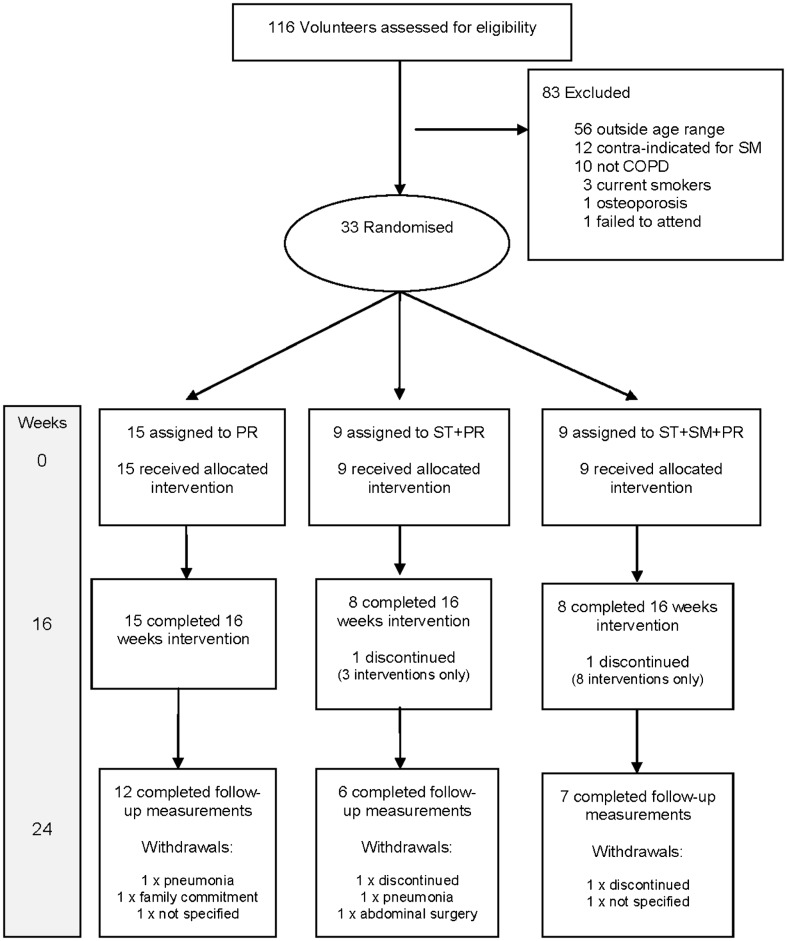
Figure 2 shows the participant flow through this trial. All participants were white Caucasian. The two participants who discontinued before completing the trial did so for reasons unrelated to the trial.
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) participant flow diagram.
Baseline characteristics for each group are listed in Table 1. Groups were similar at baseline for all characteristics except gender (P = 0·02) and anxiety (HAD anx; P = 0·02). The mean age of the participants across all groups was 65·5±4 years.
Table 1.
Baseline characteristics by group*
| Group 1 PR n = 15 | Group 2 ST+PR n = 9 | Group 3 ST+SM+PR n = 9 | All groups N = 33 | |
|---|---|---|---|---|
| Gender (F:M)† | 14:1 | 5:4 | 4:5 | 23:10 |
| Age (years) | 64·5 (4·1) | 67·6 (3·5) | 65·0 (4·1) | 65·5 (4·0) |
| Systolic BP | 132·1 (14·8) | 134·4 (16·1) | 137·9 (15·0) | 134·3 (14·9) |
| Diastolic BP | 76·9 (11·6) | 77·7 (11·3) | 79·7 (11·1) | 77·8 (11·1) |
| FEV1 (l) | 1·54 (0·57) | 1·57 (0·47) | 1·64 (0·30) | 1·57 (0·47) |
| FVC (l) | 2·14 (0·70) | 2·40 (0·83) | 2·28 (0·43) | 2·25 (0·67) |
| SGRQ | 44·7 (15·4) | 49·3 (20·3) | 34·7 (17·2) | 43·3 (17·7) |
| HAD anx† | 7·3 (3·8) | 8·4 (3·5) | 3·5 (3·2) | 6·6 (4·0) |
| HAD dep | 4·6 (2·4) | 6·8 (4·4) | 4·7 (1·7) | 5·2 (3·0) |
| 6MWT (m) | 444 (105) | 480 (77) | 534 (85) | 479 (97) |
All values except for gender are given as means with standard deviation in parentheses.
Different at baseline (P = 0·02).
BP: blood pressure; FEV1: forced expiratory volume in 1st second; FVC: forced vital capacity; HAD anx: hospital anxiety and depression scale – anxiety score; HAD dep: hospital anxiety and depression scale – depression score; PR: pulmonary rehabilitation; SGRQ: St George’s respiratory questionnaire; SM: spinal manipulation; ST: soft tissue therapy; 6MWT: 6-minute walking test.
Table 2 shows an ITT analysis of the change in outcome measures compared to baseline for each group at 16 and 24 weeks. Table 3 shows an ITT analysis of the difference in change in outcome measures between groups at 16 and 24 weeks fitted as an ANCOVA with baseline as a covariate and standard errors calculated using a non-parametric bootstrap. The major significant finding was a difference between groups for FVC at 24 weeks (P = 0·04). For participants in the ST+SM+PR group compared to PR only, there was a significant increase in FVC at 24 weeks (0·40 l, 98·33% CI: 0·02, 0·79; P = 0·03). There was also a difference between groups for distance walked (6MWT) at 16 (P = 0·01) and 24 weeks (P = 0·03). However, the changes in 6MWT for the ST+PR and ST+SM+PR groups individually compared to PR only were not significant at either 16 or 24 weeks (P = 1·0 and 0·2; P = 0·8 and 0·4 respectively). There were no differences between groups for the SGRQ or HAD.
Table 2.
Mean change in outcome measures and 95% CI compared to baseline# by intervention group at 16 and 24 weeks [intention-to-treat (ITT)]
| Assessment week | ||||||
|---|---|---|---|---|---|---|
| 16 | 24 | |||||
| Group† | PR | ST+PR | ST+SM+PR | PR | ST+PR | ST+SM+PR |
| Systolic BP | −3·6 (−13·5, 6·3) | −10·6 (−19·6, −1·5) | −8·3 (−20·5, 3·8) | −7·1 (−17·3, 3·0) | −6·9 (−15·8, 2·1) | −10·1(−25·6, 5·4) |
| Diastolic BP | −3·5 (−12·6, 5·6) | −7·7 (−17·1, 1·8) | −4·7 (−13·5, 4·2) | −4·7 (−13·3, 4·0) | −8·1 (−17·8, 1·5) | −8·0 (−19·6, 3·6) |
| FEV1 (l) | −0·042 (−0·113, 0·029) | −0·021 (−0·115, 0·072) | −0·020 (−0·136, 0·096) | −0·077 (−0·164, 0·011) | −0·089 (−0·175, −0·003) | −0·020 (−0·144, 0·104) |
| FVC (l) | 0·10 (−0·14, 0·35) | 0·45 (0·13, 0·77) | 0·37 (0·22, 0·53) | 0·10 (−0·14, 0·34) | 0·32 (−0·05, 0·68) | 0·53* (0·26, 0·81) |
| SGRQ | −4·6 (−9·8, 0·5) | −0·7 (−7·2, 5·8) | −3·3 (−16·3, 9·7) | −8·1 (−14·6, −1·6) | 1·0 (−3·5, 5·5) | −4·3 (−19·9, 11·3) |
| HAD anxiety | −0·1 (−1·5, 1·3) | −0·3 (−2·4, 1·8) | 0·5 (−0·7, 2·0) | −0·9 (−2·8, 1·1) | −0·8 (−3·7, 2·2) | 0·2 (−1·3, 1·7) |
| HAD depression | −0·9 (−1·7, −0·2) | −1·3 (−3·8, 1·1) | −1·9 (−4·2, 2·2) | −1·3 (−2·1, −0·4) | −0·8 (−2·7, 1·2) | −0·7 (−4·4, 3·1) |
| 6MWT (m) | 22·7 (−6·1, 51·4) | 5·8 (−25·1, 36·7) | 51·7 (29·8, 73·6) | 12·1 (−18·0, 42·2) | −16·4 (−55·1, 22·2) | 35·0 (−1·5, 71·5) |
See Table 1 for baseline values.
Pulmonary rehabilitation (PR): group 1; soft tissue therapy+pulmonary rehabilitation (ST+PR): group 2; soft tissue therapy+spinal manipulation+pulmonary rehabilitation (ST+SM+PR): group 3.
BP: blood pressure; FEV1: forced expiratory volume in 1st second; FVC: forced vital capacity; SGRQ: St George’s respiratory questionnaire; HAD: hospital anxiety and depression scale; 6MWT: 6-minute walking test.
Statistically significantly different (P = 0·04).
Table 3.
Mean difference in change and 98·33% CI in outcome measures between groups at 16 and 24 weeks [intention-to-treat (ITT)] ‡
| Group comparison† | |||||||
|---|---|---|---|---|---|---|---|
| 16 weeks | |||||||
| Outcome measure | ST+PR vs PR | P value* | ST+SM+PR vs PR | P value* | ST+SM+PR vs ST+PR | P value* | P value+ |
| Systolic BP | −5·4 (−2·0, 9·1) | −1·0 (−12·3, 10·3) | 4·4 (−10·3, 19·1) | 0·7 | |||
| Diastolic BP | −3·6 (−15·4, 8·3) | 1·0 (−9·1, 11·1) | 4·6 (−8·7, 17·9) | 0·4 | |||
| FEV1 (l) | 0·021 (−0·103, 0·145) | 0·022 (−0·117, 0·161) | 0·001 (−0·148, 0·151) | 0·9 | |||
| FVC (l) | 0·32 (−0·10, 0·74) | 0·26 (−0·07, 0·58) | −0·06 (−0·42, 0·29) | 0·1 | |||
| SGRQ | 5·0 (−6·1, 16·0) | −1·0 (−15·6, 13·6) | −6·0 (−23·6, 11·6) | 0·5 | |||
| HAD anxiety | −0·12 (−2·94, 2·70) | 0·53 (−2·01, 3·07) | 0·64 (−2·88, 4·17) | 0·9 | |||
| HAD depression | 0·63 (−1·73, 2·98) | −0·04 (−3·17, 3·10) | −0·66 (−4·25, 2·92) | 0·8 | |||
| 6MWT (m) | −15·3 (−62·6, 31·9) | 1·0 | 33·0 (−11·3, 77·2) | 0·2 | 48·3 (8·9, 87·6) | 0·01 | 0·01 |
| Group comparison† | ||||||||
|---|---|---|---|---|---|---|---|---|
| 24 weeks | ||||||||
| Outcome measure | ST+PR vs PR | P value* | ST+SM+PR vs PR | P value* | ST+SM+PR vs ST+PR | P value* | P value+ | |
| Systolic BP | 2·0 (−9·5, 13·5) | 1·3 (−12·7, 15·5) | −0·7 (−14·7, 13·4) | 0·9 | ||||
| Diastolic BP | −2·7 (−15·2, 9·8) | −0·8 (−9·9, 8·3) | 1·9 (−11·6, 15·4) | 0·9 | ||||
| FEV1 (l) | −0·012 (−0·147, 0·122) | 0·056 (−0·106, 0·220) | 0·069 (−0·089, 0·227) | 0·6 | ||||
| FVC (l) | 0·16 (−0·29, 0·62) | 1·0 | 0·40^ (0·02, 0·79) | 0·03 | 0·24 (−0·23, 0·71) | 0·6 | 0·04 | |
| SGRQ | 10·9^ (−0·4, 22·1) | −0·1 (−16·0, 15·7) | −11·0 (−29·5, 7·5) | 0·07 | ||||
| HAD anxiety | 0·29 (−3·62, 4·20) | 0·41 (−2·71, 3·52) | 0·12 (−4·21, 4·44) | 0·9 | ||||
| HAD depression | 1·38 (−0·90, 3·66) | 0·63 (−3·07, 4·32) | −0·75 (−4·81, 3·30) | 0·3 | ||||
| 6MWT (m) | −23·9 (−77·0, 29·2) | 0·8 | 34·6 (−20·7, 89·8) | 0·4 | 58·4 (4·70, 112·2) | 0·02 | 0·03 | |
Fitted as an ANCOVA with baseline as covariate with standard errors calculated using a non-parametric bootstrap.
Pulmonary rehabilitation (PR): group 1; soft tissue therapy+pulmonary rehabilitation (ST+PR): group 2; soft tissue therapy+spinal manipulation+pulmonary rehabilitation (ST+SM+PR): group 3; values are mean and (standard error).
BP: blood pressure; FEV1: forced expiratory volume in 1st second; FVC: forced vital capacity; SGRQ: St George’s respiratory questionnaire; HAD: hospital anxiety and depression scale; 6MWT: 6-minute walking test.
For difference between groups corrected for multiple comparisons using Bonferroni correction for three comparisons.
For difference between all three groups.
Above minimum clinically important difference (MCID).
As there is no accepted MCID for change in spirometry measurements following non-pharmacological intervention, we adopted the threshold used for a meaningful response to bronchodilator medication55 and set our threshold for a meaningful clinical change at >200 ml or >12% of baseline for FVC. The minimum increase in the distance walked on a 6MWT, considered to represent a clinical effect in patients with COPD, is 35 m or 10% of baseline.56 Table 4 lists the proportion of participants in each group (%) that showed statistically significant improvements from baseline above the MCID. There was a significant difference between groups for percentage above MCID for FVC at 16 and 24 weeks (P = 0·04 and 0·03 respectively). The NNT for reaching the MCID for FVC was 2·05 (1·41, 11·87) at 16 weeks and 1·80 (1·32, 6·82) at 24 weeks favoring ST+SM+PR over PR only. There was no significant difference between groups for percentage above MCID for 6MWT at 16 (P = 0·07) and 24 weeks (P = 0·7).
Table 4.
Percentage of participants (CI) with a change in forced vital capacity (FVC) from baseline above the minimum clinically important difference (MCID)^
| PR (n = 15) | ST+PR (n = 9) | ST+SM+PR (n = 9) | ||||
|---|---|---|---|---|---|---|
| Weeks | 16 | 24 | 16 | 24 | 16 | 24 |
| FVC | 40 (16, 68) | 33 (12, 62) | 78 (40, 97) | 67 (30, 93) | 89 (52, 100) | 89 (52, 100) |
MCID: minimum clinically important difference; FVC: forced vital capacity.
Pulmonary rehabilitation (PR): group 1; soft tissue therapy+pulmonary rehabilitation (ST+PR): group 2; soft tissue therapy+spinal manipulation+pulmonary rehabilitation (ST+SM+PR): group 3; values are mean and (standard error).
There were no major or moderate AEs42,43 reported following the administration of 131 ST and 272 SM interventions. Two mild AEs were reported by participants in the ST+PR group. Both were isolated events and consisted of muscle soreness that lasted for 48 hours following intervention, resolving without the need for additional medical intervention. The two participants went on to complete the full number of interventions without further incident.
Discussion
The results reported in this trial support the proposition that adding MT (ST and SM) to a PR exercise program for people with COPD produces benefits in lung function that continue after these interventions have been withdrawn. With an NNT of 1·80 at 24 weeks these improvements could also be considered as clinically significant. As the first study to report on the ongoing effects of combining MT and exercise, we believe it offers a clear opportunity to study the clinical implications of the combination.
Standard PR (PR only in this trial) is generally not recognized for delivering clinically meaningful increases in pulmonary function measures.10,57 The increase in FVC reported here for the ST+SM+PR group 8 weeks after all intervention had ceased (24 weeks) is therefore a unique finding. The pattern of clinically significant improvements in lung function reported here suggest that further investigation of this combination of interventions is warranted for people with COPD. Despite improvements in lung function there were no improvements in quality of life measures such as the SGRQ or HAD.
It is possible that SM+PR might be sufficient to produce results similar to the combination of ST+SM+PR. However, our rationale for using ST+SM and not SM alone (with PR) is based on the premise that to effect a change in lung function, it is beneficial to target not only the joints of the thoracic cage but also the soft tissues associated with those joints.
What could be the mechanism underlying the increase in FVC produced by combining MT (ST and SM) and PR? We have previously suggested that a synergistic effect, resulting from the combination of these interventions, is the most likely cause of the increases.41 This explanation relies on respiratory muscle remodeling and its role in maintaining CWR.58 Respiratory muscle remodeling is the product of respiratory muscle fatigue and dynamic hyperinflation and results in an uncoupling in the relationship between respiratory effort and tidal volume.16 This phenomenon, referred to as neuromechanical uncoupling,20 occurs when respiratory efforts are not rewarded appropriately in terms of tidal volume generated during each breath21 and is considered a strong determinant of dyspnea in COPD.16,20,59
Neuromechanical uncoupling is also responsible for an increase in end-expiratory lung volume and a progressive reduction in inspiratory capacity under load.60 Breathing at higher lung volumes increases the effort of breathing.21 In an attempt to optimize their force-generating capacity and increase fatigue resistance, the respiratory muscles adapt by dropping sarcomeres and contracting.58 This finding is supported by the presence of upregulation of IL-6 gene expression in respiratory muscles such as the intercostal muscles.61 As IL-6 expression is induced by muscle contraction,62 upregulation is seen as evidence that an adaptive response to the increase in contractile demand has occurred.61 The resultant ‘fragile balance’ between respiratory muscle overload and respiratory muscle adaptation63 produces a change in the thixotropic properties of these muscles. This change perpetuates the increase in CWR.58,64
When applied to the thoracic cage MT may release some of the rib cage’s natural elastic recoil, temporarily easing one of the elements perpetuating CWR. With a ‘temporary’ improvement in chest wall recoil, the effort of breathing becomes less. Under these conditions, low intensity exercise could act as a catalyst for shifting the ‘fragile balance’ between respiratory muscle overload and respiratory muscle adaptation in a favorable direction. This would benefit muscle remodeling, thereby relieving part of the mechanism underlying neuromechanical uncoupling. Given sufficient time and the right configuration of MT and exercise, the process has the potential to improve respiratory function. We hypothesize that the increases in FVC reported here indicate that this process may have occurred.
A possible reason as to why PR alone (standard PR) does not produce similar increases in FVC is because low intensity exercise, performed with a rigid chest wall, may not be able to sufficiently shift the balance between respiratory muscle overload and respiratory adaptation that is typical of COPD. This is highlighted by results from two studies that reported an immediate worsening of air trapping in people with COPD following the administration of soft tissue techniques that appeared to shift this balance in the wrong direction.31,32
It is worth noting that, notwithstanding the screening process for contraindications conducted before MT being administered, no moderate or major AEs were reported in any participant who received MT.
Limitations and future directions
While these results are encouraging, they need to be considered in light of the following limitations. A sample size calculation was not used in this trial because Phase II trials are usually designed to test a new procedure or new combination of procedures in preparation for larger Phase III trials.65 For this study, recruitment did not reach the pragmatically planned complement of 45. Enrollment was terminated early for two reasons. First, a slower than expected flow of patients through the hospital’s PR unit due to staffing issues resulted in an unexpectedly protracted recruitment period. Second, a higher than predicted number of potential participants had to be excluded from the trial as they were above the permissible age limit. The decision to terminate recruitment early was based on time constraints imposed by the extension granted to the original ethics approval. Interpretation of the results must therefore be restrained in light of any consideration regarding adequate statistical power.
The intervention ‘dose’ may not have been ideal. Two sessions per week (to align with the hospital’s PR schedule) may not have been the optimal dose of MT for people with COPD. Some PR guidelines recommend three exercise sessions per week as a minimum.2 Had we administered MT and exercise three times per week, there may have been a different level of synergy between the interventions. Restricting measurements of ventilation to FEV1 and FVC limited our ability to analyze the effect of the interventions on lung function in more depth.
It is possible that the results were affected by the groups not being totally matched. However, gender and anxiety (the two characteristics that were different at baseline between the Groups) were not significant predictors of any outcome measures after including baseline values in the ANCOVA.
In light of the results reported in this trial, further research in the field, such as a larger phase III clinical trial, is warranted.
Conclusion
This study provides some evidence that MT benefits lung function in people with COPD. Although the underlying mechanisms responsible for this outcome are not yet fully understood, the most likely explanation is the synergistic effect resulting from combining MT and PR. These results support the call for a larger trial that measures the effect of MT on COPD.
Disclaimer Statements
Contributors Roger Engel was involved in conceiving, designing, and conducting the trial and in preparing the final manuscript. Peter Gonski was involved in designing and conducting the trial and in preparing the final manuscript. Ken Beath was involved in the statistical analysis of the results and in preparing the final manuscript. Subramanyam Vemulpad was involved in designing the trial and in preparing the final manuscript.
Funding The project was funded by Macquarie University's Higher Degree Research Fund.
Conflicts of interest The authors declare that they have no conflicts of interest.
Ethics approval The trial was approved by the Human Ethics committees of Macquarie University (HE23MAR2007-D05054) and the South Eastern Sydney and Illawarra Area Health Service (07/41).
References
- 1.Australian Lung Foundation . The COPDX Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2012. Version 2.34; 2012 [cited 2013 Nov 22]. Available from: http://www.copdx.org.au/ [DOI] [PubMed] [Google Scholar]
- 2.Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–413. [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. [DOI] [PubMed] [Google Scholar]
- 4.National Clinical Guideline Centre . Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. 2004 [updated 2010; cited 2013 Aug 16]. Available from: http://www.ncgc.ac.uk/Guidelines/Published/20. [Google Scholar]
- 5.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006. [Update of Cochrane Database Syst Rev. 2002; (3):CD003793; PMID:12137716]. [DOI] [PubMed] [Google Scholar]
- 6.Pitta F, Troosters T, Probst VS, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134:273–80. [DOI] [PubMed] [Google Scholar]
- 7.Laviolette L, Bourbeau J, Bernard S, Lacasse Y, Pepin V, Breton MJ, et al Assessing the impact of pulmonary rehabilitation on functional status in COPD. Thorax. 2008;63:115–21. [DOI] [PubMed] [Google Scholar]
- 8.Oga T, Nishimura K, Tsukino M, Hajiro T, Sato S, Ikeda A, et al Longitudinal changes in health status using the chronic respiratory disease questionnaire and pulmonary function in patients with stable chronic obstructive pulmonary disease. Qual Life Res. 2004;13:1109–16. [DOI] [PubMed] [Google Scholar]
- 9.Bestall JC, Paul EA, Garrod R, Garnham R, Jones RW, Wedzicha AJ. Longitudinal trends in exercise capacity and health status after pulmonary rehabilitation in patients with COPD. Respir Med. 2003;97:173–80. [DOI] [PubMed] [Google Scholar]
- 10.Celli BR, MacNee W, ATS/ERS Task Force . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. [DOI] [PubMed] [Google Scholar]
- 11.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–77. [DOI] [PubMed] [Google Scholar]
- 12.Sewell L, Singh SJ, Williams JE, Collier R, Morgan MD. Can individualized rehabilitation improve functional independence in elderly patients with COPD? Chest. 2005;128:1194–200. [DOI] [PubMed] [Google Scholar]
- 13.Reardon J, Casaburi R, Morgan M, Nici L, Rochester C. Pulmonary rehabilitation for COPD. Respir Med. 2005;99:S19–27. [DOI] [PubMed] [Google Scholar]
- 14.Kesten S, Casaburi R, Kukafka D, Cooper CB. Improvement in self-reported exercise participation with the combination of tiotropium and rehabilitative exercise training in COPD patients. Int J Chron Obstruct Pulmon Dis. 2008;3:127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theander K, Jakobsson P, Jorgensen N, Unosson M. Effects of pulmonary rehabilitation on fatigue, functional status and health perceptions in patients with chronic obstructive pulmonary disease: a randomized controlled trial. Clin Rehabil. 2009;23:125–36. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell DE, Ora J, Webb KA, Laveneziana P, Jensen D. Mechanisms of activity-related dyspnea in pulmonary diseases. Respir Physiol Neurobiol. 2009;167:116–32. [DOI] [PubMed] [Google Scholar]
- 17.Pride NB, Macklem PT. Lung mechanics in disease. In: Fishman AP, editor. Handbook of physiology. Bethesda, MD: American Physiological Society; 1986. p. 659–92. [Google Scholar]
- 18.Ranieri VM, Giuliani R, Cinnella G, Pesce C, Brienza N, Ippolito EL, et al Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilation. Am Rev Respir Dis. 1993;147:5–13. [DOI] [PubMed] [Google Scholar]
- 19.Izumizaki M, Iwase M, Ohshima Y, Homma I. Acute effects of thixotropy conditioning of inspiratory muscles on end-expiratory chest wall and lung volumes in normal humans. J Appl Physiol. 2006;101:298–306. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD. 2007;4:225–36. [DOI] [PubMed] [Google Scholar]
- 21.Pepin V, Saey D, Laviolette L, Maltais F. Exercise capacity in chronic obstructive pulmonary disease: mechanisms of limitation. COPD. 2007;4:195–204. [DOI] [PubMed] [Google Scholar]
- 22.Ranieri VM, Giuliani R, Mascia L, Grasso S, Petruzzelli V, Bruno F, et al Chest wall and lung contribution to the elastic properties of the respiratory system in patients with chronic obstructive pulmonary disease. Eur Respir J. 1996;9:1232–39. [DOI] [PubMed] [Google Scholar]
- 23.Aliverti A, Kayser B, Macklem PT. A human model of the pathophysiology of chronic obstructive pulmonary disease. Respirology. 2007;12:478–85. [DOI] [PubMed] [Google Scholar]
- 24.Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol. 1993;41:705–21. [DOI] [PubMed] [Google Scholar]
- 25.Homma I, Hagbarth KE. Thixotropy of rib cage respiratory muscles in normal subjects. J Appl Physiol. 2000;89:1753–58. [DOI] [PubMed] [Google Scholar]
- 26.Izumizaki M, Shibata M, Homma I. Factors contributing to thixotropy of inspiratory muscles. Respir Physiol Neurobiol. 2004;140:257–64. [DOI] [PubMed] [Google Scholar]
- 27.Langer D, Hendriks E, Burtin C, Probst V, van der Schans C, Paterson W, et al A clinical practice guideline for physiotherapists treating patients with chronic obstructive pulmonary disease based on a systematic review of available evidence. Clin Rehabil. 2009;23:445–62. [DOI] [PubMed] [Google Scholar]
- 28.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogduk N, Mercer S. Selection and application of treatment. In: Refshauge K, Gass E, editors. Musculoskeletal physiotherapy: clinical science and evidence-based practice, 2nd edn. Oxford, UK: Butterworth-Heinemann; 2004. p. 245–57. [Google Scholar]
- 30.Engel R, Vemulpad S. The role of spinal manipulation, soft-tissue therapy, and exercise in chronic obstructive pulmonary disease: a review of the literature and proposal of an anatomical explanation. J Altern Complement Med. 2011;17:797–801. [DOI] [PubMed] [Google Scholar]
- 31.Noll DR, Degenhardt BF, Johnson JC, Burt SA. Immediate effects of osteopathic manipulative treatment in elderly patients with chronic obstructive pulmonary disease. J Am Osteopath Assoc. 2008;108:251–9. [PubMed] [Google Scholar]
- 32.Noll DR, Johnson JC, Baer RW, Snider EJ. The immediate effect of individual manipulation techniques on pulmonary function measures in persons with chronic obstructive pulmonary disease. Osteopath Med Prim Care. 2009;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noll DR, Shores J, Bryman PN, Masterson EV. Adjunctive osteopathic manipulative treatment in the elderly hospitalized with pneumonia: a pilot study. J Am Osteopath Assoc. 1999;99:143–6. [DOI] [PubMed] [Google Scholar]
- 34.Noll DR, Shores JH, Gamber RG, Herron KM, Swift J. Benefits of osteopathic manipulative treatment for hospitalized elderly patients with pneumonia. J Am Osteopath Assoc. 2000;100:776–82. [PubMed] [Google Scholar]
- 35.Noll DR, Degenhardt BF, Morley TF, Blais FX, Hortos KA, Hensel K, et al Efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized patients with pneumonia: a randomized controlled trial. Osteopath Med Prim Care. 2010;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howell RK, Allen TW, Kappler RE. The influence of osteopathic manipulative therapy in the management of patients with chronic obstructive lung disease. J Am Osteopath Assoc. 1975;74:757–60. [PubMed] [Google Scholar]
- 37.Masarsky CS, Weber M. Chiropractic management of chronic obstructive pulmonary disease. J Manipulative Physiol Ther. 1988;11:505–10. [PubMed] [Google Scholar]
- 38.Dougherty PE, Engel RM, Vemulpad S, Burke J. Spinal manipulative therapy for elderly patients with chronic obstructive pulmonary disease: a case series. J Manipulative Physiol Ther. 2011;34:413–7. [DOI] [PubMed] [Google Scholar]
- 39.Engel RM, Vemulpad S, Beath K. Short term effects of combining manual therapy and exercise on moderate chronic obstructive pulmonary disease (COPD): a randomized pilot study. J Manipulative Physiol Ther. 2013;36:490–6. [DOI] [PubMed] [Google Scholar]
- 40.Zanotti E, Berardinelli P, Bizarri C, Civardi A, Manstretta A, Rossetti S, et al Osteopathic manipulative treatment effectiveness in severe chronic obstructive pulmonary disease: a pilot study. Complement Ther Med. 2012;20:16–22. [DOI] [PubMed] [Google Scholar]
- 41.Engel RM, Vemulpad S. The effect of combining manual therapy with exercise on the respiratory function of normal individuals: a randomized control trial. J Manipulative Physiol Ther. 2007;30:509–13. [DOI] [PubMed] [Google Scholar]
- 42.Carnes D, Mars TS, Mullinger B, Froud R, Underwood M. Adverse events and manual therapy: a systematic review. Man Ther. 2010;15:355–63. [DOI] [PubMed] [Google Scholar]
- 43.Carnes D, Mullinger B, Underwood M. Defining adverse events in manual therapy: a modified Delphi consensus study. Man Ther. 2010;15:2–6. [DOI] [PubMed] [Google Scholar]
- 44.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2013 [updated 2013 Feb; cited 2013 Aug 20]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. [Google Scholar]
- 45.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al Standardisation of spirometry. Eur Respir J. 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- 46.Jones PW, Forde Y. St George’s Respiratory Questionnaire manual. London: University of London; 2008. [Google Scholar]
- 47.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85B:25–31. [DOI] [PubMed] [Google Scholar]
- 48.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–7. [DOI] [PubMed] [Google Scholar]
- 49.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale – a review of validation data and clinical results. J Psychosom Res. 1997;42:17–41. [DOI] [PubMed] [Google Scholar]
- 50.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 51.American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 52.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256–70. [DOI] [PubMed] [Google Scholar]
- 53.Alison J, Barrack C, Cafarella P, Frith P, Hanna C, Hill C. The Pulmonary Rehabilitation Toolkit on behalf of The Australian Lung Foundation. 2009 [cited 2013 Aug 20]. Available from: www.pulmonaryrehab.com.au. [Google Scholar]
- 54.StataCorp Stata statistical software: release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 55.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. [DOI] [PubMed] [Google Scholar]
- 56.Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32:637–43. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Aymerich J, Serra I, Gomez FP, Farrero E, Balcells E, Rodríguez DA, et al Physical activity and clinical and functional status in COPD. Chest. 2009;136:62–70. [DOI] [PubMed] [Google Scholar]
- 58.Clanton TL, Levine S. Respiratory muscle fiber remodeling in chronic hyperinflation: dysfunction or adaptation? J Appl Physiol. 2009;107:324–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Donnell DE, Webb KA. Physiological basis of dyspnoea. In: Donner CF, Ambrosino N, Goldstein R, editors. Pulmonary rehabilitation, Ch. 13. London, UK: Oxford University Press; 2005. [Google Scholar]
- 60.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:770–7. [DOI] [PubMed] [Google Scholar]
- 61.Casadevall C, Coronell C, Ramirez-Sarmiento AL, Martinez-Llorens J, Barreiro E, Orozco-Levi M, et al Upregulation of pro-inflammatory cytokines in the intercostal muscles of COPD patients. Eur Respir J. 2007;30:701–7. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–8. [DOI] [PubMed] [Google Scholar]
- 63.Orozco-Levi M. Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation? Eur Respir J. 2003;46:41–51s. [DOI] [PubMed] [Google Scholar]
- 64.Caron MA, Debigare R, Dekhuijzen PN, Maltais F. Comparative assessment of the quadriceps and the diaphragm in patients with COPD. J Appl Physiol. 2009;107:952–61. [DOI] [PubMed] [Google Scholar]
- 65.Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]